Abstract
The role of global climate change in the decline of biodiversity and the emergence of infectious diseases remains controversial, and the effect of climatic variability, in particular, has largely been ignored. For instance, it was recently revealed that the proposed link between climate change and widespread amphibian declines, putatively caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd), was tenuous because it was based on a temporally confounded correlation. Here we provide temporally unconfounded evidence that global El Niño climatic events drive widespread amphibian losses in genus Atelopus via increased regional temperature variability, which can reduce amphibian defenses against pathogens. Of 26 climate variables tested, only factors associated with temperature variability could account for the spatiotemporal patterns of declines thought to be associated with Bd. Climatic predictors of declines became significant only after controlling for a pattern consistent with epidemic spread (by temporally detrending the data). This presumed spread accounted for 59% of the temporal variation in amphibian losses, whereas El Niño accounted for 59% of the remaining variation. Hence, we could account for 83% of the variation in declines with these two variables alone. Given that global climate change seems to increase temperature variability, extreme climatic events, and the strength of Central Pacific El Niño episodes, climate change might exacerbate worldwide enigmatic declines of amphibians, presumably by increasing susceptibility to disease. These results suggest that changes to temperature variability associated with climate change might be as significant to biodiversity losses and disease emergence as changes to mean temperature.
Keywords: chytridiomycosis, climate change, conservation, El Niño, emerging infectious disease
Global climate change affects both climate averages and variability (1), but the biologic effects of changes in climatic variability have been largely neglected (2, 3). Despite convincing evidence of global climate change (1), its contribution to the unprecedented decline of biodiversity and emergence of infectious diseases remains controversial (4–9). For instance, a positive multidecadal correlation between increasing air temperature and putative amphibian extinctions at several locations across the globe led many authors to conclude that climate change was driving amphibian declines, presumably by increasing disease risk from the deadly amphibian chytrid fungus, Batrachochytrium dendrobatidis (Bd) (10–13). However, it was recently revealed that these multidecadal correlations between temperature and amphibian declines were temporally confounded, so that virtually any variable that increased in the 1980s and early 1990s would be correlated positively with these amphibian losses (8). Hence, these correlations provide, at best, tenuous evidence for a link between climate change and amphibian extinction risk.
If there is indeed a true causal relationship between climate and amphibian declines, then fluctuations around temporal trends in climatic variables and declines should also correlate positively (SI Appendix, Fig. S1). If so, this would provide more convincing evidence that climate contributed to these widespread biodiversity losses because it would reduce the likelihood that a third variable could explain the correlation. That is, there are many fewer alternative, noncausal explanations for a positive association between short-term intradecadal fluctuations around climatic trends and putative extinctions than there are for the positive multidecadal correlation between temperature and declines (7, 8) (SI Appendix, Fig. S1).
This approach of detrending the data and looking for residual associations between climate and demographic- or disease-related variables is commonly used to isolate the intradecadal effects of climatic fluctuations (7, 14–16). Moreover, detrending the amphibian decline data should reduce temporal autocorrelation, and, if Bd is a factor in these declines, it should also help to control for the likely intrinsic, epidemic spread of the pathogen, which itself could be a driver of the multidecadal increase in amphibian declines (8, 17–19). Here, we use global and regional estimates of climate averages and variation to simultaneously test several climate-related hypotheses for widespread amphibian declines and their dependence on temporal detrending. In other systems, such as human–cholera and human–malaria, intrinsic population dynamics or multidecadal control efforts concealed the true influence of extrinsic factors, such as climate forcing (7, 14, 15). Hence, we predict that a climatic footprint on amphibian declines will be more apparent after controlling for interdecadal trends in both predictors and responses.
We use two amphibian decline datasets and a hypothetico-deductive approach to test among various climate-based hypotheses for declines. The first dataset, compiled by La Marca et al. (20), is the year (between 1980 and 1998) that each “species” in the frog genus Atelopus was observed for the last time [last year observed (LYO)], presumably owing to the species going extinct or at least undergoing a dramatic decline. Genus Atelopus is endemic to Costa Rica, Panama, Colombia, Ecuador, Peru, Bolivia, Venezuela, Suriname, Guyana, Brazil, and French Guiana and, since 1980, 67 of 108 extant “species” have ostensibly gone extinct (20). Of these 108 extant species, 32 are undescribed (20). The second dataset, described by Lips et al. (17), is the year that each of 59 species (all described) in genera Atelopus and Telmatobius (4 species found in Ecuador and Peru) began to decline [year of decline (YOD)]. Because of the small number of Telmatobius species in this database, we only refer to genus Atelopus hereafter for brevity.
Although LYO and YOD represent possibly the best available datasets on the timing of a putative modern day mass extinction (8), these estimates of the timing of declines unquestionably have error. Evidence suggests, however, that this error is generally small (8). For instance, the YOD dataset, which was purposefully limited to well-documented declines (17), largely matched the LYO dataset with a median difference of zero. Furthermore, the error in these datasets seems to be predominantly random (8, 20). Random error tends to increase false-negative rather than false-positive findings, suggesting that any significant relationships between climate and these datasets are unlikely to be spurious (21). The fact that we detect striking relationships between climate and amphibian declines, despite this known random error, thus supports the robustness of our findings.
Results and Discussion
Temporal Patterns of Declines.
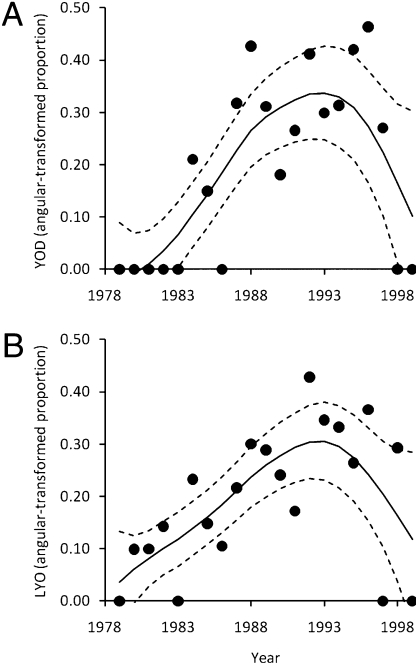
In the process of detrending the YOD and LYO data using a generalized additive model (GAM), we found that Atelopus declines were nonlinear, increasing exponentially during the 1980s but decreasing precipitously in the 1990s (Fig. 1). This nonlinearity was also detected with polynomial regression, and the polynomial fit was similar to the GAM fit (SI Appendix, Fig. S2). Further, jackknife analyses revealed that this nonlinearity was generally insensitive to the removal of individual data points (SI Appendix, Table S1).
Fig. 1.
Time series of annual proportion of Atelopus species that began to decline (YOD) and that were observed for the last time (LYO) fit with the GAM. Shown are the final fitted cubic spline functions (solid lines) and associated 95% confidence bands (dashed lines). The relationship between year and both YOD (A) and LYO (B) was significantly nonlinear (df = 3.997, coefficient = 0.019, P = 0.006, R2 = 68.17; and df = 3.997, coefficient = 0.011, P = 0.020, R2 = 58.34, respectively).
This nonlinear pattern is precisely what would be expected with epidemic spread of a recently introduced pathogen. Given that Bd is widely believed to have caused many of the amphibian declines in Latin America, that Bd was only found in Latin America around the time that amphibian declines began and thereafter, and that Bd-related declines spread through the environment (8, 17, 18, 22), a plausible interpretation for this nonlinear pattern is that declines initially increased rapidly because there were many susceptible (nontolerant) host species, but eventually declined as susceptible host species went extinct, leaving only relatively resistant and/or tolerant species. If this nonlinear pattern of declines through time represents only introduction and spread of Bd, then this intrinsic dynamic accounted for 68% and 59% of the annual variation in YOD and LYO, respectively (Fig. 1). However, given that this geographic region has experienced a multidecadal increase in tem-perature that is likely influencing the temporal trends in declines (11), detrending might also eliminate some component of any climate forcing. Thus, although it is plausible that the nonlinear multidecadal pattern predominantly represents spatiotemporal spread of a pathogen, we cannot rule out that the detrending process also controls for some component of any multidecadal climatic factor. Importantly, understanding the cause of this nonlinear pattern of declines does not affect our conclusions regarding intradecadal climate effects on amphibian declines. Regardless of the cause, detrending helps to isolate the intradecadal temporal dynamics from the long-term interdecadal patterns and reduces the number of alternative explanations for a positive association with climate relative to the nondetrended scenario (SI Appendix, Fig. S1).
Ultimate Climatic Hypothesis: An El Niño Signature.
Several researchers have detected positive relationships between the strength of global El Niño events and both infectious disease and biodiversity losses (14, 15, 23), and thus we hypothesized that, after controlling for temporal trends, the strength of El Niño would be a positive predictor of YOD and LYO. El Niño is the Pacific Ocean signature of a global, coupled ocean–atmospheric phenomenon (24, 25), measured by the continuous metric Niño 3.4, and its climatic effects on the geographic range of Atelopus are pronounced and often stronger than in many other parts of the world. El Niño years in this region tend to be warm and wet and are typically followed by cool and dry La Niña years (24, 25).
Wavelet analyses uncovered matching periodicities for Niño 3.4, YOD, and LYO (SI Appendix, Fig. S3), and regression an-alyses revealed that Niño 3.4 in the previous year (1-year lag) was a significant positive predictor of both YOD and LYO (Fig. 2A), but only after temporally detrending these response variables (YOD: n = 19, r = 0.618, P = 0.005; LYO: n = 19, r = 0.556, P = 0.013; SI Appendix, Table S2). Classifying each year as an El Niño year, a La Niña year, or neither accounted for 43% and 59% of the variation in the detrended YOD and LYO data, respectively (SI Appendix, Table S2). Moreover, these relationships with El Niño remained significant when undescribed species were excluded from the LYO analyses (SI Appendix, Table S3). The combination of putative epidemic spread of Bd (Fig. 1) and lag El Niño accounted for ≈82% and 83% of the variation in annual YOD and LYO data, respectively. The fact that an El Niño signature is only apparent after detrending YOD or LYO data suggests that, in this region, climate seems to influence whether there are more or fewer amphibian declines than would be predicted by the putative intrinsic, epidemic spread of Bd alone. Previous researchers did not detect an El Niño signature for Atelopus declines, most likely because they did not temporally detrend the LYO data (11). Although we continue to present results for both YOD and LYO, we focus on the LYO data hereafter because YOD and LYO are highly correlated (8) (Fig. 2), and YOD data exist for only a subset of the Atelopus species (17).
Fig. 2.
Time series of Niño 3.4, temperature anomalies, AVMD of anomalies, and detrended YOD and LYO. YOD and LYO represent the annual proportion of Atelopus species that began to decline and that were observed for the last time, respectively. Note the concordance of the peaks and valleys of each dataset. All time series were loess smoothed. Niño 3.4 and temperature anomalies are presented as monthly data, whereas AVMD, YOD, and LYO are presented as annual data. Thirty-six and 14 species remain extant at the end of the time series for the LYO and YOD datasets, respectively.
Proximate Climatic Hypotheses.
Although El Niño seems to be the ultimate climatic phenomenon affecting Atelopus declines, we further sought to differentiate among several climate-based factors associated with El Niño events that have been proposed as proximate drivers of enigmatic amphibian declines (26). The chytrid-thermal-optimum hypothesis proposes that increased cloud cover due to warmer oceanic temperatures leads to convergence of daytime and nighttime temperatures (i.e., a reduced diurnal temperature range) on the optimum temperature for Bd growth (11). Several mean-climate hypotheses have also been proposed for amphibian declines. These include predictions that mean temperature, mean precipitation, or an interaction between the two will be significant predictors of amphibian losses, either by directly causing declines, accelerating decomposition of leaf litter habitat, or altering interactions with natural enemies (27–31).
Finally, there is the climate-variability hypothesis, which proposes that temporal variability in temperature partially drives amphibian disease dynamics (32), although temperature variability could con-tribute directly to declines as well. This hypothesis is partly based on observations that disease outbreaks are often associated with extreme temperature events (33) and partly on evidence that temporal variability in temperature can cause suboptimal immunity in amphibians, potentially increasing their susceptibility to Bd or other infections (32). Many components of amphibian innate and adaptive immune systems depend on external temperature, but it takes time for amphibians to adjust these systems to temperature changes. Thus, changes in immune responses tend to lag behind short-term (daily to weekly) temperature changes, with increases in temperature generating suboptimal immunity (32, 34). Drops in temperature on a monthly or seasonal time scale might also be particularly important, because ectotherms seem to take longer to acclimate to a temperature decrease than to a temperature increase (35). For a variety of immune cells and proteins, low temperatures can dramatically reduce their production and/or activity levels, including (but probably not limited to) peripheral leukocyte levels (32, 34), T and B cell proliferation (35), macrophage endocytosis (36), and abundance of antimicrobial skin peptides (37), the latter of which are known to be important for defending against Bd (22). Potential increases in host susceptibility with drops in temperature are of particular concern for Bd epidemics because decreases in temperature might benefit this relatively cold-tolerant pathogen (22, 32, 38). In addition, drops in temperature are also thought to stimulate the release of Bd zoospores (39). Hence, several lines of evidence suggest that temperature variability might be important in Bd outbreaks and related mass mortality events.
To test among these proximate climate hypotheses for Atelopus declines, we gathered 13 climate variables for the region inhabited by Atelopus. First, we tested whether these climate variables, with or without a 1-year lag, were correlated positively with YOD and LYO, and we assessed the dependence of these relationships on temporal detrending of the predictors and responses. For the most part, climate predictors were not significant unless we incorporated a 1-year lag and detrended the response variables (SI Appendix, Table S2). These analyses provided no support for the chytrid-thermal-optimum hypothesis (SI Appendix, Table S2). Although several mean climate variables were significant positive predictors of Atelopus declines (SI Appendix, Table S2), their importance was not supported in subsequent analyses described below. The annual mean of the absolute value of monthly differences in temperature (AVMD) and diurnal temperature range (DTR), the only climate variability factors tested, were both positively correlated with detrended LYO the following year (SI Appendix, Table S2), supporting the climate variability hypothesis for Atelopus declines. Furthermore, removing undescribed species from the LYO dataset did not change these findings (SI Appendix, Table S3).
To identify suites of factors predictive of the LYO data, we conducted best-subset model selection using an information-theoretic-criterion approach. When only LYO was detrended, lag AVMD and lag DTR were positive predictors in 30 and 25 of the top 30 models, respectively (SI Appendix, Table S4). When both LYO and the climate variables were detrended, lag AVMD and lag DTR were positive predictors in 28 and 21 of the top 30 models, respectively (SI Appendix, Table S5). In addition, lag AVMD and lag DTR had the highest and second-highest weighted mean regression coefficients (mean of the 30 models and weighted for model adjusted R2), respectively (SI Appendix, Tables S4 and S5). The best subset model results for detrended YOD were similar to those for detrended LYO (SI Appendix, Tables S6 and S7). In general, no clear predictors consistently emerged from the best-subset analysis unless the response variables were detrended (SI Appendix, Tables S8–S11), suggesting that putative, intrinsic epidemic spread of Bd might conceal effects of climate forcing. Lag AVMD was consistently the best predictor in the best-subset models based on detrended responses. It accounted for 29.5% of the annual variation in detrended LYO (Fig. 2C), whereas the combination of lag AVMD and lag DTR (detrended) accounted for 55.2% of this variation. Lag AVMD and lag DTR remained the best two predictors when undescribed species were excluded from the LYO dataset, further supporting the robustness of these findings.
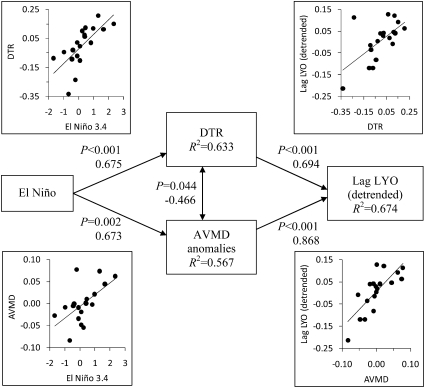
We hypothesized that the shift from warm El Niño years to cool La Niña years drives temperature variability, thereby increasing the proportion of species observed for the last time. A path analysis supported this hypothesis (Fig. 3). Niño 3.4 was a positive predictor of both AVMD and DTR, which were both positive predictors of detrended LYO the following year (Fig. 3).
Fig. 3.
Results of a path analysis testing for relationships among Niño 3.4, AVMD, DTR, and detrended LYO the following year. Probability values, standardized coefficients, and scatter plots, respectively, are provided next to each path. The scatter plots are based on the residuals from the relationship between AVMD and DTR. The model χ2 was 0.298 (df = 1, P = 0.585), indicating that the model was a good fit to the data.
Amphibian declines known to be associated with Bd have occurred more often in warm years (8, 11, 40), at high elevations (especially above 2,400 m; refs. 17 and 22), and in cool months (38, 41). If AVMD, DTR, and Bd were responsible for Atelopus declines, then AVMD and DTR should be positively associated with annual temperature and elevation but negatively associated with monthly temperature. As predicted, AVMD and DTR were positively associated with annual temperature (β = 0.569, F1,17 = 8.13, P = 0.011; and β = 0.552, F1,17 = 7.01, P = 0.018, respectively; Fig. 4A). This is likely a product of global warming generally increasing temperature maxima but not minima in this region (11), creating greater variation in daily and monthly temperatures. AVMD and DTR also increased with elevation (F3,2328 = 88.52, P < 0.001; and F3,2328 = 1,160.4, P < 0.001, respectively; Fig. 4B). Finally, AVMD (monthly temperature: parameter = 86.460, F1,9 = 19.10, P = 0.002; monthly temperature2: parameter = −87.135, F1,9 = 19.41, P = 0.002) and DTR (monthly temperature: β = −0.223, F1,10 = 0.522, P = 0.486) were the only tested climatic variables that were negatively associated with regional monthly temperature (Fig. 4C and SI Appendix, Table S12). Furthermore, this relationship between DTR and monthly temperature became significantly more negative as elevation increased, consistent with Bd-related declines occurring in cool seasons and at high elevations (monthly temperative × elevation: F3,40 = 10.48, P < 0.001; Fig. 4C; see SI Appendix, Table S13 for parameters). None of the other 11 tested climate variables were positively associated with annual temperature and elevation but negatively associated with monthly temperature (SI Appendix, Table S12). Hence, factors reflecting temperature variability were the only proximate climate variables that were entirely consistent with the spatiotemporal patterns of declines known to be caused by Bd and that could predict annual levels of Atelopus declines.
Fig. 4.
Testing for concordance between the spatiotemporal patterns of Bd-related declines and AVMD and DTR. Relationship between AVMD (•) or DTR (○) and (A) annual temperature, (B) elevation, and (C) monthly temperature. Data points with different letters are significantly different from one another (P < 0.05) according to a Fisher least significant difference test. Capital letters correspond to DTR, and lowercase letters correspond to AVMD.
Conclusions
Although there is little concrete evidence directly linking Bd infections to widespread Atelopus declines, our results offer further circumstantial evidence that Bd was an important factor. First, the temporal pattern of Atelopus declines is consistent with what would be expected on the basis of epidemic spread. Second, global and regional climatic relationships with Atelopus declines were only detectable once we controlled for this presumed epidemic spread. Third, we tested many proposed climate hypotheses for amphibian declines, including several unrelated to disease, but only found support for the climatic variability hypothesis, which was derived from studies of ectotherm immunity and disease. Finally, the proximate climatic factors that were the best predictors of Atelopus declines exhibited spatiotemporal patterns matching the spatiotemporal patterns of known Bd-related declines. Given these four results, it seems likely that Bd was associated with at least some of the Atelopus declines.
Evidence for a link between climate and Atelopus declines persisted after we detrended both the climate predictors and the decline data, providing less temporally confounded evidence of a causal relationship between these variables than available previously. Of the tested climate-based hypotheses for Atelopus dec-lines, only the climate-variability hypothesis was supported here, consistent with previous findings that temperature variability com-promises amphibian immune defenses (32) and that disease outbreaks frequently occur during extreme temperature events (33). Increases in climate variation are often linked to increases in the intensity and frequency of extreme events, which are projected to increase with anthropogenic climate change (1). These extreme events might do little to change mean climatic values, but, as sug-gested by our results, their impact on variability might have profound effects on species interactions and fitness.
In contrast to the other climate hypotheses for amphibian declines, which emphasize either climate alone or the host or parasite in isolation, the climate-variability hypothesis specifically makes predictions about the amphibian–Bd interaction. We suspect that, to thoroughly understand climatic effects on Bd-related declines, investigators will need to explicitly test how climate affects interactions between Bd and amphibian hosts, as well as consider other factors altering host–parasite dynamics or host fitness directly (39, 42–44). Additionally, the influence of climate on amphibians will almost certainly depend on the local scales at which organisms experience climatic conditions. We conducted our analyses at the regional level because this is presently the geographic scale with the best available data on the timing of presumed amphibian extinctions; however, understanding the interplay between regional and local conditions will be an important challenge for understanding amphibian declines and climatic effects on disease.
Our analyses revealed that climatic signals were usually apparent only after controlling for presumed epidemic spread of Bd-related declines. This result is consistent with the notion that El Niño events and associated variability in monthly and daily temperatures elevated mortality above that caused by epidemic spread alone. Hence, it seems that both spread and climate influence these presumed amphibian–Bd interactions and thus they do not seem to be mutually exclusive factors (45). These findings suggest that conservation efforts should first be targeted at minimizing Bd introductions, but that they might secondarily be targeted at El Niño years and regions with particularly high current or predicted temperature variability. Given that AVMD and DTR were positively associated with regional temperatures that are increasing in this region (11) and that ensemble climate modeling suggests that temperature variability in tropical and subtropical regions and the frequency of Central Pacific El Niño events will increase with projected climate change (1, 24), global climate change might indeed contribute to increases in tropical, and perhaps worldwide, enigmatic amphibian declines. Hence, amphibian chytridiomycosis seems to be consistent with the conventional, although controversial, wisdom that climate change will increase infectious diseases (6, 7). However, most of the Atelopus species of interest are unfortunately extinct and thus unavailable for experimental manipulations; thus, further evidence of a causal link between climate change and this present-day putative mass extinction event might be impossible to obtain.
Whether temperature variability and extremes can influence disease dynamics and biodiversity in general is a crucial question that remains to be answered. Although increases in temperature induced by global warming are expected to be small in tropical relative to temperate regions, recent evidence suggests that the greatest extinction risk from global warming might be for ectotherms in the hyperdiverse tropics because of their adaptations to relatively narrow and stable temperature ranges (46, 47). Effects of temporal variation in temperature might also be generalizable across ectotherm classes, given many conserved components of ectotherm innate and adaptive immune responses (32–34). If so, elevated temperature variability caused by climate change could diminish the defenses of ectothermic hosts generally, potentially representing a common, but underappreciated, link between climate change and both disease and the loss of biodiversity.
Methods
Data Collection.
For most analyses, we focused on LYO and YOD data from 1980 to 1998 because most declines occurred after 1980 (Fig. 1), there is no convincing evidence that Bd was widely present in the region occupied by Atelopus before the late 1970s, and, as the datasets approach the present time, confidence that species are “extinct” decreases. The proportions of species that declined and that were observed for the last time each year were calculated by dividing the number of declines or presumed extinctions by the number of extant species in a given year. The denominator for the proportion of declines per extant species included species with a year of decline between 1980 and 1998 plus species that we knew were extant during this period (i.e., species with last year observed after 1998). Both the denominator and numerator excluded species with ranges of years for their year of decline (see ref. 8). Analyses were conducted on the arcsine-square-root-transformed proportions using normal errors and the general linear model because we were interested in using structural equation modeling (path analysis) to test mechanistic hypotheses for the relationship between climate and declines (Fig. 3) and we are unaware of structural equation modeling software that can handle nonnormal errors. So, for consistency, all analyses were conducted with normal errors and arcsine-square-root transformations.
Global climate data on Niño 3.4 were gathered from the International Research Institute for Climate and Society's data library. We used a 5-month rolling average of Niño 3.4 for all analyses, figures, and tables that included this variable. Twelve regional climate variables (temperature anomalies, maximum and minimum temperature, cloud cover, precipitation, wet day frequency, vapor pressure, frost frequency, cloud cover × temperature interaction, precipitation × temperature interaction, diurnal temperature range, and the absolute value of monthly differences in temperature) were gathered or calculated from the Climate Research Unit (CRUTEM3, CRU TS 2.1), University of East Anglia (48) for the region described by Rohr et al. (8), which approximates the geographic range where Atelopus did or does inhabit. Most of the climate data were interpolated to 0.5° resolution using all quality climate stations in the region (48) (exception: “temperature anomalies” is only available at the 5° resolution). The climate data have passed extensive quality control measures, meet the quality standards of the Intergovernmental Panel on Climate Change (48), and arguably represent the most comprehensive and precise historical estimates of local climate across the region inhabited by Atelopus. Annual means were calculated using the monthly means provided in the database, except for AVMD, which was calculated as the average difference in average temperature between adjacent months.
Temperature anomaly data were used when possible to avoid bias associated with different countries estimating average monthly temperatures using different methods and formulae. However, the anomaly data were incomplete, which would have greatly affected estimates of AVMD. Consequently, for AVMD calculations based on anomalies, missing data were replaced with the 30-year mean of that month and grid cell. In the CRU TS 2.1 dataset, each 0.5° by 0.5° grid cell has a mean elevation that was used for our elevation analyses. Finally, temperature-dependent estimates of annual Bd growth were calculated as described in Rohr et al. (8).
Detrending Predictors and Responses.
We did not have any a priori predictions for the relationships between year and YOD, LYO, or the climate predictors, so we used the GAM, weighted by the number of extant species per year, to test for significant nonlinearities between year and these variables. YOD and LYO were arcsine-square-root-transformed before detrending. If there was significant nonlinearity, we detrended the variables using residuals from the GAM. If there was not significant nonlinearity in the GAM, the variable was detrended using residuals from a standard linear regression model. The only predictor that was significantly nonlinear through time was DTR, and thus it was the only predictor that was detrended using GAM rather than standard linear regression (SI Appendix, Tables S5, S7, S9, and S11). To ensure that the results were not dependent on the procedure used to detrend the amphibian decline data, we compared results using GAM to results using polynomial regression, and we jackknifed the GAM analyses to determine the sensitivity of the nonlinear fit to the presence of individual data points (SI Appendix, Fig. S2 and Table S1).
Testing for a Relationship Between El Niño and YOD or LYO.
We used wavelet analysis software, available at http://paos.colorado.edu/research/wavelets/, to assess whether the periodicity of El Niño, YOD, and LYO differed. We applied a Morlet wavelet function, used a global wavelet spectrum as the estimator of the “true” power spectrum, padded the time series with zeros (to bring the number of years up to the next-higher power of 2) to accelerate the Fourier transform and to reduce errors associated with the edges of the time series, and tested the periodicity against a white-noise background (49). Additional details on these analyses are in SI Appendix, Fig. S3.
Scale-dependent correlation analysis is likely the most powerful approach for detecting transitory coupling between climatic factors and amphibian declines (15), but we unfortunately did not have enough temporal YOD or LYO data to successfully use this methodology. Consequently, we relied on the less-powerful standard regression approach, whereby we tested whether Niño 3.4 (a continuous variable), detrended or not, could predict detrended or nondetrended YOD and LYO in the same or following year. We also classified years as El Niño, La Niña, or neither (SI Appendix, Table S14) and tested whether this classification was predictive of YOD and LYO. These analyses were weighted by the number of extant species per year, given that extant species declined through the time series.
Many lags are possible between climate and amphibian declines (e.g., ref. 30). However, we chose to arbitrarily select only a 1-year lag for all of our analyses because we did not want the search for a lag to become a “fishing expedition” that biased our research by identifying the lag that best supported our hypothesis. A 1-year lag has previously been proposed for detecting climatic effects on Atelopus declines (11).
Evaluating Support for Proximate Climate Hypotheses.
We first used correlation analysis to test whether the 12 climate variables (see Data Collection above) plus temperature-dependent Bd growth score, with or without a 1-year lag, were positively correlated with YOD and LYO and the dependence of these relationships on temporal detrending of the predictors and responses. Second, we identified suites of factors predictive of the YOD and LYO data using best-subset model selection based on adjusted R2, which penalizes models with more predictors. The maximum allowable number of predictors per model was capped at three, and the analyses were conducted on all combinations of detrended and nondetrended predictors and responses. Third, we used path analysis with maximum likelihood estimation and submodel deviances to test the hypothesis that El Niño events drive temperature variability, which increase Atelopus declines. Path analysis was conducted with Statistica 8.0 (Statsoft). Finally, we used regression analysis to determine which proximate climate variables had similar spatiotemporal patterns as known Bd-related declines.
Supplementary Material
Acknowledgments
We thank the A. R. Blaustein laboratory, P. J. Hudson, P. T. J. Johnson, K. M. Kriger, H. McCallum, and P. Stiling for thoughts on this article. This work was supported by National Science Foundation Grant DEB 0516227, US Department of Agriculture Grant NRI 2006-01370 (to J. R. R.), and US Environmental Protection Agency Science to Achieve Results Grant R833835 (to J. R. R and T. R. R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912883107/-/DCSupplemental.
References
- 1.Raisanen J. CO2-induced changes in interannual temperature and precipitation variability in 19 CMIP2 experiments. J Clim. 2002;15:2395–2411. [Google Scholar]
- 2.Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc Natl Acad Sci USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascual M, Dobson AP, Bouma MJ. Underestimating malaria risk under variable temperatures. Proc Natl Acad Sci USA. 2009;106:13645–13646. doi: 10.1073/pnas.0906909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife: Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 5.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvell CD, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 7.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 8.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay SI, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosch J, Carrascal LM, Duran L, Walker S, Fisher MC. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc Biol Sci. 2007;274:253–260. doi: 10.1098/rspb.2006.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 12.Laurance WF. Global warming and amphibian extinctions in eastern Australia. Austral Ecol. 2008;33:1–9. [Google Scholar]
- 13.D’Amen M, Bombi P. Global warming and biodiversity: Evidence of climate-linked amphibian declines in Italy. Biol Conserv. 2009;142:3060–3067. [Google Scholar]
- 14.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 15.Rodo X, Pascual M, Fuchs G, Faruque ASG. ENSO and cholera: A nonstationary link related to climate change? Proc Natl Acad Sci USA. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn KG, Campbell-Lendrum DH, Armstrong B, Davies CR. Malaria in Britain: Past, present, and future. Proc Natl Acad Sci USA. 2003;100:9997–10001. doi: 10.1073/pnas.1233687100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lips KR, Diffendorfer JE, Mendelson JR, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:441–454. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 19.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Marca E, et al. Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus) Biotropica. 2005;37:190–201. [Google Scholar]
- 21.Parmesan C, Singer MC. Amphibian extinctions: Disease not the whole story. [Accessed March 12, 2010];2008 Available at: http://biology.plosjournals.org/perlserv/?request=read-response&doi=10.1371/journal.pbio.0060072#r2213. [Google Scholar]
- 22.Kilpatrick AM, Briggs CJ, Daszak P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends Ecol Evol. 2010;25:109–118. doi: 10.1016/j.tree.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Kiesecker JM, Blaustein AR, Belden LK. Complex causes of amphibian population declines. Nature. 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- 24.Yeh S, et al. El Niño in a changing climate. Nature. 2009;461:511–515. doi: 10.1038/nature08316. [DOI] [PubMed] [Google Scholar]
- 25.McPhaden MJ, Zebiak SE, Glantz MH. ENSO as an integrating concept in Earth science. Science. 2006;314:1740–1745. doi: 10.1126/science.1132588. [DOI] [PubMed] [Google Scholar]
- 26.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 27.McMenamin SK, Hadly EA, Wright CK. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proc Natl Acad Sci USA. 2008;105:16988–16993. doi: 10.1073/pnas.0809090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitfield SM, et al. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proc Natl Acad Sci USA. 2007;104:8352–8356. doi: 10.1073/pnas.0611256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawler JJ, et al. Projected climate-induced faunal change in the Western Hemisphere. Ecology. 2009;90:588–597. doi: 10.1890/08-0823.1. [DOI] [PubMed] [Google Scholar]
- 30.Alford RA, Bradfield KS, Richards SJ. Ecology: Global warming and amphibian losses. Nature. 2007;447:E3–E4. doi: 10.1038/nature05940. [DOI] [PubMed] [Google Scholar]
- 31.Rohr JR, Madison DM. Dryness increases predation risk in efts: Support for an amphibian decline hypothesis. Oecologia. 2003;135:657–664. doi: 10.1007/s00442-003-1206-7. [DOI] [PubMed] [Google Scholar]
- 32.Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ. Negative effects of changing temperature on amphibian immunity under field conditions. Funct Ecol. 2006;20:819–828. [Google Scholar]
- 33.Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. The effects of anthropogenic global change on immune functions and disease resistance. Ann N Y Acad Sci. doi: 10.1111/j.1749-6632.2010.05454.x. in press. [DOI] [PubMed] [Google Scholar]
- 34.Maniero GD, Carey C. Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. J Comp Physiol B. 1997;167:256–263. doi: 10.1007/s003600050072. [DOI] [PubMed] [Google Scholar]
- 35.Bly JE, Clem LW. Temperature-mediated processes in teleost immunity: In vitro immunosuppression induced by in vivo low temperature in channel catfish. Vet Immunol Immunopathol. 1991;28:365–377. doi: 10.1016/0165-2427(91)90127-x. [DOI] [PubMed] [Google Scholar]
- 36.Plytycz B, Jozkowicz A. Differential effects of temperature on macrophages of ectothermic vertebrates. J Leukoc Biol. 1994;56:729–731. doi: 10.1002/jlb.56.6.729. [DOI] [PubMed] [Google Scholar]
- 37.Matutte B, Storey KB, Knoop FC, Conlon JM. Induction of synthesis of an antimicrobial peptide in the skin of the freeze-tolerant frog, Rana sylvatica, in response to environmental stimuli. FEBS Lett. 2000;483:135–138. doi: 10.1016/s0014-5793(00)02102-5. [DOI] [PubMed] [Google Scholar]
- 38.Kriger KM, Hero JM. Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. J Zool. 2007;271:352–359. [Google Scholar]
- 39.Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA. Life-history trade-offs influence disease in changing climates: Strategies of an amphibian pathogen. Ecology. 2008;89:1627–1639. doi: 10.1890/06-1842.1. [DOI] [PubMed] [Google Scholar]
- 40.Berger L, et al. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J. 2004;82:434–439. doi: 10.1111/j.1751-0813.2004.tb11137.x. [DOI] [PubMed] [Google Scholar]
- 41.Retallick RWR, McCallum H, Speare R. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol. 2004;2:1965–1971. doi: 10.1371/journal.pbio.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffel TR, Martin LB, Rohr JR. Parasites as predators: Unifying natural enemy ecology. Trends Ecol Evol. 2008;23:610–618. doi: 10.1016/j.tree.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Rohr JR, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- 44.Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecol Appl. 2008;18:1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- 45.Rachowicz LJ, et al. The novel and endemic pathogen hypotheses: Competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–1448. [Google Scholar]
- 46.Tewksbury JJ, Huey RB, Deutsch CA. Putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- 47.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell TD, Jones PD. An improved method of constructing a database of monthly climate observations and associated high-resolution grids. Int J Climatol. 2005;25:693–712. [Google Scholar]
- 49.Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.