Abstract
The Toll signaling pathway is required for the innate immune response against fungi and Gram-positive bacteria in Drosophila. Here we show that the endosomal proteins Myopic (Mop) and Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) are required for the activation of the Toll signaling pathway. This requirement is observed in cultured cells and in flies, and epistasis experiments show that the Mop protein functions upstream of the MyD88 adaptor and the Pelle kinase. Mop and Hrs, which are critical components of the ESCRT-0 endocytosis complex, colocalize with the Toll receptor in endosomes. We conclude that endocytosis is required for the activation of the Toll signaling pathway.
The innate immune system senses a wide range of pathogens through distinct pattern recognition receptors (PRRs). The engagement of these receptors leads to the activation of specific signaling pathways resulting in the expression of antimicrobial genes. Drosophila, which lacks an adaptive immune response, relies on the innate immune system as the major defense mechanism against pathogens. Systemic immune responses of Drosophila are regulated by at least two distinct signaling pathways, the IMD (immune deficiency) and the Toll pathways. Signaling through these two pathways culminates in the activation of different NFκB transcription factors, Relish in the IMD pathway and Dif in the Toll pathway (1). The binding of these transcription factors to target promoters leads to the expression of various antimicrobial peptides (AMPs) in the fat body, the Drosophila analog of the mammalian liver. Both the IMD and Toll pathways are evolutionarily conserved between insects and mammals. The Drosophila IMD pathway is homologous to the mammalian tumor necrosis factor receptor pathway and TRIF-dependent Toll-like receptor (TLR) pathway, whereas the Toll signaling pathway is similar to the interleukin-1 receptor (IL-1R) and MyD88-dependent TLR pathways (1, 2).
The IMD signaling pathway is activated by the binding of meso-diaminopimelic acid-type peptidoglycans, which are characteristic of Gram-negative bacteria, to the Drosophila peptidoglycan recognition proteins PGRP-LC or PGRP-LE (3–5). IMD is a death domain-containing adaptor and functions downstream of the PGRPs to activate the Tak1 complex that, in turn, activates the Drosophila IκB kinase (IKK) complex, consisting of IKKβ (also known as Ird5) and IKKγ (also known as Kenny) (6–9). The IKK complex then phosphorylates Relish, the Drosophila p100/p105 NFκB homolog (6). The Relish protein is processed by the Dredd caspase, and the N-terminal Rel DNA-binding domain of Relish induces antimicrobial gene expression, including Attacin and Cecropin genes (10).
The Toll signaling pathway is activated by fungi and bacteria through a cascade of extracellular proteolytic cleavage events that culminate in the proteolytic processing of the Spätzle proprotein by the Spätzle processing enzyme (SPE) (11, 12). The mature Spätzle ligand binds to the ectodomain of the Toll receptor, and induces receptor dimerization (13). Activated Toll receptors recruit a preformed plasma membrane-associated complex consisting of MyD88 and Tube (14, 15). Pelle, a serine-threonine kinase similar to mammalian IL-1R-assocated kinases, is then recruited to the active Toll receptor by Tube. Signaling through the trimeric complex composed of MyD88, Tube, and Pelle leads to the phosphorylation of Cactus, the Drosophila IκB homolog. This phosphorylation is a signal for Cactus degradation, which leads to the release of the transcription factor Dif. Dif then enters the nucleus to activate transcription of antimicrobial genes, including the gene encoding the antifungal peptide Drosomycin (16, 17). The Toll pathway is also required for the dorsal-ventral embryonic development in Drosophila (18). The majority of the intracellular signaling components of the Toll pathway are shared by both physiological processes. Dorsal, another Drosophila NFκB homolog, is a critical target of Toll signaling in dorsal-ventral patterning (19). Whereas Dorsal is not required for the activation of antimicrobial genes of the Toll pathway in adult flies, Dif is not required for normal dorsal-ventral patterning during development (20).
Unlike the Drosophila Toll receptor, the mammalian Toll-like receptors (TLRs) directly recognize pathogen molecules. This recognition can occur at the plasma membrane (TLR4), or in endosomes (TLR3, TLR7, and TLR9) (21). TLR4 binds to lipopolysaccharide (LPS) at the plasma membrane, and both TLR4 and LPS traffic to endosomes, which contain hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) (22). Hrs is a subunit of the endosomal sorting machinery required for transport (ESCRT) complex, which facilitates the transfer of cargo proteins to lysosomes for degradation (23). A recent report indicates that activated TLR4 initiates the TIRAP-MyD88-dependent NFκB signaling pathway from the plasma membrane, whereas the TRAM-TRIF-dependent IRF3 signaling pathway is activated from early endosomes (24). Thus, the subcellular localization and dynamic trafficking of TLRs are required to activate distinct innate immunity signaling pathways in mammals.
Here we report the results of a targeted RNAi screen to identify kinase and phosphatase genes required for signal-dependent Cactus degradation in the Drosophila Toll signaling pathway. Pelle was the only kinase identified in our screen. Our screen for phosphatases led to the identification of mop, a recently discovered member of the protein tyrosine phosphatase (PTP) family (25). We show that RNAi knockdown of mop inhibits Spätzle-dependent Cactus degradation in Drosophila S2 cells and induction of AMP genes in vitro and in vivo. Surprisingly, mutations in the putative PTP domain of mop do not affect Toll activation by Spätzle. We show that Mop colocalizes to the early endosome along with Hrs, a subunit of ESCRT-0 complex. We further demonstrate that Mop, Toll, and Hrs are present in the same complex. We conclude that the endocytosis machinery is required for signal-dependent Cactus degradation and AMP induction. Thus, endosomes play an essential role in Drosophila Toll activation.
Results
Identification of Novel Components of the Toll Signaling Pathway.
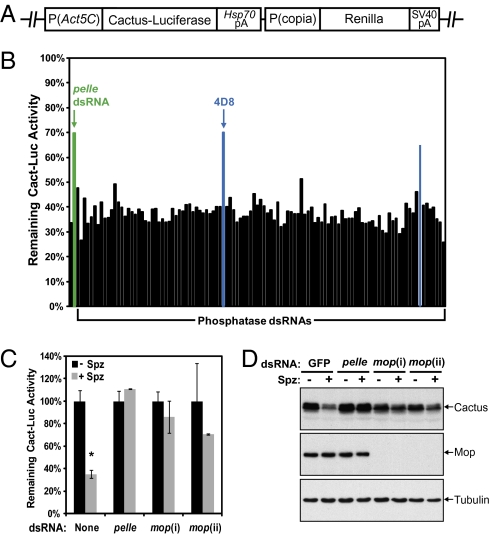
The Drosophila Toll pathway has been extensively characterized in both the dorsal-ventral patterning of the early Drosophila embryo and in the innate immune response. However, the signal-dependent regulation of Cactus degradation downstream of the Pelle kinase in the Toll pathway is not understood. To identify components of the Toll pathway that function upstream of Cactus degradation, we developed a luciferase-based RNAi screening system in Drosophila S2 cells. The firefly luciferase gene was fused to the C terminus of the Cactus ORF (Cact-Luc), and the fusion protein stably expressed under the control of the Actin 5C promoter (Fig. 1A). Activation of the Toll pathway with recombinant mature Spätzle protein, which contains the C-terminal 106 amino acid residues, induced rapid degradation of both chimeric Cact-Luc and endogenous Cactus protein (Fig. 1 B and C and Fig. S1A), and activation of Drosomycin transcription within 30 min (Fig. S1B).
Fig. 1.
Mop is required for Spätzle-induced Cactus degradation. (A) A schematic diagram of the Cact-Luc construct. The Cactus-firefly luciferase chimera is driven by the Actin 5C (Act5C) promoter. The Renilla luciferase, which is controlled by the copia promoter, serves as an internal control. (B) The degradation of Cact-Luc protein was determined by measuring the luciferase activity before and after one-hour Spätzle (Spz) stimulation in S2/Cact-Luc cells pretreated with individual dsRNA targeting Drosophila phosphatase genes. pelle dsRNA (green bar), which served as a positive control, inhibited the degradation of Cact-Luc. Two candidate phosphatase genes (blue bars) were identified in the primary screen. (C) S2/Cact-Luc cells were treated with dsRNAs targeting GFP (control), pelle, and two different regions of mop (i and ii) to silence the expression of endogenous proteins. The degradation of Cact-Luc was determined as in B. (D) Similar to C, except wild-type S2 cells were used. RNAi treated cells were divided into two groups, and one was stimulated with Spätzle for 20 min. The levels of Cactus and Mop were detected by immunoblotting with antibodies specific to Cactus, Mop, and tubulin, respectively. GFP dsRNA was used as a negative control. Data are reported as mean ± SD (*, P < 0.01; compared with the no Spz control).
A dsRNA library, containing 563 dsRNAs targeting all known and predicted Drosophila kinase and phosphatase genes, was screened in stable S2/Cact-Luc cells. The library was analyzed twice to identify genes required for Spätzle-dependent Cact-Luc degradation. Among the 476 specific dsRNAs designed against Drosophila kinases, pelle dsRNA was the only one that resulted in reproducible inhibition of signal-dependent reduction of the Cact-Luc luciferase activity. We also screened 87 dsRNAs that target Drosophila phosphatase genes and identified two dsRNAs inhibited Spätzle-dependent Cact-Luc degradation, one of which (4D8) was confirmed by using an independent dsRNA from another RNAi library (Fig. 1B). The 4D8 dsRNA targets a recently identified Drosophila gene myopic (mop), which was implicated in epidermal growth factor receptor (EGFR) signaling (25). Two additional mop dsRNAs (i and ii) targeting different regions of the mop gene also significantly inhibited Spätzle-dependent reduction of luciferase activity in the Cact-Luc cells (Fig. 1C). Consistent with the Cact-Luc luciferase assay, mop knockdown by these two dsRNAs caused strong inhibition of signal-dependent degradation of the endogenous Cactus protein in S2 cells (Fig. 1D). We conclude that mop is required for Cactus degradation in response to Spätzle induction of the Toll signaling pathway.
Mop Is Required for the Toll, but Not the IMD Pathway.
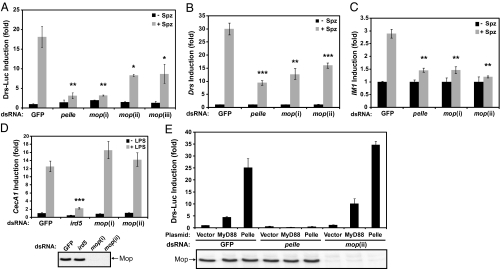
To compare the function of mop in the two Drosophila innate immune response pathways, we analyzed the induction of antimicrobial peptide genes downstream of the Toll or IMD pathway in S2 cells. The activity of the Toll signaling pathway was monitored by using a Drosomycin-luciferase reporter. As expected from previous studies, mature Spätzle protein efficiently activates the Drosomycin reporter (13) (Fig. 2A). Pelle knockdown by RNAi decreased the induction of the reporter from 18.2-fold to 2.1-fold. Three different dsRNAs targeting nonoverlapping regions of the ORF (i and ii) and the 3′ UTR (iii) of the mop gene also markedly inhibited the induction of the Drosomycin reporter (Fig. 2A). Consistent with the requirement of mop in Cactus degradation, we conclude that mop is required for the induction of antimicrobial genes of the Toll pathway.
Fig. 2.
Mop is required for the Toll, but not the IMD pathway. (A) RNAi targeting GFP (control), pelle, and three different regions of mop (i, ii, and iii) was used to knock down endogenous proteins. To measure Drosomycin induction, the Drs-Luc plasmid was transfected into the RNAi-treated cells, which were then stimulated with Spätzle (Spz) for 20 h followed by luciferase assays. The luciferase activity was normalized to unstimulated GFP RNAi treated cells treated with the same dsRNA. (B) S2 cells were treated with dsRNA against GFP, pelle, or two nonoverlapping regions of mop (i and ii). RNAi-treated cells were stimulated with Spätzle for 6 h before RNA isolation and cDNA synthesis. The relative levels of Drosomycin mRNA were measured by real-time quantitative PCR (qPCR). (C) Similar to B, except IM1 primers were used in the qPCR analysis. (D) RNAi-treated cells (GFP, ird5, or mop) were stimulated with LPS for 3 h and lysed to isolate RNA for qRT-PCR by using primers specific to CecA1 (Upper). The efficiency of mop RNAi was confirmed by immunoblotting with a Mop-specific antibody (Lower). (E) RNAi treatment using GFP, pelle, or mop dsRNAs was carried out in S2 cells. The Drs-Luc reporter plasmid was transfected into the RNAi cells together with empty vector or expression constructs for MyD88 or Pelle. Cells were collected 20 h after transfection for luciferase assays (Upper) and immunobloted by using an anti-Mop antibody to confirm RNAi efficiency (Lower). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with the GFP RNAi Spz control.
We further analyzed the induction of antimicrobial peptide genes in S2 cells by quantitative RT-PCR (qRT-PCR). Consistent with the results obtained with the Drosomycin reporter, dsRNAs against pelle and mop significantly decreased the induction of the endogenous Drosomycin gene by Spätzle (Fig. 2B). We also examined the expression of IM1, another Toll-specific gene. Induction of the endogenous IM1 by Spätzle was strongly reduced by two different dsRNAs against mop, similar to the effect observed with the pelle knockdown (Fig. 2C).
To determine whether mop is also required for the IMD pathway, S2 cells were stimulated by the peptidoglycan contaminant present in LPS and the induction of the IMD-specific antimicrobial gene, Cecropin A1 (CecA1), was assayed by qRT-PCR. As expected, RNAi targeting ird5, the Drosophila IKK homolog that functions in the IMD pathway, strongly inhibited CecA1 activation by peptidoglycan (Fig. 2D). By contrast, both dsRNAs targeting mop efficiently knocked down protein expression, but they did not affect CecA1 induction through the IMD pathway (Fig. 2D). We conclude that the Mop protein functions specifically in the Toll innate immunity signaling pathway.
We have demonstrated that mop is required for efficient Spätzle-dependent Cactus degradation, indicating that it functions upstream in the Toll signaling pathway. Two adaptors, MyD88 and Tube, and one kinase, Pelle, are known to be required downstream of the Toll receptor activation and upstream of Cactus degradation. To identify the position of Mop in the Toll signaling pathway, we examined the requirement of Mop in cells activated by over-expressing specific components of the pathway. As expected, transient expression of MyD88 or Pelle alone was sufficient to activate the Drosomycin reporter in S2 cells (Fig. 2E). Consistent with previous reports (26), activation of the Drosomycin reporter in response to exogenous MyD88 or Pelle was strongly inhibited by pelle dsRNA. In contrast, mop RNAi did not inhibit Drosomycin reporter activation by MyD88 or Pelle even though the mop was efficiently knocked down (Fig. 2E). We conclude that Mop functions at the same level or upstream of MyD88 in the Toll signaling pathway.
mop Functions in the Toll Pathway in Vivo.
To address the functions of Mop in vivo, two Drosophila lines (f02540 and d10921) carrying transposons inserted in the 5′ UTR and the first intron of mop were obtained from the Exelixis collection at Harvard Medical School. Mutant homozygous larvae did not survive beyond the second instar stage, consistent with the observation that mop plays an essential role during development (25). Two mutant mop alleles were isolated through imprecise excision of a transposon inserted in the first intron. One mutant allele lacks the entire second exon, and the other is deleted for both first and second exons. The lethality of these mutant lines could be rescued by a mop transgene, indicating that mop is an essential gene.
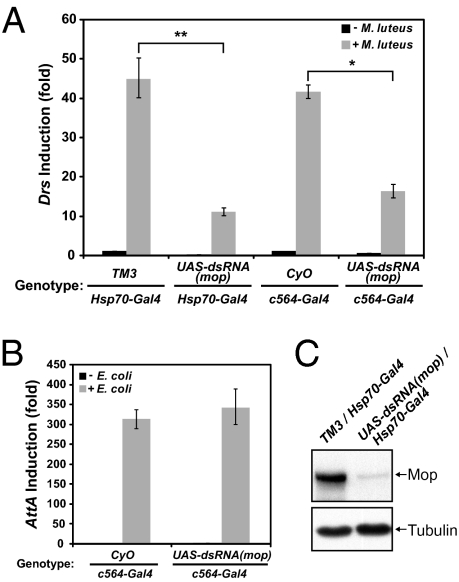
We then generated transgenic flies carrying a mop dsRNA construct driven by the Gal4 UAS promoter (27). Ubiquitous expression of Gal4 with the Actin or Tubulin driver in UAS-dsRNA(mop) flies also resulted in lethality, indicating that mop dsRNA efficiently reduced mop expression in vivo. An Hsp70-GAL4 line was used to induce mop dsRNA, and heat shock treatment efficiently knocked down the protein level of Mop (Fig. 3C). RNAi and control flies were pricked with Gram-positive bacteria (Micrococcus luteus), and the induction of Drs transcription was significantly inhibited by mop RNAi (Fig. 3A). Similar inhibition of Drs induction was observed when mop dsRNA was expressed in the fat body by the c564-Gal4 driver (Fig. 3A). In contrast, expression of mop dsRNA in the fat body did not affect the induction of Attacin by Escherichia coli (Fig. 3B). Consistent with results obtained with cultured S2 cells, we conclude that mop is required for Toll activation but not for the IMD pathway in the fly.
Fig. 3.
Mop is required for Toll activation in vivo. (A) Adult flies with indicated genotypes were divided into two groups, and one was pricked with M. luteus. Flies carrying the Hsp70-Gal4 allele were the progeny from the same cross and were subject to heat-shock treatment before the septic injury. Progeny from the other cross contained c564-Gal4, which expresses Gal4 in the adult fat body. Total RNA extracts were prepared and used for quantitative RT-PCR (qRT-PCT) assays with Drosomycin (Drs) primers. CyO and TM3 are the balancers of second and third chromosomes, respectively. (B) Similar to A, except E. coli was used in the septic injury. RNA was isolated and Attacin A (AttA) primers were used for qRT-PCR. (C) Flies carrying Hsp70-Gal4 were heat-shocked, and protein extracts from whole flies were analyzed by Western blotting with antibodies specific to Mop or tubulin. *, P < 0.01; **, P < 0.001.
Bro1 Domain of Mop Is Required for Toll Signaling.
The mop gene encodes a putative phosphatase of the protein tyrosine phosphatase (PTP) family. In addition to C-terminal PTP domain, Mop contains a conserved N-terminal Bro1 domain, which implicates endosomal localization. Mop was recently reported as an endosomal protein (25). We detected the endogenous Mop protein present in punctate and round structures throughout the cytoplasm (Fig. S2A). Exogenous Flag-tagged Mop was also localized to round vesicular structures (Fig. S2B). The majority of the Mop vesicles were positive for Hrs-GFP and Hrs-Flag proteins, a subunit of the ESCRT-0 complex, and Rab5, an early endosomal protein (Fig. S2 C–E). In addition, the Mop protein did not colocalize with markers for the endoplasmic reticulum, the Golgi apparatus, lysosomes, or mitochondria (Fig. S3).
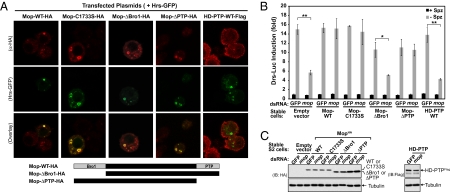
To investigate the role of the conserved Bro1 and PTP domains of Mop in the Toll signaling pathway, wild-type or mutant Mop proteins were stably expressed in S2 cells. Initially, we examined the subcellular localization of these epitope-tagged proteins (Fig. 4A). A point mutation in the putative phosphatase catalytic domain, C1733S, did not alter the subcellular distribution of Mop. The mutant protein was present in endosomes and colocalized with Hrs-GFP. Surprisingly, a mutant Mop protein lacking the entire N-terminal Bro1 domain (ΔBro1) also localized to endosomes as did Hrs-GFP. In addition, deletion of the C-terminal PTP domain (ΔPTP) did not affect the subcellular localization of Mop. The human Mop ortholog, HD-PTP, was recently shown to be an endosomal protein (28), and we find that the HD-PTP protein colocalized with the human Hrs-GFP in HeLa cells (Fig. S4). However, when expressed in S2 cells, HD-PTP displayed a diffuse localization in the cytoplasm and did not colocalize with Hrs (Fig. 4A). Similar to the Drosophila homolog, the endosomal localization of HD-PTP in HeLa cells does not require either the Bro1 or PTP domain (Fig. S4). We conclude that neither the Bro1 nor PTP domain is required for the localization Mop or HD-PTP to endosomes.
Fig. 4.
Functions of the Bro1 and PTP domains of Mop. (A) S2 cells stably expressing HA-tagged wild-type (WT) mop, three different mutant alleles of mop (C1728S, ΔBro1, and ΔPTP), or Flag-tagged human HD-PTP were transfected with a Hrs-GFP expression plasmid and then stained with anti-HA for Mop (red) or anti-Flag for HD-PTP (red) and imaged with confocal microscopy. (B) S2 cells carrying the empty expression plasmid or S2 cells stably expressing different Mop or HD-PTP proteins described in A were treated with dsRNA specifically targeting the 3′ UTR of the endogenous mop gene. To determine Drosomycin induction, a Drs-Luc reporter plasmid was transfected into the RNAi-treated cells, which were then stimulated with Spätzle for 20 h followed by luciferase assays. (C) Protein extracts prepared from Spätzle-stimulated cells in B were analyzed with immunoblotting by using anti-HA and anti-Flag antibodies, respectively. Membranes were reblotted with anti-tubulin antibody as a control. *, P < 0.01; **, P < 0.001.
Next we examined whether the mutant Mop proteins or the HD-PTP protein can function in the Toll signaling pathway. The protein level of endogenous Mop was reduced with a dsRNA [mop(iii)] targeting the predicted 3′ UTR region of endogenous mop, which is not present in the exogenous mop and HD-PTP transcripts. Treatment of cells with this dsRNA, but not GFP dsRNA, inhibited induction of the Drosomycin reporter by Spätzle in the untransformed S2 cells and in S2 cells bearing the stably integrated empty expression vector (Figs. 2, A and 4B). Expression of the wild-type Mop-HA protein fully rescued the inhibition of endogenous mop knockdown (Fig. 4B). We conclude that mop(iii) dsRNA inhibits Toll pathway activation specifically through mop and not through an off-target effect. Remarkably, the point mutation in the putative phosphatase catalytic motif, C1733S, did not affect the ability of the mop transgene to rescue the knockdown of the endogenous mop. This result is further supported by the finding that mutant mop lacking the entire PTP domain can also rescue the inhibition of Toll pathway activation observed upon knockdown of the endogenous Mop protein (Fig. 4B). We conclude that the putative phosphatase activity of Mop is not required for its function in the Toll pathway. In contrast, the Bro1 domain was necessary for rescuing the inhibition caused by RNAi targeting the endogenous mop. In addition, expression of HD-PTP did not rescue the inhibition of Drosomycin reporter activation by Spätzle (Fig. 4B). This observation is consistent with our finding that HD-PTP does not localize to endosomes in S2 cells (Fig. 4A). All of these exogenous expressed proteins were present at similar levels in stable cells (Fig. 4C). Therefore, the inability to replace the function of endogenous Mop is not due to poor expression.
Role of Endosomes in Toll Signaling.
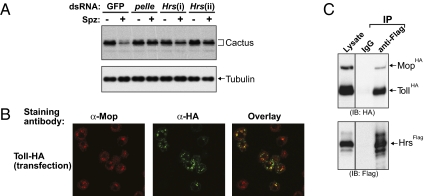
The observation that the Mop protein colocalizes with Hrs in endosomes prompted us to address the possible involvement of Hrs in Toll signaling. As shown in Fig. 5A, Hrs RNAi by two nonoverlapping dsRNAs (i and ii) inhibited Spätzle-dependent Cact-Luc degradation in a manner similar to that observed with pelle RNAi. Knocking down Hrs also inhibited the degradation of endogenous Cactus protein upon Toll activation (Fig. 5B). These observations are consistent with the hypothesis that endocytosis is required for Toll pathway signaling and suggest that an early step of endocytosis is required for proper signaling.
Fig. 5.
Endosomes and Toll signaling. (A) S2 cells stably expressing the Cact-Luc chimeric protein were treated without or with dsRNAs targeting GFP, pelle, or Hrs (i or ii). Degradation of Cact-Luc was measured by luciferase assays after 1 h of Spätzle stimulation. The remaining luciferase activity was normalized to that of unstimulated cells. (B) S2 cells treated with GFP, pelle, or Hrs (i or ii) dsRNAs were stimulated with Spätzle for 20 min. Cell lysates were resolved by SDS/PAGE and analyzed by immunoblotting with a Cactus-specific antibody. The membrane was reprobed with an anti-tubulin antibody as a loading control. (C) S2 cells were transfected with a Toll-HA expression plasmid and then stained with anti-Mop (red) and anti-HA (green) antibodies. Toll-HA was detected at the plasma membrane and in intracellular vesicles. The yellow signals indicate the locations where Toll-HA and endogenous Mop are colocalized. (D) Protein extracts prepared from S2 cells transfected with Hrs-3xFlag, Mop-HA, and Toll-HA expression plasmids were used in immunoprecipitation experiments with either anti-Flag antibody or control IgG. Cell lysates and precipitated proteins were resolved by SDS/PAGE followed by immunoblotting with anti-HA (Upper) or anti-Flag (Lower) antibodies.
The Toll receptor is present at the plasma membrane in early Drosophila embryos (29, 30). The requirement of endosomal protein for Cactus degradation led us to examine the subcellular distribution of the Toll receptor itself. HA-tagged Toll protein localizes to the plasma membrane and to intracellular punctate and round structures in S2 cells. Notably, significant colocalization of intracellular Toll protein with Mop was observed (Fig. 5C). To determine whether Toll interacts with endosomal proteins, Flag-tagged Hrs was expressed together with HA-tagged Toll and Mop in S2 cells. Anti-Flag antibody was used to immunoprecipitate Hrs. Both Toll and Mop were present in the Hrs immunocomplex (Fig. 5D). We conclude that the Toll receptor, Mop, and Hrs are present in the early endosomal complex (ESCRT-0).
Discussion
The Toll signaling pathway is essential for the Drosophila innate immune response to infections by fungi or Gram-positive bacteria (1). Most of the Toll signaling components were identified through genetic screens for mutants defective in embryonic dorsal-ventral patterning. Here we describe Mop, a putative protein tyrosine phosphatase, as a regulator of the Toll pathway. Mop is an endosomal protein that colocalizes with Hrs, a subunit of the ESCRT-0 complex. Knocking down mop by RNAi inhibits Toll pathway activation both in vitro and in vivo. Epistasis studies show that Mop functions upstream of MyD88 and Pelle, at the same level as the Toll receptor. We also demonstrate that Hrs is required for signal-dependent Cactus degradation and that Hrs is present in a complex together with Mop and the Toll receptor. Our findings strongly suggest endocytosis plays an essential role in Drosophila Toll signaling.
Mop contains two conserved domains, an N-terminal Bro1 domain and a C-terminal PTP domain. Although Mop ortholog has not been identified in the yeast genome, the Bro1 domain itself is evolutionarily conserved from yeast to humans. The yeast Bro1 protein is a component of the ESCRT machinery and is localized to endosomes through the interaction with Snf7, an ESCRT-III subunit. Bro1 recruits the Doa4 deubiquitinating enzyme to endosomes and also functions as a cofactor to activate Doa4, which removes the ubiquitin moiety of ubiquitinated membrane proteins before the cargos invaginate into MVB vesicles. The presence of the Bro1 domain in Mop suggests that Mop is an endosomal protein, which is supported by our data. However, mutant Mop protein lacking the entire Bro1 domain (Mop ΔBro1) still localizes to endosomes. The C-terminal region of the yeast Bro1 protein, outside the Bro1 domain, also contributes to its endosomal location (31). It is likely that the Mop protein is targeted to endosomes through the nonconserved sequences between two domains and/or the Bro1 domain. This hypothesis is consistent with our finding that HD-PTP, the human Mop homolog, is endosomal in HeLa cells but is distributed throughout the cytoplasm in Drosophila S2 cells. Although Mop ΔBro1 localizes to endosomes, it cannot complement the function of wild-type Mop in Toll pathway activation. The Bro1 domain may target Mop to the specific endosomal domain or recruit other proteins involved in endocytosis or signaling.
We have also found that Mop proteins bearing a mutation in the putative phosphatase catalytic motif or missing the phosphatase domain can substitute for the endogenous Mop protein, indicating that the putative phosphatase activity is not required for Toll signaling. Our immunoprecipitation experiments show that Hrs, Mop, and Toll are present in the same complex. Mop may act as an adaptor to interact with different proteins to facilitate endocytosis. During the preparation of this work, Mop was reported as an endosomal protein required for EGFR signaling during photoreceptor differentiation in Drosophila eye imaginal disk (25). Genetic evidence suggests that activated Drosophila EGFR is ubiquitylated and sorted through the endocytosis machinery for lysosomal degradation by a mechanism similar to the mammalian EGFR (32). A mop allele carrying a point mutation in the putative phosphatase catalytic motif functions as well as the wild-type allele in EGFR signaling of eye discs (25). These observations show that the putative phosphatase activity of Mop is not required for Toll or EGFR signaling. A recent paper demonstrated that the Human Mop homolog, HD-PTP, does not possess enzymatic activity (33).
The Toll signaling pathway has been characterized extensively during Drosophila embryonic development. The Toll protein has been shown to be present at the plasma membrane in the syncytial blastoderm (29, 30). The majority of MyD88 and Tube also localize to the plasma membrane. However, a significant fraction of these could be detected as punctate structures in syncytial embryos (14, 30, 34). By contrast, Pelle is distributed throughout the embyo (14). Mop and Hrs are required for Spätzle-dependent Cactus degradation, and both are essential endosomal proteins. These observations suggest that endocytosis of the Toll receptor is necessary for normal Toll signaling. Expression of chimeric Tube or Pelle proteins fused to the N-terminal 90 amino acid residues of Src activates Toll signaling without ligand binding in Drosophila embryos (14). The N-terminal region of Src contains a bipartite targeting sequence including the myristylation signal, and the Src protein is known to shuttle between the plasma membrane and endosomes (24). It is possible that the signal is initiated from endosomes under those experimental conditions.
Endocytosis is a dynamic process that regulates various signaling pathways in both a positive and negative manner. Mutations in the Drosophila tumor suppressor gene lethal giant discs (lgd) result in endosomal defects and overactivation of the Notch signaling pathway (35–37). In addition to the Toll signaling, endocytic pathway is required for EGFR activation and Wingless signaling (25, 38). Mammalian TLR4 induces TRAM-TRIF-dependent IRF3 activation from endosomes after initiating the TIRAP-MyD88-dependent NFκB signaling at the plasma membrane (24). The findings that Mop and Hrs are required for Toll signaling suggest that endocytosis has an evolutionarily conserved role in Drosophila Toll and mammalian TLR4 signaling. However, it is interesting to note that endocytosis is required for IRF3, but not NF-κB signaling in mammalian cells, but is required for NF-κB signaling in Drosophila.
Materials and Methods
Plasmid Constructs and dsRNAs.
The firefly luciferase coding sequence was joined in-frame to the 3′ end of the cactus ORF in the pPAC2 vector, which contains the Drosophila Act5C promoter and Hsp70 polyadenylation site. A DNA fragment containing the Renilla luciferase gene driven by the copia promoter and followed by the SV40 polyadenylation site was then inserted into the SacI site to generate the Cact-Luc plasmid. Other plasmids and dsRNAs are described in SI Materials and Methods.
RNAi Screen of Drosophila Kinase and Phosphatase Genes.
A library containing 563 dsRNAs targeting all known or predicted Drosophila kinase and phosphatase genes was provided by Lawrence Lum (University of Texas Southwestern Medical Center). In addition, 178 dsRNAs against kinase genes were synthesized from DNA templates of a Drosophila RNAi library (Open Biosystems) by using MEGAscript T7 RNA Synthesis Kit (Ambion) and purified with MEGAclear Purification Kit (Ambion). RNAi screening was carried out in 96-well format. For details, see SI Materials and Methods.
Fly Stocks and Infection.
The pWIZ-mop-dsRNA plasmid was used to generate transgenic fly lines (27). The expression of mop dsRNA was controlled by the UAS-GAL4 system. For details on infection, see SI Materials and Methods.
Immunofluorescence Staining.
S2 cells were fixed, permeablized, and incubated overnight with the appropriate antibodies. Samples were mounted in Vectashield medium with DAPI (Vector Laboratories) and imaged by using a Zeiss LSM 510 META laser scanning confocal microscope. See SI Materials and Methods for details.
Other detailed methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Nicholas Gay, Gudrun Ihrke, Neal Silverman, and Steven Wasserman for reagents. We thank Erin Song, Tehyen Chu, and other members of the S.K. laboratory for invaluable advice. We are grateful to Emiko Morimoto, Bill McCallum, and Sean Buchanan for valuable comments on the manuscript. This work was supported by National Institutes of Health Grants R01-AI020642 (T.M.) and R01-GM63692 (Z.J.C), and by Howard Hughes Medical Institute (to Z.J.C). H.-R.H. is an Irvington Institute Postdoctoral Fellow of the Cancer Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1004031107/DCSupplemental.
References
- 1.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 2.Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko T, et al. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko T, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 5.Leulier F, et al. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol. 2003;4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- 6.Silverman N, et al. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:2461–2471. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Y, Wu LP, Anderson KV. The antibacterial arm of the drosophila innate immune response requires an IkappaB kinase. Genes Dev. 2001;15:104–110. doi: 10.1101/gad.856901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgel P, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 9.Rutschmann S, et al. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat Immunol. 2000;1:342–347. doi: 10.1038/79801. [DOI] [PubMed] [Google Scholar]
- 10.Stoven S, et al. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci USA. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang IH, et al. A Spätzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell. 2006;10:45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Chamy LE, Leclerc V, Caldelari I, Reichhart JM. Sensing of 'danger signals' and pathogen-associated molecular patterns defines binary signaling pathways 'upstream' of Toll. Nat Immunol. 2008;9:1160–1165. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber AN, et al. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 14.Towb P, Galindo RL, Wasserman SA. Recruitment of Tube and Pelle to signaling sites at the surface of the Drosophila embryo. Development. 1998;125:2443–2450. doi: 10.1242/dev.125.13.2443. [DOI] [PubMed] [Google Scholar]
- 15.Sun H, Towb P, Chiem DN, Foster BA, Wasserman SA. Regulated assembly of the Toll signaling complex drives Drosophila dorsoventral patterning. EMBO J. 2004;23:100–110. doi: 10.1038/sj.emboj.7600033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belvin MP, Jin Y, Anderson KV. Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 1995;9:783–793. doi: 10.1101/gad.9.7.783. [DOI] [PubMed] [Google Scholar]
- 17.Reach M, et al. A gradient of cactus protein degradation establishes dorsoventral polarity in the Drosophila embryo. Dev Biol. 1996;180:353–364. doi: 10.1006/dbio.1996.0308. [DOI] [PubMed] [Google Scholar]
- 18.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo—shaping and transducing a morphogen gradient. Curr Biol. 2005;15:R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987;238:692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- 20.Ip YT, et al. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- 21.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husebye H, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 24.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura GI, Roignant JY, Wassef M, Treisman JE. Myopic acts in the endocytic pathway to enhance signaling by the Drosophila EGF receptor. Development. 2008;135:1913–1922. doi: 10.1242/dev.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Bristow BN, Qu G, Wasserman SA. A heterotrimeric death domain complex in Toll signaling. Proc Natl Acad Sci USA. 2002;99:12871–12876. doi: 10.1073/pnas.202396399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: Use of intron spacers. Methods. 2003;30:322–329. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 28.Doyotte A, Mironov A, McKenzie E, Woodman P. The Bro1-related protein HD-PTP/PTPN23 is required for endosomal cargo sorting and multivesicular body morphogenesis. Proc Natl Acad Sci USA. 2008;105:6308–6313. doi: 10.1073/pnas.0707601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto C, Gerttula S, Anderson KV. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: Importance of transmembrane signaling for dorsal-ventral pattern formation. Development. 1991;111:1021–1028. doi: 10.1242/dev.111.4.1021. [DOI] [PubMed] [Google Scholar]
- 30.Towb P, Bergmann A, Wasserman SA. The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development. 2001;128:4729–4736. doi: 10.1242/dev.128.23.4729. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, et al. Structural basis for endosomal targeting by the Bro1 domain. Dev Cell. 2005;8:937–947. doi: 10.1016/j.devcel.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Drosophila cbl is essential for control of cell death and cell differentiation during eye development. PLoS One. 2008;3:e1447. doi: 10.1371/journal.pone.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gingras MC, et al. HD-PTP is a catalytically inactive tyrosine phosphatase due to a conserved divergence in its phosphatase domain. PLoS One. 2009;4:e5105. doi: 10.1371/journal.pone.0005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galindo RL, Edwards DN, Gillespie SK, Wasserman SA. Interaction of the pelle kinase with the membrane-associated protein tube is required for transduction of the dorsoventral signal in Drosophila embryos. Development. 1995;121:2209–2218. doi: 10.1242/dev.121.7.2209. [DOI] [PubMed] [Google Scholar]
- 35.Childress JL, Acar M, Tao C, Halder G. Lethal giant discs, a novel C2-domain protein, restricts notch activation during endocytosis. Curr Biol. 2006;16:2228–2233. doi: 10.1016/j.cub.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher CM, Knoblich JA. The conserved c2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila. Dev Cell. 2006;11:641–653. doi: 10.1016/j.devcel.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Jaekel R, Klein T. The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev Cell. 2006;11:655–669. doi: 10.1016/j.devcel.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Seto ES, Bellen HJ. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.