Abstract
Beta diversity is an important component of large-scale patterns of biodiversity, but its explicit examination is more difficult than that of alpha diversity. Only recently have data sets large enough been presented to begin assessing global patterns of species turnover, especially in the fossil record. We present here an analysis of beta diversity of a Maastrichtian (71–65 million years old) assemblage of dinosaurs from the Western Interior of North America, a region that covers ≈1.5 × 106 km2, borders an epicontinental sea, and spans ≈20° of latitude. Previous qualitative analyses have suggested regional groupings of these dinosaurs and generally concluded that there were multiple distinct faunal regions. However, these studies did not directly account for sampling bias, which may artificially decrease similarity and increase turnover between regions. Our analysis used abundance-based data to account for sampling intensity and was unable to support any hypothesis of multiple distinct faunas; earlier hypothesized faunal delineations were likely a sampling artifact. Our results indicate a low beta diversity and support a single dinosaur community within the entire Western Interior region of latest Cretaceous North America. Homogeneous environments are a known driver of low modern beta diversities, and the warm equable climate of the late Cretaceous modulated by the epicontenental seaway is inferred to be an underlying influence on the low beta diversity of this ancient ecosystem.
Keywords: biogeography, macroecology, paleoecology, Cretaceous
Alpha diversity is defined by Whittaker (1, 2) as species richness on the local or habitat scale, and beta diversity is defined as the difference in the types of species found in different areas of alpha diversity. Alpha and beta diversity together make up species richness at the landscape scale, called gamma diversity. Because beta diversity measures turnover across an area, it is closely related to the numbers of endemic species within each community, which in turn can be used to assess biotic provinciality. Quantitative estimates of modern beta diversity recover surprisingly low beta diversity values despite broad taxonomic and geographic sampling, regardless of the motility of the group in question (3–5). Instead, beta diversity seems most correlated with climate evenness (4–6).
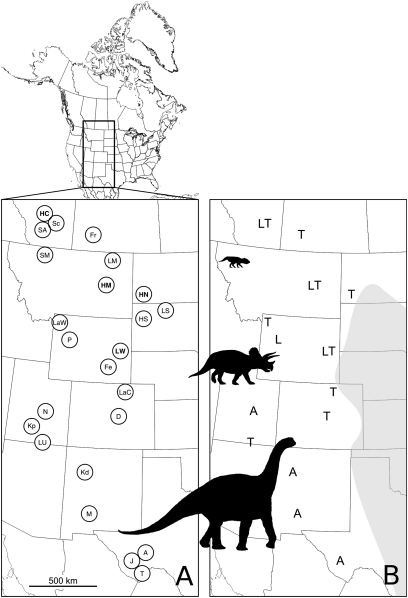
We present beta diversity estimates for an ancient terrestrial ecosystem. The Western Interior of North America is perhaps the most intensely sampled dinosaur-bearing region in the world (7). The terrestrially deposited rock sequence comprises sparsely exposed Aptian (121–112 Ma) formations to extensively exposed Maastrichtian (71–65 Ma) formations. These Maastrichian rocks are largely floodplain deposits along the western shores of the epicontinental Western Interior Seaway (Fig. 1). The rich Maastrichtian deposits have been a focus of research on patterns of dinosaur distributions and biogeography on a subcontinental scale (7–13). We used this dinosaur assemblage for our analysis of beta diversity in the fossil record because it is currently the only data set large enough and the only region with extensive previous work on endemism and provinciality. Previous studies have concentrated on dinosaur faunal provinciality at local scales on the basis of presence/absence data. Specifically, these analyses have suggested high levels of endemism at local, formational scales (13). The most widely accepted community hypothesis divides the dinosaur fauna into three zones: a northern Leptoceratops zone; a southern Alamosaurus zone; and an interior Triceratops zone (7, 8). The boundaries of these three regions have been slightly modified as more fossils have been found, and later studies incorporated these new dinosaur as well as previous pterosaur (Quetzalcoatlus) finds (8).
Fig. 1.
Approximate locations of Maastrichtian-aged dinosaur-bearing formations from the Western Interior of North America used in this analysis. (A) Abbreviations of formation names are as follows: A, Aguja; D, Denver; Fe, Ferris; Fr, Frenchman; HM, Hell Creek Montana; HN, Hell Creek North Dakota; HS, Hell Creek South Dakota; HC, Horsehshoe Canyon; J, Javelina; Kp, Kaiparowits; Kd, Kirtland; LM, Lance Montana; LS, Lance South Dakota; LU, Lance Utah; LW, Lance Wyoming; LaC, Laramie Colorado; Law, Laramie Wyoming; M, McRae; N, North Horn; P, Pinyon Conglomerate; Sc, Scollard; SA, St. Mary's River Alberta; SM, St. Mary's River Montana; T, Tornillo Texas. (B) Locations of occurrences of the three indicator taxa, Alamosaurus (A), Leptoceratops (L), and Triceratops (T). Greyed area to the east is the approximate location of the inland seaway.
The hypothesized provincial delineations lead to the prediction that beta diversity for this region as a whole should be relatively high. However, previous work was based on the occurrence of a few well-known individual genera that have well-defined geographic boundaries within relatively small spatial scales. Such high levels of endemism would be unprecedented for any modern large-bodied terrestrial fauna.
We use modern approaches to search for statistical support for areas of high endemism. These approaches included rarefaction and species estimators to calculate beta diversity and minimum spanning trees (MST) and nonmetric multidimensional scaling (NMDS) to search for readily apparent provinces (see Materials and Methods for full explanation). If there are strongly delineated provinces present, we predict that beta diversity for the Maastrichtian Western Interior dinosaur fauna is high and that the NMDS/MST ordination will be fragmented into discrete clusters of sites.
Results
Using observed values of generic richness, with alpha diversity calculated from the average richness of all formations (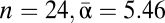 ), beta diversity is relatively high at βH1 = 8.24 (Table 1). This corroborates the high endemism found by previous workers but takes no account of sampling effects. There is an obvious and unsurprising correlation between generic richness and sample size for this dataset (R2 = 0.79, P << 0.001; Fig. S1), illustrating the bias of differential sampling intensity. Only 11 of the 24 formations have more than 10 specimens recorded, and thus low alpha diversities are due to insufficient sample sizes rather than actual conditions. This range of sampling intensity causes beta diversity estimates to be higher than those obtained from evenly, well-sampled data (5).
), beta diversity is relatively high at βH1 = 8.24 (Table 1). This corroborates the high endemism found by previous workers but takes no account of sampling effects. There is an obvious and unsurprising correlation between generic richness and sample size for this dataset (R2 = 0.79, P << 0.001; Fig. S1), illustrating the bias of differential sampling intensity. Only 11 of the 24 formations have more than 10 specimens recorded, and thus low alpha diversities are due to insufficient sample sizes rather than actual conditions. This range of sampling intensity causes beta diversity estimates to be higher than those obtained from evenly, well-sampled data (5).
Table 1.
Results for rarefaction and alternate methods of species estimation for the highlighted formations and full region
| Locality | No. of specimens | Generic richness (observed) | Rarefaction | Chao 1 | ACE | Jackknife 1 | Average estimated richness* |
| Hell Creek Montana | 211 | 17 | 14.38 | 19.58 | 15.66 | 18.2 | 17.81 |
| Hell Creek North Dakota | 268 | 9 | 6.68 | 8.5 | 10.15 | 8.58 | 9.08 |
| Horseshoe Canyon Alberta | 120 | 14 | 12.85 | 28.99 | 14.94 | 18.73 | 20.88 |
| Lance Wyoming | 111 | 14 | 13.88 | 14.58 | 14.35 | 15.39 | 14.77 |
| Average α† | 177.5 | 13.5 | 11.95 | 17.91 | 13.77 | 15.22 | 15.64 |
| Western Interior‡ | 997 | 45 | 21.95 | 40.55 | 28.02 | 31.72 | 33.43 |
| βH1 | — | 3.33 | 1.84 | 2.26 | 2.03 | 2.08 | 2.14 |
*Average estimated richness is the mean value of Chao 1, ACE, and Jacknife 1.
†Values for the average α were calculated using only the four listed formations.
‡Values for the entire Western Interior region were calculated using all 24 localities from the dataset.
To reduce this bias when calculating the average alpha diversity, we eliminated all formations with fewer than 100 specimens. This eliminated all but four formations, namely Hell Creek (Montana), Hell Creek (North Dakota), Horseshoe Canyon (Alberta), and Lance (Wyoming). Formations with more than 100 specimens are expected to give the most robust values of actual alpha diversity and are therefore more informative than keeping formations with fewer specimens. We believed that using only formations with more than 100 specimens was an appropriate compromise, maintaining high numbers of specimens while retaining enough localities for reasonable estimates. Average alpha diversity uncorrected for sampling bias (Sobs) for these four localities was 13.5. However, for the estimates of gamma diversity, we retained all formations for the calculation. This method would likely overestimate gamma diversity, because there were many genera known for this region that have not been found within these four formations.
When beta diversity was recalculated with an average alpha diversity calculated from only the four formations mentioned above, beta diversity dropped greatly to βH1 = 3.33. However, the sampling intensities for each formation and the region in total vary greatly, from n = 111 (Lance Wyoming) to n = 268 (Hell Creek North Dakota). Rarefaction methods were used to compensate for these large differences (Fig. 2). After all samples were rarefied, generic richness for each formation dropped comparatively little (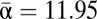 ), whereas gamma diversity decreased by nearly half. Consequently, beta diversity also showed a large decrease to βH1 = 1.84.
), whereas gamma diversity decreased by nearly half. Consequently, beta diversity also showed a large decrease to βH1 = 1.84.
Fig. 2.
Comparison of methods of sample size correction. (A) Smoothed curves for rarefaction using Coleman's random placement method, up to n = 100. (B) Smoothed curves for Chao 1 values up to n = 100. (C) ACE values. (D) Jacknife 1 values.
One criticism of rarefaction is that, although accounting for sample size, it does not reflect different rates of increase in species richness due to differences in underlying species evenness (14, 15). For example, an area with high species evenness will show a faster rate of increase than an area with a low evenness, because of very rare species taking much longer to be found. A way to compensate for this problem is by extrapolating to an estimate of total species richness, using species estimators.
Nonparametric estimators, although designed to be independent of sample size, are still affected by sampling to some extent. To correct for this, we ran estimators 1,000 times on a randomized subsample from each locality with more than 100 specimens. Although the estimated richness from the three methods differed considerably, the overall result was a higher estimated alpha diversity (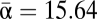 ) and a lower βH1 value. The Chao 1, abundance-based coverage estimator (ACE), and Jacknife 1 estimations yielded beta diversities of 2.26, 2.03, and 2.08, respectively.
) and a lower βH1 value. The Chao 1, abundance-based coverage estimator (ACE), and Jacknife 1 estimations yielded beta diversities of 2.26, 2.03, and 2.08, respectively.
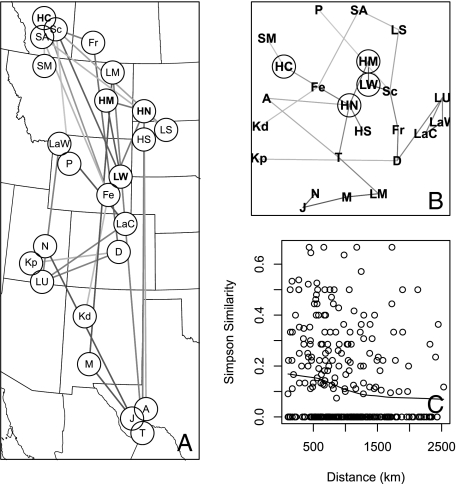
Finally, NMDS networks offer a test of similarity between all sites. If there were three distinct provinces as hypothesized, there would be at least three discrete clusters of sites within the network. If each individual site has large numbers of endemic taxa, the NMDS would show overdispersal, with all sites plotting in a ring to maximize site-to-site dissimilarities. The NMDS network plot is significantly different from either of these possibilities (Fig. 3B). Sites are scattered evenly throughout the plot space, and no clusters are present, with little patterning in regard to endemism. The same network plotted on geographic space (Fig. 3A) emphasizes the lack of regional clusters. In general, dinosaur faunal similarity between sites is poorly associated with geographic distance (Fig. 3C), because pairwise values of generic similarity show little decay over distance.
Fig. 3.
Relative pairwise similarities between localities. (A) Map of the localities used in the analysis with an MST added to indicate the relative similarities between localities. The more similar two localities are to one another on the basis of the species present, the darker the line connecting them will be. Note that no clusters are formed, and many localities are more similar to far-ranging ones than neighboring localities. (B) Plot of the relative positions of an NMDS of the localities, showing no apparent clusters. (C) Pairwise dissimilarity of sites in comparison with their geographic distance from one another. As dissimilarity increases, the sites are interpreted to be less similar to one another. The line represents a locally weighted sum of squares function. The sites show a slight decay over distance, although a linear regression is not significantly different from 0. These graphs indicate low beta diversities for this assemblage. Abbreviations as defined in Fig. 1.
Discussion
We find no evidence to support distinct faunal regions of dinosaurs during the Maastrichtian stage of the Western Interior of North America. Although our estimates of beta diversity are much lower than what might be expected from direct observation of the fossil record, the effect of uneven sampling can be very large. When sampling the richness of a region, one should not expect to find all of the species present, with rare species often not detected even in large samples (16).
Another possible explanation for low beta diversity might be the time scale used. Studies on modern community associations are limited to relatively brief periods of sampling time. Moreover, the Maastrichtian stage represents approximately 6 million years, and time averaging effects are undoubtedly confounding the data. Work on shorter time scales during the Pleistocene epoch has shown that mammal species move independently, reorganizing community compositions in time scales of only 350,000 years (17, 18). Even the large faunal exchange between North and South American mammals during the Great American Interchange occurred over ≈3 million years, with continental-scale migrations of mammals from shrews to mastodonts (19). Although the climate of the Late Cretaceous was more stable than that of the Holocene epoch, the suggestion that large, motile animals might maintain cohesive units seems unlikely.
Harrison et al. (3) obtained beta diversity levels in British birds between βH1 = 3.3 and 5.7, higher levels than we calculated here (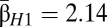 ) and over a much smaller latitudinal range. Our results indicate less turnover across a greater distance and add further evidence for a lack of dinosaurian endemism and provinciality during the Maastrichtian stage along the Western Interior Seaway. Novotny et al. (5) present a lower beta diversity for tropical insects than we found for dinosaurs, noting that their study sampled a relatively climatically homogeneous landscape. One possible implication is that the low levels of beta diversity within Maastrichtian dinosaurs are due to climactic factors. During the latest Cretaceous epoch the global climate was much warmer, with more equable temperatures and a greatly reduced latitudinal temperature gradient (20, 21). The average yearly temperature at the equator for the Maastrichtian stage has been suggested to be only slightly warmer than it is today, whereas polar regions were estimated to have been 15–25 °C higher than they are today, with mean temperatures for the coldest months likely above freezing (21).
) and over a much smaller latitudinal range. Our results indicate less turnover across a greater distance and add further evidence for a lack of dinosaurian endemism and provinciality during the Maastrichtian stage along the Western Interior Seaway. Novotny et al. (5) present a lower beta diversity for tropical insects than we found for dinosaurs, noting that their study sampled a relatively climatically homogeneous landscape. One possible implication is that the low levels of beta diversity within Maastrichtian dinosaurs are due to climactic factors. During the latest Cretaceous epoch the global climate was much warmer, with more equable temperatures and a greatly reduced latitudinal temperature gradient (20, 21). The average yearly temperature at the equator for the Maastrichtian stage has been suggested to be only slightly warmer than it is today, whereas polar regions were estimated to have been 15–25 °C higher than they are today, with mean temperatures for the coldest months likely above freezing (21).
Although this analysis does not support distinct communities of dinosaurs, some dinosaurs probably were restricted in their ranges. For example, the sauropod Alamosaurus likely did not live in the northern-most regions of the Western Interior region. This taxon has not been recovered north of Utah despite its large size and conspicuousness. However, it seems counterintuitive that an animal as large as Alamosaurus would not have dispersed at low levels to regions other than the restricted area it is found over its several million year existence. Modern species ranges often have one or more high-density peaks, where the species is highly abundant, and tails, where the species can still be found but at very low densities (22). The fossil record of Alamosaurus likely shows these high-density regions, and because of random chance, individuals that may have lived in the low-density regions of their ranges are simply not preserved. Therefore, although we may be seeing signatures of generic and species ranges within the fossil record, we are by no means seeing the entire region in which they lived. Rather than faunal provinces per se, previous research has likely recovered these high-density areas for specific taxa.
Our results suggest that any one region of the Western North American Seaway would have had much higher dinosaur generic richness than that observed, with approximately 16 genera of dinosaur on average. At the continental scale, levels of beta diversity among dinosaur assemblages are comparable to modern terrestrial faunas with low endemicity. These results suggest that dinosaurs were not as restricted in their ranges as once thought and that the fauna as a whole was largely homogeneous.
Materials and Methods
This study has made use of recent developments in the extensive cataloguing of dinosaur remains at the online and open Paleobiology Database (PaleoDB.org). All location and abundance data were downloaded from the Paleobiology Database on January 14, 2009, using the taxon name ”Dinosauria” and a time span of “Campanian” to ”Maastrichtian,” with the following parameters: Continent = “North America”; Abundance Value = TRUE; State = TRUE; and Formations = TRUE. The majority of this data set originated from the work of Carrano (23) to collect and collate the record of Dinosauria throughout the Mesozoic era. The downloaded data were further filtered manually in OpenOffice.org Calc to exclude any taxon unidentifiable to genus. All avian taxa were excluded, and Mexican and Alaskan faunas were removed to keep our data more comparable to previous work. Generic-level identifications were used for the same reason. As well, most dinosaur genera of this age are monospecific, so this more coarse taxonomic rank still accurately reflects our understanding of dinosaur species-level diversity. Formations were divided up by state and province, approximating the divisions of Lehman (7). However, we found similar results when formations were not divided by state. In determining values of species richness for the entire data set, all formations for which at least one genus with available abundance data was present were used. Dinosaur fossil records for many individual formations were too fragmentary to be used in an analysis of this type. To have adequate sample sizes for the rarefaction and species estimation, we used only sets that had more than 100 specimens, limiting us to four assemblages. Addtionally, although there were two cases of bonebeds included, these were eliminated because of their confounding effects on the various statistical methods. Although records include both instances of specimens and individuals, representing isolated elements and relatively complete skeletons, respectively, we did not differentiate so as to retain as much data as possible. Absolute values for species diversity were lower when analyses were run with only specimen records, although beta diversity values were nearly unchanged (Table S1).
Diversity can be partitioned into different components at different levels of scale. Whittaker (1, 2) defined alpha diversity as species richness on the local or habitat scale, with beta diversity as the differences between areas of alpha diversity. Together, alpha and beta make up gamma diversity, or species richness at the landscape scale.
By rearranging this relationship, Whittaker (24) originally defined beta diversity as
Although the measure is useful, its interpretation at times can be confusing. Because of the way it is calculated, the minimum value (complete similarity) is 1, whereas the maximum value (complete dissimilarity) is equal to the number of regions of alpha diversity used. Instead, Harrison et al. (3) suggested the modification
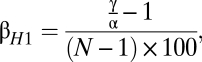 |
which then gives a more intuitive value between 0 and 100, with 0 being complete similarity and 100 complete dissimilarity between sites. Although many other methods of calculating beta diversity have been suggested, Whittaker's measure has remained one of the simplest and most commonly used (14).
Data analysis and all figures were done using the R Statistical package (25), with the packages ecodist (26), fossil (27), PBSmapping (28), proj4 (29), and shapefiles (30). For each formation, randomizations were run 1,000 times. Rather than true rarefaction, Coleman curves were calculated, using Coleman's “random placement” method, which provides results virtually indistinguishable from rarefaction but is computationally much simpler (14, 31–33). Values reported for species estimators are the statistical average, calculated from 1,000 randomizations. For the species estimators, values are from estimates calculated at n = 100. The estimates were calculated at this level to compensate for any bias from sampling intensity. For the full data tables and R code used for the analysis and figure generation, please consult Datasets S1 and S2 and SI Appendices S1 and S2.
Supplementary Material
Acknowledgments
We thank Meaghan A. Vavrek for assistance with manuscript preparation; and two anonymous reviewers for comments on the manuscript. Funding for this project was provided by a National Sciences and Engineering Research Council Canada Graduate Scholarship (PhD) (to M.J.V.), a Canada Research Chair (to H.C.E.L.), and by National Sciences and Engineering Research Council Discovery Grant 204548 (to H.C.E.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913645107/DCSupplemental.
References
- 1.Whittaker RH. Evolution and measurement of species diversity. Taxon. 1972;21:213–251. [Google Scholar]
- 2.Whittaker R. In: Evolutionary Biology. Hecht M, Steere W, Wallace B, editors. Vol. 10. New York: Plenum Press; 1977. pp. 250–268. [Google Scholar]
- 3.Harrison S, Ross SJ, Lawton JH. Beta diversity on geographic gradients in Britain. J Anim Ecol. 1992;61:151–158. [Google Scholar]
- 4.Condit R, et al. Beta-diversity in tropical forest trees. Science. 2002;295:666–669. doi: 10.1126/science.1066854. [DOI] [PubMed] [Google Scholar]
- 5.Novotny V, et al. Low beta diversity of herbivorous insects in tropical forests. Nature. 2007;448:692–695. doi: 10.1038/nature06021. [DOI] [PubMed] [Google Scholar]
- 6.Pitman NCA, Terborgh J, Silman MR, Nuñez VP. Tree species distributions in an Upper Amazonian forest. Ecology. 1999;80:2651–2661. [Google Scholar]
- 7.Lehman TM. Late Maastrichtian paleoenvironments and dinosaur biogeography in the western interior of North America. Palaeogeogr Palaeoclimatol Palaeoecol. 1987;60:189–217. [Google Scholar]
- 8.Lehman T. In: Mesozoic Vertebrate Life: New Research Inspired by the Paleontology of Philip J. Currie. Tanke D, Carpenter K, editors. Bloomington, IN: University of Indiana Press; 2001. pp. 310–328. [Google Scholar]
- 9.Sloan RE. Cretaceous and Paleocene terrestrial communities of western North America. In: Yochelson E.L., editor. Proceedings of the North American Paleontological Convention. Kansas: Allen Press; 1969. pp. 427–453. [Google Scholar]
- 10.Sloan RE. In: Athlon, Essays on Palaeontology in Honour of L. S. Russell (Life Sciences Miscellaneous Publication) Churcher CS, editor. Toronto: Royal Ontario Museum; 1976. pp. 134–154. [Google Scholar]
- 11.Russell DA. A census of dinosaur specimens collected in western Canada. Natl Museum Canada Natural History Papers. 1967;36:1–13. [Google Scholar]
- 12.Russell DA. A vanished world—the dinosaurs of western Canada. Natl Museum Canada Natural History Series. 1977;4:1–142. [Google Scholar]
- 13.Sampson SD, et al. Provincialism in Late Cretaceous terrestrial faunas: New evidence from the Campanian Kaiparowits Formation of Utah. J Vertebr Paleontol. 2004;24:108A. [Google Scholar]
- 14.Magurran AE. Measuring Biological Diversity. Oxford: Blackwell; 2004. [Google Scholar]
- 15.Fager EW. Diversity: A sampling study. Am Nat. 1972;106:293–310. [Google Scholar]
- 16.Siemann E, Tilman D, Haarstad J. Insect species diversity, abundance and body size relationships. Nature. 1996;380:704–706. [Google Scholar]
- 17.Potts R, Deino A. Mid-Pleistocene change in large mammal faunas of East Africa. Quat Res. 1995;43:106–113. [Google Scholar]
- 18.Jablonski D, Sepkoski JJ., Jr Paleobiology, community ecology, and scales of ecological pattern. Ecology. 1996;77:1367–1378. [PubMed] [Google Scholar]
- 19.Marshall LG, Butler RF, Drake RE, Curtis GH, Tedford RH. Calibration of the Great American Interchange. Science. 1979;204:272–279. doi: 10.1126/science.204.4390.272. [DOI] [PubMed] [Google Scholar]
- 20.Barron EJ. A warm, equable Cretaceous: The nature of the problem. Earth Sci Rev. 1983;19:305–338. [Google Scholar]
- 21.Amiot R, et al. Latitudinal temperature gradient during the Cretaceous Upper Campanian-Middle Maastrichtian: δ18O record of continental vertebrates. Earth Planet Sci Lett. 2004;226:255–272. [Google Scholar]
- 22.McGill B, Collins C. A unified theory for macroecology based on spatial patterns of abundance. Evol Ecol Res. 2003;5:469–492. [Google Scholar]
- 23.Carrano M. 2000. Taxonomy and classification of non-avian Dinosauria: Online Systematics Archive 4: The Paleobiology Database. Available at: http://paleodb.org. Accessed on January 14, 2009. [Google Scholar]
- 24.Whittaker RH. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr. 1960;30:279–338. [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 26.Goslee S, Urban D. ecodist: Dissimilarity-Based Functions for Ecological Analysis. 2007 (R package version 1.1.3). Available at: http://cran.r-project.org/web/packages/ecodist/index.html. [Google Scholar]
- 27.Vavrek M. fossil: Palaeoecological and Palaeogeographical Analysis Tools. 2010 (R package version 0.3.0). Available at http://cran.r-project.org/web/packages/fossil/index.html. [Google Scholar]
- 28.Schnute JT, Boers N, Haigh R. PBS Mapping 2: User's Guide. Fisheries and Oceans Canada, Nanaimo, BC, Canada: Canadian Technical Report of Fisheries and Aquatic Sciences 2549; 2004. [Google Scholar]
- 29.Urbanek S. proj4: A Simple Interface to the PROJ.4 Cartographic Projections Library. 2008. (R package version 1.0-4). Available at http://cran.r-project.org/web/packages/proj4/index.html. [Google Scholar]
- 30.Stabler B. shapefiles: Read and Write ESRI Shapefiles. 2006. (R package version 0.6). Available at http://cran.r-project.org/web/packages/shapefiles/index.html. [Google Scholar]
- 31.Coleman BD. On random placement and species-area relations. Math Biosci. 1981;54:191–215. [Google Scholar]
- 32.Coleman BD, Mares MA, Willig MR, Hsieh YH. Randomness, area, and species richness. Ecology. 1982;63:1121–1133. [Google Scholar]
- 33.Colwell RK, Coddington JA. Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc Lond B Biol Sci. 1994;345:101–118. doi: 10.1098/rstb.1994.0091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.