Abstract
IL-23 is an important molecular driver of Th17 cells and has strong tumor-promoting proinflammatory activity postulated to occur via adaptive immunity. Conversely, more recently it has been reported that IL-17A elicits a protective inflammation that promotes the activation of tumor-specific CD8+ T cells. Here we show the much broader impact of IL-23 in antagonizing antitumor immune responses primarily mediated by innate immunity. Furthermore, the majority of this impact was independent of IL-17A, which did not appear critical for many host responses to tumor initiation or metastases. IL-23–deficient mice were resistant to experimental tumor metastases in three models where host NK cells controlled disease. Immunotherapy with IL-2 was more effective in mice lacking IL-23, and again the protection afforded was NK cell mediated and independent of IL-17A. Further investigation revealed that loss of IL-23 promoted perforin and IFN-γ antitumor effector function in both metastasis models examined. IL-23-deficiency also strikingly protected mice from tumor formation in two distinct mouse models of carcinogenesis where the dependence on host IL-12p40 and IL-17A was quite different. Notably, in the 3′-methylcholanthrene (MCA) induction of fibrosarcoma model, this protection was completely lost in the absence of NK cells. Overall, these data indicate the general role that IL-23 plays in suppressing natural or cytokine-induced innate immunity, promoting tumor development and metastases independently of IL-17A.
Keywords: innate immunity, tumor, inflammation, IL-2, NK cell
The cytokines IL-12 and IL-23 are members of a small family of proinflammatory heterodimeric cytokines (1). Both cytokines share a common p40 subunit that is covalently linked to a p35 subunit to form IL-12 or to a p19 subunit to form IL-23 (2, 3). IL-12 and IL-23 are expressed predominantly by activated dendritic cells (DC) and phagocytic cells, and are stimulated by pathogens, CD40L, and TLR ligands (ref. 3; reviewed in ref. 4). Receptor complexes are comprised of an IL-12Rβ1 subunit that is completed with the IL-12Rβ2 subunit for IL-12, or the novel subunit IL-23R for IL-23 (5, 6). Cellular receptor expression reportedly includes T cells, natural killer (NK) cells, and NKT cells, with lower levels of IL-23R complexes also found on monocytes, macrophages, and DC populations (6).
There is mounting evidence that IL-12 and IL-23 drive divergent immunological pathways (reviewed in ref. 7). Whereas IL-12 leads to development of IFN-γ-producing Th1 cells and enhanced anti-microbial and cytotoxic responses, IL-23 appears to promote the proliferation and inflammatory function of Th17 cells (8). IL-17-IL-17R ligation results in the release of inflammatory factors such as IL-1, IL-6, IL-8, TNF-α, prostaglandin E2, and several chemokines to further the inflammatory cascade (9). The Th17 cell has been implicated as central to inflammatory conditions in psoriasis, ischemic injury, inflammatory bowel disease, and autoimmune inflammation (7, 10, 11). However, the importance of IL-17A and Th17 cells in autoimmune diseases remains controversial (12–14), as does the existence of the Th17 cell in vivo (15). IL-23 induces chronic inflammation through the stimulation of innate myeloid effector cells and stromal activation, whereas the Th17 cell plays a decisive role to orchestrate inflammatory tissue destruction. However, to date, the role of IL-17 and IL-23 in tumor immunity is comparatively poorly understood, and it has not been clear whether the same IL-17/IL-23 axis regulates the tumor microenvironment.
The role of IL-23 in tumorigenesis was clearly demonstrated in mice lacking IL-23p19 that were almost completely resistant to 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin papilloma (16). These studies reported a significant increase in infiltrating CD8+ T cells in carcinogen-treated skin of IL-23p19–deficient mice [compared with wild-type (WT) counterparts] coincident with reduced IL-17, MMP9, and CD31 expression, and lower numbers of Gr-1+ and CD11b+ cells. Thus the local balance of IL-12 and IL-23 in the tumor microenvironment appeared to regulate immune surveillance by cytotoxic T cells in situ. Additional experiments using transplanted syngeneic tumors in IL-23–deficient mice supported this view (16). Subsequently, Stat3 signaling within the tumor microenvironment has been shown to induce IL-23, while inhibiting IL-12, thereby shifting the balance of tumor immunity toward carcinogenesis. Stat3 induces expression of IL-23, which is mainly produced by tumor-associated macrophages, via direct transcriptional activation of the IL-23/p19 gene (17). Despite the clear link between IL-23 and Th17 cells, a role for endogenous IL-17 in tumor control has only recently been examined. Surprisingly, the results published thus far are completely contradictory with two studies reporting a tumor-suppressing role of IL-17 (18, 19) and two studies reporting a tumor-promoting role of IL-17 via IL-6/Stat3 signaling (20, 21). Most disturbingly, this profile of IL-17 activity was opposite even when examined in the context of variants of the same B16 melanoma (19, 20).
To clarify whether IL-23-mediated tumor control was T-cell specific and involved IL-17, we have assessed natural or cytokine-induced innate immunity in several models of experimental metastases and carcinogenesis. Clearly, IL-23 does very generally and effectively suppress innate antitumor responses, though quite frequently, natural host immunity to tumors is not critically dependent upon IL-17A.
Results
Host IL-23p19 Suppresses NK Cell-Mediated Control of Lung Metastases.
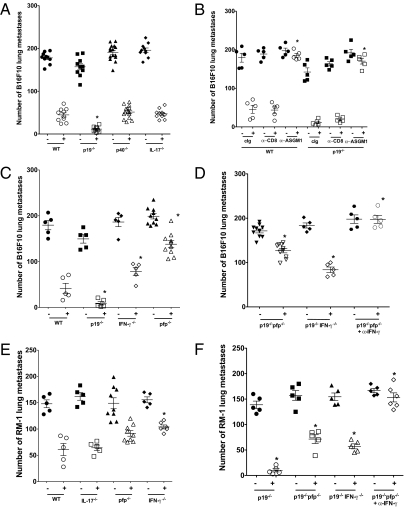
Experimental metastases of B16F10 melanoma was initially assessed by dose response in WT compared with IL-23p19−/−, IL-12p40−/−, IL-12p35−/−, and IL-17A−/− mice (Fig. 1A and Fig. S1 A–C). As increasing doses were tested, there was a significantly reduced level of B16F10 lung metastases in IL-23p19−/− mice and a significantly increased level of B16F10 lung metastases in IL-12p35−/− mice compared with WT and the other strains. The lack of significant impact of the loss of IL-17A suggested host control of B16F10 lung metastases was IL-17A independent. The comparative metastatic burden in WT and IL-12p40–deficient mice suggested that loss of IL-12 countered the loss of IL-23 in p40-deficient mice. Similar results were obtained with the B16 variant (Fig. S2). By neutralizing IL-23p19 using mAb in WT and gene-targeted mice, it was clear that the suppression of metastases in the absence of IL-23 was independent of IL-17A, T, and B cells (Fig. 1B). Importantly, blockade with anti-IL-12p40 increased lung metastases in IL-23p19–deficient mice, and blockade with anti-IL-23p19 or anti-IL-12p40 reduced lung metastases in IL-12p35−/− mice (Fig. 1C). The data illustrated the antitumor activity of IL-12 and the protumor activity of IL-23. By depleting NK cells in WT or IL-23p19–deficient mice (with anti-asialoGM1 or anti-NK1.1), it was clear that host control of B16F10 metastases was NK cell dependent, but CD8+ T-cell independent (Fig. 1D). Importantly, the equivalent levels of lung metastases between anti-asialoGM1- or anti-NK1.1-treated WT and anti-asialoGM1- or anti-NK1.1-treated IL-23p19–deficient mice suggested that the activity of IL-23 was not independent of NK cells. A very similar pattern of host IL-23p19 promotion of lung metastases was also observed in RM-1 prostate carcinoma and 3LL lung carcinoma models (Fig. S3), where NK cells have been shown to control metastatic burden (22, 23), though IL-23p19, IL-12p40, and IL-17A did not appear critical for MC38 colon adenocarcinoma metastases (Fig. S2).
Fig. 1.
Host IL-23p19 suppresses NK cell-mediated control of lung metastases. Groups of 5–15 WT, IL-23p19−/−, IL-12p40−/−, IL-12p35−/−, IL-17−/−, and RAG-1−/− mice were injected i.v. with either (A–C) 2 × 105 or (D) 1 × 105 B16F10 melanoma cells. In B and C, mice were additionally treated with control Ig (cIg), anti-IL-12p40 (α-p40), or anti-IL-23p19 (α-p19) cells by treatment with antibodies (500 μg i.p.) on day −1, 0, and 7 relative to tumor inoculation. In D, mice were additionally depleted of CD8+ T cells (αCD8) or NK (αASGM1 or αNK1.1) cells by treatment with antibodies (100 μg i.p.) on day −1, 0, and 7 relative to tumor inoculation. Fourteen days after tumor inoculation, the lungs of these mice were harvested and fixed, and the colonies counted and recorded for individual mice as shown. Asterisks indicate the groups that are significantly different from untreated or cIg-treated mice in each strain. Mann–Whitney, *P < 0.05.
IL-2 Immunotherapy Is More Effective in the Absence of IL-23p19.
We have previously shown that early, high-dose IL-2 can substantially reduce lung metastases in WT mice in a NK cell-, perforin (pfp), and IFN-γ-dependent manner (23); however, those studies did not specifically evaluate the B16F10 tumor model. By using a very high dose of B16F10 tumor cells, we showed that high-dose IL-2 significantly reduced B16F10 lung metastases in WT mice (47.6 ± 8.3 vs. 177.8 ± 7.3; P < 0.0079), and a comparable reduction in metastases was observed in either IL-12p40– or IL-17A–deficient mice (Fig. 2A). However, even more striking was the markedly reduced metastases observed in IL-2–treated IL-23p19-deficient mice (13.2 ± 3.1 vs. 144.4 ± 8.6; Fig. 2A). This greater suppression of metastases in IL-23p19−/− mice was even more obvious when dose was titered down from 100,000 to 10,000 U IL-2 (Fig. S4).
Fig. 2.
Host IL-23p19 suppresses IL-2 therapy of tumor metastases. Groups of 5–15 WT, IL-23p19−/−, IL-12p40−/−, IL-17−/−, pfp−/−, IFN-γ−/−, IL-23p19−/−xpfp−/−, or IL-23p19−/−xIFN-γ−/− mice were injected i.v. with either (A–D) 7.5 × 105 B16F10 melanoma cells or (E and F) 2 × 105 RM-1 tumor cells. Mice received PBS (−) or 100,000 U IL-2 (+) i.p. on day -1 before tumor inoculation. Some IL-23p19−/−xpfp−/− mice were additionally treated with anti-IFN-γ mAb (100 μg i.p.) on days −1, 0, and 7 to neutralize IFN-γ. Fourteen days after tumor inoculation, the lungs of these mice were harvested and fixed, and the colonies counted and recorded for individual mice as shown. Asterisks indicate the groups that are significantly different from IL-2-treated WT mice or IL-2–treated IL-23p19−/− mice (for those strains on p19−/− background). Mann–Whitney, *P < 0.05.
IL-23p19 Suppresses NK Cell-Mediated Control of Metastases by Perforin and IFN-γ.
IL-2-mediated suppression of B16F10 lung metastases was NK cell dependent and CD8+T cell independent in both WT and IL-23p19−/− mice, with IL-2 completely ineffective in either strain in the absence of NK cells (Fig. 2B). We next examined the relative role of NK cell perforin and IFN-γ effector mechanisms in IL-2-mediated control of B16F10 metastases. IL-2 was only partially effective in mice deficient for either perforin or IFN-γ compared with WT or IL-23p19−/− mice (Fig. 2C). To determine what role these effector molecules played in host control of metastases in the absence of host IL-23p19, we generated mice deficient in both IL-23p19 and perforin or IFN-γ. In the absence of IL-23p19, perforin and IFN-γ collectively accounted for the IL-2-mediated host response to B16F10, because only mice deficient for both IL-23p19 and perforin and neutralized for IFN-γ using mAb displayed a complete loss of IL-2 antimetastatic activity (Fig. 2D). The same qualitative result was obtained in the RM-1 tumor model with IL-2 therapy (Fig. 2 E and F).
Carcinogen-Induced Tumors Are IL-23p19 Dependent.
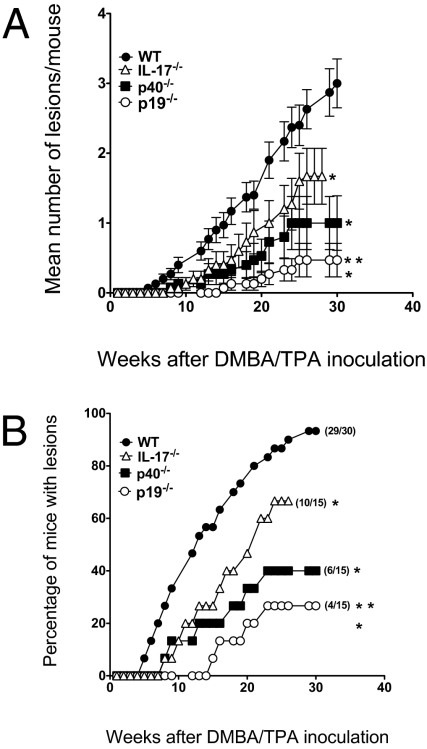
Only one study has illustrated the ability of IL-23p19 to promote tumor initiation, and this was in the context of DMBA/TPA-induced skin papillomas (16). We confirmed these published results by comparing papilloma incidence and number in C57BL/6 WT mice compared with either IL-23p19−/− or IL-12p40−/− mice (Fig. 3). Similar to reported by Langowski et al. (16), IL-23p19−/− mice were profoundly resistant to DMBA/TPA-induced skin papilloma, with less than 30% of mice developing lesions. Although IL-12p40−/− were also resistant compared with WT mice, our data suggested some balance existed between the levels of IL-12 and IL-23 in controlling papilloma formation. Langowski et al. (16) showed that IL-17 mRNA was barely detectable in the skin of DMBA/TPA-treated IL-23p19–deficient and IL-12p40–deficient mice compared with WT mice, but did not evaluate papilloma formation in IL-17−/− mice. We found that IL-17A−/− mice were resistant to DMBA/TPA-induced skin papilloma, but slightly more susceptible than IL-23p19−/− mice (Fig. 3). These findings suggest that IL-23p19 may only in part promote skin papilloma formation via IL-17A, and that other IL-17A-independent factors must contribute to this process.
Fig. 3.
IL-23p19 promotes the formation of DMBA/TPA-induced skin papillomas. Groups of 15–30 female WT, IL-23p19−/−, IL-12p40−/−, and IL-17−/− mice were injected with 25 μg DMBA, and 1 week later, twice weekly with 4 μg TPA for 20 weeks as described in Materials and Methods. Mice were subsequently monitored for skin papilloma development for 25 weeks (n = 15 mice/group). Results are shown as (A) the mean lesion number per mouse over time and (B) percentage of mice in the group with papillomas over time. Significant differences in mean lesion number per mouse were observed by the unpaired Mann–Whitney U test (*, gene-targeted vs. WT, P < 0.05; **, p19−/− vs. p17−/−, P < 0.05). Significant differences in proportion with papilloma at any one-time point were determined by the Fisher's exact test (*, gene-targeted vs. WT, P < 0.05; **, p19−/− vs. p17−/−, P < 0.05).
Although IL-23p19 appeared critical in epithelial tumorigenesis, the DMBA/TPA model is well recognized as a mouse model of inflammation-induced cancer (24). Therefore, we wished to determine the importance of IL-23p19 in the MCA induction of fibrosarcoma model where host immunity has been demonstrated as a critical factor in suppressing tumor initiation (25, 26) and progression (27), and indeed innate NK and NKT cells preventing the former (26, 28). Strikingly, even in this setting, the loss of host IL-23p19 caused a profound reduction in the initiation of fibrosarcomas by MCA (Fig. 4). Tumor incidence was reduced at the highest dose and tumor onset was delayed at all doses in IL-23p19−/− mice compared with WT mice. Similarly, a reduction in tumor incidence was observed in WT mice neutralized with an anti-IL-23p19 mAb compared with those treated with a control mAb (Fig. S5). When comparing this data with historical data that we have generated in the same model (25), it is clear that IL-23 is the most active host immune tumor-promoting factor we have observed thus far. Interestingly, in this carcinogenesis model, mice lacking either IL-12p40 or IL-17A displayed a similar tumor incidence compared with WT mice (Fig. S6)—a result clearly distinct from the DMBA/TPA model. This data first suggested that loss of IL-12 is dominant over loss of IL-23 activity in this mouse model of cancer, and second, in this context, that IL-23 action is independent of IL-17A. To further determine what cell type was preventing tumor initiation in the absence of IL-23p19, we additionally examined MCA induction in IL-23p19−/− mice depleted of either CD8+ T cells or NK cells for 6 weeks after MCA inoculation (Fig. 5). Consistent with previously published work in WT mice (28), depletion of NK cells, but not CD8+ T cells, completely abolished the protective effect of IL-23p19 deficiency (Fig. 5). These data, in concert with that in Figs. 1 and 2, indicate that IL-23 can suppress the surveillance, antimetastatic, and immunotherapeutic activity of NK cells independent of IL-17A activity.
Fig. 4.
IL-23p19 promotes the formation of MCA-induced fibrosarcomas. Groups of 10–25 male WT and IL-23p19−/− mice as indicated were injected with either 5, 25,100, or 400 μg MCA as described in Materials and Methods and subsequently monitored for tumor development over 300 days (proportion of mice with tumors in each group shown in each panel). Mice were injected with 400 μg MCA (A and B), 100 μg (C and D), 25 μg (E and F), or 5 μg (G and H). Results are shown as the growth curves of individual mice with sarcoma in each group after MCA inoculation. Proportion of mice developing lethal tumors is shown on right of each panel. Statistical differences in tumor-free mice as determined by Fisher's exact test: (A and B) WT vs. p19−/− (P = 0.0009); (C and D) WT vs. p19−/− (P = 0.2443); (E and F) WT vs. p19−/− (0.6614); and (H and I) WT vs. p19−/− (0.4737).
Fig. 5.
IL-23p19 suppression of NK cells promotes the formation of MCA-induced fibrosarcomas. Groups of 14–15 male WT and IL-23p19−/− mice as indicated were injected with 400 μg MCA as described in Materials and Methods and subsequently monitored for tumor development over 300 days (proportion of mice with tumors in each group shown). WT or IL-23p19−/− mice were additionally treated with control Ig (cIg) or depleted of CD8+ T cell or NK cells weekly from the time of MCA inoculation to day 42. Results are shown as the growth curves of individual mice with sarcoma in each group after MCA inoculation. Statistical differences as determined by Fisher's exact test: (A–D) p19−/− + cIg vs. p19−/− + anti-CD8 (ns), p19−/− + cIg vs. p19−/− + anti-ASGM1 (P = 0.0027).
Discussion
This study illustrates that IL-23p19 can suppress tumor metastases and initiation controlled by NK cell-mediated immunity. In particular, NK cell perforin and IFN-γ effector functions appear to be suppressed by host IL-23p19, and suppression is independent of host IL-17A. Much emphasis has been placed on the ability of IL-23p19, IL-1, and IL-6 to regulate Th17 cell differentiation (reviewed in ref. 4), but clearly from our studies it is clear that IL-23p19 has tumor-promoting activities that are independent of IL-17A itself. Several recent reports have contradicted one another and our data herein with respect to the role of IL-17 in natural host response to B16 melanoma variants (19, 20). Martin-Orozco et al. (19) reported that B16F10 melanoma was far more metastatic in IL-17A–deficient mice than WT mice, whereas Wang et al. (20) demonstrated slower s.c. B16 tumor growth in IL-17A–deficient mice compared with WT mice. Regardless of whether we were examining lung metastases or s.c. growth, we saw no critical role for host IL-17A (Fig. 1 and Fig. S7). It is notable that the former study used their own mixed 129 × C57BL/6 or C57BL/6 backcrossed (n = 6) strains of IL-17A-deficient mice compared with F1 and C57BL/6 WT controls, respectively (19). Given that NK cells are critical for B16F10 clearance from the lung, and 129 have a completely unique NK gene complex and weak NK cytotoxic activity compared with the C57BL/6 strain, this aspect of the study by Martin-Orozco et al. (19) is very complicated to interpret. We also have been unable to reproduce another report of greatly enhanced s.c. and metastatic tumor growth of MC38 in IL-17A-deficient mice (same mice as used herein; Fig. S2) (18). We do not dispute that IL-17A may be very important in controlling tumors in the context of T-cell responses, but the current literature is very misleading with respect to what role IL-17A might play in controlling the natural, innate immune response to tumors, and clearly IL-23p19 is far more important.
Only in the DMBA/TPA induction of papilloma model was host IL-17A shown to play any key role, and even in this model it did not account for all of the antitumor activity of IL-23p19. IL-23p19 had previously been shown to promote tumor incidence and growth in the DMBA/TPA model (16). Although others had demonstrated that γδ+T cells and NKG2D activation receptor were primarily responsible for preventing DMBA/TPA-induced skin papilloma (29, 30), Langowski et al. (16) demonstrated some evidence to suggest that IL-23p19 promoted tumor initiation by suppressing CD8+ T-cell infiltration early after carcinogen application. The key experiment to deplete either γδ+ T cells or CD8+ T cells in IL-23p19–deficient mice treated with DMBA/TPA remains to be performed, but it is clear from our work that IL-23p19 does promote MCA-induced fibrosarcoma, by suppressing the activity of NK cells rather than CD8+ T cells. It is likely that the impact of IL-23 on different effector lymphocyte populations (innate and adaptive) may be very model dependent.
The molecular mechanism by which IL-23 might suppress NK cell effector functions, and whether this suppression is direct or indirect, remains unclear. Most simplistically, IL-23p19 deficiency may simply allow greater IL-12p70 formation that can then stimulate the effective IFNγ production and cytotoxicity by NK cells. Blockade of IL-12p40 in IL-23p19–deficient mice increased tumor metastases, so clearly IL-12 is effective in suppressing tumor formation in IL-23–deficient mice. However, a direct or indirect tumor-promoting effect of IL-23 cannot be ruled out. Despite a report of IL-23 protein expressed in tumor-associated macrophages (TAM) (17), the means to use intracellular staining to accurately determine which subsets of host cells make IL-23p19 remain limited. Our metastases data suggested that IL-23p19 was suppressive in the absence of T and B cells, implying the possibility that NK cells, myeloid cells, and tumor cells might act in concert. Using a GFP reporter mouse, small populations of CD11b myeloid cells or CD11c DC, but not NK cells, reportedly constitutively express the IL-23R (31), but our attempts to stain mouse IL-23R on the surface of all cell types (including tumors) by flow cytometry were unsuccessful. Thus, establishing whether TAM IL-23p19 acts indirectly via small populations of other myeloid cells or tumors will require better reagents and further study. Though IL-23 activities were evidently independent of IL-17, another cytokine made by the Th17 lineage, IL-22, is also produced by cells of the innate immune system. A subset of mucosal NK cells express IL-22 in response to IL-23, and unlike conventionally described NK cells, these do not express high levels of IFN-γ or cytotoxicity (32). Future experiments need to evaluate the role of IL-22 and whether IL-23 serves to promote the numbers or function of some innate NK cell subsets over others.
In all mouse tumor models, the loss of endogenous IL-12p35 activity resulted in more tumor formation, and the loss of host IL-23p19 fewer tumors (ref. 16 and herein). The comparison of two different models of tumor initiation (MCA fibrosarcoma and DMBA/TPA skin) now demonstrates that the balance between IL-12 and IL-23 activity can be quite distinct depending upon the model examined. Additionally, the neutralization of host IFN-γ has been reported to be tumor suppressive (33) or promoting (34). Thus, the use of therapeutic strategies to enhance IL-12 activity and abrogate IL-23 activity must consider these potential differences in tumor microenvironment. Despite a report that patients with defects in IL-12Rβ1 or IL-12p40 have NK cells defective in IFN-γ production (35), our study of the NK cell homeostasis indicates that IL-23p19−/− mice have normal numbers and proportions of all of the mature NK cell subsets (Fig. S8). The revelation that IL-2 therapy was superior in IL-23p19–deficient hosts, and independent of IL-17, suggests clearly that anti-human IL-23p19 mAbs may be of great value in improving the current clinical use of IL-2 in a variety of human cancer patients. It is possible, although it remains to be formally tested, that other therapies for cancer may also be more effective with IL-23p19 neutralization. Notably, though we have illustrated an important tumor-promoting activity of host IL-23, studies in mice where IL-23 has been administered systemically, or ectopically delivered in the context of tumors or other cellular vehicles, have indicated IL-23 can enhance antitumor immunity and suppress tumor growth (36–40). Thus host and exogenous/therapeutic IL-23 may be very distinct entities. With anti-human p19 and anti-human p40 mAbs in the clinic for the treatment of psoriasis, we will have potentially some of the most interesting trials in human cancer immune surveillance in place.
Materials and Methods
Mice.
Inbred wild-type C57B6/J (C57BL/6 WT), C57BL/6 IL-12p40–deficient (IL-12p40−/−), C57BL/6 IL-12p35–deficient (IL-12p35−/−), C57BL/6 perforin-deficient (pfp−/−), C57BL/6 IFN-γ–deficient (IFN-γ−/−), and C57BL/6 RAG-1-deficient (RAG-1−/−) mice were bred and maintained at the Peter MacCallum Cancer Centre (Peter Mac) as described previously (34). The above C57BL/6 gene-targeted mice were all backcrossed at least 10 generations to C57B6/J, except C57BL/6 pfp−/− mice, which were derived using C57B6/J ES cells. C57BL/6 IL-23p19–deficient (IL-23p19−/−) mice were backcrossed by speed congenics to C57B6/J (microsatellite analysis confirmed the resultant line 99.99% C57B6/J) and obtained from Schering–Plough BioPharma (12). C57BL/6 IL-17A–deficient (IL-17−/−) mice were backcrossed nine generations to C57B6/J (41). The syngeneic background of the IL-12p40−/−, IL-23p19−/−, and IL-17−/− strains was confirmed by skin grafting against C57BL/6 WT controls (as donor or recipients). C57BL6 IL-23p19−/− x pfp−/− and IL-23p19−/− x IFN-γ−/− mice were generated by intercross of the appropriate strains above at Peter Mac. Mice 6–12 weeks of age were used in all experiments that were performed according to Peter Mac Animal Experimental Ethics Committee guidelines.
Metastatic Tumor Models.
To examine metastatic tumor growth, WT or gene-targeted mice were inoculated i.v. with increasing doses of B16F10 melanoma, 3LL lung carcinoma, or RM-1 prostate carcinoma cells. Mice were monitored and harvested, and lung metastases were quantified as described previously (22, 23). Some mice were treated with control Ig (Mac-4) or anti-IFN-γ (H22, kindly provided by Robert Schreiber, St. Louis), anti-CD8 (53.6.7), anti-NK1.1 (PK136), or anti-asialoGM1 (Wako Chemicals), as indicated in the legends, to neutralize IFN-γ or deplete cell subsets as previously described (23, 27, 28, 42). Anti-agp3 (cIg, 4D2), anti-IL-23p19 (16E5), and anti-IL-12p40 (C17.8) were all grafted on the same murine IgG1 background and kindly provided by AMGEN, Inc. To examine cytokine immunotherapy of metastases, some groups of mice received either PBS or recombinant IL-2 (Chiron Corporation; 10,000–100,000 U i.p. on day −1 relative to tumor inoculation as indicated).
Carcinogenesis Models.
MCA model.
Groups of 10–25 male WT, IL-23p19−/−, and IL-12p40−/− mice were inoculated s.c. in the hind flank with 5, 25, 100, or 400 μg of 3-methylcholanthrene (MCA; Sigma–Aldrich) in 0.1 mL of corn oil as described (25). Some WT mice received control Ig or weekly depletion of CD8+ T cells (53.6.7) or NK cells (anti-asialoGM1) from the time of MCA inoculation to day 42 (100 μg i.p.). Development of fibrosarcomas was monitored weekly over the course of 250 days. Tumors >3 mm in diameter and demonstrating progressive growth were recorded as positive. Measurements were made with a caliper square as the product of two perpendicular diameters (cm2), and individual mice are represented.
DMBA/TPA tumor model.
Two-stage chemical carcinogenesis by DMBA/TPA was as reported (25). Initiation was accomplished by pipette application of 25 μg of DMBA (Sigma) in acetone onto the shaved-back skin of groups of 11–15 8-week-old female mice. Promotion was performed twice weekly with 4 μg of TPA (Sigma) from week 1 to week 20. Mice were assessed weekly for papilloma development for up to 30 weeks, and lesions were counted and measured.
Statistical Analysis.
Significant differences in metastases or papillomas were determined by a Mann–Whitney U test, and differences in tumor incidence were determined by the Fisher's exact test. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors thank Michelle Stirling for maintenance of the mice, and Jennifer Towne (AMGEN, Inc.) for providing the anti-agp3, anti-IL-12, and anti- IL-23 mAbs. This work was supported by the Prostate Cancer Foundation of Australia, the National Health and Medical Research Council of Australia (NH&MRC), and in part by AMGEN, Inc. M.W.L.T. and D.M.A. were supported by NH&MRC Peter Doherty Fellowships. M.J.S. received support from a NH&MRC Australia Fellowship.
Footnotes
Conflict of interest statement: The authors M.J.S. and M.W.L.T. declare a conflict of interest. AMGEN Inc. has in part supported the work in this manuscript.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1003251107/DCSupplemental.
References
- 1.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 3.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 4.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Presky DH, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luger D, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor RA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haak S, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 17.Kortylewski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth MJ, et al. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- 23.Smyth MJ, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200:1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore RJ, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 25.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smyth MJ, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 29.Girardi M, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. [PubMed] [Google Scholar]
- 30.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 31.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao M, et al. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69:2010–2017. doi: 10.1158/0008-5472.CAN-08-3479. [DOI] [PubMed] [Google Scholar]
- 34.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 35.Guia S, et al. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008;111:5008–5016. doi: 10.1182/blood-2007-11-122259. [DOI] [PubMed] [Google Scholar]
- 36.Kaiga T, et al. Systemic administration of IL-23 induces potent antitumor immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J Immunol. 2007;178:7571–7580. doi: 10.4049/jimmunol.178.12.7571. [DOI] [PubMed] [Google Scholar]
- 37.Overwijk WW, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shan BE, Hao JS, Li QX, Tagawa M. Antitumor activity and immune enhancement of murine interleukin-23 expressed in murine colon carcinoma cells. Cell Mol Immunol. 2006;3:47–52. [PubMed] [Google Scholar]
- 39.Hu J, et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 40.Yuan X, Hu J, Belladonna ML, Black KL, Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 41.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 42.Smyth MJ, Kelly JM, Baxter AG, Körner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J Exp Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.