Abstract
Min proteins of the Escherichia coli cell division system oscillate between the cell poles in vivo. In vitro on a solid-surface supported lipid bilayer, these proteins exhibit a number of interconverting modes of collective ATP-driven dynamic pattern formation including not only the previously described propagating waves, but also near uniformity in space surface concentration oscillation, propagating filament like structures with a leading head and decaying tail and moving and dividing amoeba-like structures with sharp edges. We demonstrate that the last behavior most closely resembles in vivo system behavior. The simple reaction-diffusion models previously proposed for the Min system fail to explain the results of the in vitro self-organization experiments. We propose the hypotheses that initiation of MinD binding to the surface is controlled by counteraction of initiation and dissociation complexes; the binding of MinD/E is stimulated by MinE and involves polymerization-depolymerization dynamics; polymerization of MinE over MinD oligomers triggers dynamic instability leading to detachment from the membrane. The physical properties of the lipid bilayer are likely to be one of the critical determinants of certain aspects of the dynamic patterns observed.
Keywords: cell division, min system, oscillations and waves, self-organization, septum localization
Assembly of the cell division septum in Escherichia coli is initiated by polymerization of FtsZ, a tubulin-like GTPase, on the membrane surface (1). The central localization of the cell division septum is controlled by a set of Min proteins (2). FtsZ polymerization is inhibited by membrane localized MinC (3), and the MinC distribution on the membrane is determined by the distribution pattern of its binding partner MinD (4, 5). MinD is an ATP-dependent membrane binding protein, whose behavior is controlled by a partner protein MinE. MinE is not a membrane binding protein itself, but interacts with membrane-bound MinD and stimulates its ATPase activity and subsequent release of MinD from the membrane (6, 7). Based on structural information (8, 9), we assume that the minimum unit of the nucleotide-bound MinD is not smaller than a dimer.
Rapid pole to pole oscillations of GFP-fusion Min proteins with a period of 40–50 s were observed in vivo (4, 10, 11). An oscillation cycle involves accumulation of MinD on the membrane near a cell pole and formation of a MinE ring on the membrane near the edge of the surface bound MinD patch (12). The MinE ring chases the receding edge of the concentration gradient of MinD toward a cell pole. As MinD disappears from the pole, the E-ring fades before reaching the pole and reassembles at the flank of the MinD gathered at the other cell pole. The MinD patch near the cell pole also contains MinE at a lower concentration compared to the E-ring. The pole-to-pole oscillation pattern of MinD results in time-averaged local minima of the membrane-bound MinD con-centration (hence also MinC) at the midcell, where FtsZ polymerizes to initiate assembly of the cell division septum (13).
In a cell-free reaction chamber with a mica surface-supported lipid bilayer at the bottom, Loose, et al. (14) observed a propagating wave pattern formed by MinD and MinE in the presence of ATP on the lipid surface. Dynamic self-assembled pattern formation by a set of defined components raised the hope that the in vivo system behavior could be understood without the critical influence of additional reaction components. However, the geometric differences between the in vivo dynamic patterns and the large waves observed in vitro made it difficult to evaluate the relationship between the two observations.
MinD is a member of the Walker type partition ATPase family (15). The other members of this family are ATP-dependent DNA binding proteins, which also exhibit different types of complex behaviors involved in plasmid and chromosome partition in prokaryotes. For example, ParA can oligomerize on DNA and interact with a partner ATPase regulator ParB, which binds to the cognate centromere-like DNA sequence, and this leads to end to end oscillation of the ParA focus along the bacterial nucleoid in vivo (16). Interaction of SopA ATPase with its partner SopB in complex with its cognate centromere-like sequence on the F-plasmid leads to partitioning of replicated copies of the plasmid to the two halves of the cell before cell division (17). Rapid oscillations driven by polymerization/depolymerization of F-actin are responsible for amoeba-type cellular motilities exhibited by, for example Dictyostelium discoideum (18) and other eukaryotic cells. Microtubules are capable of synchronous assembly-disassembly oscillations (19) and are involved in many dynamic processes in eukaryotic cells. A variety of types of pattern formation can be found in many areas in nature (20). However, the mechanisms of many mesoscale self-assembled bio-patterning reactions remain yet to be explored.
A simple class of reaction-diffusion model was proposed to explain biomorphogenesis in general by Alan Turing in 1952 (21). For the Min system, Turing-style models have been proposed to describe the evolution of the dynamic protein distribution on the lipid membrane and in the solution next to it using a set of differential equations (reaction-diffusion equations, refs. 14, 22–24) or by stochastic approaches (25). The number of differential equations in the deterministic models reflects the proposed number of the different molecular species in the solution and on the membrane. The “reaction” terms in the equations describe transmutations of these molecular species with a set of hypothetical cooperativity parameters and kinetic constants, which are chosen to generate the desired system behavior. Although the general interactions of Min proteins have been known from experiments, there are no experimentally derived constraints on the details of the reaction steps, the cooperativity and kinetic parameters for each of the steps, or the number and the types of the distinct molecular states of the two proteins involved. This necessitated the exercise be limited to simpler scenarios involving as small a number of distinct molecular states as feasible, for example, typically dividing the protein molecules into only two molecular states, membrane-bound molecules and those free in the cytosol, with an assumption that all MinD molecules in the system are in the state of competence for membrane binding. Some models also explored the possibility of protein polymerization on the membrane surface (26), which would be more readily handled by stochastic approaches (27). Other models attempted to incorporate reaction steps related to the ATPase cycle, thus introducing a MinD state not competent for membrane binding (23). The “diffusion” terms for the solution and the surface-bound components describe diffusional changes of the local concentrations of the components in the bulk solution and on the membrane surface, respectively. However, in order for the bulk diffusion coefficients to be a critical determinant of the membrane surface pattern generated, the system must be bulk diffusion-limited and spatial inhomogeneities in solution above the surface must develop.
To advance our understanding of the Min system oscillation mechanism, we investigated MinD–MinE dynamic pattern formation on a lipid bilayer in a cell free system. Here we describe several other classes of Min system self-organized dynamic behavior and their interconversion processes that revealed fundamental aspects of the reaction mechanism that have to be accounted for to build a realistic model of the system behavior. These behaviors include, but are not limited to, spatially near homogeneous oscillation of membrane-bound MinD and MinE surface density (oscillation), propagating wave patterns (waves), filaments growing at one end and disassembling at the other end (snakes), and mobile amoeba-like compact structures (amoebas), which consist of a MinD-rich core (D-core) surrounded by a MinE-rich ring (E-ring). The amoeba behavior displays clear resemblance to the in vivo system dynamics.
Here, we demonstrate that the concentration heterogeneity of reaction components in the solution above the membrane surface does not contribute significantly to the spatial pattern formation on the membrane surface. In other words, according to our results, a surface pattern formation model must be able to tolerate a spatially uniform solution concentration of the reaction components. We propose that biochemical timing mechanisms, including those involving protein polymerization-depolymerization dynamics, com-bined with mechanical stress feedback of the protein–membrane interaction critically contribute to the Min system dynamics. At present, however, we believe there are too many unconstrained details to warrant the proposal of a quantitative model, and we limit ourselves to propose a set of speculative hypotheses to explain a number of mechanistic aspects revealed in this study.
Results
Our observation setup consists of a lipid bilayer supported by a fused silica slide surface of a 25 μm deep flow cell and fluorescently labeled proteins in a reaction buffer containing 2.5 mM ATP. We used three different compositions of supported lipid bilayers: E. coli polar lipids (E. coli lipid bilayer), synthetic lipid mixture mimicking the head group composition of the E. coli polar lipids but with a synthetic mixture of 70% double-unsaturated and 30% monounsaturated aliphatic tail groups (18:2 bilayer), and a synthetic lipid mixture with 100% of the monounsaturated aliphatic tail groups (18:1 bilayer). The difference between lipid bilayers was in the lipid raft structure (28–31) and fluidity of the membrane (SI Domain Structures in the Supported Lipid Bilayer, Fig. S1 A and B). EGFP-MinD fusion protein and Alexa647 labeled MinE (1.06 μM and 1.36 μM, respectively, in the solution) were illuminated on the flow cell surface using prism-type total internal reflection fluorescence microscopy with 488 nm and 633 nm lasers. EGFP-MinD (green) and Alexa647-MinE (red) fluorescence images were captured in parallel and displayed according to their color in all our figures and supporting movies. Experiments were typically carried out under constant flow of the sample solution with a cross-section average flow velocity of 0.5 mm s−1 unless otherwise noted. The constant supply of the sample assures no bulk depletion of the proteins takes place, and any significant development of reaction component concentration heterogeneity near the membrane surface is suppressed (SI Start of the Oscillation; Observation Position Effect, also see SI Flow Cell).
As expected from previous studies (29, 32), MinD binds to lipid bilayers containing anionic lipids in the presence of ATP until an “apparent steady state” is reached. The binding level at low MinD concentration was proportional to the free protein concentration. Above 1.5 μM MinD, the surface protein concentration approached saturation (Fig. S2A). The major fraction of the surface bound MinD was in rapid exchange with solution according to fluorescence recovery after photobleaching (FRAP) and buffer wash experiments (SI Membrane Binding Properties of MinD in the Absence of MinE). However, both experiments showed multiphasic kinetics indicating the presence of multiple molecular forms or states (Fig. S2 B and C). Considering the slow ATP hydrolysis rate of MinD in the absence of MinE (6), MinD release from the membrane is unlikely to be obligatorily coupled to ATPase turnover.
Oscillation and Waves of MinD/E on a Lipid Bilayer Surface.
Spatially homogeneous oscillation of the surface protein densities is the most common mode observed at the beginning of an experiment in the middle of a flow cell coated with E. coli lipid bilayer when MinD and MinE are infused into the flow cell together with ATP. In the middle of the flow cell, oscillation initiates after a time lag of roughly a half of the oscillation period during which only small amounts (less than a few percent of the saturation density) of proteins bind to the surface (Fig. 1D and Fig. S3). Under the experimental conditions used in this study, there is no significant depletion of the bulk concentration of proteins (SI Start of the Oscillation; Observation Position Effect). MinD, together with MinE, usually starts binding to the surface from a large number of spots as expanding circular zones until they cover entire field of view, and the surface MinD concentration rapidly increases to a level comparable to the saturation level of MinD binding in the absence of MinE (Fig. 1A). The rate of MinD binding in the first oscillation cycle is close to the binding rate in the absence of MinE, but in the second and subsequent oscillation cycles the MinD binding rate increases to about twice that of the first cycle (Fig. S4). This indicates that either a larger fraction of the MinD population is in the membrane binding competent states, a different molecular state with faster binding capacity is generated in the presence of MinE, or both. After staying near the saturation level for a few minutes, MinD starts dissociating from the surface of the entire field of view.
Fig. 1.
Oscillations and waves on E. coli lipid bilayer. MinD is green, MinE is red. (Scale bars: 5 μm on all still-images.) The time stamps are in seconds, the sequential frames are sorted from left to right in the rows going from top to the bottom, the flow direction is from the left to the right. (A) Arrival of MinD in a form of many circular zones during oscillation. Centers of circular zones are marked with crosses. (B) Wave. Direction of wavefront propagation is labeled with arrows. (C) Colocalized waves and oscillating spot (labeled with arrow). Frames taken with intervals equal to the period of waves (Fig. S3A). Notice that the oscillation period is twice longer than the wave period. (D) Time-course of MinD and MinE surface fluorescence intensities (red and green curves, left scale) and their ratio (black, right scale) during oscillation measured at a fixed location on E. coli lipid bilayer in the middle of the flow cell. The time lag between arrival of the bulk protein and beginning of protein binding is labeled with a blue flag (also see Fig. S8B). One camera unit (c.u.) corresponds to a molecular surface density of approximately 1.8 μm−2 as described in SI Estimation of the Protein Surface Concentrations. (E) Time derivatives of the MinD and MinE surface concentration curves in D.
MinE arrives at the surface at the same time as MinD, but at a slower rate. The peak of the MinE binding rate at the start of the cycle coincides with the peak of the MinD binding rate (Fig. 1E). After the initial peak, the MinE binding rate decreases to approximately half and stays relatively constant. Just before the start of MinE dissociation, its binding rate often displays another sharp peak, especially if the surface MinD (and MinE) reaches a high level (compare red curves in Fig. 1E and Fig. S5B). The sharp MinE maximum occurs after the bound MinD level drops at least 40% from the maximal level. In a wave or oscillation, the peak surface density of MinE was typically two- to fourfold less than the peak surface density of MinD at its own peak (Fig. 1D and SI Estimation of the Protein Surface Concentrations). The ratio of MinD to MinE surface concentrations has a maximum near the time of MinD peak and reaches a minimum at the time of MinE peak and under other special conditions (Fig. S5A). After the sharp MinE peak, both proteins dissociate from the surface in parallel with multiexponential kinetics.
After several periods of oscillation, spatial inhomogeneity on the membrane surface develops; oscillation starts from a smaller number of spots, but the expanding circular protein binding zones still merge to uniform coverage. However, if binding initiation zones become spaced too far apart, a continuous wavefront followed by parallel lines of the MinE maxima and receding edges start to appear, converting the oscillation to a propagating wave pattern (Fig. 1B). The wave amplitude is typically about half of the oscillation amplitude, but the MinD/MinE ratio is about the same as in the oscillation. The wave period is about half of the oscillation period (Figs. 1C, Fig. S3A, and Movie S1). The velocity of the waves (0.4–0.7 μm s−1) is roughly half of the velocity of the expansion of the initial circular binding zones in the preceding oscillation (0.8–1.5 μm s−1, Movie S1 and SI Microscope, Camera, and Illumination). Otherwise, the qualitative appearances of the time trajectories of the surface protein concentrations at fixed locations are similar. The very stable waves have been observed after several hours into an experiment with buffer flow in a flow cell coated with E. coli lipid bilayer after all amoeba related frozen structures dissolved (compare steady waves in Movie S2 with waves in “labyrinth” in Movie S3). We have not noticed any direct correlation between wave and buffer flow directions in the middle of the flow cell (see SI Start of the Oscillation; Observation Position Effect for special cases). Stopping the sample solution flow during the reaction did not disturb the ongoing dynamic pattern for a number of cycles. We note here that after the start of the first oscillation cycle, the surface protein densities between the peaks do not fall below approximately 10% of the peak, and this background level often slowly increases after each cycle of oscillation with a roughly constant MinD/MinE ratio close to 1 (Fig. 1D). We will discuss the possible functional significance of this residual protein on the surface later.
Amoebas.
The dynamic and relatively sparsely populated amoebas are the terminal and most stable MinDE self-organization mode on 18:2 bilayers at 22–23 °C (Movies S4 and S5) and on 18:1 bilayers at an elevated temperature (25 °C, Movie S6). The MinD-rich region inside of a typical amoeba (D-core, Fig. 2A) has a MinD surface density approximately 60% of the peak MinD density in a typical wave (Fig. S6A). The MinD surface density decreases across the thin E-ring surrounding an amoeba. The surface density of MinE in the E-ring appears to be close to the peak MinE density in a wave, although it could be higher because we do not have an accurate estimate of the thickness of the ring due to the limited resolution of the microscope. The MinD/MinE ratio of the D-core is similar to the initial phase of the oscillation or waves and that of the E-ring is close to approximately 1, although accurate estimation of this value is difficult at present. Amoebas are surrounded by an outside area of lower surface protein density similar to the low surface density phase of the oscillation or waves. The typical amoeba E-ring diameter is 3–5 μm (E-rings of the larger amoebas usually consist of multiple domains or pseudopodia of 3–5 μm size). Smaller amoebas tend to shrink and fade away: the D-core becomes smaller and fades away before the E-ring disappears; the E-ring becomes smaller as the D-core shrinks, and it also fades away a few seconds after the D-core without collapsing to a spot (Fig. 2C). The larger amoebas grow even larger and divide into two or more smaller amoebas by “E-ring septation” (Fig. 2D and Movie S4). When the amoeba size grows faster and septation for even-sized daughters does not catch up, the amoeba starts multiple divisions simultaneously by developing multiple septa along the periphery to split off smaller offsprings (Fig. 2B and Movie S5). The speed of traveling amoebas could be up to 0.15 μm s−1. The smaller amoebas tend to split off at the trailing edge of the traveling larger amoebas; these small amoebas usually disappear quickly in the fashion described above. On quickly traveling amoebas, the E-rings are fainter near the leading edge and brighter near the trailing edge, but we have not noticed other systematic morphological asymmetry. Both the D-core and E-ring are not only spatially dynamic, but their protein component concentrations are dynamically maintained by the balance of assembly and disassembly, which takes place on a rough time scale of 3.5–6.2 s for MinD and 16–20 s for MinE based on FRAP experiments (SI FRAP Experiments and SI Summary of the Fluorescence Recovery Time Data).
Fig. 2.
Amoebas. (A) Group of amoebas on the 18:1 bilayer at an elevated temperature (25 °C) from Movie S6. Three panels show MinD cores (green), MinE rings (red), and overlay. (B) Formation and multiple division of a herd of amoebas on the 18:2 bilayer from a wave. Direction of wave propagation is labeled with arrows; disappearing amoebas are crossed. (C) A small amoeba shrinking and disappearing on the 18:2 bilayer. (D) Slow isolated amoebas growing, dividing, and disappearing on the 18:2 bilayer.
Amoebas can start by septation from the trailing edge of a wave. The propagating wave can slow down or stall and develop into a group of amoebas. In one dramatic example shown in Fig. 3A and at the beginning of Movie S7, a propagating wave front stalled and slowly regressed for a short distance. In the process, a thin and sharp MinE-rich line formed at this receding front, although the trailing edge of the wave with a high MinE concentration caught up, slowed down, and the “trailing” MinE-rich zone became sharper, trapping a MinD-rich band between the two MinE-rich lines. When the distance between the two E-lines approached approximately 5 μm, the E-lines started to develop septations at roughly equal intervals to generate a line of individual amoebas. This process is, however, typically less organized (Fig. 2B).
Fig. 3.
Transitional states. (A) Wave transforms into amoebas on the 18:2 bilayer (Movie S7). Wavefront propagation is labeled with blue arrows; E-line propagation is labeled with red arrows; one of the septations of the E-line is labeled with a pair of white arrows. (B) High density of amoebas transforms into a continuous mesh structure on the 18:2 bilayer (Movie S7). Similar looking patterns have been observed as segregation patterns of different phospholipids on lipid monolayers (33). (C) Group of frozen amoebas on E. coli lipid bilayer. (D) Amoeba-like mesh formed from a wave on an E. coli lipid bilayer.
A high density of amoebas has been seen to develop after several cycles of oscillation or a passing wave (Fig. 3B). These transitions took place near the end of a cycle when the surface MinE density was high. The high-density amoebas derived from oscillation sometimes grew in size and the E-rings fused into a continuous E-mesh with holes of D-cores. In the process, the average surface MinD concentration reached as high as the peak value during normal oscillation. With time, the D-core shrank as the E-mesh became thicker. The peak average surface MinE concentration reached approximately 40% above the peak value during normal oscillation. Eventually, the D-core faded away, and the E-mesh disappeared like at the end of an oscillation cycle. Multiple ways of the mode transition have been observed within the course of an experiment (Movie S7).
The double-unsaturated lipids, used for amoeba observation, are not present in E. coli membranes, but the room temperature of our experiments (22–23 °C) was substantially lower than 37 °C growth temperature of E. coli culture used for extraction of the polar lipids (Avanti). The immobilization of the lipid bilayer on fused silica surface might also make the lipid bilayer less fluidic: on the E. coli lipid bilayer, waves have been observed to transform into a group of frozen amoebas (Fig. 3C) or E-mesh (Fig. 3D), which did not grow or propagate. New waves were seen propagating near frozen amoebas without influencing their interior or generating new amoebas. Frozen amoebas and E-mesh have been seen to act as a start site of new waves. Other amoeba-related structures observed on E. coli lipid bilayers are discussed in SI Labyrinth on E. coli Lipid Bilayer, SI Giant Amoeba on E. coli Lipid Bilayer, and SI Step-Like Wave Propagation (Figs. S7 and S8).
We believe that the amoeba behavior most closely resembles the in vivo dynamics of the Min system: dynamic amoebas were the dominant self-organized mode and the characteristic amoeba domain size approaches the size of an E. coli cell. If one were to imagine opening up an E. coli cell at the tip of one pole and flatten the membrane to a plane centered at the other pole where nearby MinD is disassembled by the E-ring, one would transform a half-cycle of the in vivo oscillation pattern to the disappearing small amoeba described above with similar spatiotemporal scale parameters. Therefore, we propose that they are topologically equivalent. The E-ring in vivo does not shrink into a point at the end of a cell pole before disappearing, in contradiction to the prediction of simple reaction-diffusion models (14, 23, 24). The equispaced septation of larger amoebas is reminiscent of the spacing between multiple E-rings in filamentous E. coli cells (5, 34), revealing similar spatial characteristic dimensions of E-rings in vivo and in vitro despite the geometrical differences of the reaction space between bacterial cells and the flow cell. The amoebas do not have clearly periodic behavior, but fast traveling amoebas are obviously avoiding recently visited areas (Movies S5 and S6) and slow amoebas are breathing (fluctuating in size) periodically (Fig. S9).
Snakes.
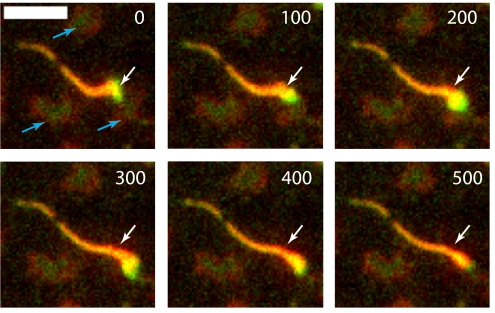
MinD filaments have been observed in vitro as polymer bundles (35). In some of our experiments at elevated MinD concentrations relative to MinE on higher melting temperature bilayers, we observed dynamic filament formation (snakes). Each snake has a MinD-rich slow growing head, which often is not firmly attached to the lipid surface, followed by a disassembling MinE-rich tail (Fig. 4 and Movie S8). Judged by the brightness of the head, it is not a single strand of MinD polymer but rather a bundle of many perhaps short polymers. The polymers might have directionality with a growing (plus) end and a depolymerizing (minus) end. Alternatively, the position of the MinE-rich tail might determine the direction of the growth. Sometimes, snakes with the MinD-rich heads at both ends have been observed. The question of whether the dynamic movement of snakes reflects a mechanism more akin to those of actin filaments and microtubules or is a spatially condensed variant of other dynamic patterning modes described above remains to be answered by future experiments.
Fig. 4.
Snake eating frozen amoeba laying on 18:1 bilayer. The position of the snake head at time 0 s is labeled with an arrow. The arrow position is the same in all frames. Blue arrows point to frozen amoebas.
Discussion
The previously proposed models of Min system self-organization start with a very limited set of experimental constraints: MinD can bind lipid membrane in the presence of ATP but not in its absence; MinE cannot bind membrane on its own, but associates with membrane-bound MinD; MinD–MinE association on the membrane leads to accelerated ATP hydrolysis by MinD and thus leads to presumed acceleration of membrane dissociation. Although these are reasonable barebone steps to be considered, experimental observations to evaluate the quantitative details of the reaction steps have been very limited and few constraints have been imposed on these models. Only bulk diffusion coefficients of proteins have been measured directly (14, 36). The surface diffusion coefficients have been estimated from FRAP experiments without analysis of exchange between membrane-bound proteins and solution (14), which turned out to be the leading cause of the fluorescence recovery according to our measurements (SI FRAP Experiments and SI Summary of the Fluorescence Recovery Time Data). All other kinetic parameters had been simply fitted to the model. The multiple patterning modes reported here demonstrate that many additional critical reaction features would have to be incorporated for an adequate model.
The simple reaction-diffusion models, like the one used in ref. 14, involve bulk protein depletion and are therefore sensitive to the bulk diffusion coefficients. The basic predictions of these models would be that in vitro MinD/E self-organization experiments might require a reaction chamber with the surface to volume ratios close to that of a cell. The published report of the in vitro MinD–MinE dynamic pattern formation and the accompanying theoretical model (14) had no experimental or theoretical data on net surface and solution protein concentrations. Nonetheless, the wave pattern reported in the paper appears qualitatively very similar to the propagating waves we observed. Then, the peak surface density of MinD also should be close to our estimates, which is below tightly packed monolayer surface coverage. Based on the reported reaction chamber depth and protein concentrations in the experiments of (14) we could exclude any significant depletion of proteins from the solution above the membrane. Because mixing of the sample solution by the micropipette (14), or the sample solution flow or flow stoppage (this study) neither contributed to, nor disturbed the established system behavior, we conclude that the volume concentration distribution in the system must have been homogeneous and constant. Thus, we conclude that the volume concentration inhomogeneities and bulk diffusion coefficients of proteins are not critical determinants for the surface pattern formation, and the system can tolerate a broad range of surface to volume ratios contrary to expectations of the simple models that assume the system is bulk phase diffusion-limited. The sharp transitions and multiple changes of the binding rate observed in the oscillation and waves (Fig. 1 C and D) would be very difficult to simulate with a simple reaction-diffusion model. Indeed, the model proposed in (14) neither reproduces the saw teeth-like MinE binding pattern nor the relative timing of the MinD and MinE arrival to and dissociation from the surface. Based on many similarities between the amoeba dynamics we observed and the in vivo Min system dynamics, we believe factors that had not been considered previously must contribute to controlling the scale parameters of the in vivo Min system dynamics. Here, we propose a speculative set of working hypotheses that could explain the reaction features observed in our study, provide a coherent perspective, and guide future investigations (Fig. 5).
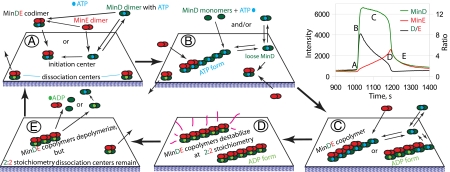
Fig. 5.
Five stages of the MinD/E membrane binding cycle; a hypothetical model. At step (A) the initiation centers are formed, overcoming the inhibitory effects of the dissociation centers left from the previous cycle. (B) The initiation center starts polymerization. On-the-surface, polymerization/depolymerization may generate a loose fraction of MinD, which is in constant exchange with solution without involving ATP hydrolysis. MinE polymerizes over MinD filaments at a near-constant rate and ATP hydrolysis may start. (C) MinD–MinE copolymer growth causes a reduction of the membrane affinity to MinD and leads to dissociation of the loose fraction of MinD. This could be a possible source of the initiation precursor downstream. At the peak of MinE surface concentration (D) the loose fraction of MinD is lost and the MinE copolymer reaches the end of MinD and the polymer destabilizes. ATP hydrolysis happens cooperatively over all units of copolymer or finishes at this moment, if ongoing from an earlier stage. The depolymerization leads to gradual protein dissociation (E) until the left over dissociation center concentration becomes low enough to allow new initiation center formation (A). 2:2 stoichiometry of the surface bound MinD:MinE archived at the peak of MinE surface concentration is maintained until the beginning of the next binding cycle (A). The above described stages are marked in the graph of one MinD/E oscillation cycle.
We propose that in the presence of MinE, protein binding to the membrane surface is controlled by counteraction of “initiation” and “dissociation” centers on the surface. The initiation center on the surface promotes MinD binding in two ways; it nucleates polymerization of more MinD molecules, which are in a state ready to bind from the solution, and it also helps generation of additional initiation centers in close proximity. The dissociation center is a specific state of the MinD–MinE complex on the membrane left from the previous cycle that prevents binding of new complexes in the vicinity without allowing polymerization of additional MinD molecules onto itself. The principal difference between the two types of complexes could be in the bound nucleotide state, although their protein composition might also be different.
We expect that preexisting initiation precursor must be a minor fraction of the total protein in solution before the first contact with lipid membrane. Most of the preexisting initiation precursor might deplete near the inlet (where the initial wave starts without a time delay, unlike in the middle of the flow cell, Fig. S3B) without significant depletion of the bulk protein in the presence of the sample flow. More initiation precursor must be generated during the first cycle of oscillation/wave near the inlet, the initiation precursor concentration downstream becomes no longer limiting, and MinD/E binding starts in the middle of the flow cell after a time lag described in Fig. 1 D and E and Fig. S3.
During the cycle MinD/E complexes would transform from the initiation to dissociation form. The start of the subsequent oscillation cycles requires a decrease of the surface density of the dissociation centers left from the previous cycle to a level that allows de novo formation of the initiation centers to start the expanding circular binding zones (Fig. 1A). This type of binding initiation has the appearance of an “autocatalytic” process; one initiation center generates more initiation centers in its neighborhood to form a circular binding zone. However, simple autocatalytic binding would predict quick surface saturation near the nucleation sites, which does not happen until approximately 20 s later, well after fusion of the binding zones. We propose that the mechanical stress in the membrane structure caused by binding of the initiation or dissociation centers affects the affinity of the membrane in the neighborhood of the complex to additional protein binding. Distortion of the membrane structure by MinD binding has been evidenced by the previous observation that unilamellar vesicles are converted to thin tubes by high-density binding of MinD (32), as well as recent fluorescence anisotropy and calorimetry studies of MinD interactions with lipid membranes (37, 38). The above “membrane stress” hypothesis also explains additional aspects of our results as discussed below. However, at present we restrain ourselves from speculating about the nature of the membrane structure changes that are involved.
The initiation center formation in the wave regime is auto-primed by the propagating wave front without requiring de novo start of the circular binding zone and thus should be able to overcome the somewhat higher density of the leftover dissociation centers. This leads to slightly lower surface density of the initiation centers and subsequent peak protein densities, slower propagation speed of the wave front in the wave regime compared to the oscillation mode that precedes the waves, and shorter periodicity of the net MinD/E binding-unbinding cycle.
The simultaneous MinE and MinD binding starts at the beginning of the oscillation or wave cycle (Fig. 1 D and E) indicating that the initiation centers either contain both proteins at a stoichiometry of perhaps 2:2 (based on the dimeric nature of the functional unit of the proteins) upon which MinD and MinE polymerize at different rates, or otherwise, the initial binding of the ATP bound MinD dimer alone immediately attracts MinE to form the initiation center (Fig. 5 A and B). The subsequent slower and near linear increase of MinE surface concentration could be most easily explained by MinE polymerization starting from a fixed number of the initiation centers (MinE FRAP was not detected during the steady increase period, which is consistent with the growing polymer model, Fig. S10). According to this scenario, the ratio of the MinE peak amplitude and the amplitude of the initial fast increase, roughly in the order of 10, would reflect the polymer length. This estimate of MinD/E polymer size matches reasonably well with the size of the small MinD polymers described in figure 2 a and b of ref 35. The majority of MinD on the surface is in fast exchange with solution (FRAP time approximately 10 s, SI Summary of the Fluorescence Recovery Time Data), but a smaller fraction did not recover after photobleaching, and we propose that this fraction is directly connected to the initiation centers as copolymers with MinE. The rapidly exchanging population could exist as separate molecular assemblies, or as MinD polymer tails on the MinD/E copolymers. We argue that the switch of the protein binding process from increasing number of the initiation centers to the polymerization-only mode takes place because increasing membrane stress slows down new initiation center additions, whereas polymerization is less sensitive to the membrane stress.
MinD starts net dissociation while MinE is still accumulating, possibly by a mechanism involving modulation of membrane physical properties by the increase of the surface MinE density and/or a bound nucleotide state dependent polymer length controlling mechanism. According to FRAP data (Fig. S10), as well as MinD/MinE stoichiometry (Fig. 1D), all MinD molecules that dissociate before the peak of MinE surface concentration likely are not directly associated with MinE, and perhaps they do not hydrolyze ATP before dissociation (6, 7).
The surface bound MinE concentration exhibits a sharp transition from the steady (or even boosted) increase to fast disassembly after the MinD level drops at least 40% from the maximum. At the time of the MinE peak the ratio of MinD to MinE surface concentrations reaches a minimum of approximately 1, which suggests that the fraction of MinD not directly in contact with MinE as copolymer was completely lost by that moment and MinE assembly reached the end of the underlying MinD polymer (Fig. 5D). The depolymerization of the MinD–MinE copolymer might be triggered at this moment. The ratio of MinD to MinE surface concentrations stays near this low basal level until the next cycle of protein binding. It is attractive to postulate that after the peak of MinE concentration all proteins remaining on the membrane are transformed into dissociation complexes (possibly ATP already hydrolyzed). As the dissociation complexes depolymerize and leave the surface, the membrane stress is reduced and the dissociation of the remaining dissociation centers slows down until their surface density becomes low enough to allow de novo generation of the initiation centers or advancement of the next wave front (Fig. 5E). At this point, there are many alternative or complementary scenarios that could explain this sudden and apparently locally coordinated transition at the peak concentration of MinE. For example, the loss of the end cap of the MinD-tail on the copolymers might trigger depolymerization through ATPase cycle control. These and other possible models almost certainly have built-in biochemical timing mechanisms to control the local time trajectory of the reaction. In either case, the gap between the classical nucleotide-hydrolysis-driven dynamics of protein filaments, such as the actin filaments and microtubules, and the more loosely organized system studied here might be surprisingly narrow.
In many experiments, we observed sequential conversion from one class of patterning behavior to another without external mani-pulations during a course of observation, in the process revealing the closely related nature among the different modes of seemingly distinct behaviors. The waves are unstable with respect to the formation of scattered amoebas, but it appears as if certain conditions need to develop for this to occur. A possible development that might lead to the amoeba transition is an increase of the residual dissociation centers to reach a certain level, functioning as a barrier for MinD binding. We assume that the biochemical processes involved in the maintenance of the D-core of amoebas are similar to those during the earlier phases of the oscillation and waves before the peak of MinE concentration. The E-rings of amoebas most likely consist of MinD/E copolymers present near the peak of MinE surface concentration in the oscillation and waves. We propose that the E-ring is a spatially confined version of the E-rich phase of the oscillation/wave that is segregated from the D-core that represents the earlier phase of the oscillation/wave. As the dissociation complexes in the E-ring disassemble, additional dissociation complexes are generated and replenished from the D-core for near steady state maintenance of the E-ring. Unlike MinE during the midphase in the oscillation and wave cycle, MinE in dynamic E-mesh and in amoebas is exchanging according to the FRAP experiments (SI Summary of the Fluorescence Recovery Time Data). Thus, we view the amoebas as a version of the waves that are spatially reorganized by membrane stress. It is possible that membrane deformation caused by the broad spatial distribution of the dissociation complexes in oscillation or waves is more energetically costly and the spatially more confined sparse amoeba pattern is the more stable mode. In addition, this type of “stress relief” mechanism could have a built in characteristic spatial scale, assuming the local stress dampens out with distance characterized by the mechanical properties of the membrane. This is an attractive way to explain the characteristic size of smaller amoebas and the equidistant septation of larger amoebas. Alternatively or in addition, diffusional properties of the protein complexes on the membrane could be the principal determinant of the characteristic spatial scale of the dynamic amoeba patterns.
The above scenario has been proposed as an example of perhaps many possibilities to explain the aspects of this reaction uncovered by our experiments. Further experimental elucidation of the apparently cooperative process at the onset of each cycle of the oscillation/wave, uncovering the nature of the initiation and dissociation centers, the nature of the event that triggers the onset of MinE dissociation, and the dissection of the organization and dynamics of the E-ring will be essential for better understanding of the behavior of this fascinating system.
Materials and Methods
Details of methods including preparation of proteins, buffers, and lipids, flow cell assembly, microscope, camera, and illumination setup, and protein surface concentration estimate are given in SI Materials and Methods. SI Text also includes description of FRAP experiments; FRAP experiments in the absence of ascorbic acid or oxygen scavenger (Fig. S11); Domain structures in the supported lipid bilayer (Movie S9); membrane-binding properties of MinD in the absence of MinE; supporting data on self-organization behavior; and movies.
Supplementary Material
Acknowledgments
We thank Min Li (National Institute of Diabetes and Digestive and Kidney Diseases) for help with protein expression, Dominic Esposito (Science Applications International Corporation-Frederick Inc.) for preparation of part of expression vectors, and Robert Craigie (National Institute of Diabetes and Digestive and Kidney Diseases) for help with editing of the manuscript. This research was supported by the Intramural Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
This Feature Article is part of a series identified by the Editorial Board as reporting findings of exceptional significance.
See Commentary article on page 8053.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911036107/DCSupplemental.
References
- 1.Michie KA, Lowe J. Dynamic filaments of the bacterial cytoskelton. Annu Rev Biochem. 2006;75:467–492. doi: 10.1146/annurev.biochem.75.103004.142452. [DOI] [PubMed] [Google Scholar]
- 2.de Boer PAJ, Crossley RE, Rothfield LI. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 3.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Z, Lutkenhaus J. Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol. 1999;34:82–90. doi: 10.1046/j.1365-2958.1999.01575.x. [DOI] [PubMed] [Google Scholar]
- 5.Raskin DM, de Boer PAJ. MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol. 1999;181:6419–6424. doi: 10.1128/jb.181.20.6419-6424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Z, Lutkenhaus J. Topological regulation of cell division in E. coli: Spatiotemporal oscillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol Cell. 2001;7:1337–1343. doi: 10.1016/s1097-2765(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 7.Lackner LL, Raskin DM, de Boer PAJ. ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J Bacteriol. 2003;185:735–749. doi: 10.1128/JB.185.3.735-749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutkenhaus J, Sundaramoorthy M. MinD and role of the deviant Walker A motif, dimerization and membrane binding in oscillation. Mol Microbiol. 2003;48:295–303. doi: 10.1046/j.1365-2958.2003.03427.x. [DOI] [PubMed] [Google Scholar]
- 9.Leonard TA, Butler PJ, Löwe J. Bacterial chromosome segregation: Structure and DNA binding of the Soj dimer- a conserved biological switch. EMBO J. 2005;24:270–282. doi: 10.1038/sj.emboj.7600530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskin DM, de Boer PAJ. Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu X, Shih Y-L, Zhang Y, Rothfield LI. The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc Natl Acad Sci USA. 2001;98:980–985. doi: 10.1073/pnas.031549298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raskin DM, de Boer PAJ. The MinE Ring: An FtsZ-independent cell structure required for selection of the correct division site in E. coli. Cell. 1997;91:685–694. doi: 10.1016/s0092-8674(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 13.Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- 14.Loose M, Fischer-Friedrich E, Ries J, Kruse K, Schwille P. Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science. 2008;320:789–792. doi: 10.1126/science.1154413. [DOI] [PubMed] [Google Scholar]
- 15.Koonin EV. A superfamily of ATPases with diverse functions containing either classical or deviant ATP. J Mol Biol. 1993;229:1165–1174. doi: 10.1006/jmbi.1993.1115. [DOI] [PubMed] [Google Scholar]
- 16.Ebersbach G, Gerdes K. Bacterial mitosis: Partitioning protein ParA oscillates in spiral-shaped structures and positions plasmids at mid-cell. Mol Microbiol. 2004;52:385–398. doi: 10.1111/j.1365-2958.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 17.Hatano T, Yamaichi Y, Niki H. Oscillating focus of SopA associated with filamentous structure guides partitioning of F plasmid. Mol Microbiol. 2007;64:1198–1213. doi: 10.1111/j.1365-2958.2007.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicker MG. Reaction-diffusion waves of actin filament polymerization/depolymerization in Dictyostelium pseudopodium extension and cell locomotion. Biophys Chem. 2000;84:87–98. doi: 10.1016/s0301-4622(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 19.Obermann H, Mandelkow EM, Lange G, Mandelkow E. Microtubule oscillations. Role of nucleation and microtubule number concentration. J Biol Chem. 1990;265:4382–4388. [PubMed] [Google Scholar]
- 20.Cross MC, Hohenberg PC. Pattern formation outside of equilibrium. Rev Mod Phys. 1993;65:851–1112. [Google Scholar]
- 21.Turing AM. The chemical basis of morphogenesis. Philos Trans R Soc Lond, B. 1952;237:37–72. [Google Scholar]
- 22.Meinhardt H, de Boer PAJ. Pattern formation in Escherichia coli: A model for the pole-to-pole oscillations of Min proteins and the localization of the division site. Proc Natl Acad Sci USA. 2001;98:14202–14207. doi: 10.1073/pnas.251216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang KC, Meir Y, Wingreen NS. Dynamic structures in Escherichia coli: Spontaneous formation of MinE rings and MinD polar zones. Proc Natl Acad Sci USA. 2003;100:12724–12728. doi: 10.1073/pnas.2135445100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meacci G, Kruse K. Min-oscillations in Escherichia coli induced by interactions of membrane-bound proteins. Phys Biol. 2005;2:89–97. doi: 10.1088/1478-3975/2/2/002. [DOI] [PubMed] [Google Scholar]
- 25.Fange D, Elf J. Noise-induced Min phenotypes in E. coli. PLOS Comput Biol. 2006;2:e80. doi: 10.1371/journal.pcbi.0020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drew DA, Osborn MJ, Rothfield LI. A polymerization-depolymerization model that accurately generates the self-sustained oscillatory system involved in bacterial division site placement. Proc Natl Acad Sci USA. 2005;102:6114–6118. doi: 10.1073/pnas.0502037102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cytrynbaum EN, Marshall BD. A multistranded polymer model explains MinDE dynamics in E. coli cell division. Biophys J. 2007;93:1134–1150. doi: 10.1529/biophysj.106.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mileykovskaya E, Dowhan W. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-n-nonyl acridine orange. J Bacteriol. 2000;182:1172–1175. doi: 10.1128/jb.182.4.1172-1175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mileykovskaya E, et al. Effects of phospholipid composition on MinD-membrane interactions in vitro and in vivo. J Biol Chem. 2003;278:22193–22198. doi: 10.1074/jbc.M302603200. [DOI] [PubMed] [Google Scholar]
- 30.Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbamem.2009.04.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barák I, Muchová K, Wilkinson AJ, O’Toole PJ, Pavlendová N. Lipid spirals in Bacillus subtilis and their role in cell division. Mol Microbiol. 2008;68:1315–1327. doi: 10.1111/j.1365-2958.2008.06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Z, Gogol EP, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc Natl Acad Sci USA. 2002;99:6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey SL, et al. Condensing and fluidizing effects of ganglioside GM1 on phospholipid films. Biophys J. 2008;94:3047–3064. doi: 10.1529/biophysj.107.119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hale CA, Meinhardt H, de Boer PAJ. Dynamic localization cycle of the cell division regulator MinE in Escherichia coli. EMBO J. 2001;20:1563–1572. doi: 10.1093/emboj/20.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suefuji K, Valluzzi R, RayChaudhuri D. Dynamic assembly of MinD into filament bundles modulated by ATP, phospholipids, and MinE. Proc Natl Acad Sci USA. 2002;99:16776–16781. doi: 10.1073/pnas.262671699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meacci G, et al. Mobility of Min-proteins in Escherichia coli measured by fluorescence correlation spectroscopy. Phys Biol. 2006;3:255–263. doi: 10.1088/1478-3975/3/4/003. [DOI] [PubMed] [Google Scholar]
- 37.Mazor S, et al. Mutual effects of MinD-membrane interaction: I. Changes in the membrane properties induced by MinD binding. Biochim Biophys Acta. 2008;1778:2496–2504. doi: 10.1016/j.bbamem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazor S, et al. Mutual effects of MinD-membrane interaction: II. Domain structure of the membrane enhances MinD binding. Biochim Biophys Acta. 2008;1778:2505–2511. doi: 10.1016/j.bbamem.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.