Abstract
According to the doctrine underlying the current radiation protection regulations each, no matter how small, exposure to ionizing radiation may be carcinogenic. However, numerous epidemiological observations demonstrate that cancer incidence and/or mortality are not elevated among inhabitants of the high- versus low-natural-background radiation areas and homes. Results of our own and other authors’ studies described in this paper bear testimony to the possibility that stimulation of the anti-neoplastic immune surveillance mediated by NK lymphocytes and activated macrophages explains, at least partially, the accumulating epidemiological and experimental evidence indicating that low-level exposures to the low-linear energy transfer (LET) radiation inhibit the development of spontaneous and artificial metastases in humans and laboratory animals, respectively. The results presented also suggest the possibility of using low-level X- and gamma-ray exposures to cure cancer and to prevent cancer metastases. For a broader perspective, the results presented may help towards relaxing the current radiation protection regulations, especially as they apply to diagnostic and therapeutic exposures of patients to the indicated forms of radiation.
Keywords: low-level X-rays, tumor lung colonies, NK cells, cytotoxic macrophages, anti-neoplastic activity
I. INTRODUCTION
Humans have always been exposed to various natural sources of ionizing radiation emitted by the isotopes present in the earth’s crust, air, water and biosphere, and also originating from the outer space. In some parts of the globe the level of this natural background radiation is significantly higher than the world average with no adverse health effects. Today, people can be additionally exposed to “man-made” radiation delivered at high doses (e.g., during radiotherapy and radiation accidents as well as after detonations of nuclear weapons) or low doses (e.g., during production and distribution of radioactive materials and use of radiation sources for industrial and medical purposes). The low-level environmental and occupational exposures are much more common and distributed over much larger populations than the high-level exposures.
Low doses and dose rates of ionizing radiation (low-level radiation) are defined as those below 0.1–0.2 Gy and below 0.05–0.1 mGy/min., respectively (UNSCEAR 1986, BEIR VII 2006). Absorption of low doses of ionizing radiation may stimulate cellular detoxification and repair mechanisms leading to reduction of the DNA damage even below the spontaneous level and decreasing the probability of neoplastic transformation (for review see: Azzam et al. 1996, Pollycove 2004, 2007, Mitchel 2007, Portess et al. 2007, Redpath and Elmore 2007, Feinendegen et al. 2008). Such exposures may also enhance immune reactions of the organism and attenuate harmful effects of higher doses of radiation (Liu et al. 1982, 1985, Tuschl et al. 1995, Safwat 2000b, Safwat et al. 2003; for review see: Liu 1989, 2004, Luckey 1980, 1999, Ju et al. 1995). These mechanisms may explain various epidemiological observations indicating that cancer incidence and mortality are not elevated among inhabitants of the high- versus low-background radiation areas (Ishii et al. 1996, Kesavan 1997, Jagger 1998; for review see: Luckey 1999, Wei and Sugahara 2000) as well as among tenants of homes with the elevated levels of radiation from 222Rn or 60Co (Cohen 1995, 1997, UNSCEAR 2000, Wang et al. 2002, Chen et al. 2004). Also, in many cohorts of nuclear workers and in the survivors of the Hiroshima and Nagasaki bombings whose absorbed doses did not exceed 0.25 Gy the incidence of leukemia and some solid tumors has been reported to be lower compared to the respective control groups (Matanoski et al. 1990, Cardis et al. 1995, Pierce et al. 1996, McKinney et al. 1998, Little et al. 1999, UNSCEAR 2000, Berrington et al. 2001, Katayama et al. 2002; for review see: Kondo 1993, Luckey 1999).
These results of epidemiological analyses encouraged many to perform experimental studies utilizing the low-level low-LET irradiations of cells and animals in strictly defined conditions. Such experiments have been providing data which have already contributed to the more detailed understanding of the mechanisms plausibly responsible for the decreased incidence of tumors among people exposed to the low-level ionizing radiation. More broadly, these data may be instrumental in testing the linear-no-threshold (LNT) hypothesis which is central to establishing radiation exposure limits for humans. The LNT hypothesis is based on the controversial assumption that the underlying biological processes triggered by low radiation doses are essentially the same as those that function after higher radiation doses (Mossman 2009, Tubiana 2008). Under the LNT hypothesis, any amount of radiation would be considered to cause cancer among some members of a very large population and cancer risk would increase linearly with increasing dose.
II. ANTI-TUMOR PROPERTIES OF THE LOW-LEVEL LOW-LET IRRADIATIONS
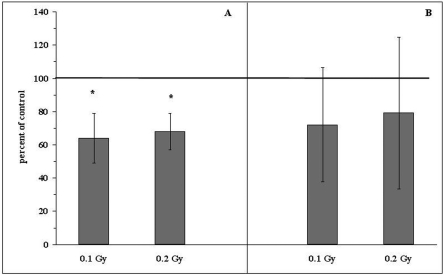
The recent evidence has demonstrated that in animals exposed to single or fractionated low total doses of X- or γ-rays the growth of primary and/or metastatic tumors is inhibited or retarded (Hosoi and Sakamoto 1993, Ishii et al. 1996, Caratero et al. 1998, Cai 1999, Hashimoto et al. 1999, Mitchel et al. 1999, 2003, Wang and Cai 2000, Sakai et al. 2003, Ina and Sakai 2004, for review see: Ju et al. 1995, Liu 2004, 2007). In many of these investigations, anti-tumor properties of the low-level exposures were detected when whole animals were irradiated prior to inoculation of neoplastic cells, indicating that the immune surveillance mechanisms might be involved (Hashimoto et al. 1999, for review see: Safwat 2000a). In their pioneering study, Hosoi and Sakamoto (1993) detected marked reductions in the numbers of both artificial and spontaneous pulmonary metastases after single whole body-irradiation (WBI) of mice with 0.15, 0.2, or 0.5 Gy X-rays. In that study the inhibitory effect was expressed when tumor cells were inoculated either a few hours before or after the exposure. Likewise, Ju et al. (1995) and Cai (1999) who irradiated mice with single doses of X-rays ranging from 0.05 to 0.15 Gy 24 hours before the intravenous (i.v.) injection of B16 melanoma or Lewis Lung Carcinoma (LLC) cells reported a significant retardation of the development of pulmonary tumor nodules. Moreover, a decreased incidence of the lung and lymph node metastases accompanied by the enhanced infiltration of the metastatic foci by lymphocytes was demonstrated by Hashimoto et al. (1999) who exposed rats to 0.2 Gy γ-rays 14 days after subcutaneous (s.c.) implantation of hepatoma cells. Importantly, a local irradiation of the developing tumors did not reduce the number of spontaneous metastases derived thereof. Likewise, Sakai et al. (2003) reported that protracted irradiation of mice with γ-rays at 1 mGy/h dose rate for over 250 days attenuated the growth of the 20-methylocholantrene-induced skin tumors. In our own experiments, two hours after cessation of both single (Fig. 1) and fractionated (Fig. 2) WBI of mice with 0.1 or 0.2 Gy X-rays the animals were i.v. injected with L1 sarcoma cells and 14 days later macroscopic tumor colonies were counted on the lungs’ surfaces (Cheda et al. 2004a,b, 2006, Nowosielska et al. 2005, 2006b, 2008, Janiak et al. 2006). We showed that development of the induced tumor metastases was significantly inhibited after single WBI with the two low doses of X-rays (Fig. 3A and Table 1). Similarly, mice exposed to the total dose of 0.1 or 0.2 Gy applied in ten equal fractions tended to have less pulmonary tumor colonies than sham-exposed control animals (Fig. 3B) (Nowosielska et al. 2008).
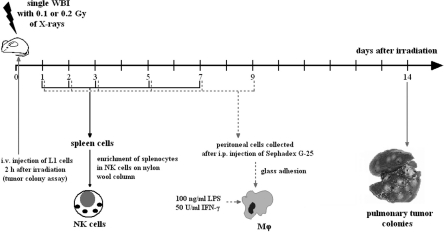
FIGURE 1.
Schematic outline of single exposures of BALB/c mice to X-rays (HS320 Pantak X-ray generator [230 kV, 20 mA] supplied with the Al and Cu filters, at 2.2 Gy/h dose rate) and times of the assessment of tumor lung colonies and activities of the NK cell-enriched splenocytes (NK cells) and peritoneal macrophages (Mϕ).
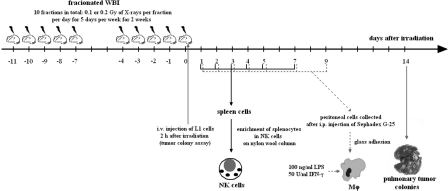
FIGURE 2.
Schematic outline of fractionated exposures of BALB/c mice to X-rays (ANDREX X-ray generator [150 kV, 3 mA], at 2.16 Gy/h dose rate) and times of the assessment of tumor lung colonies and activities of the NK cell-enriched splenocytes (NK cells) and peritoneal macrophages (Mϕ).
FIGURE 3.
Relative numbers (percentages of the control values indicated as solid line at 100%) of the induced tumor colonies in the lungs of BALB/c mice exposed to single (A) or fractionated (B) total doses of 0.1 or 0.2 Gy X-rays and two hours later i.v. injected with L1 sarcoma cells. Mean values ± SD (bars) are shown. *indicates statistically significant (p<0.05) difference from the control (100%) value.
TABLE 1.
Relative numbers (percentage of the control value) of the pulmonary L1 sarcoma colonies and cytotoxic activity of the NK cell-enriched splenocytes (NK cells) and IFN-γ- and LPS-stimulated peritoneal macrophages (Mϕ) after single WBI (Fig. 1) of mice with 0.1 or 0.2 Gy X-rays and i.p. injection with anti-asialo GM1 antibody or CGN.
| Pulmonary colonies [% of the control value] |
Cytotoxic activity of NK cells [% lysis] |
Cytotoxic activity of Mϕ [% lysis] |
|||||
|---|---|---|---|---|---|---|---|
| Group | PBS | Ab | CGN | PBS | Ab | PBS | CGN |
| Control | 100 | 147* | 401* | 7.2 | 0.5* | 29.4 | 14.6* |
| 0.1 Gy | 64* | 155* | 352* | 11.1* | 0.3* | 40.4* | 16.3* |
| 0.2 Gy | 68* | 137* | 387* | 11.4* | 0.3* | 42.1* | 17.5* |
The parameters were examined on the 14th (pulmonary colonies), 2nd (NK cells), and 3rd (Mϕ) days after single WBI. Control – sham-exposed mice; 0.1 Gy – mice exposed to a single WBI with 0.1 Gy X-rays; 0.2 Gy – mice exposed to a single WBI with 0.2 Gy X-rays; PBS – mice i.p. injected with phosphate buffered saline; Ab – mice i.p. injected with anti-asialo GM1 antibody; CGN – mice i.p. injected with CGN.
indicates statistically significant (p<0.05) difference from the control/PBS value.
The above results suggest that the inhibitory effects of the WBI with low doses of X- or γ-rays on the development of both primary and secondary tumor foci may result from stimulation by such exposures of anti-cancer immune mechanisms rather than from a direct impairment of the viability and/or function of the neoplastic cells.
III. LOW DOSE-INDUCED STIMULATION OF THE ANTI-NEOPLASTIC IMMUNITY
Numerous experimental investigations have demonstrated that exposures to low doses of ionizing radiation spur functions of the anti-neoplastic immune surveillance system. For example, Ina and coworkers (Ina and Sakai 2005a, Ina et al. 2005) showed that chronic irradiation of C57BL/6 mice with γ-rays at 1.2 mGy/h dose rate resulted in the activation of T and B lymphocytes, including the plaque forming cells (PFC), as well as in the total depletion of the abnormal T CD3−CD4+ lymphocytes. In another study, the same authors demonstrated that chronic low-dose-rate (0.35 or 1.2 mGy/h) irradiation with γ-rays enhanced survival of the MRL-lpr/lpr mice carrying a deletion in the apoptosis-regulating Fas gene leading to a severe autoimmune disease and reduced life-span. This effect was accompanied by significant elevations in the number of CD4+CD8+ T cells in the thymus and CD8+ T cells in the spleen as well as by a marked down-regulation of the abnormal CD3+↑CD45R/B220+ and CD45R/B220+↓CD40+ splenocytes accompanied by a drastic attenuation of the total-body lymphadenopathy, splenomegaly, proteinuria, and kidney and brain disorders (Ina and Sakai 2004,Ina and Sakai 2005b).
1. NK cell-mediated activity
Primary cellular effectors of the non-specific anti-tumor surveillance system are natural killer (NK) lymphocytes and activated macrophages (Mϕ) (Nathan 1991, Farias-Eisner et al. 1994, Liu et al. 1994a,b, Moretta et al. 1994; for review see: Barao and Ascensao 1998, Al-Sarireh and Eremin 2000). Stimulation of the NK cell-mediated cytotoxicity after a single irradiation of mice was described by Liu et al. (1994b) and by Kojima et al. (2002, 2004). The former group detected the effect 24 hours after exposure to 0.075 Gy X-rays and the latter – between the fourth and sixth hours post irradiation with 0.5 Gy γ-rays. Other authors (Ju et al. 1995) reviewed the enhanced cytocidal function of murine NK-type lymphocytes 2–6 days after a single exposure of the animals to 0.075 Gy X-rays. These findings were corroborated by the results of our own studies demonstrating that single WBI (Fig. 1) of mice with 0.1 or 0.2 Gy X-rays increased the cytolytic function of NK lymphocytes obtained from the spleen (NK cell-enriched splenocytes), as measured by the classic 51Cr release from the YAC-1 tumor target cells; this effect was detectable between the first and third days post-irradiation and was most pronounced on the second day after the exposure (Table 1 and 2) (Cheda et al. 2004a,b, 2006, Nowosielska et al. 2005, 2006a, 2008, Janiak et al. 2006). We also showed that fractionated WBI of mice (Fig. 2) with either of the above two low doses of X-rays led to the significant upregulation of the cytotoxic function of the NK cell-enriched splenocytes (Table 3); in this case the enhanced cytotoxicity was most pronounced between the first and fourth days post-exposure and declined to the baseline level on the seventh day (Nowosielska et al. 2008). When mice were intraperitoneally (i.p.) injected with anti-asialo GM1 antibody (a classical blocker of the NK cells activity) 24 hours before single WBI, the NK-type activity tested two days later was totally suppressed and this inhibition could not be reversed by a single WBI with 0.1 or 0.2 Gy X-rays (Table 1) (Cheda et al. 2004b, 2006, Nowosielska et al. 2005, Janiak et al. 2006). Moreover, i.p. injection of the anti-asialo GM1 antibody resulted in the significant increase in the numbers of the tumor colonies developing in the lungs of both irradiated and sham-exposed mice (Table 1).
TABLE 2.
Immunological parameters in mice exposed to single WBI (Fig. 1) with 0.1 or 0.2 Gy X-rays.
| Parameter | Control | 0.1 Gy | 0.2 Gy |
|---|---|---|---|
| Cytotoxicity (NK cells) [% lysis] | 7.2 | 11.1* ↑ | 11.4* ↑ |
| Expression of FasL by NK cells | 3.5 | 4.9* ↑ | 4.9* ↑ |
| Cytotoxicity (Mϕ) [% lysis] | 29.3 | 40.4* ↑ | 42.1* ↑ |
| Production of NO by Mϕ [μM NO2−/l] | 6.2 | 21.4* ↑ | 20.4* ↑ |
| Production of IFN-γ by NK cells [pg/ml] | 46 | 75* ↑ | 75* ↑ |
| Production of IL-1β by Mϕ [pg/ml] | 584 | 1005* ↑ | 1168* ↑ |
| Production of IL-2 by splenocytes [pg/ml] | 19 | 31* ↑ | 35* ↑ |
| Production of IL-12 by Mϕ [pg/ml] | 400 | 1881* ↑ | 1844* ↑ |
| Production of TNF-α by Mϕ [pg/ml] | 6600 | 8320* ↑ | 9570* ↑ |
The parameters were examined on the 2nd (NK cell-enriched splenocytes – NK-cells) and 3rd (PHA-stimulated splenocytes and IFN-γ and LPS-stimulated peritoneal macrophages – Mϕ) days after single WBI. Control – sham-exposed mice; 0.1 Gy – mice exposed to a single WBI with 0.1 Gy X-rays; 0.2 Gy – mice exposed to a single WBI with 0.2 Gy X-rays.
indicates statistically significant (p<0.05) difference from the results obtained in the control group.
TABLE 3.
Immunological parameters in mice exposed to fractionated WBI (10 fractions in total: 0.01 or 0.02 Gy per fraction per day for 5 days per week for 2 weeks; Fig.2) with total doses of 0.1 or 0.2 Gy X-rays.
| Parameter | Control | 0.1 Gy | 0.2 Gy |
|---|---|---|---|
| Cytotoxicity (NK cells) [% lysis] | 7.0 | 11.9* ↑ | 12.3* ↑ |
| Cytotoxicity (Mϕ) [% lysis] | 24.7 | 42.6* ↑ | 42.9* ↑ |
| Production of NO by Mϕ [μM NO2−/l] | 10.7 | 30.7* ↑ | 36.8* ↑ |
| Production of IFN-γ by NK cells [pg/ml] | 43 | 87* ↑ | 89* ↑ |
| Production of IL-1β by Mϕ [pg/ml] | 532 | 768* ↑ | 897* ↑ |
| Production of IL-2 by splenocytes [pg/ml] | 19 | 30* ↑ | 41* ↑ |
| Production of IL-12 by Mϕ [pg/ml] | 399 | 2342* ↑ | 2898* ↑ |
| Production of TNF-α by Mϕ [pg/ml] | 6875 | 33783* ↑ | 44810* ↑ |
The parameters were examined on the 3rd (PHA-stimulated splenocytes), 4th (NK cell-enriched splenocytes – NK cells) and 5th (IFN-γ- and LPS-stimulated peritoneal macrophages – Mϕ) days after fractionated WBI. Control – sham-exposed mice; 0.1 Gy – mice exposed to total dose of 0.1 Gy X-rays; 0.2 Gy – mice exposed to total dose of 0.2 Gy X-rays.
indicates statistically significant (p<0.05) difference from the results obtained in the control group.
Cytotoxic activity of NK lymphocytes is mediated by the extra-cellularly secreted perforin which creates ‘pores’ in the target cell’s membrane whereby the concomitantly released granzymes enter and kill the target (Lord et al. 2003, Smyth et al. 2005). Another potential cytotoxic mechanism consists in the activation of the Fas receptor on the surface of tumor cells upon binding of the specific ligand (FasL) whose expression is upregulated on the activated NK lymphocytes (Reyburn et al. 1997, for review see: Barao and Ascensao 1998). In our experiments suppression of the perforin activity by concanamicin A (CMA) significantly inhibited cytotoxic function of the NK cell-enriched splenocytes collected from both irradiated and non-irradiated mice; the suppression was lower but still significantly expressed when the anti-FasL antibody was added to the incubation medium (Table 4) (unpublished data). Also, surface expression of FasL was significantly increased on the NK-type splenocytes obtained from mice two days after a single WBI (Fig. 1) with 0.1 and 0.2 Gy X-rays, i.e. at the time when the cytotoxic function of these cells was maximally stimulated (Table 2) (Nowosielska et al. 2005, 2006a, Janiak et al. 2006). These results clearly indicate that both secretion of perforin by and expression of FasL on the surface of the effector cells were responsible for the demonstrated by us enhanced cytotoxic function of the NK cell-enriched splenocytes obtained from mice exposed to single irradiations with low doses of X-rays.
TABLE 4.
Inhibition of the cytotoxic activity [expressed in % lysis] of the NK cell-enriched spleno-cytes (NK cells – inhibited by CMA and anti-FasL Ab) and the IFN-γ- and LPS-stimulated peritoneal macrophages (Mϕ – inhibited by CGN and AG) on the 2nd and 3rd days after single WBI (Fig. 1) with X-rays, respectively.
| Cytotoxic activity of NK cells [% lysis] |
Cytotoxic activity of Mϕ [% lysis] |
|||||||
|---|---|---|---|---|---|---|---|---|
| Groups | CM | CMA | anti-FasL Ab | CMA + anti-FasL Ab | PBS | CGN | AG | CGN + AG |
| Control | 7.2 | 2.4* | 4.5* | 0.6* | 29.4 | 14.6 a | 4.2 a | 3.8 a |
| 0.1 Gy | 11.1^ | 3.5*^ | 6.8*^ | 0.4* | 40.4 b | 16.3 a | 8.3 ab | 7.9 ab |
| 0.2 Gy | 11.4^ | 3.5*^ | 7.2*^ | 1.1*^ | 42.1 b | 17.5 a | 9.5 ab | 8.7 ab |
Control – sham-exposed mice; 0.1 Gy – mice exposed to a single WBI with 0.1 Gy X-rays; 0.2 Gy –mice exposed to a single WBI with 0.2 Gy X-rays.
CM – NK cells incubated in culture medium without blockers; CMA – NK cells incubated with CMA; anti-FasL Ab – NK cells incubated with anti-FasL antibody; CMA + anti-FasL Ab – NK cells incubated with CMA and anti-FasL antibody.
indicates statistically significant (p<0.05) difference between NK cells collected from irradiated mice and the respective NK cells obtained from non-irradiated mice.
indicates statistically significant (p<0.05) difference within each sham-irradiated or irradiated group between NK cells incubated with CMA and/or anti-FasL antibody and NK cells incubated without blockers (CM group).
PBS – Mϕ obtained from mice pretreated with PBS; CGN – Mϕ obtained from mice pretreated with CGN; AG – Mϕ obtained from mice pretreated with PBS and incubated in vitro in the presence of AG; CGN + AG – Mϕ obtained from mice pretreated with CGN and incubated in vitro in the presence of AG.
indicates statistically significant (p<0.05) difference within sham-irradiated or irradiated groups between Mϕ incubated with AG and/or obtained from mice pretreated with CGN and Mϕ collected from mice pretreated with PBS and incubated without AG.
indicates statistically significant (p<0.05) difference between Mϕ collected from irradiated mice and the respective Mϕ obtained from non-irradiated mice.
2. Macrophage-mediated activity
Activated macrophages (Mϕ) kill susceptible tumor cells by means of a number of cytotoxic factors of which nitric oxide (NO) plays a prominent role (Nathan 1991, Cui et al. 1994, Farias-Eisner et al. 1994, Jenkins et al. 1995, Xie and Fidler 1998). Ibuki and Goto (1995) were among the first to demonstrate that irradiation of mice with 0.04 Gy γ-rays stimulated production of NO by the IFN-γ- and LPS-treated peritoneal Mϕ collected on the day of exposure. This effect was associated with the significant enhancement of the cytotoxic function of Mϕ against the P815 tumor cells. Up-regulated secretion of NO was also described by Pandey et al. (2005) who five times in a row irradiated C57BL/6 mice with 0.04 Gy γ-rays (with the 24-hour intervals between the irradiations) and assessed production of this cytocidal factor by the ConA-stimulated adherent splenocytes on the third day after cessation of the exposures; this effect was associated with the increased phagocytic function of peritoneal exudate cells obtained from the irradiated animals.
The above results were corroborated and extended by us in experiments demonstrating that single exposures (Fig. 1) of mice to 0.1 or 0.2 Gy X-rays led to the significant up-regulation of the cytolytic activity (measured in the 3H-thymidine release assay) of the untreated and IFN-γ and LPS-stimulated peritoneal Mϕ against both the L1 (Table 1 and 2) and P815 tumor cells; the effect was most pronounced on the third day and sustained until the ninth day post-irradiation (Cheda et al. 2005, 2006, Janiak et al. 2006, Nowosielska et al. 2006a,b, 2008). Moreover, fractionated WBI of mice (Fig. 2) with either of the two low total doses of X-rays resulted in the significant enhancement of the cytotoxic function of peritoneal Mϕ (Table 3) against the L1 cells; the effect was most pronounced between the second and fifth days post-irradiation and declined thereafter (Nowosielska et al. 2008). The up-regulated cytotoxicity was accompanied by the elevated production of NO (Table 2 and 3) (Cheda et al. 2004a, 2005, 2006, Janiak et al. 2006, Nowosielska et al. 2006a,b, 2008) and reactive superoxide anions in the IFN-γ and LPS-treated (NO) or untreated (superoxide anions) peritoneal Mϕ (Cheda et al. 2005, Janiak et al. 2006). Interestingly, in these experiments the kinetics of the NO production closely followed the changes in the cytolytic activity of the peritoneal Mϕ.
Notably, i.p. injection of mice with carrageenan (CGN – a lysosome-disrupting and phagocyte-damaging compound) (Frank et al. 2003) 24 hours before single WBI resulted in the almost total abrogation of the synthesis of NO in the collected peritoneal Mϕ regardless of whether the cells were obtained from the irradiated or sham-exposed mice (Cheda et al. 2006, Janiak et al. 2006, Nowosielska et al. 2006b, 2008). This finding supports a possible involvement of NO in the Mϕ-mediated anti-tumor effect of the low-level exposures to X-rays. However, peritoneal Mϕ collected from the CGN-treated animals still exhibited cytotoxic activity in vitro, even in the absence of IFN-γ and LPS in the incubation medium. This observation can be explained by triggering of the synthesis of NO in the effector peritoneal Mϕ by the target cells (Nowosielska et al. 2006b) and/or involvement of other stimulatory mechanisms in the Mϕ-mediated function. Indeed, suppression of the activity of the inducible nitric oxide synthase (iNOS) by aminoguanidine (AG – a classical inhibitor of iNOS) markedly reduced the cytolytic function of the effector peritoneal Mϕ obtained from both the sham-exposed and irradiated mice (Table 4) (Nowosielska et al. 2006b).
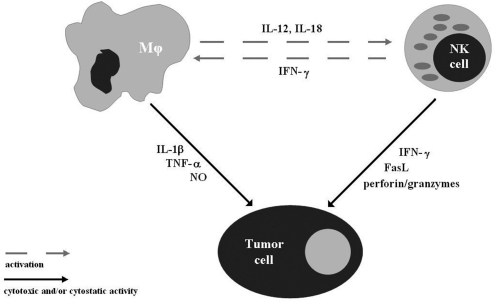
We also demonstrated that injection of mice with CGN, in addition to the submaximal abrogation of the synthesis of NO in the collected Mϕ, totally abolished the inhibitory effect of the irradiations with both 0.1 and 0.2 Gy X-rays on the growth of the induced tumor metastases: the number of the pulmonary colonies developing in the irradiated mice pre-treated with CGN did not differ from that obtained in the control animals (Table 1). Interestingly, CGN appeared to be a more potent suppressor of the anti-neoplastic effect of the low-level exposure to X-rays than the anti-asialo GM1 antibody (Cheda et al. 2004b, 2006, Nowosielska et al. 2005, 2008, Janiak et al. 2006): the suppressive effect of the former was severalfold greater than that of the latter. This observation may be explained by a possible shut-down or reduction by CGN of the cytotoxic functions of Mϕ and, indirectly, NK cells. In fact, it has been shown that several cytokines produced by Mϕ, such as IL-12 and IL-18, are potent modulators of the activity of NK lymphocytes (for review see Young and Ortaldo 2006) and inhibition of the function of the former cells may affect that of the latter cells in vivo (Fig. 4).
FIGURE 4.
Schematic outline of the possible interactions between NK cells, macrophages, and tumor cells.
3. Cytokines produced by NK cells and macrophages
Notably, the results obtained in our studies indicated that neither the i.p. injection of mice with CGN nor addition of AG to the culture medium led to the complete abrogation of the cytotoxic activity of peritoneal Mϕ even when the two blockers were used simultaneously. Likewise, both CMA and the anti-FasL antibody were unable to totally suppress the cytolytic function of the NK cell-enriched splenocytes (Table 4). These findings suggest that the residual cytotoxic activity of the two types of the effector cells may result from the production and secretion of additional cytotoxic and/or cytostatic factors likely to be involved in elimination of neoplastic cells. In fact, both NK lymphocytes and activated Mϕ produce a number of cytokines which mediate anti-neoplastic functions of these cells, i.e. either directly suppress proliferation of and/or kill tumor targets (e.g., IL-1β, IFN-γ, TNF-α) or paracrinely stimulate neighboring cells to secrete cytocidal factors (e.g., IL-1β, IL-12, IFN-γ). In fact, researchers in Japan and China, after exposure of mice and rats to single WBI with X-or γ-rays at doses ranging from 0.04 to 0.25 Gy, detected up-regulated secretion and/or expression of mRNAs for IL-1β, IL-12, TNF-α (by Mϕ), IFN-γ (by NK cells), and IL-2 (by splenocytes) (DeBlaker-Hohe et al. 1995, Miller et al. 2003, Liu et al. 1994b, 2001, Fu et al. 1996, 1997, Zhang et al. 1996, Gong et al. 1997, Zhang et al. 1998a,b, 1999, Bai et al. 1998, Ibuki and Goto 1999, Hashimoto et al. 1999, Shan et al. 2007, for review see: Al-Sarireh and Eremin 2000, Belardelli and Ferrantini 2002, Liu 2007). In contrast to single irradiations, almost no evidence exists in the literature on triggering of the expression of cytokines by multiple low-level exposures to ionizing radiation. Indeed, Pandey et al. (2005) were unable to detect any stimulation of the production of IFN-γ in splenocytes collected and assayed on the third day after completion of the fractionated (0.04 Gy per day for 5 days) irradiation of the C57BL/6 mice with γ-rays.
These observations were generally corroborated by the results of our own investigations in which peritoneal Mϕ and NK cell-enriched spleno-cytes (Table 2 and 3) were assayed for their capacity to produce the selected cytokines using experimental procedures identical to the ones previously utilized by us for testing of the cytotoxic activity of these cells. Thus, tumor target cells (P815 or L1 for peritoneal Mϕ and YAC-1 for NK cell-enriched splenocytes) or PHA (for splenocytes) were included in the incubation wells and then the cell-free supernatants were assayed by the ELISA methodology for the levels of IL-1β, TNF-α, IL-12 (synthesized by peritoneal Mϕ), IL-2 (produced by splenocytes), and IFN-γ (secreted by NK cell-enriched splenocytes) (Cheda et al. 2008). In this study, we demonstrated that both single (Fig. 1) and fractionated (Fig. 2) irradiations of mice with total absorbed doses of 0.1 or 0.2 Gy X-rays significantly stimulated peritoneal Mϕ to produce IL-1β (Table 2 and 3) (Cheda et al. 2008). Notably, the kinetics of the low-level X-ray-induced production of IL-1β was similar to that detected for the cytotoxic activity and production of NO by peritoneal Mϕ obtained after single (Cheda et al. 2005, 2006, Janiak et al. 2006, Nowosielska et al. 2006a,b) or fractionated (Nowosielska et al. 2008) irradiations of mice with 0.1 or 0.2 Gy X-rays. Stimulation of the synthesis of IL-1β was accompanied by the enhanced production of TNF-α after both single (Fig. 1) and fractionated (Fig. 2) irradiations of mice, although the stimulatory effect of the latter exposures on the secretion of TNF-α was much stronger than that detected after the former exposures (Table 2 and 3) (Cheda et al. 2005, 2008, Janiak et al. 2006, Nowosielska et al. 2006a). The radiation-induced up-regulated production of IL-1β and TNF-α coincided with the elevated secretion of IL-12 (Table 2 and 3) (Cheda et al. 2008). Moreover, both single (Fig. 1) and fractionated (Fig. 2) WBI of mice with 0.1 or 0.2 Gy X-rays significantly stimulated synthesis of IL-2 and IFN-γ in the unseparated and the NK cell-enriched splenocytes, respectively (Table 2 and 3) (Cheda et al. 2008). Importantly, the time course of the changes in the production of IFN-γ after a single but not fractionated irradiation of mice with either of the two low doses of X-rays closely resembled the kinetics of the previously demonstrated by us (Cheda et al. 2004a,b, 2006, Nowosielska et al. 2005, 2006a, 2008, Janiak et al. 2006) enhanced cytotoxicity of the NK cell-enriched splenocytes obtained from the similarly exposed animals.
4. NK cell- and macrophage-mediated activities after in vitro irradiation
In contrast to the single and fractionated WBI of mice with low doses of X-rays, we showed that the in vitro irradiations of NK cell-enriched splenocytes and peritoneal Mϕ did not significantly affect the NK cell- and Mϕ-mediated functions (Table 5). This observations suggests that the above described anti-tumor properties of both single and fractionated irradiations with low total doses of X-rays result from the stimulated interaction of many components of the complex immune surveillance system rather than from boosting of function(s) of a single cell population.
TABLE 5.
Effects of the in vitro irradiation of NK cell-enriched splenocytes (NK cells) and the IFN-γ- and LPS-stimulated peritoneal macrophages (Mϕ) with low doses of X-rays.
| NK cells |
Mϕ |
|||
|---|---|---|---|---|
| Groups | Cytotoxicity [% lysis] | Production of IFN-γ [pg/ml] | Cytotoxicity [% lysis] | Production of NO [μM NO2−/l] |
| Control | 6.0 | 46.1 | 25.2 | 12.5 |
| 0.1 Gy | 5.5 | 51.3 | 24.6 | 11.8 |
| 0.2 Gy | 5.8 | 53.7 | 24.9 | 13.1 |
Control – sham-exposed NK cells or Mϕ; 0.1 Gy – NK cells or Mϕ exposed to single irradiation with 0.1 Gy X-rays; 0.2 Gy – NK cells or Mϕ exposed to single irradiation with 0.2 Gy X-rays.
IV. IMPLICATIONS
Results of the epidemiological and experimental studies presented in this paper indicate that low-level exposures to X- and γ-rays may suppress the development and progression of tumors and that these effects can be associated with stimulation by such irradiations of anti-neoplastic functions of the immune system. This type of the radiation-evoked hormetic effect (for review see: Luckey 1980, Webster 1993, Calabrese and Baldwin 2002, Pollycove 2007, Feinendegen et al. 2008) may have several implications.
Firstly, the described experimental data provide clues for, at least partial, explanation of the inhibitory effects of the low-level exposures to the low-LET radiation on the development and progression of tumors. Indeed, these low-dose data contradict the commonly applied LNT model-related assumption that the underlying biological processes that function after low radiation doses are essentially the same as those that function after higher radiation doses (for review see: Averbeck et al. 2006, Tubiana and Aurengo 2006, Tubiana et al. 2006a,b, Tubiana 2008, 2009).
Secondly, after careful preclinical and clinical trials, low-level exposures to X- or γ-rays could be employed as a new modality in the treatment of cancer. Indeed, the half or total body exposures to low doses of X-rays have been already tested in experimental therapeutic protocols as complements to the standard chemo- and/or radiotherapy of ovarian and colon cancer, as well as non-Hodgkin lymphoma (Choi et al. 1979, Sakamoto et al. 1997, for review see Cuttler and Pollycove 2003). As indicated recently by Tubiana in his review of the current data (Tubiana 2009), the risk of the second primary malignancies associated with therapeutical application of the total doses of radiation lower than 0.1–0.2 Gy is negligible.
Thirdly, the results of the investigations described above as well as other supporting radiation-adaptive-response data may in a broader perspective contribute to relaxing the current stringent radiation protection regulations. This includes regulations that govern diagnostic and therapeutic applications of ionizing radiation as well as those related to occupational and environmental radiation exposures. Current regulations are based on the controversial LNT hypothesis (ICRP 1990, 2006; Mossman 2009) which imposes avoiding exposures to even very low doses and dose rates of radiation. This practice not only elevates the costs of the enforcement of the protective measures, but may also have –opposite to the intentions – adverse health effects (for review see Jaworowski 2000, 2009).
REFERENCES
- Al-Sarireh B, Eremin O. Tumour-associated macrophages (TAMS): disordered function, immune suppression and progressive tumour growth. J R Coll Surg Edinb. 2000;45:1–16. [PubMed] [Google Scholar]
- Averbeck D, Testard I, Boucher D. Changing views on ionising radiation-induced cellular effects. Int J Low Radiation. 2006;3:117–134. [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- Bai O, Liu SZ, Mu Y. Effect of low dose radiation on Th1 and Th2 of thymocytes and splenocytes in mice. Chin J Radiol Med Prot. 1998;18:106–109. [Google Scholar]
- Barao I, Ascensao JL. Human natural killer cells. Arch Immunol Ther Exp. 1998;46:213–229. [PubMed] [Google Scholar]
- Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumour immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: mortality from cancer and other causes 1897–1997. Br J Radiol. 2001;74:507–519. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- Cai L. Research of the adaptive response induced by low-dose radiation: Where have we been and where should we go? Hum Exp Toxicol. 1999;18:419–425. doi: 10.1191/096032799678840291. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Radiation hormesis and cancer. Hum Ecol Risk Assess. 2002;8:327–353. [Google Scholar]
- Caratero A, Courtade M, Bonnet L, Planel H, Caratero C. Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology. 1998;44:272–276. doi: 10.1159/000022024. [DOI] [PubMed] [Google Scholar]
- Cardis E, Gilbert ES, Carpenter L, Howe G, Kato I, Armstrong K, Beral V, Cowper G, Douglas A. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995;142:117–32. [PubMed] [Google Scholar]
- Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat Environ Biophys. 2008;47:275–283. doi: 10.1007/s00411-007-0147-7. [DOI] [PubMed] [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Lisiak E, Marciniak M, Nowosielska EM, Janiak MK. Inhibition of the development of pulmonary tumour nodules and stimulation of the activity of NK cells and macrophages in mice by single low doses of low-LET radiation. Int J Low Radiation. 2004a;1:171–179. [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska EM, Marciniak M, Janiak MK. Single Low Doses of X-Rays Inhibit the Development of Experimental Tumor Metastases and Trigger the Activities of NK Cells in Mice. Radiat Res. 2004b;161:335–340. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Nowosielska EM, Janiak MK. Stimulatory effect of a single low-level irradiation with X-rays on functions of murine peritoneal macrophages. Nukleonika. 2005;50(Suppl. 2):S13–S16. [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Nowosielska EM, Janiak MK. Immune mechanism of the retarded growth of tumor nodules in mice exposed to single low-level irradiations with X-rays. Centr Eur J Immunol. 2006;31:44–50. [Google Scholar]
- Chen WL, Luan YC, Shieh MC, Chen ST, Kung HT, Soong KL, Yeh YC, Chou TS, Mong SH, Wu JT, Sun CP, Deng WP, Wu MF, Shen ML. Is chronic radiation an effective prophylaxis against cancer? J Am Physicians Surg. 2004;9:6–10. [Google Scholar]
- Choi NC, Timothy AR, Kaufman SD, Carey RW, Aisenberg AC. Low dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin’s lymphoma. Cancer. 1979;43:1636–1642. doi: 10.1002/1097-0142(197905)43:5<1636::aid-cncr2820430512>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Test the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;86:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Problems in the radon vs lung cancer test of linear no-threshold theory and a procedure for resolving them. Health Phys. 1997;72:623–628. doi: 10.1097/00004032-199704000-00015. [DOI] [PubMed] [Google Scholar]
- Cui S, Reichner JS, Mateo RB, Albina JE. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994;54:2462–2467. [PubMed] [Google Scholar]
- Cuttler JM, Pollycove M. Can cancer be treated with low doses of radiation? J Am Phys Surg. 2003;8:108–111. [Google Scholar]
- DeBlaker-Hohe DF, Yamauchi A, Yu CR, Horvath-Arcidiacono JA, Bloom ET. IL-12 synergises with IL-2 to induce lymphokine-activated cytotoxicity and perforin and granzyme gene expression in fresh human NK cells. Cell Immunol. 1995;165:33–43. doi: 10.1006/cimm.1995.1184. [DOI] [PubMed] [Google Scholar]
- Farias-Eisner R, Sherman MP, Aeberhard E, Chaudhuri G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci USA. 1994;91:9407–9411. doi: 10.1073/pnas.91.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinendegen L, Hahnfeldt P, Schadt EE, Stumpf M, Voit EO. Systems biology and its potential role in radiobiology. Radiat Environ Biophys. 2008;47:5–23. doi: 10.1007/s00411-007-0146-8. [DOI] [PubMed] [Google Scholar]
- Frank J, Born K, Barker JH, Marzi I. In vivo effect of tumor necrosis factor alpha on wound angiogenesis and epithelialization. Eur J Trauma. 2003;29:208–219. [Google Scholar]
- Fu H, Li X, Chen Y, Zhang Y, Liu SZ. Mechanism of suppressive effect of low dose radiation on cancer cell dissemination in mice. J Radiat Res Radiat Process. 1997;15:40–43. [Google Scholar]
- Fu H, Li X, Li Y, Liu SZ. Whole-body low dose irradiation suppresses cancer cell dissemination in mice. Chin J Radiol Med Prot. 1996;16:307–309. [Google Scholar]
- Gong S, Liu J, Liu S, Zhang Y, Liu SZ. Effect of splenocyte extracellular fluid irradiated with low dose X-rays on thymocyte apoptosis after larger dose X-irradiation in mice. J Radiat Res Radiat Process. 1997;15:36–39. [Google Scholar]
- Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–724. [PubMed] [Google Scholar]
- Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26:177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Ibuki Y, Goto R. Contribution of inflammatory cytokine release to activation of resident peritoneal macrophages after in vivo low-dose γ-irradiation. J Radiat Res. 1999;40:253–262. doi: 10.1269/jrr.40.253. [DOI] [PubMed] [Google Scholar]
- Ibuki Y, Goto R. Augmentation of NO production and cytolytic activity of Mϕ obtained from mice irradiated with a low dose of γ-rays. J Radiat Res. 1995;36:209–220. doi: 10.1269/jrr.36.209. [DOI] [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection) Recommendations of the International Commission on Radiological Protection, Publication 60. Elsevier; London: 1990. [Google Scholar]
- ICRP (International Commission on Radiological Protection) Low-dose Extrapolation of Radiation Related Cancer Risk Publication 99. Elsevier; London: 2006. [Google Scholar]
- Ina Y, Sakai K. Prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice. Radiat Res. 2004;16:168–173. doi: 10.1667/rr3120. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains; Analysis of immune cell populations surface molecules. Int J Radiat Biol. 2005a;81:721–729. doi: 10.1080/09553000500519808. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Further study on prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole-life irradiation. Radiat Res. 2005b;163:418–423. doi: 10.1667/rr3316. [DOI] [PubMed] [Google Scholar]
- Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by lifelong low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163:153–158. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hosoi Y, Yamada S, Ono T, Sakamoto K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat Res. 1996;146:582–585. [PubMed] [Google Scholar]
- Jagger J. Natural background radiation and cancer death in Rocky Mountain states and Gulf Coast states. Health Phys. 1998;7:428–430. doi: 10.1097/00004032-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Janiak MK, Wrembel-Wargocka J, Cheda A, Nowosielska EM, Lisiak E, Bilski M. Modulation of the antitumour functions of murine NK cells and macrophages after single low-level exposures to X-rays. Int J Low Radiation. 2006;3:178–191. [Google Scholar]
- Jaworowski Z.2000Beneficial radiation and regulations Proc of 8th International Conference on Nuclear Engineering (ICONE 8) Baltimore, USAPaper No. 8790. American Society of Mechanical Engineers; New York [Google Scholar]
- Jaworowski Z. Radiation hormesis – a remedy for fear. BELLE News. 2009;15:14–20. doi: 10.1177/0960327110363974. [DOI] [PubMed] [Google Scholar]
- Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Role of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju GZ, Liu SZ, Li XY, Liu WH, Fu HQ. Effect of high versus low dose radiation on the immune system. In: Hagen U, Harder D, Jung H, Streffer C, editors. Radiation Research 1895–1995. Proc of the Tenth Int Congress of Radiation Research, 709–714. Würzburg, Germany: 1995. [Google Scholar]
- Katayama H, Matsuura M, Endo S, Hosoi M, Othaki M, Hayakawa N. Reassessment of the cancer mortality risk among Hiroshima atomic-bomb survivors using a new dosimetry system, ABS2000D, compared with ABS93D. J Radiat Res. 2002;43:53–64. doi: 10.1269/jrr.43.53. [DOI] [PubMed] [Google Scholar]
- Kesavan PC. Indian research on high levels of natural radiation: pertinent observations for further studies. In: Wei L, Sugahara T, Tao Z, editors. High levels of natural radiation, 1996: Radiation dose and health effects Proc of 4th International Conference on High Levels of Natural Radiation; Beijing, China. Amsterdam, Netherlands: Elsevier; 1997. pp. 112–117. [Google Scholar]
- Kojima S, Ishida H, Takahashi M, Yamaoka K. Elevation of glutathione induced by low-dose gamma rays and its involvement in increased natural killer activity. Radiat Res. 2002;157:275–280. doi: 10.1667/0033-7587(2002)157[0275:eogibl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kojima S, Nakayama K, Ishida H. Low dose γ-rays activate immune functions via induction of glutathione and delay tumor growth. J Radiat Res. 2004;45:33–39. doi: 10.1269/jrr.45.33. [DOI] [PubMed] [Google Scholar]
- Kondo S. Health effects of low level radiation. Kinki University Press; Osaka, Japan: 1993. [Google Scholar]
- Little MP, Weiss HA, Boice JD. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiat Res. 1999;152:280–292. [PubMed] [Google Scholar]
- Liu SZ, Han ZB, Liu WH. Changes in lymphocyte reactivity to modulatory factors following low dose ionizing radiation. Biomed Environ Sci. 1994a;7:130–135. [PubMed] [Google Scholar]
- Liu SZ, Jin SZ, Liu XD, Sun YM. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol. 2001;2:8–15. doi: 10.1186/1471-2172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SZ, Su X, Zhang YC, Zhao Y. Signal transduction in lymphocytes after low dose radiation. Chin Med J. 1994b;107:431–436. [PubMed] [Google Scholar]
- Liu SZ, Xiao PX, Ma SY, Xu GZ, Tian CH, Yu HY, Zhang LM. A study of the immune status of inhabitants in an area of high natural radioactivity in Guangdong. Chin J Radiol Med Prot. 1982;2:64–68. [Google Scholar]
- Liu SZ, Xu GZ, Li XY, Xia FQ, Yu HY, Qi J, Wang FL, Wang SK. A restudy of immune functions of the inhabitants in a high natural radioactivity area in Guangdong. Chin J Radiol Med Prot. 1985;5:124–127. [Google Scholar]
- Liu SZ. Radiation hormesis. A new concept in radiological science. Chin Med J. 1989;102:750–755. [PubMed] [Google Scholar]
- Liu SZ. Cancer control related to stimulation of immunity by low dose radiation. Proc. of 14th Pacific Basin Nuclear Conference; Honolulu, USA. La Grange Park, Illinois: American Nuclear Society; 2004. pp. 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SZ. Cancer Control Related to Stimulation of Immunity by Low-Dose Radiation. Dose Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SJ, Rajotte RV, Korbutt GS, Blezckley RC. Granzyme B: a natural born killer. Immunol Rev. 2003;193:31–38. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Hormesis of Ionizing Radiation. CRC Press; Boca Raton, Florida, USA: 1980. [Google Scholar]
- Luckey TD. Nurture with ionising radiation: a provocative hypothesis. Nutr Cancer. 1999;34:1–11. doi: 10.1207/S15327914NC340101. [DOI] [PubMed] [Google Scholar]
- Matanoski GM, Santos-Burgoa C, Schwartz L. Mortality of a cohort of workers in the styrene-butadiene polymer manufacturing industry (1943-1982) Environ Health Perspect. 1990;86:107–117. doi: 10.1289/ehp.9086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney LC, Aquilla EM, Coffin D, Wink DA, Vodovotz Y. Origin and functions of human natural killer cells. J Leukocyte Biol. 1998;64:459–466. doi: 10.1002/jlb.64.4.459. [DOI] [PubMed] [Google Scholar]
- Miller GM, Kim DW, Andres ML, Green LM, Gridley DS. Changes in the activation and reconstitution of lymphocytes resulting from total-body irradiation correlate with slowed tumor growth. Oncology. 2003;65:229–241. doi: 10.1159/000074476. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBAH mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ. Low doses of radiation reduce risk in vivo. Dose-Response. 2007;5:1–10. doi: 10.2203/dose-response.06-109.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L, Ciccone E, Poggi A, Mingari MC, Moretta A. Origin and functions of human natural killer cells. Int J Clin Lab Res. 1994;24:181–186. doi: 10.1007/BF02592459. [DOI] [PubMed] [Google Scholar]
- Mossman KS. Policy decision-making under scientific uncertainty: Radiological risk assessment and the role of expert advisory groups. Health Phys. 2009;97:101–106. doi: 10.1097/HP.0b013e3181a7abf2. [DOI] [PubMed] [Google Scholar]
- Nathan C. Mechanisms and modulation of macrophage activation. Behrings Inst Mitt. 1991;88:200–207. [PubMed] [Google Scholar]
- National Research Council (NRC Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation) Health Risks from Exposure to Low Levels of Ionizing Radiation (BEIR VII, Phase 2) National Academy Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Modulation of the growth of pulmonary tumour colonies in mice after single or fractionated low-level irradiations with X-rays. Nukleonika. 2008;53(Suppl. 1):S9–S15. [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Janiak MK. A single low-dose irradiation with X-rays stimulates NK cells and macrophages to release factors related to the cytotoxic functions of these cells. Centr Eur J Immunol. 2006a;31:51–57. [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Lisiak E, Janiak MK. Low-level exposures to ionising radiation modulate the anti-tumour activity of murine NK cells. Nukleonika. 2005;50(Suppl. 2):S21–S24. [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Lisiak E, Janiak MK. Enhanced cytotoxic activity of macrophages and suppressed tumor metastases in mice irradiated with low doses of X- rays. J Radiat Res. 2006b;47:229–236. doi: 10.1269/jrr.0572. [DOI] [PubMed] [Google Scholar]
- Pandey R, Shankar BS, Sharma D, Sainis KB. Low dose radiation induced immunomodu-lation: effect on macrophages and CD8+ T cells. Int J Radiat Biol. 2005;81:801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950–1990. Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- Pollycove M. Radiobiological basis of low dose irradiation in prevention and therapy of cancer. Proc. of 14th Pacific Basin Nuclear Conference; Honolulu, USA. La Grange Park, Illinois: American Nuclear Society; 2004. pp. 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose-Response. 2007;5:26–38. doi: 10.2203/dose-response.06-112.Pollycove. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portess DI, Bauer G, Hill MA, O’Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Elmore E. Radiation-induced neoplastic transformation in vitro, hormesis and risk assessment. Dose-Response. 2007;5:123–130. doi: 10.2203/dose-response.06-010.Redpath. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H, Mandelboim O, Vales-Gomez M, Sheu EG, Pazmany L, Davis DM, Strominger JL. Human NK cells: their ligands, receptors and functions. Immunol Rev. 1997;155:119–125. doi: 10.1111/j.1600-065x.1997.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Safwat A, Bayoumy Y, El-Sharkawy N, Shaaban K, Mansour O, Kamel A. The potential palliative role and possible immune modulatory effects of low-dose total body irradiation in relapsed or chemo-resistant non-Hodgkin’s lymphoma. Radiother Oncol. 2003;69:33–36. doi: 10.1016/s0167-8140(03)00247-0. [DOI] [PubMed] [Google Scholar]
- Safwat A. The immunology of low-dose total-body irradiation: more questions than answers. Radiat Res. 2000a;153:599–604. doi: 10.1667/0033-7587(2000)153[0599:tioldt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Safwat A. The role of low-dose total body irradiation in treatment of non-Hodgkin’s lymphoma: a new look at an old method. Radiother Oncol. 2000b;56:1–6. doi: 10.1016/s0167-8140(00)00167-5. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, Oda T, Iwasaki T, Fujita K, Yamada T, Tanooka H. Suppression of carcinogenic processes in mice by chronic low dose rate gamma-irradiation. Int J Low Radiation. 2003;1:142–146. [Google Scholar]
- Sakamoto K, Myogin M, Hosoi Y. Fundamental and clinical studies on cancer control with total or upper half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;16:161–175. [Google Scholar]
- Shan YX, Jin SZ, Liu XD, Liu Y, Liu SZ. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: dose-response relationship, mechanisms and implications. Radiat Environ Biophys. 2007;46:21–29. doi: 10.1007/s00411-006-0076-x. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A. Dose–effect relationship and estimation of the carcinogenic effects of low doses of ionising radiation: the Joint Report of the Académie des Sciences (Paris) and of the Académie Nationale de Médecine. Int J Low Radiation. 2006;2:135–153. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. Recent reports on the effect of low doses of ionizing radiation and its dose–effect relationship. Radiat Environ Biophys. 2006a;44:245–251. doi: 10.1007/s00411-006-0032-9. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of linear no threshold for assessing the effects of low doses. J Radiol Prot. 2006b;26:317–324. doi: 10.1088/0952-4746/26/3/N01. [DOI] [PubMed] [Google Scholar]
- Tubiana M. The 2007 Marie Curie prize: the linear no threshold relationship and advances in our understanding of carcinogenesis. Int J Low Radiation. 2008;5:173–204. [Google Scholar]
- Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol. 2009;91:4–15. doi: 10.1016/j.radonc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Tuschl H, Steger F, Kovac R. Occupational exposure and its effect on some immune parameters. Health Phys. 1995;68:59–66. doi: 10.1097/00004032-199501000-00007. [DOI] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Sources and Effects of Ionizing Radiation, Annex I. 2000. Epidemiological Evaluation of Radiation-Induced Cancer; pp. 297–450. [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Report to the General Assembly, with Annexes. 1986. Genetic and Somatic Effects of Ionizing Radiation; p. 170. [Google Scholar]
- Wang GJ, Cai L. Induction of cell-proliferation hormesis and cell-survival adaptive response in mouse hematopoietic cells by whole-body low-dose radiation. Toxicol Sci. 2000;53:369–376. doi: 10.1093/toxsci/53.2.369. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lubin JH, Wang L, Zhang S, Boice JD, Cui H, Zhang S, Conrath S, Xia Y, Shang B, Brenner A, Lei S, Metayer C, Cao J, Chen KW, Lei S, Kleinerman RA. Residential radon and lung cancer risk in a high-exposure area of Gansu Province, China. Am J Epidemiol. 2002;155:554–564. doi: 10.1093/aje/155.6.554. [DOI] [PubMed] [Google Scholar]
- Webster EW. Hormesis and radiation protection. Invest Radiol. 1993;28:451–453. doi: 10.1097/00004424-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Wei L, Sugahara T. An introductory overview of the epidemiological study on the population at the high background radiation areas in Yangjiang, China. J Radiat Res. 2000;41(Suppl):1–7. doi: 10.1269/jrr.41.s1. [DOI] [PubMed] [Google Scholar]
- Xie K, Fidler IJ. Therapy of cancer metastasis by activation of the inducible nitric oxide synthase. Cancer Met Rev. 1998;17:55–75. doi: 10.1023/a:1005956721457. [DOI] [PubMed] [Google Scholar]
- Young HA, Ortaldo J. Cytokines as critical co-stimulatory molecules in modulating the immune response of natural killer cells. Cell Res. 2006;16:20–24. doi: 10.1038/sj.cr.7310004. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen Z, Liu SZ. Effects of low dose irradiation on the splenic macrophage function in mice. J N Bethune Univ Med Sci. 1996;22:131–133. [Google Scholar]
- Zhang Y, Li X, Li X, Liu SZ. Stimulatory effect of low dose radiation on the immune function in tumor-bearing mice. J N Bethune Univ Med Sci. 1999;25:592–594. [Google Scholar]
- Zhang Y, Sun Y, Li X, Mu Y, Liu SZ. An experimental study on the enhanced macrophage function in tumor-bearing mice treated with low dose radiation. J Radiat Res Radiat Proces. 1998a;16:249–252. [Google Scholar]
- Zhang Y, Sun Y, Li X, Mu Y, Liu SZ. Low dose radiation increases the mRNA transcriptional levels of IL-1beta and TNF-alpha in peritoneal macrophages of tumor-bearing mice. J N Bethune Univ Med Sci. 1998b;24:565–567. [Google Scholar]