Abstract
Obsessive-compulsive disorder (OCD) is a common, heritable and disabling neuropsychiatric disorder. Theoretical models suggest that OCD is underpinned by functional and structural abnormalities in orbitofronto-striatal circuits. Evidence from cognitive and neuroimaging studies (functional and structural magnetic resonance imaging (MRI) and positron emission tomography (PET)) have generally been taken to be supportive of these theoretical models; however, results from these studies have not been entirely congruent with each other. With the advent of whole brain-based structural imaging techniques, such as voxel-based morphometry and multivoxel analyses, we consider it timely to assess neuroimaging findings to date, and to examine their compatibility with cognitive studies and orbitofronto-striatal models. As part of this assessment, we performed a quantitative, voxel-level meta-analysis of functional MRI findings, which revealed consistent abnormalities in orbitofronto-striatal and other additional areas in OCD. This review also considers the evidence for involvement of other brain areas outside orbitofronto-striatal regions in OCD, the limitations of current imaging techniques, and how future developments in imaging may aid our understanding of OCD.
Keywords: Neuroimaging, Brain structure, MRI, Region-of-interest analysis, Voxel-based morphometry, Multivoxel analysis, Meta-analysis, Neuropsychology, Obsessive-compulsive disorder, Fronto-striatal loops, Orbitofrontal cortex, Parietal cortex, Response inhibition, Set shifting, Decision-making, Human
1. Introduction
Obsessive-compulsive disorder (OCD) is a chronically debilitating disorder with a lifetime prevalence of 2–3% (Robins et al., 1984; Karno et al., 1988; Weissman et al., 1994). It is characterised by two sets of symptoms: obsessions, which are unwanted, intrusive, recurrent thoughts or impulses that are often concerned with themes of contamination and ‘germs’, checking household items in case of fire or burglary, order and symmetry of objects, or fears of harming oneself or others and compulsions, which are ritualistic, repetitive behaviours or mental acts carried out in relation to these obsessions e.g., washing, household safety checks, counting, rearrangement of objects in symmetrical array or constant checking of oneself and others to ensure no harm has occurred. These symptoms are time-consuming and cause marked distress and impairment (DSM-IV; American Psychiatric Association, 1994). Suppression of compulsive behaviours leads to high levels of anxiety, and OCD is chronically disabling within the realms of both social and occupational functioning (Leon et al., 1995; Koran et al., 1996). OCD was named by the World Health Organisation in 1996 as one of the top 10 causes worldwide of ‘years lived with illness-related disability’ (Murray and Lopez, 1996), indicating its serious impact on quality of life. The burden of OCD at a population level is considerable; e.g., a study in the US estimated the economic cost of OCD to be $8.4 billion in 1990 (DuPont et al., 1995).
Relatively little is known about the neurobiology and aetiological origins of OCD (Chamberlain et al., 2005). There is strong evidence that OCD has a genetic basis, with levels of monozygotic twin concordance reported to be between 63% and 87%, and first-degree relatives showing increased rates of OCD of 10–22.5% compared with the normal population risk of 2–3% (Inouye, 1965; Carey and Gottesman, 1981; Rasmussen and Tsuang, 1984; Pauls et al., 1995; Nestadt et al., 2000b; Hanna et al., 2005). Additionally, linkage studies have pointed to a locus on chromosome 9 (though encompassing a large number of genes) (Hanna et al., 2002; Willour et al., 2004) and complex segregation analysis suggests both Mendelian dominant forms of transmission and polygenic contributions (Cavallini et al., 1999; Nestadt et al., 2000a). However, to date, although there are intriguing findings concerning, for example, the serotonin transporter gene (SERT) (Hu et al., 2006), candidate gene association studies have not yet provided sufficient consistent evidence to confirm specific gene involvement in OCD. This may be partly due to heterogeneity within the clinical diagnostic category of OCD—it is hoped that this may be circumvented by the use of endophenotypic strategies in the future using objective and reliable measures, such as might be taken from neuroimaging (Menzies et al., 2007).
In this review, we focus on the degree of consistency between studies and the extent to which findings have been replicated. It is important to consider at the outset that inconsistent findings do not necessarily indicate a lack of validity of the model under investigation, but instead could reflect confounding factors resulting from study design. For example, not all studies of OCD control rigorously for experimental factors such as the age, IQ, handedness and gender of participants. In the context of imaging in particular, this may be of considerable importance since it is evident that age has marked effects on brain structure (Ge et al., 2002; Lemaitre et al., 2005; Smith et al., 2007). Another key difficulty in OCD is that many patients have co-morbidities, in particular depression or anxiety disorders, which would be predicted to have confounding effects upon cognitive and imaging findings (Chamberlain and Sahakian, 2006; see Section 4.3.2). For example, as many as one-third of OCD patients have concurrent major depressive disorder (MDD) at the time of evaluation, and the number suffering from a depressive episode at some point over their lifetime is estimated to be considerably higher (Rasmussen and Eisen, 1992; Weissman et al., 1994).
The current dominant model of OCD focuses on abnormalities in cortico-striatal circuitry, with particular emphasis on the orbitofronto-striato-thalamic circuits (Saxena et al., 1998, 2001a; Graybiel and Rauch, 2000). This review aims to examine the neurobiological foundations of this influential model of OCD and to assess how well it is supported by evidence from neuropsychological and neuroimaging studies. We consider additional regions which have been implicated in OCD by cognitive and imaging studies, including recent findings from whole brain-based structural imaging studies using techniques such as voxel-based morphometry (VBM) and multivoxel analysis. Finally we propose an updated model for OCD that includes structural brain abnormalities not limited exclusively to orbitofronto-striatal circuitry, which may account more comprehensively for cognitive and imaging findings in OCD.
2. The orbitofronto-striatal model of OCD
2.1. Anatomical evidence for involvement
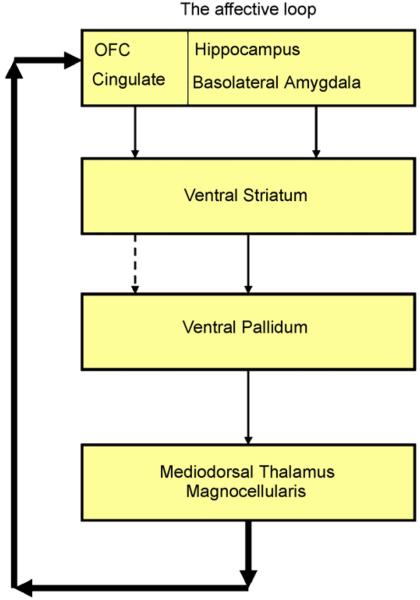
Early work based on anatomical studies in primates documented the existence of several relatively specialised brain circuits, the so-called ‘fronto-striatal loops’, organised in parallel and linking the basal ganglia to the frontal cortex (Alexander et al., 1986). It was suggested that these circuits each play a relatively specific functional role, based on the connections within each circuit to relatively discrete areas of frontal cortex. The existence of a lateral orbitofrontal loop was proposed, involving projections from the orbitofrontal cortex (OFC) to the head of the caudate and ventral striatum, then to the mediodorsal thalamus via the internal pallidus and finally returning from the thalamus to the OFC. More recently, researchers have modified the model of this circuit to include the hippocampus, anterior cingulate and basolateral amygdala, all of which are extensively connected with the OFC, and ascribed an affective function to this circuit, based on our current knowledge of the functional significance of these limbic regions in affective states and emotional perception (Lawrence et al., 1998; Phillips et al., 2003) (see Fig. 1).
Fig. 1.
Diagram of the affective orbitofronto-striatal circuit. Dysfunction in this circuit is proposed to underlie OCD. After Lawrence et al. (1998).
2.2. Orbitofrontal cortical function
Evidence from lesion studies in animals and humans strongly suggests that the OFC plays a crucial role in emotional and motivational aspects of behaviour (Rolls, 2004; Elliott and Deakin, 2005; Kringelbach, 2005). This was famously illustrated by the case of Phineas Gage, a railway workman who sustained OFC damage in a blasting accident involving a metal rod, and subsequently demonstrated profound changes in emotional behaviour (Harlow, 1868). More recent studies have again shown that patients with orbitofrontal lesions consistently show behavioural changes relating to inappropriate affect, disinhibition and poor decision-making (Eslinger and Damasio, 1985; Bechara et al., 1994; Damasio et al., 1994).
Functional imaging studies have provided evidence supporting a role for the OFC in ascribing and monitoring changes in reward value, including awareness of the anticipation of expected rewards and the probability such rewards will occur (Tremblay and Schultz, 1999, 2000; Hikosaka and Watanabe, 2004). Further work has suggested that medial and lateral regions of the OFC may have dissociable functions, with the lateral OFC being especially likely to be activated when a response previously associated with reward has to be suppressed, indicating it may play an inhibitory role (Elliott et al., 2000).
In agreement with these imaging findings, orbitofrontal lesions in animals and humans lead to reward-related learning deficits in tasks such as reversal learning (McEnaney and Butter, 1969; Jones and Mishkin, 1972; Rolls et al., 1994). It is suggested that impairments in reversal learning reflect an inability to detect alterations in reinforcement contingencies, i.e., changes in the motivational value of stimuli, and to then modify behaviour accordingly. It has also been argued that the OFC is important in inhibiting previously important, but now inappropriate responses, which would also lead to task impairments following a reversal of reinforcement contingency (Schoenbaum et al., 2002; Chudasama and Robbins, 2003). The neuropsychological evidence for orbitofrontal function also suggests this area is necessary for completion of reversal learning tasks and decision-making tasks which require an assessment of the reward value of possible options (Clark et al., 2004; Elliott and Deakin, 2005).
2.3. Orbitofronto-subcortical circuitry in OCD
The cortico-striato-thalamic circuit involving the OFC has repeatedly been implicated in the neuropathology of OCD; for review see Saxena (2003). Early positron emission tomography (PET) studies measuring brain function via cerebral glucose metabolism showed significantly elevated metabolic rates in the whole cerebral hemispheres, the heads of the caudate nuclei and the orbital gyri in patients with OCD (Baxter et al., 1987, 1988). In addition, the hypermetabolism in the orbital gyri was still present in patients after controlling for global differences in hemisphere metabolism. These findings have been replicated for the OFC in PET studies using both resting and symptom provocation designs (Nordahl et al., 1989; Swedo et al., 1989; Sawle et al., 1991; McGuire et al., 1994; Rauch et al., 1994; Cottraux et al., 1996), though it is of note that some studies have not found OFC hypermetabolism in OCD (Martinot et al., 1990; Perani et al., 1995; Busatto et al., 2000; Saxena et al., 2001b). These inconsistencies between studies with regard to orbitofrontal metabolism could be due to demographic differences in sample groups across studies, for example, in gender, handedness or IQ; and different thresholds for exclusion of co-morbidities, for example, many studies have included patients with a diagnosis of depression or other psychiatric disorders e.g., Swedo et al. (1989). For a summary of findings from PET studies comparing OCD patients and healthy controls see Table 1 and the meta-analysis by Whiteside et al. (2004), which reported consistent abnormalities between patients and controls in the orbital gyrus and the head of the caudate nucleus.
Table 1.
Significant case-control differences between OCD patients and healthy controls reported from PET studies
| Author | Year | Sample | Design | Direction | Region | Coordinates (if provided) |
p-Value | Notes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | Space | ||||||||
| Baxter et al. | 1987 |
N = 14 controls, 14 OCD14 UPD |
FDG PET, resting |
OCD>UPD and controls | L cerebral hemisphere |
Region of interest identified (Bonferroni correction for multiple tests on first seven patients). Regions with corrected p<0.05 (two- tailed) were then analysed in second seven patients (p<0.05 one-tailed) |
|||||
| OCD>UPD and controls | R cerebral hemisphere |
||||||||||
| OCD>UPD and controls | L caudate head | ||||||||||
| OCD>UPD and controls | R caudate head | ||||||||||
| OCD>UPD and controls | L orbital gyrus | ||||||||||
| OCD>UPD and controls | R orbital gyrus | ||||||||||
| OCD>controls | L orbital gyrus to L hemisphere ratio |
||||||||||
| Baxter et al. | 1988 |
N = 10 controls, 10 OCD |
FDG PET, resting |
OCD>healthy controls | L cerebral hemisphere |
0.022 | Region of interest p<0.05 (one-tailed) uncorrected |
||||
| OCD>healthy controls | R cerebral hemisphere |
0.028 | |||||||||
| OCD>healthy controls | L caudate head | 0.015 | |||||||||
| OCD>healthy controls | R caudate head | 0.02 | |||||||||
| OCD>healthy controls | L orbital gyrus | 0.005 | |||||||||
| OCD>healthy controls | R orbital gyrus | 0.006 | |||||||||
| OCD>healthy controls | L orbital gyrus to L hemisphere ratio |
0.025 | |||||||||
| OCD>healthy controls | R orbital gyrus to R hemisphere ratio |
0.02 | |||||||||
| Nordahl et al. | 1989 |
N = 30 controls, 8 OCD |
FDG PET, resting |
L anterior OFC | 0.017 | ||||||
| R anterior OFC | 0.002 | ||||||||||
| R posterior OFC | 0.0015 | ||||||||||
| Anterior medial OFC |
0.034 | ||||||||||
| Superior occipital | 0.07 (trend) | ||||||||||
| L parietal | 0.06 (trend) | ||||||||||
| R parietal | 0.04 | ||||||||||
| L parieto-occipital | 0.01 | ||||||||||
| Swedo et al. | 1989 |
N = 18 controls, 18 OCD |
FDG PET, resting |
OCD>healthy controls | R prefrontal | Many regions of interest. No multiple comparisons correction so as to favour Type II error over Type I |
|||||
| OCD>healthy controls | L prefrontal | <0.01 | |||||||||
| OCD>healthy controls | L orbitofrontal | <0.05 | |||||||||
| OCD>healthy controls | L premotor | <0.05 | |||||||||
| OCD>healthy controls | R sensorimotor | <0.05 | |||||||||
| OCD>healthy controls | R inferior temporal | <0.05 | |||||||||
| OCD>healthy controls | L paracentral | <0.05 | |||||||||
| OCD>healthy controls | R cerebellar | <0.05 | |||||||||
| OCD>healthy controls | R thalamus | <0.05 | |||||||||
| OCD>healthy controls | R anterior cingulate | <0.05 | |||||||||
| OCD>healthy controls | L anterior cingulate | <0.01 | |||||||||
| Perani et al. | 1995 |
N = 15 controls, 11 OCD |
FDG PET, resting |
OCD>healthy controls | Anterior cingulate | <0.0001 | Region of interest spheres, Bonferroni corrected to <0.002 |
||||
| OCD>healthy controls | Mid cingulate | <0.001 | |||||||||
| OCD>healthy controls | Posterior cingulate | <0.001 | |||||||||
| OCD>healthy controls | Putamen/Pallidum | <0.001 | |||||||||
| OCD>healthy controls | Thalamus | <0.001 | |||||||||
| Saxena et al. | 2001 |
N = 27 OCD, 27 MDD, 17 OCD+MDD, 17 controls |
OCD>healthy controls | L thalamus | −20 | −22 | 18 | TAL | <0.001 | Whole brain coordinates reported here, L and R thalamus regions of interest also found to be significant at <0.05 |
|
| OCD>healthy controls | R thalamus | 16 | −18 | 20 | TAL | 0.002 | |||||
| Kwon et al. | 2003 |
N = 14 controls, 14 OCD |
FDG PET, resting |
OCD>healthy controls | R OFC | 26 | 28 | −24 | MNI | <0.005 |
p<0.005 uncorrected but with cluster size threshold of >50 contiguous voxels |
| OCD>healthy controls | L insula | −42 | −8 | 4 | MNI | <0.005 | |||||
| Healthy controls>OCD | L inferior parietal | −48 | −54 | 56 | MNI | <0.005 | |||||
| Healthy controls>OCD | L parieto-occipital | −10 | −84 | 32 | MNI | <0.005 | |||||
| Van den Heuval et al. |
2004 |
N = 10 controls, 11 OCD |
Oxygen-15 PET, symptom provocation |
OCD>healthy controls | L amygdala | −18 | −6 | −18 | TAL | <0.001 |
p<0.001 uncorrected but with cluster size threshold of >5 contiguous voxels |
| Healthy controls>OCD | L DLPFC | −18 | 44 | 32 | TAL | <0.001 | |||||
| Healthy controls>OCD | R caudate | 18 | 10 | 14 | TAL | <0.001 | |||||
| Stein et al. | 2006 |
N = 11 controls, 5 OCD |
PET, disgust inducing task |
OCD>healthy controls | L insula | 42 | −4 | 0 | MNI | 0.002 | Region of interested, p value at voxel level FWE corrected for volume |
| Harrison et al. | 2006 |
N = 9 controls, 7 OCD |
PET, Cognitive Stroop |
Healthy controls>OCD | L postcentral gyrus | −24 | −30 | 48 | MNI | <0.001 |
p<0.001, >20 contiguous voxels. Multivariate analysis also conducted, finding abnormalities in similar areas |
| Healthy controls>OCD | L medial frontal gyrus |
−26 | 66 | 16 | MNI | <0.001 | |||||
| Healthy controls>OCD | L medial frontal gyrus |
−6 | −58 | −8 | MNI | <0.001 | |||||
| OCD>healthy controls | L caudate | −16 | −6 | 16 | MNI | <0.001 | |||||
Further evidence for OFC involvement in OCD comes from the finding that treatment with selective serotonin reuptake inhibitors (SSRIs), a current first-line treatment for OCD (though cognitive therapy is also effective), has been shown to be associated with a down-regulation of 5HT-1D autoreceptors in the OFC in animal studies. Moreover, this down-regulation occurs over a time period of 8 weeks, compatible with the time course for therapeutic effects of SSRIs in OCD (el Mansari et al., 1995; Bergqvist et al., 1999). However, our understanding of the role of serotonin in OCD is complicated by the fact that approximately 50% of patients with OCD do not respond to SSRIs (Greist et al., 1995). Additionally there is indirect evidence for a role of other neurotransmitters in OCD, such as dopamine (Denys et al., 2004). For example dopamine D2 receptor antagonists have been used with some success to augment the effects of SSRIs in treating OCD (Westenberg et al., 2007).
Findings from PET studies have been less consistent for the caudate, particularly once measures have been normalised to a reference value, e.g., the cortical mean, to correct for global metabolic differences between groups. A review of this literature found that whereas increased function in the frontal cortex was evident in OCD, the available structural and functional neuroimaging literature did not consistently verify dysfunction of the caudate in the disorder (Aylward et al., 1996).
However, there is indirect evidence for basal ganglia involvement in OCD from findings that patients who suffer focal lesions in the striatum or the area it projects to, the pallidum, often then exhibit striking obsessive-compulsive behaviours (Rapoport and Wise, 1988; Laplane et al., 1989). Additionally the ventral caudate has been shown to be a promising site for deep brain stimulation treatment of refractory OCD (Aouizerate et al., 2004). Furthermore there is an emerging literature of striatal cognitive dysfunction in OCD, for example in implicit learning (Rauch et al., 2007). It has been suggested that discrepancies in basal ganglia findings in OCD may have resulted from heterogeneity within the disorder both in terms of diagnostic classification and the underlying brain pathology (Saxena, 2003; see Section 4.1).
There are also numerous functional magnetic resonance imaging (fMRI) studies of OCD investigating brain activity during symptom provocation and executive function paradigms, both in cohorts of patients alone, and in case-control studies comparing OCD patients with healthy volunteers. Previous reviews of OCD have detailed such studies in narrative/tabular form (Saxena, 2003; Friedlander and Desrocher, 2006), indicating support for orbitofronto-striatal abnormalities in OCD, However, it is often difficult to directly compare fMRI studies due to differences in anatomical labelling systems and localisation methods. Despite the wide range of paradigms employed by these studies, we wanted in some way to synthesise this large body of literature, and to test for any anatomical commonality across regions which have been reported to show abnormal activation (either increased or decreased) in OCD patients compared with healthy controls. A quantitative voxel-level meta-analysis was performed on fMRI case-control studies of OCD using activation likelihood estimation (ALE) software. In ALE, a group of studies is tested for concordance by modelling each reported focus of activation as the centre of a Gaussian probability distribution. These are then summed to create a statistical map that estimates the activation likelihood, as determined by the whole group of studies, for each voxel across the whole brain. Studies were included if they reported coordinates from contrasts assessing activation differences between OCD patients and healthy controls during task performance (Shapira et al., 2003; Ursu et al., 2003; Cannistraro et al., 2004; Mataix-Cols et al., 2004; Fitzgerald et al., 2005; Maltby et al., 2005; Nakao et al., 2005a; Schienle et al., 2005; van den Heuvel et al., 2005; Viard et al., 2005; Remijnse et al., 2006; Lawrence et al., 2007; Rauch et al., 2007; Roth et al., 2007; Yucel et al., 2007). Studies not directly assessing case-control activation differences between patients and healthy volunteers were not included. A full description of the method can be found elsewhere (Turkeltaub et al., 2002; Laird et al., 2005c). Briefly, the spatial normalisation template was noted for each study and coordinates were then automatically transformed into a single standard space template (Talairach and Tournoux, 1988) using the icbm2tal transform (Lancaster et al., 2007). The studies were then inserted into a database in BrainMap (Fox and Lancaster, 2002; Laird et al., 2005b) for further analysis. Each included focus was blurred with a kernel width (FWHM) of 12 mm, and the ALE statistic was computed for every voxel in the brain. Statistical significance was determined by a permutation test corrected for multiple comparisons; 5000 permutations of randomly generated foci were performed, using the same smoothing kernel and the same number of foci used in computing the ALE values. The final ALE maps were thresholded at p<0.05 (FDR-corrected) and overlaid onto a template generated by spatially normalising the ICBM template to Talairach space (Kochunov et al., 2002; Laird et al., 2005a). To our knowledge this is the first such meta-analysis of fMRI data of OCD.
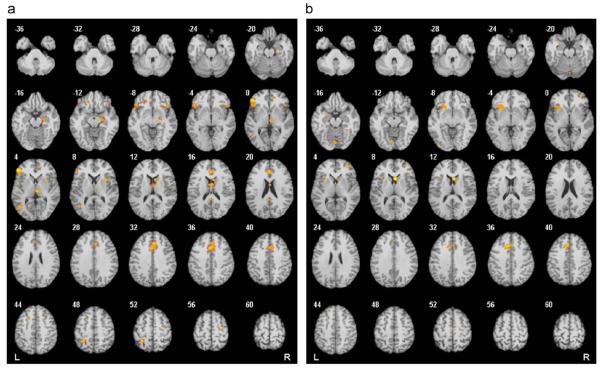
The results of this meta-analysis (Fig. 2 and Table 2) provide clear support for abnormalities of orbitofrontostriatal regions in OCD. However, there are also consistent foci of activation abnormalities in lateral frontal, anterior cingulate, middle occipital and parietal cortices and cerebellum, suggesting that more distributed large-scale brain systems may be involved in OCD. However, it must be borne in mind that combining studies using many different paradigms is an oversimplification, justified by the desire to know overall if there are consistent abnormalities in OCD, but troubled by the difficulty of interpreting what this activation might mean in terms of related cognitive function on task performance. Future meta-analysis of fMRI data on OCD will seek to address this issue of task heterogeneity in more detail.
Fig. 2.
Results from a quantitative voxel-level meta-analysis of fMRI studies reporting case-control differences for OCD across a range of paradigms: (a) areas where activation was greater in OCD patients than healthy controls (p<0.05) and (b) areas where activation was greater in healthy controls than OCD patients (p<0.05). R and L markers denote side of brain, numbers denote z dimension of each slice in MNI space. See Table 2 for full details of anatomical coordinates.
Table 2.
Significant foci of case-control abnormalities in fMRI studies of OCD from an ALE meta-analysis
| Cluster | Volume (mm3) | Weighted centre |
Maximum ALE value |
ALE maxima and submaxima |
Region/approximate BA | ||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | ||||
| Activation in OCD patients>activation in healthy controls | |||||||||
| 1 | 4912 | 3.0 | 22.8 | 31.0 | 0.0080 | −2 | 14 | 34 | L anterior cingulate BA 32 |
| 0.0073 | 0 | 32 | 18 | L anterior cingulate BA 32 | |||||
| 0.0069 | 6 | 28 | 34 | R medial frontal gyrus BA 6 | |||||
| 0.0064 | 8 | 18 | 38 | R anterior cingulate BA 32 | |||||
| 2 | 2960 | −44.8 | 29.5 | −0.4 | 0.0094 | −46 | 36 | 2 | L inferior frontal gyrus BA 45 |
| 0.0061 | −44 | 22 | −6 | L inferior frontal gyrus BA 47 | |||||
| 3 | 1064 | 22.1 | −15.8 | −12.9 | 0.0062 | 22 | −16 | −14 | R parahippocampal gyrus BA 28 |
| 4 | 888 | 2.1 | −3.8 | 15.6 | 0.0066 | 4 | −4 | 16 | R thalamus |
| 5 | 768 | −27.0 | −47.9 | 49.9 | 0.0078 | −28 | −48 | 50 | L parietal (precuneus) BA 7 |
| 6 | 704 | 6.2 | −21.1 | 3.2 | 0.0074 | 6 | −22 | 4 | R thalamus |
| 7 | 648 | −40.4 | −64.2 | 4.5 | 0.0069 | −42 | −64 | 4 | L mid occipital gyrus BA 37 |
| 8 | 640 | 32.2 | 10.1 | 7.8 | 0.0074 | 32 | 10 | 8 | R claustrum |
| 9 | 592 | 43.3 | 21.1 | −4.8 | 0.0059 | 44 | 22 | −6 | R inferior frontal gyrus BA 47 |
| 10 | 440 | 8.1 | 10.0 | 13.8 | 0.0060 | 8 | 10 | 14 | R caudate |
| 11 | 360 | −16.1 | 31.6 | −10.0 | 0.0058 | −16 | 32 | −10 | L medial frontal gyrus BA 10 |
| 12 | 320 | 12.6 | 42.5 | 0.6 | 0.0059 | 12 | 42 | 0 | R anterior cingulate BA 32 |
| 13 | 312 | −0.6 | −44.8 | 18.1 | 0.0059 | −2 | −44 | 18 | L posterior cingulate BA 30 |
| 14 | 304 | 24.7 | 20.7 | −7.7 | 0.0057 | 24 | 20 | −8 | R claustrum |
| 15 | 288 | −16.9 | 12.3 | 43.8 | 0.0058 | −18 | 12 | 44 | L medial frontal gyrus BA 32 |
| 16 | 256 | 25.1 | −16.8 | 54.8 | 0.0058 | 24 | −18 | 56 | R precentral gyrus BA 6 |
| Activation in healthy controls>activation in OCD patients | |||||||||
| 1 | 1920 | −26.3 | 17.9 | −2.8 | 0.0048 | −20 | 16 | −6 | L putamen |
| 0.0048 | −24 | 16 | −2 | L putamen | |||||
| 0.0044 | −34 | 18 | −2 | L inferior frontal BA 47 | |||||
| 0.0041 | −32 | 18 | 10 | L insula BA 13 | |||||
| 2 | 1864 | −6.2 | 20.7 | 36.7 | 0.0061 | −6 | 22 | 38 | L anterior cingulate BA 32 |
| 3 | 928 | 5.3 | 13.6 | 9.6 | 0.0071 | 6 | 14 | 10 | R caudate |
| 4 | 624 | 32.5 | 47.5 | 2.6 | 0.0053 | 32 | 48 | 2 | R inferior frontal BA 44 |
| 5 | 496 | 0 | −79.4 | −15.7 | 0.0048 | 0 | −80 | −16 | R cerebellum |
| 6 | 280 | −24.5 | −3.2 | −18.4 | 0.0041 | −24 | −8 | −16 | L parahippocampal gyrus/amygdala |
| 0.0041 | −24 | 2 | −20 | L uncus BA 28 | |||||
Abbreviations: L, Left; R, Right; BA, Brodmann area.
Talairach coordinates are reported.
2.4. How might deficits in the regions within this frontostriatal circuit relate to the expression of OCD symptoms?
The precise relationship between OCD symptom expression and dysregulation of the orbitofronto-striatal circuit has yet to be well-characterised. Graybiel and Rauch (2000) have put forward a cortico-basal ganglia model of OCD based on findings that the basal ganglia influence both motor pattern generators in the spinal cord and brainstem as well as putative ‘cognitive pattern generators’ in the cerebral cortex. They suggest that activity in frontostriatal loops may be involved in establishing cognitive habits, just as they are in the development of motor habits. Saxena et al. (1998, 2001a) have explored this model further, proposing that OCD is mediated by an imbalance between the direct (excitatory) and indirect (inhibitory) pathways within this circuit, which leads to emergence of obsessive-compulsive behaviours. Multiple tiers of evidence implicate the OFC in the representation of rewards and punishments (O'Doherty et al., 2001; Murray et al., 2007), in anxiety and emotional processing (Zald and Kim, 1996b; Kalin et al., 2007) and in inhibitory control (Elliott et al., 2000). Since compulsions are classically posited to reduce anxiety, and suggest underlying inhibitory deficits (Chamberlain et al., 2005), it is plausible that OFC dysfunction plays an important role in the manifestation of OCD symptoms. Indeed, PET studies show that OFC activity in OCD patients is increased compared to controls in the resting state (see Section 2.3 and Table 1) and furthermore, that this normalises following successful treatment (Saxena et al., 1999).
3. Evidence from cognitive studies of OCD
There are interesting paradoxes when considering support for the orbitofronto-striatal model from cognitive studies of OCD. Below, consistent findings of impairment in response inhibition and attentional set-shifting are contrasted with inconsistent reports on decision-making. Deficits in spatial working memory (van der Wee et al., 2003) and implicit learning (Rauch et al., 2007) have also been reported.
3.1. Response inhibition
Using objective computerised neuropsychological tests, several studies have reported response inhibition deficits in OCD, using tasks such as go/no-go and stop-signal reaction time (SSRT) which examine motor inhibitory processes, and also the Stroop task, a putative test of cognitive inhibition (Hartston and Swerdlow, 1999; Bannon et al., 2002, 2006; Penades et al., 2005, 2007; Chamberlain et al., 2006, 2007b). For example, response inhibition deficits have been reported in OCD patients when performing the SSRT, which measures the time taken to internally suppress pre-potent motor responses (Chamberlain et al., 2006). Unaffected first-degree relatives of OCD patients are also impaired on this task compared with unrelated healthy controls, suggesting that response inhibition may be an endophenotype (or intermediate phenotype) for OCD (Chamberlain et al., 2007b; see Section 5). Interestingly, performance of the SSRT is thought to be critically dependent upon an intact right inferior frontal gyrus in patients with frontal lesions, but not correlated with the extent of damage to the right orbitofrontal gyrus (Aron et al., 2003, 2004). However, functional neuroimaging studies of motor inhibition have generally identified a more extensive system of regions including orbitofrontal, anterior cingulate, dorsolateral and medial frontal, temporal and parietal cortices, the cerebellum and the basal ganglia (Godefroy et al., 1996; Humberstone et al., 1997; Garavan et al., 1999; Rubia et al., 1999, 2000, 2001a, 2001c; Horn et al., 2003). Notably, many of these areas have not yet been targets of investigation by neuroimaging in OCD and so the existence of structural abnormalities in these regions in OCD is unknown.
3.2. Set shifting
Deficits in cognitive set shifting are also evident in OCD. These have been concerned with two quite different forms of shift: affective set shifting, where the affective or reward value of a stimulus changes over time (e.g., a rewarded stimulus is no longer rewarded), and attentional set shifting, where the stimulus dimension (e.g., shapes or colours) to which the subject must attend is changed. Work in primates has suggested a double dissociation between these types of shift, with affective shifts being dependent upon the OFC and attentional shifts requiring the lateral prefrontal cortex (Roberts et al., 1992; Dias et al., 1996; Hornak et al., 2004).
At first glance, the literature suggests impairments in OCD in both affective and attentional shift domains as exemplified by the Object Alternation Task (OAT) and the CANTAB intra-dimensional/extra-dimensional (ID/ED) set shifting task, respectively (Veale et al., 1996; Abbruzzese et al., 1997; Aycicegi et al., 2003; Watkins et al., 2005; Chamberlain et al., 2006). However, there have been recent doubts concerning the specific sensitivity of the OAT to orbitofrontal damage since other frontal lobe lesions may affect this task (Freedman et al., 1998). The sensitivity of the OAT to cognitive dysfunction in OCD is also questionable; 1 study showed intact performance on this task in OCD patients (Katrin Kuelz et al., 2004). Using a probabilistic learning and reversal task where subjects must acquire and then reverse a two-choice visual discrimination, a task reported to be dependent on the integrity of the OFC (Fellows and Farah, 2003), a recent study also reported intact performance in (largely medicated) patients with OCD (despite impairment in other cognitive domains) (Chamberlain et al., 2007a). This finding suggests either that this probabilistic reversal learning task is not sufficiently sensitive to identify what are likely therefore to be subtle changes in orbitofrontal regions in OCD; that OCD patients do not have cognitive deficits on orbitofrontal-based cognitive tasks; or that SSRIs affect performance on this task. Of note, a reversal learning fMRI study reported reduced activation of OFC, dorsolateral prefrontal cortex, anterior prefrontal cortex and insula in OCD patients during affective switching compared with healthy controls (Remijnse et al., 2006), indicating that there may be abnormalities in patients during reversal learning tasks at a brain functional level. However, it must be appreciated that this study involved considerable patient co-morbidity which could account for aberrant activations.
Deficits of attentional set shifting in OCD have been found in several neurocognitive studies using the CANTAB ID/ED set shifting task (Veale et al., 1996; Watkins et al., 2005; Chamberlain et al., 2006, 2007b). This deficit is most consistently reported at the ED stage (in which the stimulus dimension, e.g., shape, colour or number, alters and subjects have to inhibit their attention to this dimension and attend to a new, previously irrelevant dimension). The ED stage is analogous to the stage in the Wisconsin Card Sorting Task where a previously correct rule for card sorting is changed and the subject has to respond to the new rule (Berg, 1948). This ED shift impairment in OCD patients is considered to reflect a lack of cognitive or attentional flexibility and may be related to the repetitive nature of OCD symptoms and behaviours. Deficits in attentional set shifting are considered to be more dependent upon dorsolateral and ventrolateral prefrontal regions than the orbital prefrontal regions included in the orbitofronto-striatal model of OCD (Pantelis et al., 1999; Rogers et al., 2000; Nagahama et al., 2001; Hampshire and Owen, 2006), again suggesting that cognitive deficits in OCD may not be underpinned exclusively by OFC pathology.
3.3. Planning
There is also evidence for dorsolateral prefrontal cortex (DLPFC) dysfunction in patients with OCD, in conjunction with impairment on a version of the Tower of London, a task often used to probe planning aspects of executive function. A neuroimaging study demonstrated planning impairments in OCD patients on this task, which were associated with decreased activation compared with matched controls in DLPFC, premotor cortex, anterior cingulate cortex, precuneus, inferior parietal cortex, caudate and putamen (van den Heuvel et al., 2005). The authors propose that their findings represent decreased responsiveness in dorsal prefronto-striatal circuits in OCD, suggesting that cognitive impairments in OCD are related to differences in brain regions outside the affective orbitofrontal loop, perhaps also including abnormalities in the dorsolateral prefronto-striatal loop (Alexander et al., 1986). This loop involves projections from the DLPFC and posterior parietal areas to the head of the caudate, then to the mediodorsal and ventral anterior thalamus via the globus pallidus and substantia nigra pars reticulata, and may be involved in spatial attention and working memory processes. Impairment on the Tower of London task has also been demonstrated in healthy first-degree relatives of OCD patients (Delorme et al., 2007).
3.4. Decision-making
Lesion studies have suggested that orbitofrontal damage often leads to impairments in decision-making (see Section 2.2), thought to reflect the function of the OFC in: (i) integrating affective information relayed from other limbic areas, (ii) attributing reward value to stimuli or behavioural response outcomes and (iii) suppressing responses which are now inappropriate. Indeed, some have even conceptualised OCD as a disorder of decision-making caused by orbitofrontal dysfunction (Sachdev and Malhi, 2005). However, using tasks such as the Iowa gambling task, which aims to simulate real-life decision-making and is known to be sensitive to frontal lobe dysfunction, there have been inconsistent findings as to whether OCD patients have decision-making impairments (Bechara et al., 1994; Cavedini et al., 2002; Nielen et al., 2002). In addition, using a different task, the Rogers et al. (1999) gamble task, decision-making has been repeatedly found to be intact in patients with OCD despite impairment on other tasks (Watkins et al., 2005; Chamberlain et al., 2007a).
In summary, there is some incongruence between cognitive findings and the orbitofronto-striatal model of OCD. Although there is reasonably well-replicated evidence that patients with OCD are impaired in response inhibition and in attentional set-shifting, regions thought to be necessary for these functions are not exclusively limited to the lateral orbitofrontal loop. In addition, there is little conclusive evidence to suggest that patients with OCD are impaired on tasks classically thought to be dependent upon the OFC, such as reversal learning and decision-making. Findings from the cognitive tests discussed in this review are summarised in Table 3, along with putative brain regions thought to be important for each cognitive process. These findings raise questions concerning the relationship between cognitive function and the orbitofrontal dysfunction identified in OCD and the potential involvement of additional brain regions in OCD pathology. This will be discussed further after a consideration of the structural MRI findings in OCD.
Table 3.
Summary of findings from neuropsychological testing of OCD patients on the cognitive tasks discussed in this review
| Cognitive process | Cognitive subdomain |
Task(s) | Putative underlying brain region(s) | Findings in OCD to date |
|---|---|---|---|---|
| Inhibitory control | Motor inhibition | Stop-signal task; Go/no-go task |
Predominantly a right-sided network including right inferior frontal gyrus, anterior cingulate, frontal, temporal and parietal cortices |
Impaired: Bannon et al. (2002, 2006); Chamberlain et al. (2006a, 2007b); Penades et al. (2007) Intact: Bohne et al. (2008)a |
| Cognitive inhibition |
Stroop task | Impaired: Hartston and Swerdlow (1999); Penades et al. (2005, 2007); Bannon et al. (2002, 2006) |
||
| Cognitive flexibility | Attentional set shifting |
CANTAB intra- dimensional/extra- dimensional task |
Ventrolateral and dorsolateral prefrontal cortex |
Impaired: Veale et al. (1996); Watkins et al. (2005); Chamberlain et al. (2006a, 2007b) |
| Simple reversal | Object alternation task |
Orbitofrontal cortex, medial prefrontal, anterior cingulate, frontal pole |
Intact: Nielen and Den Boer (2003)b Impaired: Abbruzzese et al. (1997); Aycicegi et al. (2003) Intact: Katrin Kuelz et al. (2004) |
|
| Probabilistic and reversal learning |
Probabilistic reversal learning |
Orbitofrontal cortex | Impaired: Remijnse et al. (2006)c Intact: Chamberlain et al. (2007a) |
|
| Executive planning | Tower of London | Dorsolateral prefrontal cortex and associated network including premotor cortex, anterior cingulate, precuneus, inferior parietal cortex, caudate and putamen |
Impaired: Veale et al. (1996); Nielen and Den Boer (2003); Van den Heuvel et al. (2005); Chamberlain et al. (2007a) | |
| Decision-making | Iowa gambling task | Orbitofrontal cortex and other frontal areas |
Impaired: Cavedini et al. (2002) Intact: Nielen et al. (2002) |
|
| Rogers gambling task |
Orbitofrontal cortex and other frontal areas |
Intact: Watkins et al. (2005); Chamberlain et al. (2007a, b) |
||
| Implicit learning | Serial reaction time task |
Caudate/ventral striatum, hippocampus, frontal areas |
Intact: no between-group behavioural difference but aberrant recruitment of brain regions in an fMRI study (Rauch et al. (2007) |
|
Likely due to relative task insensitivity (go/no-go).
Unclear how data for subjects ‘failing’ stages were dealt with in this study; exclusion of data for subjects failing a stage is unduly conservative.
Fewer points accumulated. 50–90% of participants had a co-morbid axis-I mood disorder—a major confound for probabilistic learning (depressive patients show deficits on this task).
4. Findings from structural MRI
4.1. Region of interest studies
The evidence in support of the orbitofronto-striatal model of OCD from PET studies (see Section 2.3) has led many groups to search within these regions for structural brain abnormalities in patients. The majority of studies have utilised a case-control region-of-interest (ROI) approach, which was until recently the main analysis method available. These studies have suggested that structural abnormalities are evident in OCD patients within the affective fronto-striatal loop. The most consistent finding has been reduced volume in OFC (Szeszko et al., 1999; Choi et al., 2004; Kang et al., 2004; Atmaca et al., 2006, 2007); significant abnormalities have also been reported elsewhere, including in the basal ganglia, thalamus, amygdala, anterior cingulate and hippocampus (Scarone et al., 1992; Robinson et al., 1995; Aylward et al., 1996; Jenike et al., 1996; Rosenberg and Keshavan, 1998; Szeszko et al., 1999, 2004a; Gilbert et al., 2000; Kwon et al., 2003; Choi et al., 2004, 2006; Kang et al., 2004; Atmaca et al., 2006, 2007).
In accordance with the orbitofronto-striatal model of OCD, reduced striatal volumes have been reported several times in patients (Robinson et al., 1995; Rosenberg et al., 1997b; Szeszko et al., 2004a), but the direction of findings is not entirely consistent; increased volume of the head of the right caudate has also been reported in OCD (Scarone et al., 1992) and a review in 1996 reported no consistent differences in caudate volume in OCD (Aylward et al., 1996).
The thalamus has also been implicated with findings of increased volume in OCD (Gilbert et al., 2000; Atmaca et al., 2006), and a subsequent reduction of thalamic volume after 12-week treatment with the SSRI paroxetine (Gilbert et al., 2000) but not following Cognitive Behavioural Therapy (CBT) (Rosenberg et al., 2000). Given that SSRIs and CBT are both recognised as first-line treatments of OCD (Stein et al., 2007), with some positing cognitive therapy to be more effective than SSRIs (Foa et al., 2005), these data suggesting a differential effect of cognitive and pharmacological treatments on brain structure are of considerable interest. However, replication is warranted when comparing findings from studies using small samples, and there is also a need to study therapy-associated effects on brain structure beyond a 12-week treatment period, particularly in the case of CBT where treatment-response time may be longer than this (Grados et al., 1999).
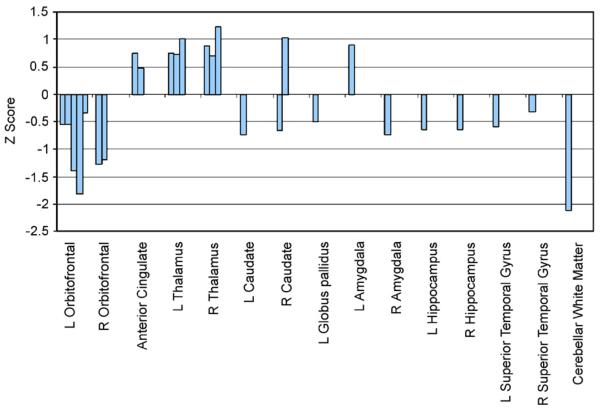
Other studies have emphasised limbic elements of the circuit with findings of significantly increased anterior cingulate volume (Szeszko et al., 2004a), decreased hippocampal volume (Kwon et al., 2003), a loss of the normal hemispheric asymmetry of the hippocampal–amygdala complex (Szeszko et al., 1999) and other amygdala volumetric differences (Szeszko et al., 1999, 2004b). Finally, reduced pituitary volume has been reported in unmedicated children with OCD (MacMaster et al., 2006) and reduced anterior superior temporal volume has also been found in patients with OCD (Choi et al., 2006). Other studies have chosen to look specifically at white matter abnormalities, with findings of reduced total white matter (Jenike et al., 1996), decreased retrocallosal white matter including in the parieto-occipital area (Breiter et al., 1994; Jenike et al., 1996), and increased size of the corpus callosum in patients with OCD (Rosenberg et al., 1997a). See Fig. 3 summarising z-scores for significant findings from previous ROI studies.
Fig. 3.
Summary of significant structural abnormalities identified by case-control, region-of-interest MRI studies of OCD patients compared with healthy controls. Positive z-scores represent increased region volume in OCD patients compared with healthy controls, negative z-scores represent decreased region volume in OCD. Each bar represents the z-score for a significant case-control difference identified from a previous study.
Given the diversity of methods used to examine the OFC, for example in defining anatomical boundaries, the repeated finding of reduced OFC volume in OCD patients appears to be remarkably robust (though this could represent a publication bias). However, these MRI findings also demonstrate inconsistencies in the direction of reported volume changes of some structures, particularly with regard to the basal ganglia. This discrepancy may be partly explained by heterogeneity within the OCD phenotype. For example, in a subgroup of patients with OCD, basal ganglia enlargement may occur as a result of antibody-mediated inflammation as part of Paediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infection (PANDAS) (Giedd et al., 2000; Peterson et al., 2000; Swedo and Grant, 2005). On the other hand, some OCD studies may have included more patients with co-morbid tic disorders such as Tourette's syndrome, in which there is evidence to suggest reduced basal ganglia volumes (Peterson et al., 1993). Additionally differences in striatal findings may be related to age differences between samples, which are known to have an effect on striatal volume (Toga et al., 2006). Further evidence is still required to fully assess the occurrence of structural abnormalities in other limbic regions such as the amygdala and hippocampus.
4.2. Limitations of the region of interest approach
While the findings from case-control ROI studies are clearly relevant in elucidating the neurobiology of OCD, there are several methodological limitations which may account for inconsistent findings. The ROI method requires that regions are manually delineated; a subjective and technically laborious process for which operators must be rigorously trained. This has restricted studies in terms of the number of regions and subjects that can be feasibly investigated. Thus in most cases analysis has focused upon one or two regions within a small sample, limiting statistical power and generalisability of results. Also, methods for manually defining anatomical landmarks differ between studies which may lead to inconsistencies in reported volume changes; and separate analyses of several regions present a multiple comparisons issue which is not always accounted for.
Additionally, an a priori hypothesis is required because researchers must choose specific regions to investigate based on prior evidence. This approach carries with it the obvious caveat of self-fulfilling findings in that if one only searches where case-control differences are expected, the potential to discover theoretically unanticipated regions is limited. There is also a question of which evidence should be used to formulate such hypotheses. The hypothesis typically employed in structural MRI studies investigating OCD centres on abnormalities in the orbitofrontostriatal circuit. This hypothesis is largely based on evidence from functional neuroimaging (see Section 2.3), initially from PET studies measuring cerebral glucose metabolism and regional cerebral blood flow (rCBF), both at rest and during symptom provocation (Baxter et al., 1987, 1988; Nordahl et al., 1989; Swedo et al., 1989; Sawle et al., 1991; McGuire et al., 1994; Rauch et al., 1994; Cottraux et al., 1996). Interestingly, following the seminal paper by Baxter et al. (1987), many of these PET studies were also heavily based on an ROI approach, only examining select regions such as those originally reported by Baxter et al., e.g., the OFC and caudate. Therefore, critically implicated regions may have been overlooked. Additionally, it may not always be the case that functional differences reflect structural abnormalities within the same brain areas, i.e., regions of functional, metabolic and structural abnormalities in OCD may not be equivalent. In summary, we advocate a cautious approach when developing a hypothesis of which brain regions to choose for any ROI-based analysis as this requires narrowing the field of investigation and so may neglect findings elsewhere in the brain.
4.3. Whole brain-based analyses
In recent years, whole brain-based analyses using voxel-level analysis methods (Bullmore et al., 1999; Ashburner and Friston, 2000) have also provided some intriguing results about OCD. A key feature of this rapid and automated method of analysis is that it examines differences in grey matter throughout the brain, without the need to pre-specify regions of interest for investigation. This unbiased approach is of value both to confirm the validity of the orbitofronto-striatal hypothesis developed in ROI studies and also to reveal grey matter differences in areas not previously considered. The potential utility of voxel-level analysis is demonstrated by schizophrenia research, where this has consistently revealed decreased grey matter concentrations in the insula, an unpredicted area not previously examined in earlier ROI studies (Wright et al., 1999; Sigmundsson et al., 2001; Hulshoff Pol et al., 2002; Kubicki et al., 2002).
The whole brain-based structural MRI studies currently available are far fewer in the field of OCD than in schizophrenia; there are three published VBM studies on OCD yet there were at least 15 published VBM studies on schizophrenia by May 2004; for review see Honea et al. (2005). We performed a quantitative voxel-level meta-analysis (using ALE software) on these structural studies of OCD. However, the available coordinates from the few studies published to date were insufficient to reveal significant foci of structural abnormalities (for methodological details of ALE see Section 2.3). Thus the evidence that can be drawn from VBM studies on OCD is only preliminary at this stage but certainly may already help to reveal whether OCD models proposing dysfunction in the orbitofrontal–striatal loop are sufficient to provide a comprehensive account of OCD.
4.3.1. VBM in OCD
The first VBM study of OCD involved 25 patients and 25 controls (Kim et al., 2001). Eight patients were medication-naive, the others were taking anti-obsessional medication or neuroleptics, but were medication-free for 4-weeks prior to scanning. Four patients had co-morbidities, 21 had OCD as their sole diagnosis. The authors reported increased grey matter density in left OFC, superior temporal gyrus, inferior parietal lobule, thalamus, right insula, middle temporal gyrus, inferior occipital cortex, and bilateral hypothalamus; and decreased grey matter density in the left cerebellum and cuneus. Although differences were reported in many regions, findings were not corrected for multiple comparisons so type I errors (false positives) may prevail and replication is required.
Pujol et al. (2004) reported a second VBM study in 72 patients and 72 healthy controls. This large study employed a modulated method of VBM, ‘optimised VBM’ (Good et al., 2001), where a study-specific template is used to reduce possible errors during image normalisation. Additionally, voxel values were modulated by Jacobian determinants to restore volume differences which have been removed during normalisation, allowing an indication of volume changes as opposed to only changes in grey matter density. Five patients were medication-naïve, 67 had previously received medication including SSRIs, clomipramine, and neuroleptics; 18 of these subjects were medication-free for 4 weeks prior to scanning. Co-morbidity with anxiety and depressive symptoms was not considered an exclusion criterion provided that OCD was the primary clinical diagnosis. The authors reported significant absolute volume decreases in right medial frontal gyrus, left medial OFC and the left insular–opercular region. They also reported significant grey matter increases relative to global grey matter volume in bilateral putamen and left anterior cerebellum. Additionally they described interregional volume correlations between these six locations in patients, with an inverse correlation between subcortical and cortical structures, positive correlation between cortical regions and positive correlation between left and right striatum. In the control group, only the latter of these was found to be significant, suggesting an abnormal anatomical connectivity in patients with OCD. Finally the authors showed an apparent preservation of bilateral ventral striatal areas (i.e., an absence of age-related volume decrease) in patients but not in controls.
Another recent whole brain study in OCD also used optimised VBM to assess grey matter volumes in 19 OCD patients and 15 healthy controls (Valente et al., 2005). Eight patients were medication-free for at least 3 weeks prior to scanning, 4 patients were taking SSRIs and seven patients were taking clomipramine. Seven patients fulfilled DSM-IV (American Psychiatric Association, 1994) criteria for MDD, seven for social phobia, five for specific phobia, three for generalised anxiety disorder, one for panic disorder and one for ADHD. Based on their a priori ROI hypothesis, the authors performed an analysis uncorrected for multiple comparisons on the orbitofrontal, anterior cingulate, striatal, thalamic and temporo-limbic regions previously implicated in imaging studies of OCD. They then performed a corrected whole brain-based VBM analysis, to search for additional volumetric abnormalities not previously assessed in ROI-based MRI studies. Both types of analysis were performed within the whole sample, and within OCD subjects without major depression (n = 12) versus healthy controls (n = 15). When assessing OCD patients without depression (uncorrected p⩽0.001), the authors reported increased grey matter in areas of left OFC and insula, left parahippocampal gyrus/uncus/amygdala, and right parahippocampal and fusiform gyri; and decreased grey matter in right OFC and left anterior cingulate/medial frontal gyri. The report of increased grey matter in left OFC was the only predicted finding that survived correction for multiple comparisons (corrected p-value of 0.043). However, it is striking that this study revealed one unpredicted region of grey matter decrease, in the angular and supramarginal gyri of the right parietal lobe (whole brain corrected p = 0.004).
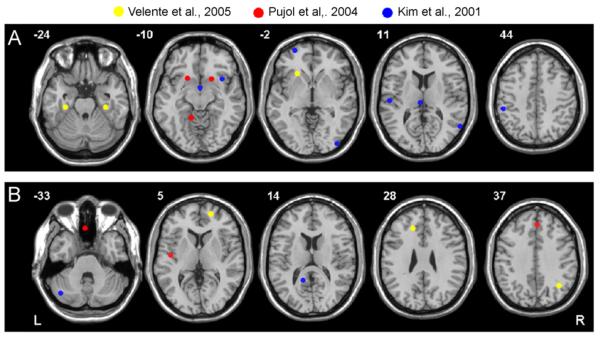
In summary, these VBM findings provide some supportive evidence for orbitofronto-striatal structural abnormalities in OCD, for example there are two further findings of reduced grey matter in orbitofrontal regions (Valente et al., 2005; Pujol et al., 2004), and there are findings of increased grey matter in striatal areas (Pujol et al., 2004), consistent with some findings from ROI structural MRI studies (see Section 4.1). However, two of the three VBM studies have reported structural changes in parietal regions; left inferior parietal lobe (Kim et al., 2001) and right supramarginal and angular gyri (Valente et al., 2005). These results suggest that the parietal lobe should be a focus for further exploration of structural abnormalities in OCD; this is echoed by findings from PET and fMRI studies as described below (see Section 5.1). Anatomical regions reported in the whole brain-based VBM analyses are shown in full in Table 4; Fig. 4 shows peak coordinates of whole brain VBM findings in OCD.
Table 4.
Voxel-based morphometry MRI analyses of OCD
| Study | Talairach coordinatesa |
Location | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Regions of increased grey matter in patients | ||||
| Kim et al. (2001) | −25 | 55 | 6 | Left orbitofrontal cortex |
| −49 | −21 | 11 | Left superior temporal gyrus | |
| −50 | −35 | 41 | Left inferior parietal lobule | |
| −5 | −23 | 14 | Left thalamus | |
| 32 | 12 | −2 | Right insula | |
| 54 | −59 | 9 | Right middle temporal gyrus | |
| 36 | −84 | −4 | Right inferior occipital cortex | |
| −1 | −1 | −6 | Bilateral hypothalamus | |
| Pujol et al. (2004) | 17 | 12 | 0 | Right ventral putamen |
| −12 | −45 | −10 | Left anterior cerebellum | |
| −19 | 13 | −4 | Left ventral putamen | |
| Valente et al. (2005) b | −22 | 19 | 7 | Left posterior orbitofrontal cortex and anterior insula |
| −31 | −23 | −19 | Left parahippocampal gyrus, uncus, amygdala | |
| 30 | −23 | −18 | Right parahippocamapal and fusiform gyri | |
| Regions of decreased grey matter in patients | ||||
| Kim et al. (2001) | −40 | −64 | −34 | Left cerebellum |
| −14 | −49 | 13 | Left cuneus | |
| Pujol et al. (2004) | 1 | 31 | 39 | Right medial frontal gyrus |
| −4 | 33 | −19 | Left gyrus rectus (medial orbitofrontal cortex) | |
| −43 | −11 | 8 | Left posterior insula | |
| Valente et al. (2005) b | 34 | −61 | 35 | Right angular and supramarginal gyrus (parietal cortex) |
| 17 | 50 | 16 | Right anterior orbitofrontal cortex | |
| −17 | 27 | 33 | Left anterior cingulate and medial frontal cortex | |
Where analyses produced coordinates in MNI space, these coordinates were then converted to Talairach space using the icbm2tal transform (Lancaster et al., 2007).
Reported coordinates are for the sample including 12 patients, after subjects with co-morbid depression were excluded.
Fig. 4.
Summary of significant structural abnormalities identified by case-control, voxel-based morphometry studies of OCD. Peak MNI coordinates of decreased and increased grey matter from whole brain VBM studies are shown on axial brain images. The plotted coordinates indicate some agreement with traditional orbitofronto-striatal models of OCD but also a rationale to explore other theoretically unpredicted regions such as parietal areas: (A) areas of increased grey matter and (B) areas of decreased grey matter. R and L markers denote side of brain, numbers denote z dimension of each slice in MNI space.
4.3.2. Limitations of VBM studies
Limitations of the VBM studies performed to date may help account for inconsistencies in anatomical regions that have been proposed by each study. Firstly, one study did not correct for multiple comparisons and so may be affected by type I error (Kim et al., 2001). Further, the majority of patients from all three studies had been medicated previously and a considerable number were taking medication at the time of scanning.
Additionally, many of the patients in the three VBM studies had co-morbidities such as MDD, social phobia or panic disorder. Since the neural correlates of these disorders are not well-characterised and may differ from those of OCD, inclusion of such cases may lead to non-replication. For example, a PET study of glucose metabolism showed significantly reduced left hippocampal metabolism in subjects with MDD alone, and subjects with OCD and concurrent MDD, but not in individuals with OCD alone (Saxena et al., 2001b). Additionally, another glucose metabolism study, where scanning was followed by 8–12 weeks treatment with the SSRI paroxetine, showed that subsequent improvement in OCD symptoms was associated with a higher pre-treatment glucose metabolism in the right caudate nucleus, whereas improvement of MDD was significantly correlated with lower pre-treatment metabolism in the amygdala and the thalamus, and higher pre-treatment metabolism in the medial prefrontal cortex and anterior cingulate. This indicated that treatment-response has different neural substrates in the two disorders (Saxena et al., 2003).
A possible additional confound within imaging studies is the inclusion of subjects with a plethora of OCD symptoms. Previous work has suggested that different symptom dimensions, e.g., contamination/washing versus symmetry/ordering, may have distinct neural substrates (Phillips et al., 2000; Mataix-Cols et al., 2004), which may lead to inconsistent findings in groups of patients with differing symptom profiles. Further research in carefully selected samples is needed to explore this fully.
From a methodological perspective, there are some criticisms about potential confounds from the preprocessing methods employed in structural VBM, such as difficulties in alignment of non-homologous brains (Crum et al., 2003) and in choosing smoothing kernel size (Jones et al., 2005). However, VBM at least provides an objective starting point for identifying brain abnormalities across the whole brain which can be further validated by more in-depth ROI studies.
4.3.3. Multivariate systems-level approaches
Theoretically, OCD has been considered to result from dysfunction within a neurocognitive circuit, the orbitofronto-striatal loop, with structural and functional abnormalities across a brain system, rather than within discrete brain regions. In contrast, the methods employed by most imaging studies to date, using either ROI or mass univariate testing of individual voxels, will best identify case-control differences in individual voxels or regions. Theoretically more appropriate methods aiming to identify systems-level brain abnormalities in OCD have only been used very recently. For example, using the structural MRI data from a sample which underwent conventional VBM in a previous study (Pujol et al., 2004), a system of abnormal regions in OCD was identified by testing the sum of t-statistics across all voxels against a chi-square distribution (Soriano-Mas et al., 2007). This system comprised bilateral medial prefrontal areas, posterior cingulate and precuneus, cerebellum and posterior insula; grey matter volume in this system could be used to predict whether a subject had a diagnosis of OCD. Likewise, a recent PET study employing both univariate and multivariate methods to identify abnormalities in OCD reported functional abnormalities in cortico-striatal regions using both methods, but demonstrated relatively greater power of the multivariate approach to reveal functional network abnormalities (Harrison et al., 2006). We have recently used a multivariate method, partial least squares (PLS) (McIntosh et al., 1996), to identify two anatomical brain systems; a parieto-cingulo-striatal system, and a predominantly fronto-temporal system including OFC and inferior frontal gyri, in which increased and decreased grey matter, respectively, were associated with impairment on a motor response inhibition task (SSRT), in both OCD patients and their unaffected first-degree relatives, compared with healthy controls (Menzies et al., 2007). We were also able to demonstrate that anatomical variation within these two systems was familial, suggesting that brain structure in these systems is a candidate endophenotype for OCD and may represent a marker of genetic risk for OCD. Multivariate techniques such PLS are particularly advantageous since they allow flexible, combined analysis of both cognitive and imaging data, yet reduce significance testing to one or a few statistics and so do not incur the stringent thresholds usually required to control multiple comparisons entailed by mass univariate analyses.
5. Additional regions putatively involved in OCD
5.1. Evidence from cognitive studies
From a cognitive perspective, there has been sparse and inconsistent documentation of impairments in OCD patients on tasks classically defined as ‘orbitofrontal-dependent’, yet paradoxically other cognitive processes, not regarded to rely so heavily on orbitofrontal function, such as set shifting, response inhibition and planning, are frequently impaired in patients. These deficits have been reported in medicated and unmedicated patients (Mataix-Cols et al., 2002; Watkins et al., 2005; Chamberlain et al., 2006) and persist over time (Nielen and Den Boer, 2003; Roh et al., 2005; Bannon et al., 2006), despite the effects of pharmacological intervention, e.g., SSRIs, and CBT, on reducing anxiety, OCD symptom expression, and metabolic hyperactivity (Benkelfat et al., 1990; Baxter et al., 1992; Swedo et al., 1992; Saxena et al., 1999). Response inhibition and set shifting deficits are also evident in unaffected first-degree relatives of patients with OCD (Chamberlain et al., 2007b) and response inhibition deficits are associated with brain system structural abnormalities in patients and unaffected relatives (Menzies et al., 2007) indicating that they may be a marker of genetic risk for OCD, and could potentially be useful in clarifying both the diagnostic classification of OCD and its underlying genetic basis; for review of the principles of endophenotypes see Gottesman and Gould (2003). In summary, deficits in set shifting, planning and response inhibition indicate that regions exclusive of the orbitofronto-striatal loop, such as dorsolateral and ventrolateral prefrontal and parietal cortex may be involved in the pathology of OCD.
5.2. Evidence from PET
A number of functional imaging studies report findings in OCD from regions outside the orbitofronto-striatal circuit (Table 1). In the symptom provocation PET study by Rauch et al. (1994), when the authors carried out an additional, whole brain analysis for increased rCBF in OCD (including correction for multiple comparisons), the areas they found which approached significance included several outside orbitofronto-striatal regions, e.g., the angular gyrus in the parietal lobe, and visual association cortex. Furthermore, in the glucose utilisation PET study by Nordahl et al. (1989), the authors report significant abnormal findings in OCD, not just within predicted regions of OFC but also a reduction of glucose metabolism within bilateral parietal lobes. They state that these are preliminary findings requiring validation since they are uncorrected for multiple comparisons. However, even despite this exploratory approach, they did not find any significant abnormalities within the basal ganglia, an area considered to be part of a clearly hypothesised circuit for OCD. Further evidence supporting the involvement of parietal areas in OCD comes from another symptom provocation PET study which reported that cerebral blood flow at the temporo-parietal junction, particularly on the right, was negatively correlated with symptom intensity (McGuire et al., 1994). Additionally, SPECT studies measuring rCBF using technetium 99m d,l_hexamethyl propyleneamine oxime (99mTc-HMPAO) indicate parietal abnormalities in OCD (Rubin et al., 1992; Lucey et al., 1995). For example, in agreement with McGuire et al. (1994), Lucey et al. (1995) found a negative correlation between an obsessive-compulsive symptom dimension and right parietal rCBF.
5.3. Evidence from fMRI
FMRI studies using symptom provocation paradigms have been carried out which do in part suggest abnormal activation of the affective loop in patients during symptom exposure (Breiter et al., 1996; Adler et al., 2000; Shapira et al., 2003; Mataix-Cols et al., 2004; Nakao et al., 2005b; Schienle et al., 2005); this is also evident in the ALE meta-analysis including some of these studies (Fig. 2). These fMRI studies also show changes in activation of theoretically unanticipated regions such as the DLPFC and parietal cortex during symptom provocation, suggesting areas outside of the orbitofronto-striatal loop also respond abnormally to symptom exposure in OCD patients. A number of fMRI studies employing cognitive tasks also report abnormalities in regions of the DLPFC and parietal cortex (Maltby et al., 2005; van den Heuvel et al., 2005; Viard et al., 2005; Remijnse et al., 2006). See Fig. 2 and Table 2 for a summary of regions reported as abnormal in case-control fMRI studies of OCD.
Summarising the findings from functional, metabolic and structural imaging studies indicates that dysfunction in the orbitofronto-striatal circuit and connected limbic structures such as the anterior cingulate and amygdala contribute to the pathology of OCD. There is convincing data suggesting that: (i) this circuit shows elevated metabolism in patients with OCD, particularly associated with expression of OCD symptoms and anxiety (see Section 2.3 and Table 1), (ii) the OFC is consistently reduced in volume in OCD (see Section 4.1 and Fig. 3) and (iii) that activation abnormalities are observed in these regions during fMRI in OCD patients compared with controls (Fig. 2 and Table 2). The causal relationship between these structural and functional observations is unknown, but interestingly functional brain changes have been shown to be dynamic and may normalise following therapeutic approaches which also reduce OCD symptoms and anxiety (Saxena et al., 1999).
However, the evidence we review above suggests that brain abnormalities are not limited exclusively to these areas. Many imaging studies have reported abnormalities in additional brain regions in OCD including the parietal lobe, particularly in the angular and supramarginal gyri (Brodmann areas 39, 40), and the dorsolateral prefrontal cortex, suggesting that parietal regions and the dorsolateral prefronto-striatal circuit may also be affected in OCD. Involvement of these candidate regions may well contribute to the deficits in OCD patients in cognitive functions not thought to be dependent upon orbitofrontostriatal circuitry. Recent whole-brain studies, though currently few in number, also lend support to the existence of additional structural abnormalities in OCD, particularly within parietal cortex (Valente et al., 2005; Kim et al., 2001).
5.4. Parietal cortex
Major efforts are currently underway to further our understanding of functional specialisations within the parietal lobe e.g., Simon et al. (2002). This region is important in a variety of executive tasks involving functions such as attention, spatial perception and working memory (Cabeza and Nyberg, 2000; Culham and Kanwisher, 2001). Given that some of these functions are consistently reported to be affected in OCD, such as attentional shifting (see Section 3.2), it is conceivable that parietal lobe dysfunction, particularly within the angular and supramarginal gyri, could contribute to the cognitive deficits evident in OCD. In support of this argument, Posner and Petersen (1990) suggested that parietal regions operate as part of a posterior attention system involved in disengaging spatial attention, and there is also evidence suggesting that activity in the parietal lobe is related to sustained attention and attentional set shifting (Nagahama et al., 1996; Le et al., 1998; Hampshire and Owen, 2006). Additionally, an fMRI study conducted in patients with ADHD revealed reduced activation of parietal areas including the angular and supramarginal gyri which was associated with attentional impairments (Tamm et al., 2006). Furthermore, the parietal lobe has also been specifically implicated in planning (Williams-Gray et al., 2007) and response inhibition (Rubia et al., 2001b; Lepsien and Pollmann, 2002; Horn et al., 2003), which are also both reported to be impaired in OCD. Of interest, in an event-related potential study, patients with OCD showed an enhanced P600 at the right temporo-parietal area and prolonged latencies at the right parietal region during a digit span test when compared with healthy controls (Charalabos et al., 2003). It is also intriguing that studies of white matter abnormalities in OCD, for example employing diffusion tensor imaging (DTI) and spectroscopy, have reported abnormalities in bilateral supramarginal gyri (Szeszko et al., 2005) and parietal white matter (Kitamura et al., 2006).
5.5. Prefrontal cortex
Frontal areas other than the OFC have also been implicated in the neuropathology of OCD by findings from cognitive studies (see Section 3). In particular the DLPFC is implicated in functions such as planning, and, together with parietal regions, has been postulated to be part of the dorsolateral prefronto-striatal loop. Interestingly, there is further suggestive evidence for a role of the DLPFC in OCD pathology, for example the putative neuronal marker N-acetyl-aspartate was significantly increased in the DLPFC in 15 treatment-naïve cases of paediatric OCD (Russell et al., 2003). Other additional regions which may well be affected in OCD include the inferior frontal gyrus/ventrolateral prefrontal cortex, known to be critical in both response inhibition (Aron et al., 2003) and attentional set-shifting (Hampshire and Owen, 2006).
There is evidence from primate studies showing anatomical connectivity between these regions which we putatively suggest are also involved in OCD. Connections have been demonstrated between parietal regions and the DLPFC (Cavada and Goldman-Rakic, 1989; Romanski et al., 1997; Roberts et al., 2007) and both regions contribute to the dorsolateral prefronto-striatal circuit. Interestingly, there is also evidence from primate studies to suggest connections between the parietal lobe and areas of the orbitofronto-striatal circuit already suggested to function aberrantly in OCD, for example with the OFC itself (Cavada and Goldman-Rakic, 1989; Zald and Kim, 1996a), the thalamus (Giguere and Goldman-Rakic, 1988) and the striatum (Yeterian and Pandya, 1993).
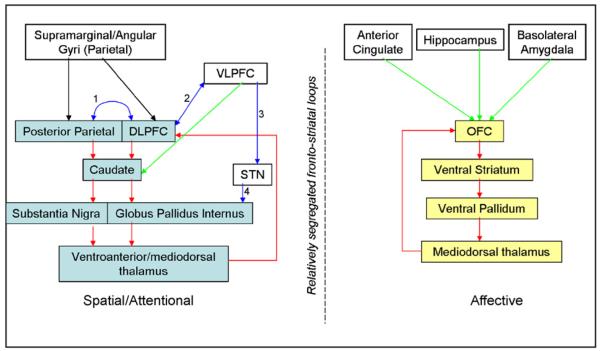
In light of the above, we propose a revised model for OCD in which the underlying pathology is not limited to orbitofronto-striatal regions and associated limbic structures such as the amygdala, but also involves abnormalities in additional brain systems, particularly including more lateral frontal and parietal regions which may be considered to represent the dorsolateral prefronto-striatal circuit defined by Alexander et al. (1986), (Lawrence et al., 1998). See Fig. 5 for a summary of putative brain regions involved in OCD and how these may be anatomically linked.
Fig. 5.
Simplified diagram summarising putative regions and circuits which may be affected in OCD. Yellow boxes indicate regions comprising the traditionally implicated orbitofronto-striatal loop. Blue boxes indicate regions comprising the dorsolateral prefronto-striatal loop. White boxes indicate additional brain regions putatively involved in OCD. Red arrows indicate connections proposed as components of fronto-striatal loops by Alexander et al. (1986). Green arrows indicate connections incorporated into fronto-striatal loops by Lawrence et al. (1998). Blue arrows indicate connections with supportive anatomical evidence as denoted by number: (1) Cavada and Goldman-Rakic (1989), (2) Takahashi et al. (2007), (3) Aron et al. (2007) and (4) Mink (1996).
6. Conclusions
6.1. Future directions
In summary there are several notable discrepancies between findings from cognitive studies, neuroimaging studies and the present theoretical model proposed to underlie OCD. The currently available evidence suggests that the orbitofronto-striatal model may not be sufficient to explain the brain basis of OCD. There are several potential reasons for this lack of concurrence. For example, some cognitive tasks may have lacked the required sensitivity and specificity to detect subtle orbitofrontal-related cognitive dysfunction in OCD patients. In a disorder such as OCD, with an early onset and most likely a prolonged developmental trajectory with the potential for formation of compensatory cognitive strategies, it is perhaps not surprising the patients are not impaired as predicted on some tasks. However it must be borne in mind that in other putative neurodevelopmental disorders, such as schizophrenia, there is broad cognitive impairment and little evidence suggesting development of compensatory cognitive strategies. Conflicting findings could also be due to confounding factors such as patient co-morbidity and inadequate matching of sample groups. Further work on task development and careful selection of patient samples may help to improve these aspects.
Nonetheless, it must be considered that non-replicated and inconsistent findings, such has been the case particularly with the VBM studies of OCD conducted so far (see Section 4.3), may in fact reflect the null hypothesis that there is no consistent structural abnormality in OCD and that cognitive impairments are not underpinned by structural abnormalities identifiable by MRI. Furthermore, it is currently impossible to determine a direction of causality between brain structure, cognition and a clinical diagnosis of OCD—longitudinal follow-up studies would be required to test relevant hypotheses. However, the existence of brain structural abnormalities and cognitive impairment in unaffected relatives of patients does at least suggest that these observations predispose to and precede the emergence of OCD. The putative vulnerabilities which might precipitate OCD in individuals at increased genetic risk of the disorder, and the extent to which vulnerability factors might be genetic or environmental in nature, can only be speculated upon at this time. It is also likely that some forms of OCD, for example associated with PANDAS or following basal ganglia lesions, might be directly related to particular structural changes within the orbitofronto-striatal loop, in this case most likely in the basal ganglia, and as such it could be expected that these patients may have their own distinct cognitive profile.
Alternatively, it may be that structural changes have not yet been found in OCD patients that fully account for their cognitive deficits because ROI studies have not yet included all of the areas mediating such deficits in the relatively restricted searches necessitated by this method. This could also be because the brain abnormalities responsible for OCD are represented at a system or network level, in which abnormalities across several different regions account for impairments in cognition. Further work involving connectivity analyses analogous to that occurring in the field of schizophrenia research (Schlosser et al., 2003; Honey et al., 2005), or employing multivariate methods would help to clarify this matter.
In conclusion, consideration of findings from neuropsychological and neuroimaging domains which are unexpected on a theoretical level may provide a framework for further investigations necessary to fully understand: (i) the neurobiological underpinnings of OCD and (ii) cognitive and brain imaging markers both of OCD itself and for genetic risk of this disorder. We would advocate a future approach where whole brain-based neuroimaging and cognitive studies direct researchers to the study of additional regions outside the orbitofronto-striatal circuit, followed by closer investigation of these regions. We would also anticipate potential adjunct contributions from connectivity and multivariate imaging analysis methods. We would predict that this strategy has the potential to be of significant benefit in comprehensively identifying brain changes in patients with OCD, thereby facilitating our understanding of the disorder.
Acknowledgements
This work was funded by the National Alliance for Research on Schizophrenia and Depression (Distinguished Investigator Award to EB), the Wellcome Trust (BJS), the Harnett Fund (University of Cambridge; MB/PhD studentship to LM), and the Medical Research Council (MB/PhD studentship to SC). ARL and SMT were supported by the Human Brain Project of the NIMH (RO1-MH074457-01A1). The Behavioural and Clinical Neuroscience Institute is supported by a joint award from the Medical Research Council and Wellcome Trust. Dr. Sahakian and Samuel Chamberlain consult for Cambridge Cognition.
Footnotes
All other authors report no competing interests.
References
- Abbruzzese M, Ferri S, Scarone S. The selective breakdown of frontal functions in patients with obsessive-compulsive disorder and in patients with schizophrenia: a double dissociation experimental finding. Neuropsychologia. 1997;35:907–912. doi: 10.1016/s0028-3932(96)00095-4. [DOI] [PubMed] [Google Scholar]
- Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. Journal of Psychiatric Research. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. Journal of Neurosurgery. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim BH, Ozdemir BH, Aydin BA, Tezcan AE, Ozler AS. Volumetric MRI assessment of brain regions in patients with refractory obsessive-compulsive disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 2006;30:1051–1057. doi: 10.1016/j.pnpbp.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Aycicegi A, Dinn WM, Harris CL, Erkmen H. Neuropsychological function in obsessive-compulsive disorder: effects of comorbid conditions on task performance. European Psychiatry. 2003;18:241–248. doi: 10.1016/s0924-9338(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Harris GJ, Hoehn-Saric R, Barta PE, Machlin SR, Pearlson GD. Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Archives of General Psychiatry. 1996;53:577–584. doi: 10.1001/archpsyc.1996.01830070021006. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Research. 2002;110:165–174. doi: 10.1016/s0165-1781(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Executive functions in obsessive-compulsive disorder: state or trait deficits? Australian and New Zealand Journal of Psychiatry. 2006;40:1031–1038. doi: 10.1080/j.1440-1614.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr., Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Archives of General Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr., Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. American Journal of Psychiatry. 1988;145:1560–1563. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr., Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, Alazraki A, Selin CE, Ferng HK, Munford P, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Archives of General Psychiatry. 1992;49:681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Archives of General Psychiatry. 1990;47:840–848. doi: 10.1001/archpsyc.1990.01810210048007. [DOI] [PubMed] [Google Scholar]
- Berg E. A simple objective technique for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bergqvist PB, Bouchard C, Blier P. Effect of long-term administration of antidepressant treatments on serotonin release in brain regions involved in obsessive-compulsive disorder. Biological Psychiatry. 1999;45:164–174. doi: 10.1016/s0006-3223(98)00154-1. [DOI] [PubMed] [Google Scholar]
- Bohne A, Savage CR, Deckersbach T, Keuthen NJ, Wilhelm S. Motor inhibition in trichotillomania and obsessive-compulsive disorder. Journal of Psychiatric Research. 2008;42(2):141–150. doi: 10.1016/j.jpsychires.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Filipek PA, Kennedy DN, Baer L, Pitcher DA, Olivares MJ, Renshaw PF, Caviness VS., Jr. Retrocallosal white matter abnormalities in patients with obsessive-compulsive disorder. Archives of General Psychiatry. 1994;51:663–664. doi: 10.1001/archpsyc.1994.03950080075010. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Archives of General Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Transactions on Medical Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Zamignani DR, Buchpiguel CA, Garrido GE, Glabus MF, Rocha ET, Maia AF, Rosario-Campos MC, Campi Castro C, Furuie SS, et al. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT) Psychiatry Research. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL. Amygdala responses to human faces in obsessive-compulsive disorder. Biological Psychiatry. 2004;56:916–920. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Carey G, Gottesman II. Twin and family studies of anxiety, phobic and obsessive disorders. In: Klein D, Radkin J, editors. Anxiety; New Research and Changing Concepts. Raven Press; New York: 1981. [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. Journal of Comparative Neurology. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cavallini MC, Pasquale L, Bellodi L, Smeraldi E. Complex segregation analysis for obsessive compulsive disorder and related disorders. American Journal of Medical Genetics. 1999;88:38–43. doi: 10.1002/(sici)1096-8628(19990205)88:1<38::aid-ajmg7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, D'Annucci A, Belotti P, Cisima M, Bellodi L. Decision-making heterogeneity in obsessive-compulsive disorder: ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsychologia. 2002;40:205–211. doi: 10.1016/s0028-3932(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychology of mood disorders. Current Psychiatry Report. 2006;8:458–463. doi: 10.1007/s11920-006-0051-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Review. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. American Journal of Psychiatry. 2006;163:1282–1284. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007a;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2007b;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalabos C, Papageorgiou CC, Rabavilas AD. Abnormal P600 in obsessive-compulsive disorder. A comparison with healthy controls. Psychiatry Research. 2003;119:133–143. doi: 10.1016/s0165-1781(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kang DH, Kim JJ, Ha TH, Lee JM, Youn T, Kim IY, Kim SI, Kwon JS. Left anterior subregion of orbitofrontal cortex volume reduction and impaired organizational strategies in obsessive-compulsive disorder. Journal of Psychiatric Research. 2004;38:193–199. doi: 10.1016/j.jpsychires.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim HS, Yoo SY, Ha TH, Chang JH, Kim YY, Shin YW, Kwon JS. Morphometric alterations of anterior superior temporal cortex in obsessive-compulsive disorder. Depression and Anxiety. 2006;23:290–296. doi: 10.1002/da.20171. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Cottraux J, Gerard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, Galy G, Millet P, Labbe C, Lavenne F, et al. A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Research. 1996;60:101–112. doi: 10.1016/0165-1781(96)02697-2. [DOI] [PubMed] [Google Scholar]
- Crum WR, Griffin LD, Hill DL, Hawkes DJ. Zen and the art of medical image registration: correspondence, homology, and quality. Neuroimage. 2003;20:1425–1437. doi: 10.1016/j.neuroimage.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Delorme R, Gousse V, Roy I, Trandafir A, Mathieu F, Mouren-Simeoni MC, Betancur C, Leboyer M. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. European Psychiatry. 2007;22:32–38. doi: 10.1016/j.eurpsy.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D, Zohar J, Westenberg HG. The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence. Journal of Clinical Psychiatry. 2004;65(Suppl. 14):11–17. [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- DuPont RL, Rice DP, Shiraki S, Rowland CR. Economic costs of obsessive-compulsive disorder. Medical Interface. 1995;8:102–109. [PubMed] [Google Scholar]
- el Mansari M, Bouchard C, Blier P. Alteration of serotonin release in the guinea pig orbito-frontal cortex by selective serotonin reuptake inhibitors. Relevance to treatment of obsessive-compulsive disorder. Neuropsychopharmacology. 1995;13:117–127. doi: 10.1016/0893-133X(95)00045-F. [DOI] [PubMed] [Google Scholar]
- Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, Huppert JD, Kjernisted K, Rowan V, Schmidt AB, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Opinion: mapping context and content: the BrainMap model. Nature Reviews Neuroscience. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cerebral Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clinical Psychology Review. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR American Journal of Neuroradiology. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. American Journal of Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. Journal of Comparative Neurology. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, Mac Master FP, Stewart CM, Rosenberg DR. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Archives of General Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier C, Rousseaux M. Non-spatial attention disorders in patients with frontal or posterior brain damage. Brain. 1996;119(Pt 1):191–202. doi: 10.1093/brain/119.1.191. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grados M, Scahill L, Riddle MA. Pharmacotherapy in children and adolescents with obsessive-compulsive disorder. Child and Adolescent Psychiatry Clinics of North America. 1999;8:617–634. [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Greist JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Archives of General Psychiatry. 1995;52:53–60. doi: 10.1001/archpsyc.1995.03950130053006. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cerebral Cortex. 2006;16(12):1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, Leventhal BL, Cook EH., Jr. Genomewide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. American Journal of Medical Genetics. 2002;114:541–552. doi: 10.1002/ajmg.10519. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Himle JA, Curtis GC, Gillespie BW. A family study of obsessive-compulsive disorder with pediatric probands. American Journal of Medical Genetics: Part B Neuropsychiatric Genetics. 2005;134:13–19. doi: 10.1002/ajmg.b.30138. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society. 1868;2:327–347. [Google Scholar]
- Harrison BJ, Yucel M, Shaw M, Kyrios M, Maruff P, Brewer WJ, Purcell R, Velakoulis D, Strother SC, Scott AM, et al. Evaluating brain activity in obsessive-compulsive disorder: preliminary insights from a multivariate analysis. Psychiatry Research. 2006;147:227–231. doi: 10.1016/j.pscychresns.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hartston HJ, Swerdlow NR. Visuospatial priming and stroop performance in patients with obsessive compulsive disorder. Neuropsychology. 1999;13:447–457. doi: 10.1037//0894-4105.13.3.447. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Long- and short-range reward expectancy in the primate orbitofrontal cortex. European Journal of Neuroscience. 2004;19:1046–1054. doi: 10.1111/j.0953-816x.2004.03120.x. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional disconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MG, van Haren NE, van der Tweel I, Staal WG, Baare WF, Kahn RS. Volume changes in gray matter in patients with schizophrenia. American Journal of Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- Humberstone M, Sawle GV, Clare S, Hykin J, Coxon R, Bowtell R, Macdonald IA, Morris PG. Functional magnetic resonance imaging of single motor events reveals human presupplementary motor area. Annals of Neurology. 1997;42:632–637. doi: 10.1002/ana.410420414. [DOI] [PubMed] [Google Scholar]
- Inouye E. Similar and dissimilar manifestations of obsessive-compulsive neuroses in monozygotic twins. American Journal of Psychiatry. 1965;121:1171–1175. doi: 10.1176/ajp.121.12.1171. [DOI] [PubMed] [Google Scholar]
- Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, O'Sullivan RL, Shera DM, Rauch SL, Keuthen N, et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Archives of General Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus—reinforcement associations. Experimental Neurology. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry. 2007;62(10):1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Kim JJ, Choi JS, Kim YI, Kim CW, Youn T, Han MH, Chang KH, Kwon JS. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:342–349. doi: 10.1176/jnp.16.3.342. [DOI] [PubMed] [Google Scholar]
- Karno M, Golding JM, Sorenson SB, Burnam MA. The epidemiology of obsessive-compulsive disorder in five US communities. Archives of General Psychiatry. 1988;45:1094–1099. doi: 10.1001/archpsyc.1988.01800360042006. [DOI] [PubMed] [Google Scholar]
- Katrin Kuelz A, Riemann D, Zahn R, Voderholzer U. Object alternation test—is it sensitive enough to detect cognitive dysfunction in obsessive-compulsive disorder? European Psychiatry. 2004;19:441–443. doi: 10.1016/j.eurpsy.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. British Journal of Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, Someya T. Parietal white matter abnormalities in obsessive-compulsive disorder: a magnetic resonance spectroscopy study at 3-Tesla. Acta Psychiatrica Scandinavica. 2006;114:101–108. doi: 10.1111/j.1600-0447.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17:922–927. [PubMed] [Google Scholar]
- Koran LM, Thienemann ML, Davenport R. Quality of life for patients with obsessive-compulsive disorder. American Journal of Psychiatry. 1996;153:783–788. doi: 10.1176/ajp.153.6.783. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, Shin YW, Kim CW, Kim YI, Youn T, Han MH, Chang KH, Kim JJ. Similarity and disparity of obsessive-compulsive disorder and schizophrenia in MR volumetric abnormalities of the hippocampus-amygdala complex. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:962–964. doi: 10.1136/jnnp.74.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005a;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005b;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Human Brain Mapping. 2005c;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, Tran Dinh S, Sette G, Danze F, Baron JC. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112(Pt 3):699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: insights from Huntington's disease. Trends in Cognitive Sciences. 1998;2:379–388. doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, An SK, Mataix-Cols D, Ruths F, Speckens A, Phillips ML. Neural responses to facial expressions of disgust but not fear are modulated by washing symptoms in OCD. Biological Psychiatry. 2007;61:1072–1080. doi: 10.1016/j.biopsych.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. Journal of Neurophysiology. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Leon AC, Portera L, Weissman MM. The social costs of anxiety disorders. British Journal of Psychiatry Supplement. 1995:19–22. [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Lucey JV, Costa DC, Blanes T, Busatto GF, Pilowsky LS, Takei N, Marks IM, Ell PJ, Kerwin RW. Regional cerebral blood flow in obsessive-compulsive disordered patients at rest. Differential correlates with obsessive-compulsive and anxious-avoidant dimensions. British Journal of Psychiatry. 1995;167:629–634. doi: 10.1192/bjp.167.5.629. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Russell A, Mirza Y, Keshavan MS, Banerjee SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, et al. Pituitary volume in pediatric obsessive-compulsive disorder. Biological Psychiatry. 2006;59:252–257. doi: 10.1016/j.biopsych.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Allilaire JF, Mazoyer BM, Hantouche E, Huret JD, Legaut-Demare F, Deslauriers AG, Hardy P, Pappata S, Baron JC, et al. Obsessive-compulsive disorder: a clinical, neuropsychological and positron emission tomography study. Acta Psychiatrica Scandinavica. 1990;82:233–242. doi: 10.1111/j.1600-0447.1990.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Alonso P, Pifarre J, Menchon JM, Vallejo J. Neuropsychological performance in medicated vs. unmedicated patients with obsessive-compulsive disorder. Psychiatry Research. 2002;109:255–264. doi: 10.1016/s0165-1781(02)00024-0. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speck-ens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- McEnaney KW, Butter CM. Perseveration of responding and nonresponding in monkeys with orbital frontal ablations. Journal of Comparative Physiological Psychology. 1969;68:558–561. doi: 10.1037/h0027639. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. British Journal of Psychiatry. 1994;164:459–468. doi: 10.1192/bjp.164.4.459. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, Del Campo N, et al. Brain. 2007 doi: 10.1093/brain/awm205. in press, doi:10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Murray C, Lopez A. The Global Burden of Disease. A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. World Health Organisation, USA. 1996 [Google Scholar]
- Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. The Journal of Neuroscience. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Matsuzaki S, Konishi J, Shibasaki H, Kimura J. Cerebral activation during performance of a card sorting test. Brain. 1996;119(Pt 5):1667–1675. doi: 10.1093/brain/119.5.1667. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cerebral Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Research. 2005a;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biological Psychiatry. 2005b;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, Hoehn-Saric R, Cullen B, Grados M, Beaty TH, Shugart YY. Complex segregation analysis provides compelling evidence for a major gene underlying obsessive-compulsive disorder and for heterogeneity by sex. American Journal of Human Genetics. 2000a;67:1611–1616. doi: 10.1086/316898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R. A family study of obsessive-compulsive disorder. Archives of General Psychiatry. 2000b;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Nielen MM, Den Boer JA. Neuropsychological performance of OCD patients before and after treatment with fluoxetine: evidence for persistent cognitive deficits. Psychological Medicine. 2003;33:917–925. doi: 10.1017/s0033291703007682. [DOI] [PubMed] [Google Scholar]
- Nielen MM, Veltman DJ, de Jong R, Mulder G, den Boer JA. Decision making performance in obsessive compulsive disorder. Journal of Affective Disorders. 2002;69:257–260. doi: 10.1016/s0165-0327(00)00381-5. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM. Cerebral glucose metabolic rates in obsessive compulsive disorder. Neuropsychopharmacology. 1989;2:23–28. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophrenia Research. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. American Journal of Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Andres S, Salamero M, Gasto C. Executive function and nonverbal memory in obsessive-compulsive disorder. Psychiatry Research. 2005;133:81–90. doi: 10.1016/j.psychres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C. Impaired response inhibition in obsessive compulsive disorder. European Psychiatry. 2007;22(6):404–410. doi: 10.1016/j.eurpsy.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, Bellodi L, Smeraldi E, Fazio F. [18F]FDG PET study in obsessive-compulsive disorder. A clinical/metabolic correlation study after treatment. British Journal of Psychiatry. 1995;166:244–250. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- Peterson B, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, Leckman JF. Reduced basal ganglia volumes in Tourette's syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43:941–949. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, King R, Cohen DJ, Gore JC, Lombroso P. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive-compulsive, and attention deficit/hyperactivity disorders. Archives of General Psychiatry. 2000;57:364–372. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Marks IM, Senior C, Lythgoe D, O'Dwyer AM, Meehan O, Williams SC, Brammer MJ, Bullmore ET, McGuire PK. A differential neural response in obsessive-compulsive disorder patients with washing compared with checking symptoms to disgust. Psychological Medicine. 2000;30:1037–1050. doi: 10.1017/s0033291799002652. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Wise SP. Obsessive-compulsive disorder: evidence for basal ganglia dysfunction. Psychopharmacology Bulletin. 1988;24:380–384. [PubMed] [Google Scholar]
- Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatric Clinics of North America. 1992;15:743–758. [PubMed] [Google Scholar]
- Rasmussen SA, Tsuang MT. The epidemiology of obsessive compulsive disorder. Journal of Clinical Psychiatry. 1984;45:450–457. [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Archives of General Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, Cannistraro PA, Wilhelm S. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biological Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. Reduced orbitofrontal–striatal activity on a reversal learning task in obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. Journal of Comparative Neurology. 2007;502:86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD, Regier DA. Lifetime prevalence of specific psychiatric disorders in three sites. Archives of General Psychiatry. 1984;41:949–958. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, Koreen A, Cole K, Bogerts B. Reduced caudate nucleus volume in obsessive-compulsive disorder. Archives of General Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Roh KS, Shin MS, Kim MS, Ha TH, Shin YW, Lee KJ, Kwon JS. Persistent cognitive dysfunction in patients with obsessive-compulsive disorder: a naturalistic study. Psychiatry Clinical Neuroscience. 2005;59:539–545. doi: 10.1111/j.1440-1819.2005.01411.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1997;379:313–332. [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biological Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, Dick EL, Bagwell WW, Mac-Master FP, Birmaher B. Corpus callosal morphology in treatment-naive pediatric obsessive compulsive disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 1997a;21:1269–1283. doi: 10.1016/s0278-5846(97)00163-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, O'Hearn KM, Dick EL, Bagwell WW, Seymour AB, Montrose DM, Pierri JN, Birmaher B. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Archives of General Psychiatry. 1997b;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Benazon NR, Gilbert A, Sullivan A, Moore GJ. Thalamic volume in pediatric obsessive-compulsive disorder patients before and after cognitive behavioral therapy. Biological Psychiatry. 2000;48:294–300. doi: 10.1016/s0006-3223(00)00902-1. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62(8):901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Review. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophrenia Research. 2001a;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001b;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001c;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Villanueva-Meyer J, Ananth J, Trajmar PG, Mena I. Regional xenon 133 cerebral blood flow and cerebral technetium 99m HMPAO uptake in unmedicated patients with obsessive-compulsive disorder and matched normal control subjects. Determination by high-resolution single-photon emission computed tomography. Archives of General Psychiatry. 1992;49:695–702. doi: 10.1001/archpsyc.1992.01820090023004. [DOI] [PubMed] [Google Scholar]
- Russell A, Cortese B, Lorch E, Ivey J, Banerjee SP, Moore GJ, Rosenberg DR. Localized functional neurochemical marker abnormalities in dorsolateral prefrontal cortex in pediatric obsessive-compulsive disorder. Journal of Child and Adolescent Psychopharmacology. 2003;13(Suppl. 1):S31–S38. doi: 10.1089/104454603322126322. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Malhi GS. Obsessive-compulsive behaviour: a disorder of decision-making. Australian and New Zealand Journal of Psychiatry. 2005;39:757–763. doi: 10.1080/j.1440-1614.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- Sawle GV, Hymas NF, Lees AJ, Frackowiak RS. Obsessional slowness. Functional studies with positron emission tomography. Brain. 1991;114(Pt 5):2191–2202. doi: 10.1093/brain/114.5.2191. [DOI] [PubMed] [Google Scholar]
- Saxena S. In: Neuroimaging and the pathophysiology of obsessive compulsive disorder (OCD) Fu C, Senior C, Russell T, Weinberger D, Murray R, editors. Neuroimaging in Psychiatry; Martin Dunitz, UK: 2003. [Google Scholar]
- Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal–subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry Supplement. 1998:26–37. [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, Phelps ME, Baxter LR., Jr. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999;21:683–693. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bota RG, Brody AL. Brain–behavior relationships in obsessive-compulsive disorder. Seminars in Clinical Neuropsychiatry. 2001a;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM, Huang SC, Wu HM, Au SC, Baxter LR., Jr. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biological Psychiatry. 2001b;50:159–170. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR., Jr. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. American Journal of Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- Scarone S, Colombo C, Livian S, Abbruzzese M, Ronchi P, Locatelli M, Scotti G, Smeraldi E. Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Research. 1992;45:115–121. doi: 10.1016/0925-4927(92)90005-o. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. International Journal of Psychophysiology. 2005;57:69–77. doi: 10.1016/j.ijpsycho.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, Stein DJ, Lang PJ, Goodman WK. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biological Psychiatry. 2003;54:751–756. doi: 10.1016/s0006-3223(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiology of Aging. 2007;28(7):1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Soriano-Mas C, Pujol J, Alonso P, Cardoner N, Menchon JM, Harrison BJ, Deus J, Vallejo J, Gaser C. Identifying patients with obsessive-compulsive disorder using whole-brain anatomy. Neuroimage. 2007;35:1028–1037. doi: 10.1016/j.neuroimage.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Baldwin DS, Bandelow B. Treatment of obsessive-compulsive disorder. CNS Spectroscopy. 2007;12:28–35. [Google Scholar]
- Swedo SE, Grant PJ. Annotation: PANDAS: a model for human autoimmune disease. Journal of Child Psychology and Psychiatry. 2005;46:227–234. doi: 10.1111/j.1469-7610.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Archives of General Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, Rapoport SI, Rapoport JL, Grady CL. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Archives of General Psychiatry. 1992;49:690–694. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2004a;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Lorch E, Madden R, Ivey J, Banerjee SP, Moore GJ, Rosenberg DR. Amygdala volume reductions in pediatric patients with obsessive-compulsive disorder treated with paroxetine: preliminary findings. Neuropsychopharmacology. 2004b;29:826–832. doi: 10.1038/sj.npp.1300399. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Archives of General Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ohki K, Kim DS. Diffusion tensor studies dissociated two fronto-temporal pathways in the human memory system. Neuroimage. 2007;34:827–838. doi: 10.1016/j.neuroimage.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. American Journal of Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neuroscience. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go–no-go task performance in primate orbitofrontal cortex. Journal of Neurophysiology. 2000;83:1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Valente AA, Jr., Miguel EC, Castro CC, Amaro E, Jr., Duran FL, Buchpiguel CA, Chitnis X, McGuire PK, Busatto GF. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biological Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, Barkhof F, van Dyck R. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Archives of General Psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van der Wee NJ, Ramsey NF, Jansma JM, Denys DA, van Megen HJ, Westenberg HM, Kahn RS. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. Neuroimage. 2003;20:2271–2280. doi: 10.1016/j.neuroimage.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychological Medicine. 1996;26:1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Viard A, Flament MF, Artiges E, Dehaene S, Naccache L, Cohen D, Mazet P, Mouren MC, Martinot JL. Cognitive control in childhood-onset obsessive-compulsive disorder: a functional MRI study. Psychological Medicine. 2005;35:1007–1017. doi: 10.1017/s0033291704004295. [DOI] [PubMed] [Google Scholar]
- Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, Aitken MR, Robbins TW. Executive function in Tourette's syndrome and obsessive-compulsive disorder. Psychological Medicine. 2005;35:571–582. doi: 10.1017/s0033291704003691. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Lee CK, Newman SC, Oakley-Browne MA, Rubio-Stipec M, Wickramaratne PJ, et al. The cross national epidemiology of obsessive compulsive disorder. The Cross National Collaborative Group. Journal of Clinical Psychiatry. 1994;55(Suppl.):5–10. [PubMed] [Google Scholar]
- Westenberg HG, Fineberg NA, Denys D. Neurobiology of obsessive-compulsive disorder: serotonin and beyond. CNS Spectroscopy. 2007;12:14–27. [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Research. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. Journal of Neuroscience. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willour VL, Yao Shugart Y, Samuels J, Grados M, Cullen B, Bienvenu OJ, 3rd, Wang Y, Liang KY, Valle D, Hoehn-Saric R, et al. Replication study supports evidence for linkage to 9p24 in obsessive-compulsive disorder. American Journal of Human Genetics. 2004;75:508–513. doi: 10.1086/423899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophrenia Research. 1999;35:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys. Journal of Comparative Neurology. 1993;332:175–197. doi: 10.1002/cne.903320204. [DOI] [PubMed] [Google Scholar]
- Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, Clarke K, Phillips ML, Kyrios M, Velakoulis D, Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Archives of General Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex, I: anatomy, neurocircuitry; and obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 1996a;8:125–138. doi: 10.1176/jnp.8.2.125. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex, II: function and relevance to obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 1996b;8:249–261. doi: 10.1176/jnp.8.3.249. [DOI] [PubMed] [Google Scholar]