Abstract
In most versions of theories of the segmentation of the vertebrate head, a premandibular segment is present rostral to the jaw-forming mandibular segment. These theories posit that in ancient fishes this segment included a gill and a gill-supporting skeleton, which then was modified to support the anterior brain. However, we find no recent evidence for existence of such a premandibular segment. Rather, new findings from studies of fate mapping and gene expression show that the “premandibular” territory is in fact the maxillary region of the mandibular arch. A signaling cascade, beginning with dorsal midline mesoderm in the gastrula and relayed through neural ectoderm and then oral ectoderm, greatly expands the skeletal derivatives of maxillary neural crest in a manner fully consistent with the Gans–Northcutt theory of the vertebrate new head.
Introduction
An idea stemming from the 19th Century is that the fundamental organization of the vertebrate head is segmental—vertebrates are segmented “from the tip of the head to the hindmost parts” (Jacobson 1993, 71). The quotation refers to mesodermal head somitomeres, proposed by Jacobson and his colleagues to be continuous with somites in the trunk and tail. Whereas head somitomeres may not exist at all (Kimmel et al. 2001; Olsson et al. 2005; Kuratani and Ota 2007;), certainly the head of a vertebrate includes segmentally organized sets of structures–notably the rhombomeres of the hindbrain and the “arches” of the pharyngeal walls. In fishes, a segmental set of pharyngeal arches form the gills and their supports. In gnathostomes, a prominent anterior pharyngeal arch, generally known as the mandibular arch, or pharyngeal arch 1, forms the upper and lower elements of the jaw. In this essay, we focus on the pharyngeal arches, examining and rejecting the hypothesis that one or more arches (pharyngeal segments) exist ahead of the segment that makes jaws – ahead of arch 1. We refer to this hypothesis as the “arch-0” problem and suggest that the problem has several causes.
As we shall argue, causes of the arch-0 problem include untoward idealism about the morphology of our ancient vertebrate and chordate ancestors, and related to this, a very old misunderstanding about the evolutionarily early nature of segmentally organized series of structures, namely that segments were “initially uniform”. An unfortunate feature of the arch-0 problem is that it has created confusion and misunderstanding in the literature about the geographical boundaries of the first pharyngeal arch. We will show that understanding the geography correctly is of central importance in solving the arch-0 problem, hence we examine this issue in some detail, including fate mapping of the derivatives of the first arch and its delineation by gene expression. Finally, we propose that the principal reason for postulating existence of an arch 0 is that the early workers, while aware of inductive interactions in neurocranial development (de Beer 1937), had not—could not have—appreciated the full consequences of cell-to-cell signaling that occurs in the embryo. The interactions of interest here begin during gastrulation between the dorsal midline mesoderm and the neural ectoderm, and are eventually relayed through the oral ectoderm to the skeletogenic neural crest. A consequence of this cascade, as foreseen in the “new head” theory of Gans and Northcutt (1983), is to greatly expand skeletal development in the anterior part of the head.
To be clear at the outset, we equate a pharyngeal arch with a pharyngeal segment or metamere, and we use this language in a restricted sense to mean that foremost in our understanding of segmentation is iteration of structure (Bateson 1894). To be segmentally organized, a series of structures must be present in repeated copies along an axis, generally the anterior-to-posterior axis. The repetitious elements, the serial or segmental homologs, do not need be perfect copies of one another for the definition to apply, and Bateson coined the terms homeosis and heterosis to describe conditions in which the homologs are substantially similar or different from one another in structure and function. We note that even though Bateson's definition is restricted to morphology, we do not think it useful to include this particular restriction in our understanding of segmental patterning; to do so would be retrogressive. Importantly, early patterns of gene expression often show clearly iterated segmental patterning in instances where later morphological change obscures the patterning.
Arch 0: An hypothesis stemming from an unrealized, idealized view of our gill-bearing ancestors
“If then the trabeculae represent visceral arch cartilages, they must be the skeletal supports of a whilom [ancient] premandibular arch, separating the originally anterior mouth from a pair of mandibular visceral clefts.” (de Beer 1937, 376)
The trabeculae cranii (“little rods of the cranium”) are transient cartilaginous elements present in the mesenchymal tissue in the roof of the embryonic oral cavity, in close association with the rudiments of ventral midbrain and forebrain that lie just above. de Beer, perhaps the most influential student of skull development and evolution in the 20th century, thought the trabeculae were derived evolutionarily from ancient gill supports. The hypothesis, meaning to explain the existence of trabeculae and other skeletal elements in the anterior part of the head, was not original, but came from one of the most influential students of skull development and evolution in the 19th century, TH Huxley (reviewed by de Beer 1937) and was championed by other important authors as well. The theory had strong support.
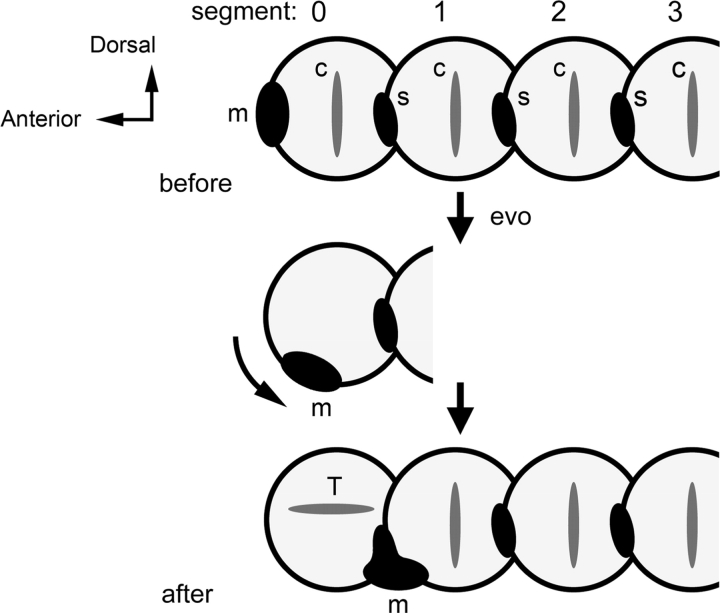
The mouth, a passage for inward flow of water for feeding and respiration, was, for arch 0 advocates, “originally anterior” (Fig. 1, before). Outward flow in the pharynx is through gill slits, segmentally arranged, and formed where pharyngeal pouches, the out pockets of the endodermal pharyngeal lining, meet pharyngeal clefts, the in pockets of overlying ectoderm. Then the mouth relocates to a new posterior-ventral position, and an ingenious part of the theory is that in its new location the mouth takes over a bilateral pair of clefts (Fig. 1, after). These clefts separate the premandibular arch, or arch 0, located immediately anterior to the newly positioned mouth and the mandibular arch, or arch 1, located immediately posterior to the mouth. The arch-0 skeletal element loses its gill bearing function, and is “pressed into the service of the brain-case” (de Beer 1937, 375).
Fig. 1.
The arch 0-hypothesis. A caricature of a segmented worm-like ancestor, as described by de Beer (1937). c, cartilaginous gill support; Evo, evolution; m, mouth; s, gill slit; T, trabecula, supporting the brain and evolving from the arch-0 cartilage. More anatomically detailed drawings of the hypothetical ancestor also are available from other sources, notably Bjerring 1977; Jollie 1984; Kuratani et al. 1997. These drawings differ substantially from one another.
We note here, and will take up more fully later in this article, that in today's literature the same preoral region posited in this case as the premandibular arch, arch 0, is more generally taken to be a subregion of arch 1, termed maxillary arch 1. The region posterior to the mouth that de Beer calls the mandibular arch, is more generally the mandibular portion of arch 1, maxillary and mandibular processes together making up the whole of the mandibular arch, arch 1. Understanding arch-1 geography correctly, for which new studies of gene expression are critical, is helpful for solving the arch-0 problem. However, just where in the head of recent vertebrates different proponents of arch-0 theory assume that arch 0 is located is not uniform. For example, Kuratani and his associates have provided the most recent argument in the literature for arch 0 (Kuratani et al. 1997) and, a few years later, describe a premandibular “region” in the chick embryo as being not the same as maxillary arch 1, but located just rostral to maxillary arch 1 (Shigetani et al. 2000). We note that in the latter paper Shigetani et al (2000), make no reference to a premandibular “arch” or “segment,” but simply to a premandibular region, and in later reviews Kuratani et al. (2001, 2004) are explicit that this “region” is not to be equated with “arch.” It could well be that the earlier enthusiasm for arch 0 (Kuratani et al. 1997) cooled down a few years later. The identity of this premandibular region is not completely clear. Lee et al. (2004), who carried out detailed fate mapping of the region in question interpret the evidence differently than do Shigetani et al., stating that it contributes to the maxillary prominence, which by our interpretation would be part of arch 1.
A current problem with arch-0 theory, as viewed retrospectively well over a century after initial formulation of the theory, is the lack of any evidence supporting the proposed ancestral state. A century, especially considering the remarkable recent progress in evolutionary developmental biology, provides a lot of time to have obtained supportive evidence for the theory by identifying an ancient creature, a putative ancestor, with the characteristics posited by the theory. New fossils, e.g., soft-bodied ancient vertebrates, myllokunmingiids, recently uncovered in China (Shu et al. 2003a, 2003b; reviewed in Janvier 2007) show segmented pharyngeal walls located rather well behind a region with no signs of overt segmentation, suggesting that if an ancestral creature ever existed that possessed pharyngeal gill-bearing segments in the anterior-most region of the head, it must have existed before vertebrates evolved, not after. As was well known to the early proponents of arch 0, the cephalochordate amphioxus, even though showing somite-derived metamerism of the body wall right up to the anterior tip of the body axis, has a well delimited pharyngeal region beginning only rather posteriorly, behind the oral region, which itself is well back from the anterior end of the head. Hence, amphioxus could not have reflected the primitive condition imagined by the proponents of arch 0. Enteropneusts, hemichordates showing pharyngeal segmentation, including cartilaginous supports of the gills, also have their segmented pharyngeal region well behind other structures, including the prominent proboscis. In particular, the gill slits of enteropneusts line up with anterior-posterior neural markers just as they do in vertebrates (Gerhart et al. 2005). New studies suggest that enteropneusts, among all recent invertebrates, might most closely resemble the chordate ancestor (Gerhart et al. 2005; Rychel and Swalla 2007; Swalla 2007). The morphologies we see are not those that an early advocate of arch-0 theory might have predicted.
Carving out segments: Paleontology provides no evidence for arch 0
A prominent group of paleontologists, working in Stockholm during the major part of the 20th century, argued that comparative anatomy and paleontology of vertebrate material supported the case for arch 0. However, the views of this school were treated with increasing disbelief as the studies progressed. The work began with Stensio in the early part of the 20th century, was then taken up by others including his very well-known and influential student Jarvik (1954, 1980), and then by Jarvik's students, notably Bjerring (1977). The ideas about head segmentation and their history are nicely reviewed by Janvier in his important “Early Vertebrates” (1996). Janvier was trained in the Stockholm environment, and in his book he treats the arch-0 problem with gentle skepticism. More recently, Olsson and his colleagues have examined this body of work as well, focusing on the philosophy of thought lying behind it (Olsson 2005; Olsson et al. 2005).
The disciples of this school saw arches, actually two of them—a terminal arch and a premandibular arch (arch “minus-1” and arch 0, if you like) ahead of the first arch. To “see” these arches in their subject matter, fossil fishes, and recent fishes with primitive skull characteristics, took some invention, understandably necessary if evolution has obliterated a supposed very clear segmental pattern that characterized an early ancestor. The best and most thoroughly documented example of such invention is Jarvik's 1954 study of the fossil fish Eusthenopteron, a very well known sarcopterygian and close relative of the early tetrapods (Fig. 2). Janvier, in his review (1996, 255), stated that this particular fossil provides “the best palaeontological support for full segmentalist theories, in particular those for the premandibular arches.” Jarvik's reconstruction (Fig. 2B) shows the principal skeletal element of arch 0 as a prominent strut similar in form to the arch-1 bone and to the serial set continuing posteriorly. The pattern clearly looks segmental.
Fig. 2.
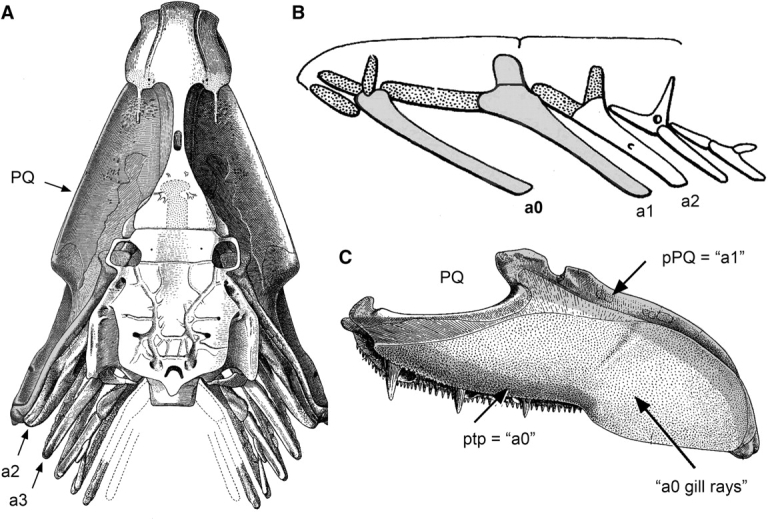
Carving out segments in the Devonian sarcopterygian Eusthenopteron, after Jarvik (1954; see also Jarvik 1980 and review by Janvier 1996). (A) Palatal reconstruction (a view of the mouth roof). (B) Jarvik's imaginative reconstruction, shown in side view with premandibular (a0), mandibular (a1), and hyoid (a2) segments indicated. (C) Reconstruction of the palatoquadrate (PQ, medial aspect). The shaded areas were taken to be derived from gill supports in a0 and a1, as shown in (B) [ptp, pterygoid process (also known as the palatine process); pPQ, the posterior region of the palatoquadate (part of which gives rise to the quadrate bone of the jaw articulation)].
The invention, in this case, was to carve two bones from a single bone, the palatoquadrate, the upper jaw bone shown in Fig. 2A and C. The palatoquadrate is no doubt a single chondral bony plate in the beautifully preserved fossil. The bone has two regions, an anterior region we term (following de Beer) the pterygoid process, and a posterior “quadrate” region. Whereas de Beer supposed the trabecula to be the cartilaginous gill support of arch 0, Jarvik saw the pterygoid process of the palatoquadrate as being the supportive element of the same arch. The posterior quadrate region of the palatoquadrate becomes the supportive element of arch 1. For the reconstruction in Fig. 2B, Jarvik throws away the middle region.
Jarvik thought it completely justifiable to effect this surgery. There is no attempt at cover-up; rather the whole intellectual deconstruction of the fossil is explained in great detail in his original publication (1954). He saw the middle region of the palatoquadrate as a set of gill rays from arch 0 that were fused together. Gill rays can be tossed out of the reconstruction because including them in the drawing only tends to obscure the basic segmental pattern.
Looking at this argument more than 50 years later, we can be more than a little incredulous. Still, we think it useful to have reviewed this story because it points up key problems in scientific inquiry: First, we expect to see arch 0 because of the paradigm in which we make our inquiry, a strictly segmentalist school in this case. In defending head-segmentation theory, Jollie argued (1984, p 326): “The assumption of a segmented head, an assumption that has not been falsified, is and has been a part of the thinking … for over one hundred years … . What does strengthen it is its anatomical (and developmental) explanatory value …. Further, there is, in my opinion, no good alternative hypothesis.” In fact, as we discuss below, the new-head theory of Gans and Northcutt, published a year earlier (1983) provides an excellent alternative hypothesis.
Second, and more importantly, even if a premandibular segment were present in a Eusthenopteron ancestor there is no compelling reason to suspect it was a gill-bearing pharyngeal arch. An error in logic here comes from an old misconception about the nature of segmental series and their origins. Namely, as it was thought, when a series of segments evolves, its components must be “originally uniform” (the quotation is from Goodrich 1930, 217; but essentially all other segmentalists shared this viewpoint). Homogeneity among segments (homeosis, using Bateson's term) would precede any segmental diversification (heterosis) within the series. As we have argued elsewhere (Kimmel et al. 2001), current evidence suggests this proposition cannot be an axiomatic truth. Hox patterning along the anterior-posterior axis precedes, phylogenetically, acquisition of body segmental patterning in invertebrates. This interpretation is independent of how many times segmentation has evolved in parallel (see De Robertis 2008, for a new review of this subject). In other words, during evolution, segmentation is imposed upon a body plan that is already diversified along the primary body axis, the AP axis. The new understanding conforms to the lack of any direct evidence from fossils or comparative anatomy that gill-bearing arches ever existed ahead of arch 2 (reviewed by Janvier 1996, 2007). Arch 0, if it ever existed, must never have been a gill-bearing arch. Jarvik's rationale for carving up the palatoquadrate as he did, is spurious.
Fate-map locations of the first-arch cartilages derived from the neural crest are conserved across gnathostomes
The discussion above shows that two skeletal elements, the pterygoid process of the palatoquadrate (Jarvik 1954) and the trabecula (de Beer 1937), figure prominently in the arch-0 problem, our analysis of which now turns to developmental biology. The trabecula is one of the first cartilages to form in the embryo, and newer work fully supports that this cartilage develops from the neural crest, as de Beer and several other early investigators believed. The trabecula, by its relatively constant position and morphology, as well as by its very early development, has been homologized among gnathostomes as diverse as shark, zebrafish, and mouse in a relatively straightforward way. On the other hand, a prominent part of the argument de Beer (1937) lays out for the existence of arch 0 involves the trabecula of the lamprey, an agnathan, and Kuratani et al. (1997, 2001 ,2004) have much more recently provided argument that the lamprey trabecula may not be homologous to that of gnathostomes. Whereas Huxley, Allis, de Beer, and Kuratani et al., among other authors, postulated that the gnathostome trabecula represents a skeletal element of arch 0, this view was not universal among proponents of arch-0 theory. There was sometimes substantial disagreement as to the assignment of particular skeletal elements to particular head segments (Allis 1923, 1938; de Beer 1931, 1937). Jarvik, for example, placed the trabecula in arch 1, and, as we have seen, considered the pterygoid process of the palatoquadrate to be a key element of arch 0. Jarvik's student Bertmar (1959), who accomplished what is perhaps the best descriptive study of head skeletal development in a bony fish, followed Jarvik's view exactly.
The postulates that either the trabecula or the pterygoid process, or both, derive from arch 0 might be tested directly by fate mapping. Accurate fate maps were not available for any species at the times arch-0 proposals were made, but they are now, and the results are quite striking. Recently, fate mapping, achieved by vitally marking postmigratory neural-crest cells within the pharyngeal arches and examining the resulting labeling in cartilages, show that the trabeculae derive from cells within maxillary arch 1, i.e., just that region proposed as the premandibular arch by Allis and de Beer. Other skeletal fates deriving from this domain include the maxillary bone in at least chickens (Lee et al. 2004) and zebrafish (Eberhart et al. 2006), and, notably in zebrafish, the pterygoid process of the palatoquadrate.
In the studies in our laboratory, the labeling was done by marking single cells in transgenic embryos. The transgene, fli1: EGFP, reports the location of the neural crest at the time of the labeling, and then remains expressed after the cartilages develop, hence providing for increased accuracy of the mapping (Supplementary Fig. 1). The resulting fate maps for the first and second arches (Fig. 3) show a general correspondence to one another, supporting segmental homology. Further, there is a topographic conservation between fate-map positions in either arch to the positions of the resulting cartilages later. For example, the ventral cartilages usually taken to be segmental homologs, Meckel's cartilage in arch 1 and the ceratohyal cartilage in arch 2, both map ventrally in their respective segments. The dorsal hyosymplectic cartilage in arch 2 comes from a dorsal position in arch 2. The fate-map domains in arch 1 appear more extensively overlapped than in arch 2. The maps of the pterygoid process and trabecula/ethmoid plate of the anterior neurocranium show essentially complete overlap in an anterior region of arch 1 (supporting statistics in Supplementary Fig. 2). This anterior region, located just above the invaginated oral ectoderm of the stomodeum, is the zebrafish's maxillary arch 1, as supported by gene-expression studies described below. Importantly, the mapping of the maxillary derivatives—the anterior neurocranial elements and the pterygoid process—and the mapping of the more posterior region of the palatoquadrate are statistically separable; the maxillary derivatives are significantly more anterior.
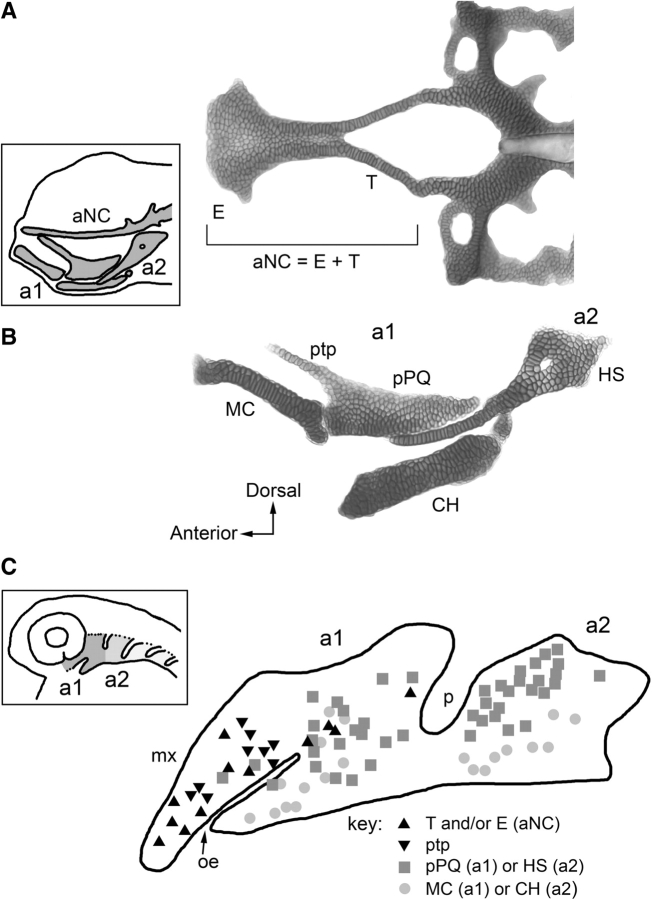
Fig. 3.
Fate map analysis of early larval zebrafish cartilages derived from the first and second pharyngeal arches. (A) Cartilages of the anterior neurocranium. Dissected preparation at 6 days postfertilization, in dorsal view. The boxed inset shows the position of the anterior neurocranium and pharyngeal cartilages in a left side view of the head. (B) Jaw (arch 1) and jaw supporting (arch 2) pharyngeal arch cartilages. (C) Fate map locations of the elements in the pharyngeal arches in the embryo at one day postfertilzation. The boxed inset shows the location of the arches in head, ventral, and posterior to the eye. The symbols in (C) represent individual cells, occasionally small groups of cells marked with a vital dye by electroporation and resulting in labeling of the cartilage elements as indicated in the key. a1, a2, arch 1 and 2; aNC, anterior neurocranium; CH, ceratohyal cartilage; E, ethmoid plate; HS, hyosymplectic cartilage; MC, Meckel's cartilage; mx, condensed crest of the maxillary region of a1; oe, oral ectodermal invagination (stomodeum); p, endodermal pharyngeal pouch 1 (hyomandibular pouch); pPQ, posterior region of the palatoquadrate; ptp, pterygoid process of the palatoquadrate; T, trabecula. Experiments by Mary Swartz, from Crump et al. (2004b, 2006) and Eberhart et al. (2006).
Data reported by Cerny et al. (2004) for axolotls and chickens agree remarkably well with these findings for zebrafish, with the notable exception that in tetrapods the pterygoid process of the palatoquadrate has been lost or greatly reduced. We note also that for reasons of their own choosing, Cerny et al. term maxillary arch 1 the “trabecular condensation,” a naming quite likely to lead to misunderstanding, and that we consider unjustified (see comments by Janvier 2007). Nevertheless, Cerny et al. found that the trabecula derives from cells located within maxillary arch 1, and the palatoquadrate in these animals (corresponding to the zebrafish posterior palatoquadrate) maps largely within mandibular arch 1. Supportive data were also reported by Lee et al. (2004), although their study did not examine labeling of specific cartilages. To the point of the arch-0 problem, Lee et al. interpret their findings rather differently than we or Cerny et al. do, concluding (223) that their “data support the concept that the maxillary prominence is not a derivative of the first pharyngeal arch.” This conclusion and related arguments made by Lee et al. have already been reviewed, and were criticized extensively elsewhere (Depew and Simpson 2006) and we will not dwell further on the study by Lee et al. here. However, we note that in making their conclusion Lee et al. do not seem to be directly addressing arch-0 theory. What they are meaning is perhaps better understood by replacing the last word “arch” in their sentence with “condensation.” The point is that their data show that cells eventually giving rise to the maxillary skeletal elements are not arriving in the maxillary region by a migration from the mandibular region of arch 1.
Hence, we have broad concordance across diverse gnathostomes concerning the skeletal fate map of arch 1. The data showing that the trabecula and, in zebrafish also the pterygoid process, derive from a preoral domain largely ahead of the posterior palatoquadrate, have direct bearing on the arch-0 problem. Mapping of the trabecula is just where de Beer would have predicted for a cartilage derived from arch 0. Nevertheless, we argue that maxillary arch 1 is not arch 0. That the location of the precursors of the trabecula and pterygoid process map within maxillary arch 1, rather than rostral to it, is evidence against any particular version of arch-0 theory that places a gnathostome “premandibular arch” ahead of maxillary arch 1, e.g., within an anterior region well-known in amniotes as the frontonasal prominence. Hence, frontonasal postmigratory crest and the resulting frontonasal prominence cannot, by fate mapping, be arch 0, if arch 0 is to give rise to the trabecula and pterygoid process. That the trabecula and pterygoid process stem from the same domain in zebrafish would seem to eliminate the version of the arch-0 hypothesis advanced by the Swedish school, namely the hypothesis that the pterygoid process comes from arch 0, and the trabecula comes from arch 1.
Domains of expression of Dlx homeobox genes serve to pattern the pharyngeal arches and define their extents
Whereas some proponents of arch-0 theory might argue that maxillary arch 1 is in fact arch 0, gene-expression studies strongly suggest the opposite. Positionally restricted expression domains of developmental regulatory genes control the patterning of the locations and the fates of elements deriving from the pharyngeal arches, as in other embryonic tissues. A large amount of RNA in-situ data for several vertebrate embryos, as well as functional studies in some cases, has accumulated during the past 15 years. Accordingly, our understanding of the patterning of arches is becoming quite refined. For the problem we address here, we can use gene-expression domains to delimit the extents of individual pharyngeal arches, in particular arch 1, with some confidence, as well as to identify subregions within these arches.
Among the very many genes that have been shown to be expressed in the pharyngeal arches, the Dlx homeobox genes are special, as we know from functional analyses, particularly from studies on targeted loss of function in the mouse, recently reviewed quite extensively by Depew and Simpson (2006). The Dlx genes combinatorially control patterning along what primitively is the dorsal-ventral axis of each arch, the axis that in arch 1 separates the maxillary from the mandibular. Here, we restrict our analysis to expression of these genes, first described in the mouse and now extended to other gnathostomes, as we illustrate for the mouse (Qiu et al. 1997; Depew et al. 2002) and zebrafish (Walker et al. 2006; Miller et al. 2007) (Fig. 4). Expression of a linked pair of Dlx homeobox genes, Dlx1 and Dlx2, fill out each of the arches at early developmental times, and expression persists as the maxillary and mandibular prominences develop in arch 1 (Fig. 4A and B). The arches also express two other linked Dlx pairs, Dlx5 and 6, and Dlx3 and 4. For these pairs, expression does not entirely fill the arches as for the Dlx1, 2 pair but expression is nested within the Dlx1, 2 expression domain—confined to inferior territories of the arches in the mouse and zebrafish alike (Fig. 4C and D). Dlx3 expression in arch 1 is within the mandibular region, and not within maxillary arch 1.
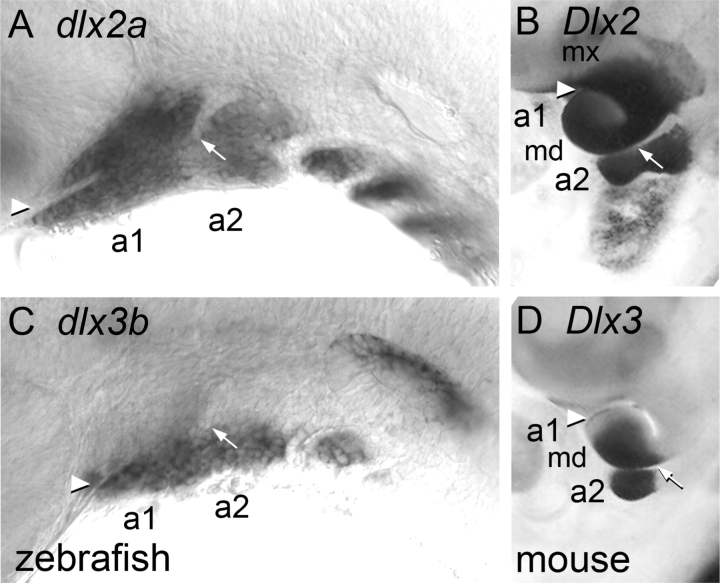
Fig. 4.
Expression of Dlx homeobox genes show features of the geography of the neural-crest-derived territories of the embryonic pharyngeal arches in diverse gnathostomes. (A and C) Zebrafish, with dorsal to the top, anterior to the left. (B and D) Mouse, with anterior to the top and dorsal to the right; with these orientations the positions of maxillary (mx) and mandibular (md) regions of arch 1 correspond between the two species. Dlx2/dlx2a expression in (A) and (B) shows the full extent of the first two arches. Dlx3/dlx3b expression in (C) and (D) is limited within the lower part of each arch (i.e., within mandibular a1 and ventral a2). Arrowhead, position of invaginated oral ectoderm, separating mx and md a1; arrow, position of the bottom of the hyomandibular pouch, separating dorsal and ventral a2. From Miller et al. (2007; zebrafish), and Qiu et al. (1997; mouse).
The similarities among the arches, particularly between arches 1 and 2, in the nesting of Dlx gene expression reveal segmental homologies and hence give strong support to the inference that the mandibular process is a domain within arch 1 and does not comprise the entire extent of arch 1, as incorrectly supposed by de Beer (1937) and more recently by Lee et al. (2004). Maxillary arch 1 is situated above the oral ectoderm in territory expressing Dlx2 but not Dlx3.
Hence, the expression patterns of specific Dlx genes can be used to critically define the extent of each arch, to confirm that the identities of maxillary versus mandibular regions in the first arch correspond across species and to see that these domains have iterated segmentally equivalent regions in arch 2 and the more posterior arches. Arch 0, as occupying the location that de Beer supposed it did, is unsupported. Superimposing results from fate maps onto maps of gene expression shows clearly that the cartilages supposed to have come from arch 0, instead arise from maxillary arch 1.
What about the crest-derived mesenchyme that is located anterior to maxillary arch 1? Gene expression in the frontonasal domain supports a hypothesis that even though we know that frontonasal mesenchyme is crest-derived (Wada et al. 2005; Eberhart et al. 2006, 2008), this region simply is not a part of the segmentally organized pharyngeal arches. In zebrafish, cells from this frontonasal location contribute to the medial ethmoid plate, a position in the cartilaginous anterior neurocranium distinct from the trabecula, but anterior neurocranial skeleton nevertheless (Wada et al. 2005; Eberhart et al. 2006). The zebrafish frontonasal mesenchyme does not express Dlx genes, but as in amniotes, zebrafish frontonasal mesenchyme does express genes shared with all or most other cranial neural crest cells, such as Sox9 and Pdgfra (Wright et al. 1995; Soriano 1997, Tallquist and Soriano 2003; Eberhart et al. 2006, 2008). The clear implication is that Dlx gene expression, a signature of the crest in the segmented pharyngeal walls, is completely nonessential for crest-derived mesenchyme in the more anterior head to form cartilage. Matching the results from fate mapping described above, that showed that the frontonasal crest does not contribute to the cartilages that de Beer, Jarvik, and others argued came from arch 0, there have been no gene-expression patterns reported that suggest that the frontonasal cells are relict survivors of arch 0. Whether crest cells will contribute to the maxillary or the frontonasal populations appears to depend on the signaling environments the cells encounter as their migrations from the dorsal neural tube come to an end. Whereas important patterning influences on the facial skeleton derived from the neural crest are well known to come from pharyngeal endoderm (Couly et al. 2002; Crump et al. 2004a, 2004b; see also Haworth et al. 2007), the signals in question appear to emanate from different sources of facial ectoderm (Shigetani et al. 2000; Lee et al. 2001; Hu et al. 2003), as we describe next, with particular focus on the oral ectoderm.
A signaling cascade, originating in midline mesoderm and including oral ectoderm, plays a critical role in specifying the fates of the neural crest of maxillary arch 1
Recently, and primarily through mutational analyses in zebrafish, we have come to realize that the patterning of maxillary arch 1, together with that of the frontonasal crest, is special, and as such might have fooled investigators of the stature of Huxley, de Beer, and Jarvik, along with others, into believing in the existence of one or more premandibular segments. A very pronounced craniofacial skeletal phenotype results from loss of function of either the Nodal-related gene ndr2 (the gene was originally called “cyclops,” CT Miller, unpublished data), or genes in the sonic hedgehog (Shh) signaling pathway (Brand et al. 1996; Wada et al. 2005; Eberhart et al. 2006), e.g., the shha gene (Fig. 5). ndr2 functions upstream to shha, hence, it is likely that loss of either function is effecting skeletal development at the same point. As is well known, Nodal signaling is critical for dorsal mesodermal functioning at the midline of the embryo at gastrula stages (Dougan et al. 2003). In response to Nodal signaling, the midline cells of the neural plate, which will form the floor of the brain after neurulation, turn on a relaying signal, Shh. These events happen long before the neural crest migrates or any skeleton is made.
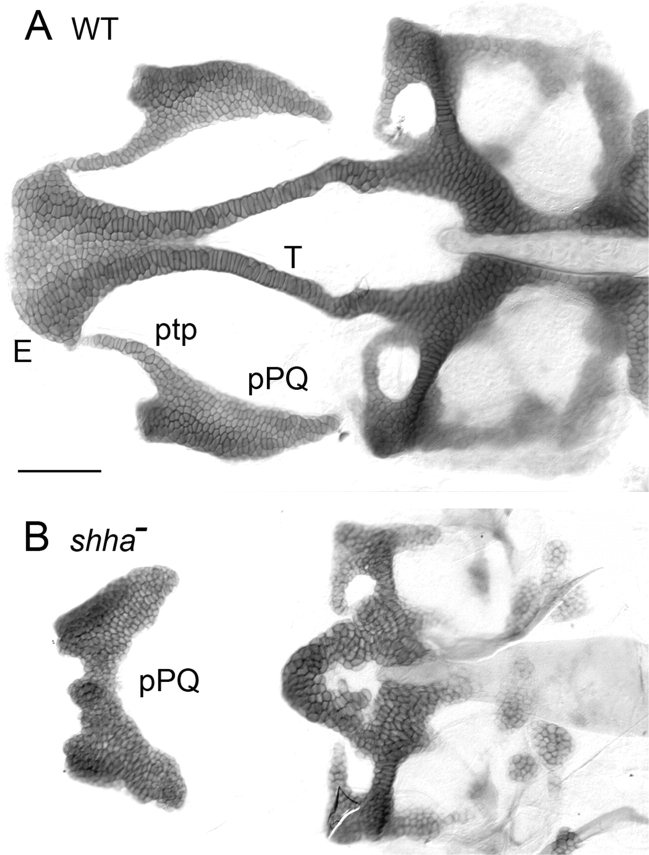
Fig. 5.
The cartilages deriving from maxillary arch 1 in zebrafish are critically dependent on Shh signaling. Flat mounts of Alcian-blue-stained neural cranial cartilages with bilateral palatoquadrates attached. (A) Wild-type embryo. (B) shha homozygous mutant. The anterior neural cranial cartilages and the pterygoid process of the palatoquadrates are deleted and the posterior regions of the palatoquadrates are fused at the midline. E, ethmoid plate; ptp, pterygoid process; pPQ, posterior palatoquadrate; T, trabecula. From Eberhart et al. (2006).
In ndr2 and shha mutants, Meckel's cartilages are variably shortened and may be fused together at the midline where a joint is normally made between the bilateral pair. The posterior regions of the palatoquadrates are usually reduced and fused together at the midline (Fig. 5). Strikingly, the anterior extensions of the palatoquadrate, their pterygoid processes, are missing altogether. The pair of trabeculae, and the ethmoid plate which together form the anterior neurocranial skeleton of the larva are missing altogether as well. These entirely missing elements, as we have detailed above, are those that normally derive from the neural crest of the frontonasal process and maxillary arch 1.
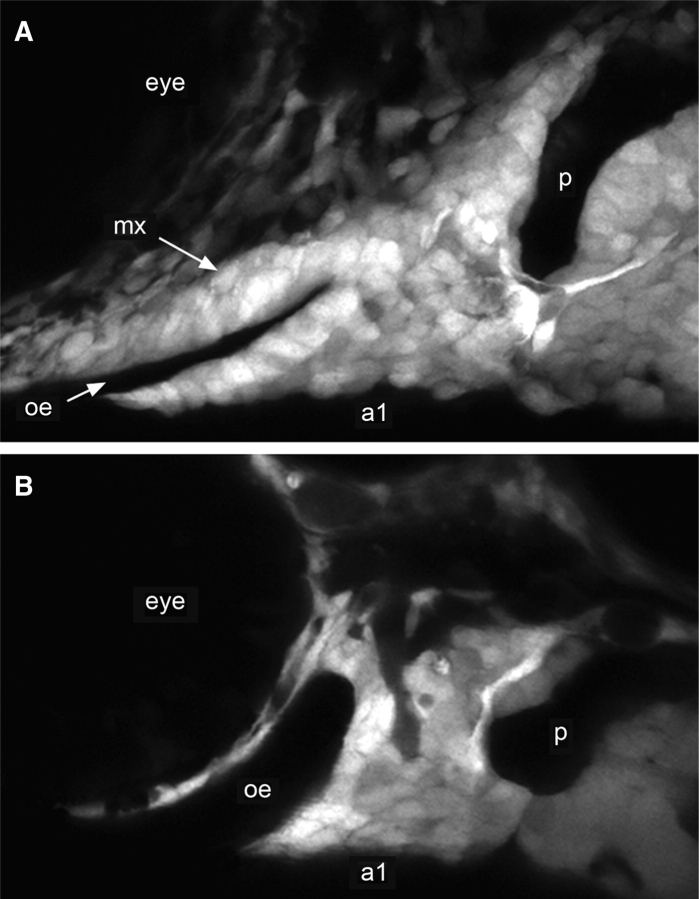
Fate-mapping studies showed that the missing elements normally come from neural crest that specifically condenses onto the ectodermal epithelium of the oral roof. Time-lapse analyzes of embryos mutant for smoothened (smo), a gene encoding a membrane receptor required in the target cell for Shh signal to function (Eberhart et al. 2006). In smo mutants, the oral ectoderm invaginates as usual, and the neural crest migrates into the maxillary first arch as usual. However, within that arch the normally observed condensation fails; rather, in smo mutants the cells disperse and maxillary arch 1 is lost. The loss of maxillary arch 1 could be effectively phenocopied by treatment of embryos with cyclopamine, a drug that blocks Hh signaling. As shown in Fig. 6, essentially no neural crest cells condense on the roof of the invaginated oral ectoderm, whereas the other postmigratory crest in arch 1 looks normal. For example, the condensation surrounding the oral ectoderm ventrally in the region of mandibular arch 1 is present, as in untreated embryos. Varying the duration of exposure to the drug revealed that the critical period for Hh signaling occurs many hours before the neural crest migrates into the pharyngeal arches (Eberhart et al. 2006). This result suggested that the skeletogenic neural crest itself, remote at the time of signaling, was not the direct target of the signal.
Fig. 6.
The maxillary (mx) portion of the neural-crest-derived mesenchyme, normally condensed on the invaginated oral ectodermal (oe) roof in (A), is critically dependent upon Hedgehog signaling, as revealed by the absence of mx in (B) after exposure of the early embryo to cyclopamine. The other arch 1 (a1) crest-derived mesenchyme is relatively normal in appearance. p, hyomandibular pouch. From Eberhart et al. (2006).
Mosaic analyses confirmed that the effective source of Shh signaling was the rudiment of the ventral brain, also a source of Shh signaling in chickens (Marcucio et al. 2005), and showed that the target of the signal was the oral ectoderm upon which the neural crest normally condenses in maxillary arch 1. To learn the source, we transplanted embryonic tissues from wild-type donors into host embryos that had been injected with morpholinos to the shha and shhb genes, lowering the functions of both genes together. In the absence of any transplant, the morpholino treatment phenocopied the skeletal and condensation defects shown in Figs 5B and 6B. On the other hand, nearly complete rescue resulted if untreated ventral brain was transplanted into such embryos, but not if prechordal plate were transplanted, another potential source of Shh. We used a similar transplantation strategy to learn the target of Shh signaling, in this case with smo mutants to identify in which tissue the receptor for the Shh signal was required. In these experiments, we learned that the smo genotype of the neural-crest cells that form maxillary arch 1 was irrelevant: smo+ neural crest transplanted into smo− embryos formed no condensation and smo− crest transplanted into smo+ hosts condensed on the oral ectoderm. These results pointed to the oral ectoderm as being the target of the Shh signal and this interpretation was fully supported by transplanting smo+ oral ectoderm into smo− hosts. In this case, the crest's maxillary condensation was rescued.
The data strongly suggest that for neural crest to condense in maxillary arch 1, and then go on to form skeletal derivatives, an early signal must pass from the ventral brain to the oral ectoderm. We can view the Shh signaling as a “priming” of the ectoderm for some later interaction with the migrating crest. Time-lapse studies suggest, further, that this later interaction can happen only when the crest reaches the immediate vicinity of the ectoderm—perhaps direct contact is required.
Further support for this model comes from study of zebrafish embryos in which an altogether different signaling pathway is reduced, the platelet-derived growth-factor (Pdgf) signaling pathway. In embryos bearing a loss-of-function mutation in the gene pdgfra, encoding Pdgf-receptor-α, crest cells can migrate but a portion of the anterior cranial neural crest cells disperse and migrate abnormally. As revealed by time-lapse analyses, these cells normally migrate over the eye rudiment that out pockets from the wall of the diencephalon and then continue around the anterior part of the primordium of the eye to reach the optic stalk, before then going on to settle on the anterior-most oral ectoderm. In pdgfra mutants, the cells stall in the region of the optic stalk, most of them never reaching the oral ectoderm where they would normally condense. Even though the crest cells are just a few cell diameters away from the oral ectoderm, they do not develop a condensation. This observation reinforces the proposal made above that the crest cells require a location in the immediate neighborhood of oral ectoderm in order to condense. Later, there is a striking midline deficiency of the cartilage of the anterior neurocranium, and in the more severe cases the ethmoid plate fails to develop and the trabeculae are extremely shortened. Loss of the condensation leads secondarily to loss of the cartilages derived from the condensation.
This signaling cascade, originating with Nodal and relayed via Shh to the oral ectoderm, then impinges back upon the neural crest. Ectodermal signals cause both the condensation of the crest on the oral ectoderm and the outgrowth of skeletal elements derived from the crest of the first arch. The molecular nature of the cues regulating condensation of the crest is unknown, but insight has been made into understanding the outgrowth of the first arch. Exquisite experiments utilizing tissue transplantation, performed by Hu et al. (2003), demonstrated that the boundary of the oral and adjacent facial ectoderm within the frontonasal process and molecularly defined by a boundary of Shh and Fgf8 expression, directs the outgrowth of skeletal elements of the first arch. Two significant findings for our discussion of arch 0 come from these analyses. First, mandibular and maxillary parts of arch 1 and the frontonasal crest were equally responsive to ectodermal signals, but the crest of arch 2 was immune to these signals. Second, the facial ectoderm maintained expression of Shh and Fgf8 when transplanted into ectopic regions of arch 1, but not into the region of arch 2. Whereas Hu et al. found that beads soaked with Shh and Fgf could not recapitulate the effects of their ectoderm transplants, other studies by Abzhanov and Tabin (2004) found that retrovirus-mediated ectopic expression of Shh and Fgf8 in the ectoderm was sufficient to cause outgrowth of cartilage. Collectively, these results suggest that specific interactions between the crest of arch 1 and the adjacent ectoderm direct skeletal outgrowth.
Conclusion: “arch 0” results from the oral-ectoderm-dependent development of maxillary arch 1
We have seen that the old evidence supporting the existence of premandibular arches is flawed, coming from authors that took undue liberties in interpreting the morphological data at hand. The workers justified their data manipulation, first, through extremely idealistic views about the nature of the early ancestor, segmented all along the body axis, with pharyngeal segments extending to the anterior tip of the animal, where the mouth was present. We mean to find no fault with the individual authors of the studies we have described, who were giants in their field, but the work comes from a different age, with different standards, and a different paradigm of understanding. Currently we have a much fuller understanding of the nature of the early ancestors of chordates, vertebrates, and gnathostomes, through phylogenetic analyses aided by molecular data, and better understanding of the morphological patterning, coming from studies of gene expression. We see no evidence for the proposed idealistic ancestor predicted by the proponents of arch 0.
In the most widely held view of arch 0, the tissue thought to comprise arch 0, is instead a preoral subdivision of arch 1—the region termed maxillary arch 1. This understanding that the maxillary region is a part of arch 1 is fully supported by new studies. Expression analyses, supported by functional analyses in the mouse and zebrafish not reviewed here show that Dlx-dependent patterning within arch 1 (inclusive of the maxillary region) in many respects resembles (and as we argue, is serially homologous with) patterning of arch 2. Arch 1, arch 2, and the more posterior arches share nested expression of Dlx genes, and the full extent of each arch is delineated by expression of the Dlx1-Dlx2 pair of genes. This expression in arch 1, critically, includes the maxillary region.
Explaining the “why” and “wherefore” of the trabecula cranii was, for several influential authors including, particularly, de Beer (1937), the key in the argument for arch 0. A principal mischief, the incorrect logic, was the equating of “visceral skeleton” with skeleton derived from the neural crest. Certainly, the neural crest forms the segmentally arranged, mostly gill-bearing cartilages along the pharyngeal wall (Schilling and Kimmel 1994). However, does forming gill supports in one region (arches 3–6 in zebrafish) preclude it from forming jaws (arch 1), opercular supports (arch 2), tooth supports (arch 7), and brain supports (maxillary arch 1) elsewhere in the series? Not at all. The old assumption for an “originally uniform series”, i.e., for homogeneity among the segments of an ancient ancestor, is unjustified, based on incorrect understanding rather than upon any direct evidence.
Furthermore, the argument for the trabecula as an arch-0 gill support is not substantiated, and in fact is argued against by our new data on the patterning of the maxillary domain in arch 1. If the trabecula had been a gill support, then, like the extant arches that do bear gills, one might expect that the epithelium chiefly responsible for patterning would be the pharyngeal endoderm. That is, there is no reason to expect that the skeletal element of arch 0 should be dependent upon oral ectoderm—and further back along the signaling cascade upon neural ectoderm and the prechordal plate—for its formation. Yet, along with another principal anterior neurocranial element, the ethmoid plate, and the element that connects this region of the brain case to the upper jaw, the pterygoid process of the palatoquadrate, we see, unique among the derivatives of the pharyngeal arches, hierarchical dependence on a midline-dependent signaling cascade.
The arguments for arch 0 (along with other features of head-segmentation theory not considered here) were justified in part for “explanatory value” anatomically and developmentally and because, at least in the view of Jollie (1984), there existed no good alternative hypothesis. Yet, the theory of the vertebrate “new head” proposed by Gans and Northcutt (1983; see Northcutt 2008) is entirely consistent with our newer understanding. The vertebrate's new head, by this theory, is a head elaborated over that present in any invertebrate ancestor, through novelties arising particularly in ectoderm. Hatta et al. (1994) suggested that an expanded role of midline signaling, that we now understand as Nodal-dependent, could also have figured prominently in the new head, anterior neural inductions being responsible in part for the expansion of the anterior brain. Among the ectodermal novelties, the neural crest was principal (Gans and Northcutt 1983) and we easily extend the theory to include new ectodermal signaling functions, dependent ultimately on the Nodal-midline and acting on the anterior neural crest. A result of the oral-epithelial/neural-crest signaling interaction was, as we have seen, the formation of skeletal supports for the brain and sense organs, in particular the forebrain, eyes, and nasal organs. Expansion of an anterior crest-derived skeleton would then be included among changes in the new head. By this interpretation, the enigmatic trabeculae are not remnant gill supports; rather they would be novelties, structures sui generis (Goodrich 1930, 239) developing by invention in Paleozoic vertebrates, possibly gnathostomes.
Supplementary data
Supplementary data are available at ICB online.
Supplementary Material
Acknowledgments
We acknowledge the lovely fate-mapping experiments of Mary Swartz. Her work provided results that have greatly helped resolve the arch-0 problem in our laboratory. We are grateful to members of the laboratory, past and present, supporters and nonsupporters of arch 0 alike, for other investigations and for ideas and debate on this subject. Outside the laboratory, Michael Depew provided excellent insight, and especially an historical perspective. Research from the laboratory was funded by NIH grants DE13834, HD22486 and DE018088.
References
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273:134–48. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Allis EP. Are the polar and trabecular cartilages of vertebrate embryos the pharyngeal elements of the mandibular and premandibular arches? J Anat. 1923;58:37–51. [PMC free article] [PubMed] [Google Scholar]
- Allis EP. Concerning the development of the prechordal portion of the vertebrate head. J Anat. 1938;72:584–607. [PMC free article] [PubMed] [Google Scholar]
- Bateson W. Materials for the study of variation treated with especial regard to discontinuity in the origin of species. London: Macmillan Company; 1894. [Google Scholar]
- Bertmar G. On the ontogeny of the chondral skull in Characidae, with a discussion on the chondrocranial base and the visceral chondrocranium in fishes. Acta Zool. 1959;40:203–364. [Google Scholar]
- Bjerring HC. A contribution to structural analysis of the head of craniate animals. Zool Scr. 1977;6:127–83. [Google Scholar]
- Brand M, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–42. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- Cerny R, Lwigale R, Ericsson R, Meulemans D, Epperlein H-H, Bronner-Fraser M. Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular”. Dev Biol. 2004;276:225–36. doi: 10.1016/j.ydbio.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–73. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004a;131:5703–16. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Eberhart JK, Kimmel CB. moz-dependent hox expression controls segment-specific fate maps of skeletal precursors in the face. Development. 2006;133:2661–69. doi: 10.1242/dev.02435. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2004b;9 doi: 10.1371/journal.pbio.0020244. E244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer GR. The development of the skull of Scyllium (Scylorhinus) canicula L. Quart J Micr Sci. 1931;74:591–645. [Google Scholar]
- de Beer GR. The development of the vertebrate skull. Oxford: Clarendon Press; 1937. [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JLR. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–5. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA. 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn. 2006;235:1256–91. doi: 10.1002/dvdy.20796. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Evo-devo: variations on ancestral themes. Cell. 2008;132:185–95. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan ST, Warga RM, Kane DA, Schier AF, Talbot WS. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development. 2003;130:1837–51. doi: 10.1242/dev.00400. [DOI] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan Y-L, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008;40:290–8. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–77. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–73. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Lowe C, Kirschner M. Hemichordates and the origin of vertebrates. Curr Opin Genet Dev. 2005;15:461–7. doi: 10.1016/j.gde.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the structure and development of vertebrates. Chicago: University of Chicago Press; 1930. [Google Scholar]
- Hatta K, Püschel AW, Kimmel CB. Midline signaling in the primordium of the zebrafish anterior central nervous system. Proc Nat Acad Sci USA. 1994;91:2061–65. doi: 10.1073/pnas.91.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth KE, Wilson JM, Grevellec A, Cobourne MT, Healy C, Helms JA, Sharpe PT, Tucker AS. Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Dev Biol. 2007;303:244–58. doi: 10.1016/j.ydbio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–58. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. Somitomeres: mesodermal segments of the head and trunk. In: Hanken J, Hall BK, editors. The skull. Chicago: University of Chicago Press; 1993. pp. 42–76. [Google Scholar]
- Janvier P. Oxford Monographs on Geology and Geophysics, 33. Oxford: Oxford University Press; 1996. Early vertebrates. [Google Scholar]
- Janvier P. Homologies and evolutionary transitions in early vertebrate history. In: Anderson J, Sues H-D, editors. Transitions in vertebrate evolution. Bloomington: Indiana University Press; 2007. pp. 57–121. [Google Scholar]
- Jarvik E. On the visceral skeleton in Eusthenopteron with a discussion of the parasphenoid and palatoquadrate in fishes. K. Svenska VetenskAkad Handl. 1954;5:1–104. [Google Scholar]
- Jarvik E. Basic structure and evolution of vertebrates. Vol. 1. London: Academic Press; 1980. [Google Scholar]
- Jollie M. The vertebrate head – segmented or a single morphogenetic structure? J Vertebr Paleontol. 1984;4:320–29. [Google Scholar]
- Kimmel CB, Miller CT, Keynes RD. Neural crest patterning and the evolution of the jaw. J Anat. 2001;199:105–20. doi: 10.1046/j.1469-7580.2001.19910105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S, Matsuo I, Aizawa S. Developmental patterning and evolution of the mammalian viscerocranium: genetic insights into comparative morphology. Dev Dyn. 1997;209:139–55. doi: 10.1002/(SICI)1097-0177(199706)209:2<139::AID-AJA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Murakami Y, Nobusada Y, Kusakabe R, Hirano S. Developmental fate of the mandibular mesoderm in the lamprey, Lethenteron japonicum: comparative morphology and development of the gnathostome jaw with special reference to the nature of the trabecula cranii. J Exp Zoolog B Mol Dev Evol. 2004;302:458–68. doi: 10.1002/jez.b.21011. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Nobusada Y, Norigome N, Shigetani Y. Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Phil Trans Roy Soc London B. 2001;356:1615–32. doi: 10.1098/rstb.2001.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S, Ota KG. Primitive versus derived traits in the developmental program of the vertebrate head: views from cyclostome developmental studies. J Exp Zoolog B Mol Dev Evol. 2007;308B doi: 10.1002/jez.b.21190. 294-314. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Bédard O, Buchtová M, Fu K, Richman JM. A new origin for the maxillary jaw. Dev Biol. 2004;276:207–24. doi: 10.1016/j.ydbio.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Fu KK, Hui JN, Richman JM. Noggin and retinoic acid transform the identity of avian facial prominences. Nature. 2001;414:909–12. doi: 10.1038/414909a. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Miller CT, Swartz ME, Khuu PA, Walker MB, Eberhart JK, Kimmel CB. Mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish. Dev Biol. 2007;308:144–57. doi: 10.1016/j.ydbio.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG. Historical hypotheses regarding segmentation of the vertebrate head. Proceedings of the Society for Integrative and Comparative Biology. 2008. January 2–6 in San Antonio, TX ( http://www.sicb.org/meetings/2008/schedule) [DOI] [PubMed]
- Olsson L. Alternatives to Darwinism in Sweden: Lamarckism and idealistic morphology, disbelief in mutations and the poverty of selection. Jahrbuch für Europäische Wissenschaftskultur. 2005;1:1–14. [Google Scholar]
- Olsson L, Ericsson R, Cerny R. Vertebrate head development: segmentation, novelties, and homology. Theory Biosci. 2005;124:145–63. doi: 10.1007/BF02814481. [DOI] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Ghattas I, Meneses JL, Christensen L, Sharpe PT, Presley R, Pedersen RA, Rubenstein JLR. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–84. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Swalla BJ. Development and evolution of chordate cartilage. J Exp Zoolog B Mol Dev Evol. 2007;308:325–35. doi: 10.1002/jez.b.21157. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–94. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Nobusada Y, Kuratani S. Ectodermally derived fgf8 defines the maxillomandibular region in the early chick embryo: epithelial-mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- Shu D, Conway Morris S, Zhang Z-F, Liu J-N, Han J, Cheng L, Zhang X-L, Yasui K, Yong L. A new species of yunnanozoan with implications for deuterostome phylogeny. Science. 2003a;299:1380–84. doi: 10.1126/science.1079846. [DOI] [PubMed] [Google Scholar]
- Shu D, et al. Head and back bone of the early Cambrian vertebrate Haikouichthys. Nature. 2003b;421:526–9. doi: 10.1038/nature01264. [DOI] [PubMed] [Google Scholar]
- Soriano P. The PDGFα receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Swalla BJ. New insights into vertebrate origins. In: Moody SA, editor. Principles of developmental genetics. Amsterdam: Elsevier Academic Press; 2007. pp. 114–28. [Google Scholar]
- Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRα in populations of cranial and cardiac neural crest cells. Development. 2003;130:507–18. doi: 10.1242/dev.00241. [DOI] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–88. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Talbot JC, Stock DW, Kimmel CB. Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev Biol. 2006;295:194–205. doi: 10.1016/j.ydbio.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.