Abstract
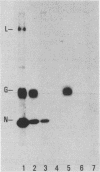
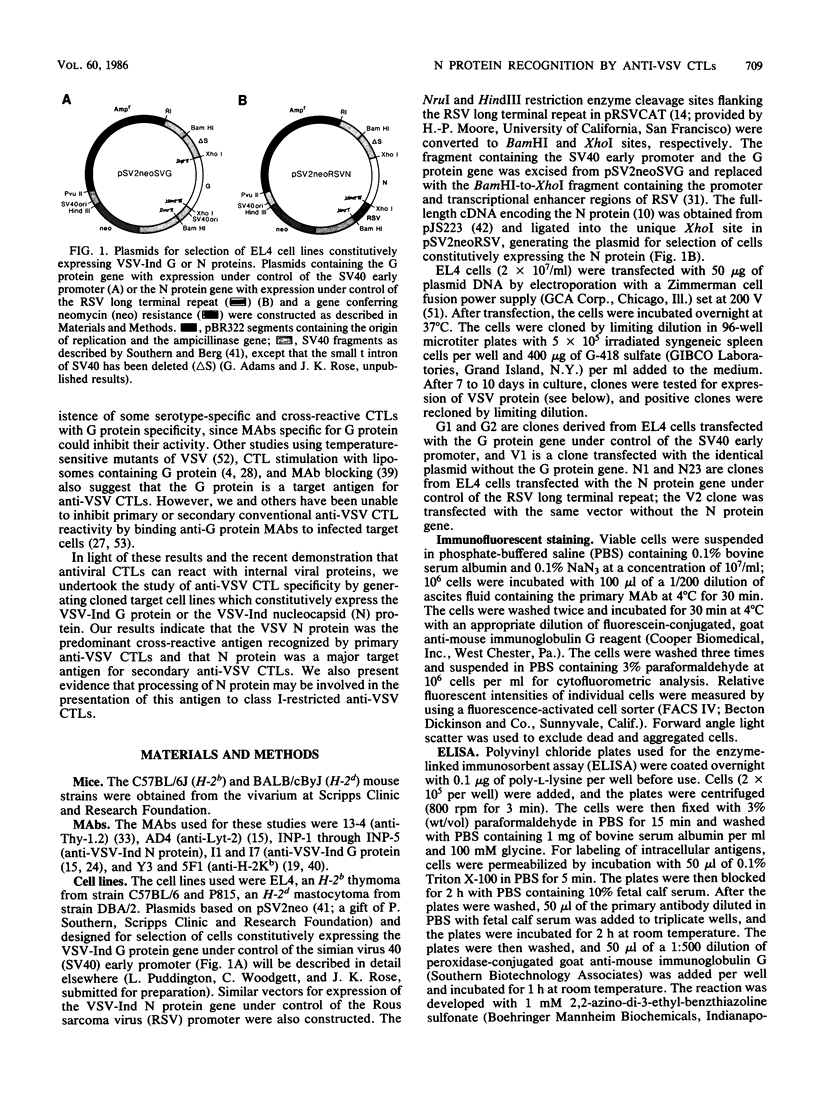
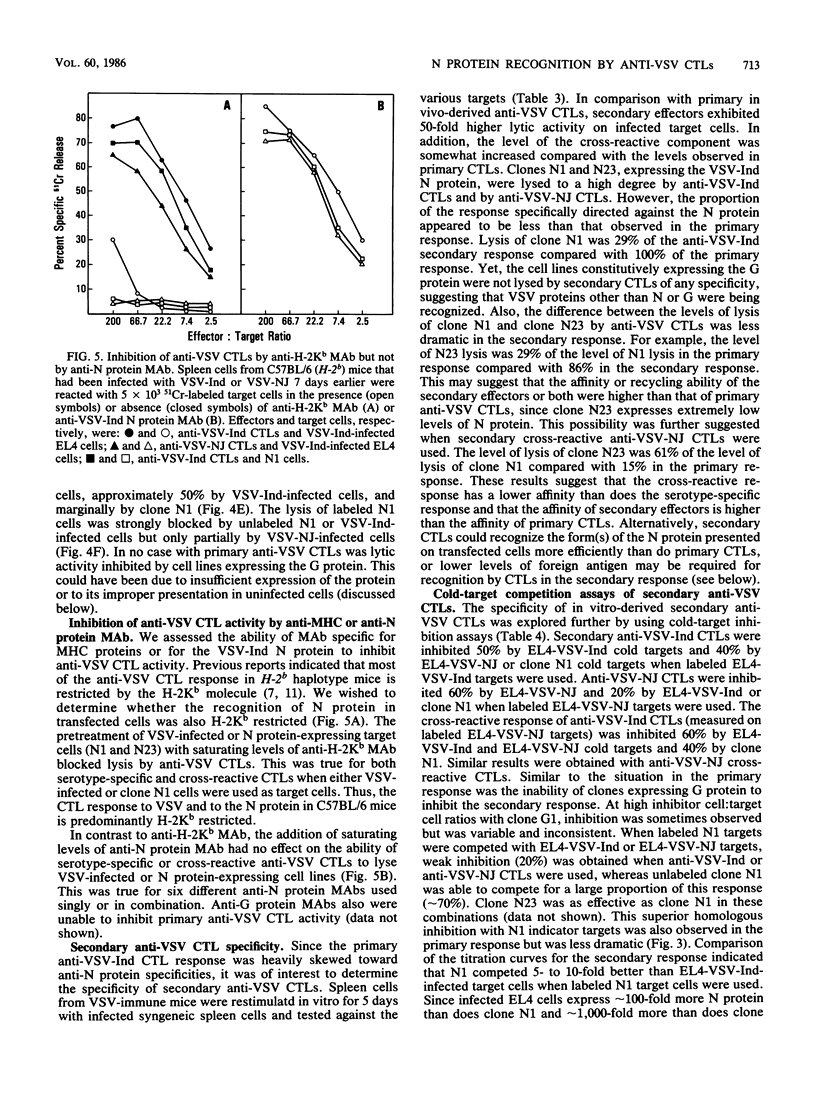
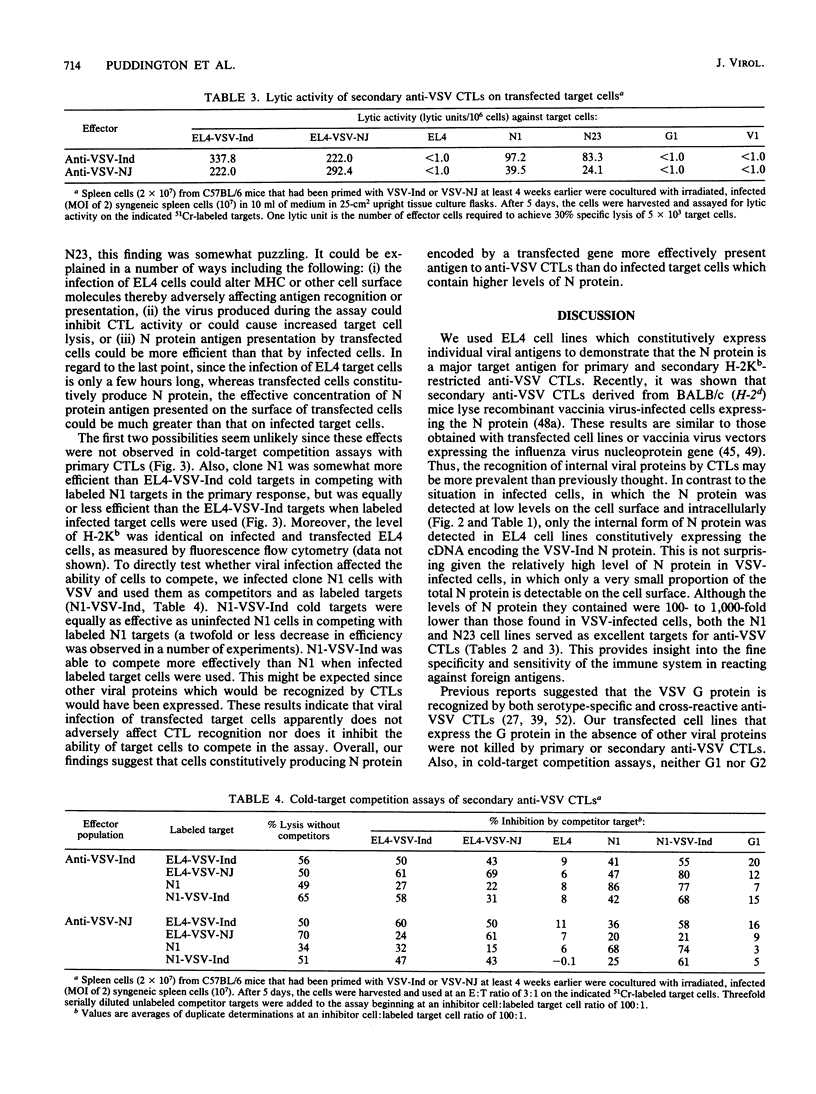
The specificity of anti-vesicular stomatitis virus (VSV)-specific cytotoxic T cells was explored with cell lines expressing VSV genes introduced by electroporation. Low levels of nucleocapsid (N) protein were detected on the surface of VSV-infected cells, but N protein could not be detected on the plasma membrane of transfected EL4 cells. Intracellular N protein was detectable by enzyme-linked immunosorbent assay or immunoprecipitation in some of the transfected cell lines but not in others, unless the transfected genes were induced by sodium butyrate. However, all of the stably transfected EL4 cell lines expressing the VSV-Indiana N protein were efficiently lysed by serotype-specific and cross-reactive anti-VSV cytotoxic T cells (CTLs). Primary cross-reactive anti-VSV CTLs appeared to be specific solely for N protein, based on cold-target competition assays using infected and transfected target cells. Cell lines expressing 100- to 1,000-fold less N protein than did VSV-infected cells were efficiently lysed by both primary and secondary anti-VSV CTLs. Cell lines expressing 100-fold less G protein than did VSV-infected cells were not lysed by either population of effectors. Significantly, cold-target competition studies with secondary CTLs demonstrated that N protein-expressing cell lines were more efficient competitors than were VSV-infected cells even though the latter expressed 100- to 1,000-fold more N protein. This was not an artifact of viral infection since infection of the transfected cell lines did not affect their ability to compete. The possibility that cell lines constitutively expressing internal virus proteins present antigen more effectively than infected cells do is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Campen T. J., Royer H. D., Hussey R. E., Poole C. B., Reinherz E. L. Molecular analysis of T cell receptor (Ti) variable region (V) gene expression. Evidence that a single Ti beta V gene family can be used in formation of V domains on phenotypically and functionally diverse T cell populations. J Exp Med. 1985 Jun 1;161(6):1326–1343. doi: 10.1084/jem.161.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- Cartwright G. S., Smith L. M., Heinzelmann E. W., Ruebush M. J., Parce J. W., McConnell H. M. H-2Kk and vesicular stomatitis virus G proteins are not extensively associated in reconstituted membranes recognized by T cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1506–1510. doi: 10.1073/pnas.79.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Chabanas A., Khoury E., Goeltz P., Froussard P., Gjerset R., Dod B., Eisen H., Lawrence J. J. Effects of butyric acid on cell cycle regulation and induction of histone H1(0) in mouse cells and tissue culture. Inducibility of H1 (0)in the late S-G2 phase of the cell cycle. J Mol Biol. 1985 May 25;183(2):141–151. doi: 10.1016/0022-2836(85)90208-6. [DOI] [PubMed] [Google Scholar]
- Clark S. S., Geier S., Nathenson S. G., Forman J. Analysis of associative recognition determinants on class I H-2Kb mutant molecules. Immunogenetics. 1985;22(4):391–397. doi: 10.1007/BF00430922. [DOI] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Becht H., Rott R. Recognition of viral antigens by human influenza A virus-specific T lymphocyte clones. J Immunol. 1985 Oct;135(4):2800–2804. [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Rosenthal K. L., Klein J., Zinkernagel R. M., Singer S. J. Selective and unidirectional membrane redistribution of an H-2 antigen with an antibody-clustered viral antigen: relationship to mechanisms of cytotoxic T-cell interactions. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4603–4607. doi: 10.1073/pnas.76.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., O'Connell K. A. Recognition by cytotoxic T lymphocytes of cells expressing fragments of the SV40 tumor antigen. J Immunol. 1983 Nov;131(5):2580–2586. [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Jarvis D. L., Cole C. N., Butel J. S. Absence of a structural basis for intracellular recognition and differential localization of nuclear and plasma membrane-associated forms of simian virus 40 large tumor antigen. Mol Cell Biol. 1986 Mar;6(3):758–767. doi: 10.1128/mcb.6.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Allen H., Gething M. J., Waterfield M. D., Klenk H. D. Recognition of viral glycoproteins by influenza A-specific cross-reactive cytolytic T lymphocytes. J Exp Med. 1980 Apr 1;151(4):945–958. doi: 10.1084/jem.151.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. Antigenic determinants of vesicular stomatitis virus: analysis with antigenic variants. J Immunol. 1983 Jan;130(1):394–398. [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. Cytotoxic T lymphocytes reactive with vesicular stomatitis virus: analysis of specificity with monoclonal antibodies directed to the viral glycoprotein. J Immunol. 1983 Mar;130(3):1408–1412. [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982 Aug;121(1):168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Loh D., Ross A. H., Hale A. H., Baltimore D., Eisen H. N. Synthetic phospholipid vesicles containing a purified viral antigen and cell membrane proteins stimulate the development of cytotoxic T lymphocytes. J Exp Med. 1979 Nov 1;150(5):1067–1074. doi: 10.1084/jem.150.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Gerhard W. Topological mapping antigenic sites on the influenza A/PR/8/34 virus hemagglutinin using monoclonal antibodies. Virology. 1981 Aug;113(1):64–72. doi: 10.1016/0042-6822(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Lukacher A. E., Morrison L. A., Braciale V. L., Malissen B., Braciale T. J. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985 Jul 1;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Mullbacher A., Marshall I. D., Blanden R. V. Cross-reactive cytotoxic T cells to alphavirus infection. Scand J Immunol. 1979;10(4):291–296. doi: 10.1111/j.1365-3083.1979.tb01353.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Rupp F., Acha-Orbea H., Hengartner H., Zinkernagel R., Joho R. Identical V beta T-cell receptor genes used in alloreactive cytotoxic and antigen plus I-A specific helper T cells. 1985 May 30-Jun 5Nature. 315(6018):425–427. doi: 10.1038/315425a0. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer J., Rosenthal A. S. Function of macrophages as antigen presenting cells. Springer Semin Immunopathol. 1980 Aug;3(2):247–264. doi: 10.1007/BF02053977. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. The role of vesicular stomatitis virus major glycoprotein in determining the specificity of virus-specific and H-2-restricted cytolytic T cells. Eur J Immunol. 1980 Apr;10(4):268–272. doi: 10.1002/eji.1830100409. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Randolph C. P. Monoclonal anti-H-2Kb antibodies detect serological differences between H-2Kb mutants. Immunogenetics. 1981;12(1-2):183–186. doi: 10.1007/BF01561661. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S., Tevethia M. J., Lewis A. J., Reddy V. B., Weissman S. M. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). IX. Analysis of TrAg in mouse cells synthesizing truncated SV40 large T antigen. Virology. 1983 Jul 30;128(2):319–330. doi: 10.1016/0042-6822(83)90259-3. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. Influenza A specific cytotoxic T-cell clones that do not recognize viral glycoproteins. Nature. 1982 Dec 16;300(5893):655–657. doi: 10.1038/300655a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J Exp Med. 1984 Aug 1;160(2):552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Holland J. Target antigens for H-2-restricted vesicular stomatitis virus-specific cytotoxic T cells. J Immunol. 1978 Aug;121(2):744–748. [PubMed] [Google Scholar]
- Zinkernagel R. M., Rosenthal K. L. Experiments and speculation on antiviral specificity of T and B cells. Immunol Rev. 1981;58:131–155. doi: 10.1111/j.1600-065x.1981.tb00352.x. [DOI] [PubMed] [Google Scholar]