Abstract
The use of stents for vascular disease has resulted in a paradigm shift with significant improvement in therapeutic outcomes. Polymer-coated drug-eluting stents (DES) have also significantly reduced the incidence of reobstruction post stenting, a disorder termed in-stent restenosis. However, the current DESs lack the capacity for adjustment of the drug dose and release kinetics to the disease status of the treated vessel. We hypothesized that these limitations can be addressed by a strategy combining magnetic targeting via a uniform field-induced magnetization effect and a biocompatible magnetic nanoparticle (MNP) formulation designed for efficient entrapment and delivery of paclitaxel (PTX). Magnetic treatment of cultured arterial smooth muscle cells with PTX-loaded MNPs caused significant cell growth inhibition, which was not observed under nonmagnetic conditions. In agreement with the results of mathematical modeling, significantly higher localization rates of locally delivered MNPs to stented arteries were achieved with uniform-field–controlled targeting compared to nonmagnetic controls in the rat carotid stenting model. The arterial tissue levels of stent-targeted MNPs remained 4- to 10-fold higher in magnetically treated animals vs. control over 5 days post delivery. The enhanced retention of MNPs at target sites due to the uniform field-induced magnetization effect resulted in a significant inhibition of in-stent restenosis with a relatively low dose of MNP-encapsulated PTX (7.5 μg PTX/stent). Thus, this study demonstrates the feasibility of site-specific drug delivery to implanted magnetizable stents by uniform field-controlled targeting of MNPs with efficacy for in-stent restenosis.
Keywords: angioplasty, biodegradable nanoparticles, magnetic targeting, restenosis, rat model
Stent angioplasty has resulted in improved therapeutic outcomes for occlusive vascular disease. However, the high rates of reobstruction related to stent-induced injury, a disease process termed in-stent restenosis, has prompted the development and introduction of drug-eluting stents (DES) that provide local delivery of potent antiproliferative agents, such as paclitaxel or sirolimus (1). Currently used DESs cause serious complications in a significant number of patients, such as late stent thrombosis that can result in myocardial infarction and sudden death (2, 3). DES are not uniformly effective in all clinical settings (4–6) and are also limited from a pharmacologic perspective because each device contains a fixed dose of a single therapeutic agent and there are no means at present to replenish their drug payload, change agents, or modify dosing.
Thus, the present investigations sought to address these challenges by exploring local drug delivery mediated by stent-targeted magnetic nanoparticles (MNPs). Our group recently reported targeting of endothelial cells loaded with biodegradable MNPs to stented arteries using the magnetizing effect of homogeneous magnetic fields on the susceptible materials, 304-grade stainless steel, and MNP-incorporated magnetite (7). This effect is mediated by high magnetic gradients induced by the uniform field throughout the structural matrix of a stent and acting on MNPs in the vicinity of the implantation site. In the present studies, we investigated the hypothesis (Fig. 1) that steel stents deployed in an artery can be targeted with MNPs formulated with an antirestenotic agent, paclitaxel (PTX), with subsequent inhibition of in-stent restenosis achieved at drug doses significantly below those provided by DESs.
Fig. 1.
Targeted local delivery of MNPs to a deployed 304-grade stainless steel stent mediated by the uniform-field–induced magnetization effect. The uniform field generated by paired electromagnets (A) both induces high gradients on the stent and magnetizes drug-loaded MNPs, thus creating a magnetic force driving MNPs to the stent struts and adjacent arterial tissue (B).
The goals of the present studies were the following: (1) to formulate and characterize PTX-loaded MNPs, (2) to investigate the antiproliferative potency of drug-impregnated MNPs in cultured arterial smooth muscle cells under magnetic vs. nonmagnetic conditions, (3) to compare the arterial uptake and biodistribution of MNPs after local delivery in a rat carotid stenting model with and without the presence of a uniform field created by regionally positioned electromagnets; and (4) to assess in rat carotid stent angioplasty studies the comparative antirestenotic efficacy of PTX-loaded MNPs locally delivered with or without a magnetic exposure.
Results
Polylactide-based, PTX-loaded MNPs formulated using a modified emulsification-solvent evaporation approach had a narrow size distribution with an average hydrodynamic diameter of 263 ± 7 nm (Fig. 2 A and B) and zeta potential of −12 ± 2 mV consistent with the negative charge of serum albumin (8) used as a colloidal stabilizer. The particles were near-spherical in shape with nanocrystalline magnetite uniformly distributed in the polymeric matrix (Fig. 2A). The high content of magnetite in the particle composition (weight fraction of 36%) provided MNPs with strong magnetic responsiveness (saturation magnetization of 14.3 emu/g) in the absence of significant remanence (Fig. 2C). The drug entrapment efficiency expressed as the ratio of the PTX amount determined in MNPs to that initially used for their preparation was 30%, corresponding to a drug loading of 2.5% wt/wt.
Fig. 2.
Physical characterization of MNPs. Transmission electron micrograph was obtained using a Tecnai G2 electron microscope (FEI) (A). Note the composite structure of MNPs and their narrow size distribution (B). MNPs exhibit a magnetic moment of 14.3 emu/g at saturation and a near-superparamagnetic behavior (C). PTX release kinetics was determined under sink conditions using a modified external sink method (D). Released drug was assayed spectrophotometrically (λ = 230 nm) in periodically replaced acceptor medium (1:1 mixture of n-heptane and 1-octanol) immiscible with an MNP aqueous suspension. Note the initial burst release followed by a sustained phase with slower kinetics.
PTX release from MNPs assayed using a modification of the external sink method (9) showed a biphasic pattern where rapid initial kinetics (60% of the drug released after 8 h under sink conditions) were followed by a sustained phase with ∼12% of the drug remaining associated with the carrier particles after 48 h (Fig. 2D). MNPs maintained their original size over the course of the release experiment, suggesting that their colloidal stability was not affected by the employed experimental conditions.
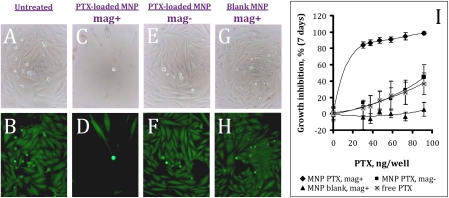
PTX-loaded MNPs efficiently inhibited growth of cultured aortic smooth muscle cells in vitro after a short incubation in the presence of a high-gradient magnetic field (average field gradient of 32.5 T/m; Fig. 3). The growth inhibitory effect exhibited steep drug dose dependence in the PTX dose range studied. The cell growth was inhibited by 84 ± 4% and 98 ± 2% at the MNP doses corresponding to 30 and 90 ng PTX/well, respectively (Fig. 3I). Contrastingly, free PTX or PTX-loaded MNPs applied under nonmagnetic conditions inhibited cell proliferation at the latter drug dose by 37 ± 13% and 45 ± 6%, respectively. The growth of cells treated with control MNPs at the equivalent amount was not inhibited (Fig. 3I). The mechanism underlying the enhancing effect of magnetic exposure on the cell growth inhibition mediated by PTX-loaded MNPs was addressed in a series of additional experiments (Section B in SI Appendix). It is noteworthy that an exposure of MNPs to the high-gradient field for 5 min before the release experiment did not cause changes in the kinetics of PTX release in comparison with untreated MNPs (Fig. S1 in SI Appendix, Section B), thus ruling out the possibility that the magnetic conditions could alter the release profile of the drug. MNP targeting via the uniform field-induced magnetization effect was studied in the rat carotid stenting model. Local delivery of MNPs to temporarily isolated rat carotid arteries in the presence of the magnetic field resulted in substantially higher amounts of MNPs localized to the stent and arterial tissue 2 and 24 h post procedure in comparison with the nonmagnetic controls (Fig. 4 A and E vs. B and F, and Fig. 4 C and G vs. D and H, respectively). Quantitative analysis demonstrated a fourfold higher MNP amount in the stented arterial segments at the earliest examined time point (5 min) following the magnetic treatment in comparison with nonmagnetic conditions (6990 ± 1220 vs. 1720 ± 1160 ng/mg arterial tissue, magnetic vs. nonmagnetic, respectively; Fig. 5A), and the ratio between the respective animal groups remained in the 5.5–9.5 range at the later time points (2 h–5 days post surgery). Rapid initial MNP clearance from the arterial tissue (78 ± 9% and 90 ± 4% of the particles eliminated after 24 h in the magnetic and nonmagnetic treatment group, respectively) was followed by an elimination phase with a notably slower rate (8 ± 4% and 6 ± 1% of MNPs was retained in the respective groups 5 days post treatment; Fig. 5A). Notably, the average amount of MNPs in the contralateral (untreated) carotid arteries in the two groups remained uniformly below the limit of quantitation at all time points. MNPs escaping their target site were taken up by the organs of the reticuloendothelial system with the highest local concentration detected in the spleen in both animal groups (Fig. 5 B and C). Importantly, the maximal off-target tissue weight-normalized MNP levels determined in the magnetic delivery group did not exceed 2% of those in the targeted arterial segment (Fig. 5 B and A).
Fig. 3.
Antiproliferative effect of PTX-loaded MNPs on A10 cells mediated by a high-gradient magnetic field (5-min exposure). Seven days post treatment A10 cells treated with MNPs at 30 ng PTX/well were observed microscopically in the bright field (A, C, E, and G) and fluorescent (B, D, F, and H) modes, and the cell viability was determined quantitatively as a function of the MNP dose (I) following staining with Calcein AM (λex/λem = 485/535 nm). Untreated cells were used as a reference. Note the profound growth inhibition of smooth muscle cells treated with PTX-loaded MNPs under magnetic conditions (C and D), as opposed to the nonmagnetic treatment (E and F). Also note that blank MNPs have no cell growth inhibitory effect in the studied dose range after the magnetic exposure (G and H). Original magnification ×100.
Fig. 4.
In vivo targeting of MNPs to stented arteries via the uniform field-induced magnetization effect. MNPs covalently labeled with BODIPY564/570 were delivered over 30 s under magnetic vs. nonmagnetic conditions (Upper and Lower rows, respectively) after deployment of a 304-grade stainless steel stent. The field was maintained for an additional 5 min. Stented carotid segments were excised, and the stent (A–D) and the luminal surface of the arteries (E–H) were examined by fluorescent microscopy 2 h (A, B, E, and F) and 24 h (C, D, G, and H) post treatment. Note the significantly enhanced retention of magnetically targeted MNPs at both time points. Original magnification ×200.
Fig. 5.
The effect of uniform field-controlled targeting on the biodistribution of MNPs. MNPs labeled with BODIPY564/570 were delivered under magnetic vs. nonmagnetic conditions after placement of a 304-grade stainless steel stent, and the amount of MNPs was determined fluorimetrically in the treated arteries (A) and peripheral organs (B anf C) 5 min, 2 h, 1 d, and 5 d post surgery (n = 5). Note the significant difference in the arterial MNP levels between the animals treated under magnetic vs. nonmagnetic conditions maintained over 5 days. Data are presented as mean ± SE.
The antirestenotic effect of PTX-impregnated MNP magnetically targeted to the stented artery was determined in comparison with stenting without MNP delivery, PTX-loaded MNPs administered under nonmagnetic conditions, and stenting followed by the i.v. injection of PTX-loaded PEGylated “stealth” nanoparticles (NPs) that exhibit prolonged circulation (Fig. S2 in SI Appendix, Section B) due to delayed elimination by the reticuloendothelial system. On the basis of the lack of cell growth effect observed with blank MNPs in cell culture studies, a blank MNP treatment group was not included in the efficacy study design. Drug-loaded MNPs locally delivered to each stented artery using the uniform field-induced magnetization effect at a calculated dose of 7.5 μg PTX significantly inhibited in-stent restenosis with a marked reduction in the neointima/media ratio to 63 ± 13% of the control levels (P < 0.05, Fig. 6 A vs. B). Furthermore, the respective nonmagnetic treatment group exhibited a statistically insignificant reduction in the neointima/media ratio (Fig. 6C). The antirestenotic efficiency of magnetically targeted MNPs was directly dose-dependent with a lower MNP dose (0.75 μg PTX/stent) inhibiting neointima formation only to a minor extent in contrast to the higher MNP dose (Fig. 6C). Tail vein administration of PEGylated PTX Ps as positive controls both at a dose equivalent to the magnetically targeted PTX and at a 20-fold higher dose did not result in significant inhibition of in-stent restenosis (Fig. 6C).
Fig. 6.
The antirestenotic effect of PTX-loaded MNPs targeted to stented carotid arteries by uniform field-induced magnetization. Animals treated with MNPs at PTX doses of 7.5 and 0.75 μg under magnetic vs. nonmagnetic conditions were killed, and the stented carotid segments were harvested 14 days post surgery. The control group was stented animals untreated with MNPs. A representative Verhoeff–van Gieson-stained section of an artery treated with 7.5 μg PTX under magnetic conditions (A) is shown in comparison with a “no treatment” control (B) (P < 0.05, Dunn’s Test Q statistic = 3.7). Original magnification 100×. Morphometric results expressed as neointima/media ratios (C) are shown as a function of the magnetic field exposure and PTX dose (n ≥ 6). Data are presented as mean ± SE.
Discussion
Magnetic Nanoparticle Formulation.
The present study demonstrates the feasibility of a unique site-specific delivery strategy based on the uniform-field–induced magnetization effect and its use for targeting drug-loaded MNPs to stented arteries. MNPs used in these proof-of-concept experiments were designed with the following combination of properties required for a safe and efficacious magnetic drug carrier: (1) strong magnetic responsiveness in the absence of magnetic memory due to the multiple small-sized iron oxide nanocrystals incorporated in each individual particle; (2) fully biocompatible components and a size suitable for intravascular delivery; (3) protection of the chemically labile drug (t1/2 ∼8 h in aqueous solution at pH 7) (10) from premature degradation; and (4) sustained drug release properties shown under perfect sink conditions using the external sink drug release method (9). Notably, sink conditions enabled by the latter release experiment design are essential for reliable measurements of the actual drug release (11). This is achieved by avoiding the considerable distortion of the apparent release kinetics reported for hydrophobic compounds, such as PTX (12), when a poor acceptor phase rapidly saturated with the drug is used as the release medium [nonsink release conditions (11, 13)].
The dual role played by the biodegradable hydrophobic polyester, polylactide, in this formulation is noteworthy. In addition to providing a sustained release depot for the encapsulated drug, PTX, the polymer maintains the composite particle structure with the multiple magnetite grains embedded in the solid polymeric matrix, thus enabling the high magnetic susceptibility of the formulation achieved without the loss of the superparamagnetic properties characteristic of ultrasmall-sized crystallites (14). Albumin, used as a colloidal stabilizer of the MNP suspension, has the advantages of being both biodegradable and nonimmunogenic (15) while efficiently preventing aggregation of PLA-based nanoparticles as previously shown with model nonmagnetic formulations (16, 17).
Magnetic Delivery of PTX-Loaded MNPs in Vitro and in Vivo.
Our in vitro studies demonstrated the essential dependence of the therapeutic effect mediated by the PTX-loaded MNPs on the presence of a high-gradient magnetic field. Notably, this profound and long-lasting cell growth inhibition was achievable with a relatively brief magnetic field exposure (5 min) and a low dose of nanoencapsulated PTX, as opposed to all control treatments. Both the duration of the magnetic exposure, practically feasible in experimental and clinical settings, and the low MNP dose enabling the potent antiproliferative action of PTX on the target cells are advantageous in the context of restenosis therapy. Both of these findings, highly relevant for a viable antirestenotic strategy, were further confirmed in vivo in the rat stenting angioplasty studies using the uniform field-induced magnetization approach.
In experiments addressing the in vivo feasibility of the uniform field-controlled magnetic targeting, the therapeutic effect was achieved with a drug dose considerably lower than its clinically used equivalent. A direct comparison of the present results with clinically used PTX eluting stents is not possible because these devices are not fabricated in a size range suitable for use in rat carotid stenting. However, in a speculative comparison, the following calculations are of interest: (1) a total drug dose of 85 μg/15 mm (length) stent was typically employed in PTX DES clinical studies (18), (2) MNP-encapsulated PTX at a dose of 7.5 μg delivered to a 5-mm-long stent was efficacious in the present experiments, (3) the higher relative dose efficiency of MNP-encapsulated PTX in our study may be hypothetically due to both the enhanced local delivery and the greater drug availability due to its more efficient release from the carrier. Thus, although MNPs released 82.3 ± 0.5% of the drug after 24 h under sink conditions, the release of PTX from the TAXUS stent plateaus at ∼10% of the drug dose with the remaining 90% permanently retained in the polymeric coating (19).
Magnetic Targeting to Stents.
The present results also provide several important insights into the mechanisms governing MNP targeting to steel stents in homogeneous magnetic fields. Quantitative assay data show that from the calculated 300 μg of MNPs administered to each stented carotid segment, 13.2 ± 2.0 μg were retained in the artery 5 min post delivery with magnetic field exposure vs. 3.4 ± 1.9 μg without a magnetic field. Thus, the arterial tissue levels of MNPs at the earliest examined time point are fourfold greater on average after magnetic treatment, which is in good agreement with the ratio provided by mathematical modeling of the uniform field-controlled targeting (SI Appendix, Section A). These results viewed in the perspective of the fluorescent MNP imaging data (Fig. 4) prompt the following observations: (1) The MNP distribution, as evident by fluorescence (Fig. 4), is relatively uniform on the struts of the stent implanted in the magnetically treated animals, with scant fluorescence noted in the stents not exposed to the field. (2) The intense MNP-associated fluorescence seen with magnetic targeting is likely due to the initial concentration of MNPs around the steel struts in the presence of a magnetic field. Although the arterial wall uptake of MNPs may passively occur without a field exposure, the magnetic conditions are required for the strong initial interaction of the MNP with the luminal surface of the stent followed by the relatively rapid redistribution of the stent-targeted MNPs into the arterial tissue and the bloodstream following termination of the field exposure. (3) The model of uniform field-controlled targeting presented in Section A of the SI Appendix suggests that the higher rates of MNP capture in the presence of a uniform field are achieved via extending the effective distance within which the magnetic force can act on the particles, thereby increasing the fraction of MNPs deposited on the stent surface. The magnetic responsiveness of an individual particle is proportionate to its magnetite content, which in its turn is directly dependent on the particle size. Thus the lower size limit of the MNPs designed for the uniform field-controlled, magnetically targeted delivery is defined by the efficiency of the MNP–stent interaction (20), whereas the upper size limit is stated by the clinical safety considerations (21). It is notable that the elimination pathway and kinetics of parenterally administered formulations are also size-dependent. Rigid particles exceeding the 200 nm threshold have previously been shown to be rapidly eliminated by the organs of the reticuloendothelial system with a significant fraction sequestered by the spleen (22, 23). This is in agreement with our observation that after magnetically targeted delivery, a fraction of MNPs loosely bound to the stented arterial segment redistributes over time to the organs of the reticuloendothelial system with higher weight-normalized MNP amounts observed in the spleen (Fig. 5; see also Table S1 in SI Appendix, Section B). However, it is notable that despite the redistribution of a sizable fraction of initially captured MNPs, magnetic delivery resulted in their protracted presence at the target site at significantly higher levels compared to nonmagnetic control conditions. These high local levels of magnetically guided MNPs extending over a period of several days translated into a significant therapeutic effect, as opposed to only marginal antirestenotic efficiency observed in the animals treated without a magnetic exposure. Importantly, the maximal possible systemic exposure in our local delivery experiments was 20 μg PTX/kg, which is three orders of magnitude lower than the maximum tolerated dose of PTX reported in the literature (24, 25).
It is noteworthy that administration of “stealth” PEGylated NP at PTX doses equivalent and 20-fold higher than the effective dosage of MNP-encapsulated PTX did not significantly inhibit in-stent restenosis in our study. Interestingly, PEGylated NP administered at a dose of 150 μg PTX (corresponding to 0.35 mg/kg) gave 4 and 10 times higher PTX plasma levels 20 min and 6 h after injection, respectively, than those observed in rat studies by others with 1.0 mg/kg PTX administered intraarterially (26). These findings are in agreement with the “stealth” properties of PEGylated NP (Fig. S2 in SI Appendix) associated with prolongation of elevated PTX levels. Thus, the essential dependence of the antirestenotic efficacy of PTX-loaded nanoparticle formulations on targeted delivery (Fig. 6) is emphasized by these comparative data supporting the therapeutic advantage of the magnetic targeting approach.
Conclusions.
This study demonstrated the feasibility of the uniform field-based targeting approach and showed its therapeutic potential in a rat carotid artery model of in-stent restenosis using a unique PTX-loaded MNP formulation. A significant antirestenotic effect was observed 14 days post magnetic treatment with a single low dose of PTX formulated in MNPs. By combining efficient drug carrier targeting to the stented blood vessel and the sustained drug release properties of the biodegradable polymer-based MNPs, this strategy could provide a safer and more efficacious alternative to current clinically used therapies.
Materials and Methods
Nanoparticle Preparation and Characterization.
Polylactide-based magnetic nanoparticles were formulated with inclusion of nanocrystalline magnetite by a modification of the emulsification-solvent evaporation method. Ferric chloride hexahydrate and ferrous chloride tetrahydrate (170 and 62.5 mg, respectively) were dissolved in ethanol (2.5 mL) and mixed with freshly prepared aqueous sodium hydroxide (0.5 N, 5 mL). The precipitate was heated for 1 min at 90 °C, cooled on ice, and separated on a magnet. The obtained magnetite was stirred with a solution of oleic acid in ethanol (150 mg in 2 mL) at 90 °C for 5 min. Free oleic acid was separated by deionized water. Magnetite was washed with ethanol, dispersed in 8 mL of chloroform, and used to dissolve (1) 100 mg of poly(D,L-lactide) (Mr 75,000–120,000; Sigma) and 10 mg paclitaxel (LC Laboratories) or (2) 90 mg of poly(D,L-lactide) and 10 mg of poly(D,L-lactide) covalently labeled with BODIPY564/570 (Invitrogen) to obtain (1) PTX-loaded MNPs or (2) fluorescent-labeled MNPs, respectively. The organic dispersion was emulsified by sonication on ice in an aqueous solution of BSA (1% wt/vol, 15 mL), and the organic solvent was evaporated under reduced pressure. MNPs were washed twice by magnetic decantation, resuspended in 6 mL of aqueous trehalose (10% wt/vol), filtered, and lyophilized. Lyophilized MNPs were kept at −80 °C and resuspended in deionized water before use.
The drug loading in MNPs was determined as follows. PTX was extracted in two steps in chloroform in the presence of sodium chloride (2.5 N). The organic solution was clarified by centrifugation after adding acetonitrile and evaporated, and the residue redissolved in acetonitrile. The amount of PTX was determined spectrophotometrically after background correction according to the formula OD282 − (OD272 + OD292)/2, where OD282, OD272, and OD292 are respective absorbances at 282, 272, and 292 nm.
Particle size measurements were performed by dynamic light scattering. Hysteresis loops of MNPs were obtained using an alternating gradient magnetometer (Princeton Measurements). An external sink method (9) was adapted for measuring the release kinetics of poorly water-soluble PTX [water solubility of 1 mg/L (27)] under sink conditions. Aliquots of MNPs (250 μl) were mixed with 250 μl of acceptor medium (1-octanol and n-heptane, 1:1 vol/vol) and placed in test tubes on a rotator at 25 °C. The organic layer was withdrawn and replaced with fresh acceptor phase at predetermined time points. PTX was assayed in the acceptor medium samples spectrophotometrically (230 nm). Cumulative release was expressed as the ratio of PTX released by a given time point to the total amount of PTX determined as above. The immiscibility of the MNP suspension with the acceptor phase and the stability of MNP size, as well as the solubility of PTX sufficient for providing sink conditions (4.3 mg/mL at 25 °C), were determined in prior experiments.
Nonmagnetic “stealth” nanoparticles loaded with PTX were formulated and characterized as described in the SI Appendix.
Cell Culture Studies.
Rat aortic smooth muscle cells (A10) were seeded at 5% confluence on 96-well plates. Varying amounts of PTX-loaded MNPs serially diluted in DMEM supplemented with 2% FBS and 20 ng/mL BB isoform of platelet derived growth factor (PDGF-BB) were added to cells for 5 min with/without a magnetic exposure (LifeSepTM 96F magnetic separator, Dexter Magnetic Technologies). The cells were then washed twice and incubated with fresh medium. Blank MNPs or free PTX applied at equivalent amounts were used as controls. PTX was first dissolved in dimethyl sulfoxide and then diluted with cell culture medium 1:2,000 to provide the final PTX concentration equal to that in PTX-loaded MNPs. Cell viability was determined after 7 days fluorimetrically using Calcein AM (Invitrogen) as described by the manufacturer.
In Vivo Targeting of Locally Delivered MNPs.
Under general anesthesia the left common carotids of male Sprague–Dawley rats (450–500 g) were injured by four passages of a Fogarty catheter before deployment of a 304-grade stainless steel stent (Circle Medical Devices). A catheter was introduced via the external carotid into the common carotid artery and positioned distal to the stent. A 15-mm segment of the common carotid artery encompassing the site of stent placement was isolated by ligatures. A uniform field (1,200 G) was generated using paired solenoid coils with iron cores connected to a DC power supply and positioned at a distance of 40 mm at both sides of the animal. Fifteen microliters of MNPs diluted accordingly to provide PTX doses of 7.5 or 0.75 μg were applied into the isolated arterial segment over 30 s, and excess MNPs were evacuated. The drug dose was calculated as the product of the volume delivered (15 μl), the concentration of MNPs in the two preparations (20 and 2 μg/μl, respectively), and the MNP drug loading (2.5% wt/wt). The ligatures were released, and the field was maintained for an additional 5 min. Animals in the nonmagnetic control group were treated as above without the magnetic field exposure. Two groups of animals serving as positive controls were administered PEGylated PTX NP (SI Appendix) by tail vein injection immediately after stent deployment at PTX doses of 7.5 or 150 μg PTX, respectively.
Rats were euthanized 14 days after treatment. The stented carotid arteries were processed as described elsewhere (28), and the morphometric measurements were carried out in a blinded fashion.
In biodistribution studies, fluorescent-labeled MNPs were delivered with or without the magnetic exposure as above. Blood and organ tissue samples, including liver, spleen, lung, and carotid arteries, were collected from animals killed 5 min, 2 h, 1 d, and 5 d post surgery. PLA-BODIPY564/570 conjugate was extracted from tissue homogenates in acetonitrile in the presence of sodium chloride (2.5 M), and the amount of MNPs was determined fluorimetrically (λex/λem of 540/575 nm).
Statistical Analysis.
Experimental data were presented as means ± SDs except where otherwise stated. The results were evaluated by Kruskal–Wallis one-way ANOVA with Dunn's post hoc analysis. Differences were termed significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Gary Yu (Advanced Polymer Materials) for providing materials used to prepare PEGylated NP. We also thank NuMED for providing rat PTCA catheters. This research was supported in part by the National Institutes of Health Grant T32-HL07915 (to M.B. and R.J.L.), an American Heart Association Scientist Development Grant (to I.F.), an American Heart Association Beginning Grant-in-Aid (to M.C.), and by the William J. Rashkind Endowment of The Children's Hospital of Philadelphia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909506107/DCSupplemental.
References
- 1.Chong PH, Cheng JW. Early experiences and clinical implications of drug-eluting stents: Part 1. Ann Pharmacother. 2004;38:661–669. doi: 10.1345/aph.1D256. [DOI] [PubMed] [Google Scholar]
- 2.Pfisterer M, et al. BASKET. Long-term benefit-risk balance of drug-eluting vs. bare-metal stents in daily practice: Does stent diameter matter? Three-year follow-up of BASKET. Eur Heart J. 2009;30:16–24. doi: 10.1093/eurheartj/ehn516. [DOI] [PubMed] [Google Scholar]
- 3.Lagerqvist B, et al. SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 4.Zähringer M, et al. Sirolimus-eluting versus bare-metal low-profile stent for renal artery treatment (GREAT Trial): Angiographic follow-up after 6 months and clinical outcome up to 2 years. J Endovasc Ther. 2007;14:460–468. doi: 10.1177/152660280701400405. [DOI] [PubMed] [Google Scholar]
- 5.Duda SH, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: The SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–338. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 6.Duda SH, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: Long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- 7.Polyak B, et al. High field magnetic gradients can target magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci USA. 2008;105:698–703. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 9.Chorny M, Fishbein I, Danenberg HD, Golomb G. Study of the drug release mechanism from tyrphostin AG-1295-loaded nanospheres by in situ and external sink methods. J Control Release. 2002;83:401–414. doi: 10.1016/s0168-3659(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 10.Dordunoo SK, Burt HM. Solubility and stability of taxol: Effects of buffers and cyclodextrins. Int J Pharm. 1996;133:191–201. [Google Scholar]
- 11.Washington C. Drug release from microdisperse systems: A critical review. Int J Pharm. 1990;58:1–12. [Google Scholar]
- 12.Závisová V, et al. Synthesis and characterization of polymeric nanospheres loaded with the anticancer drug paclitaxel and magnetic particles. J Magn Magn Mater. 2009;321:1613–1616. [Google Scholar]
- 13.Washington C. In: Microencapsulation. Methods and Industrial Applications. Benita S, editor. Vol. 73. New York: Marcel Dekker; 1996. pp. 155–181. [Google Scholar]
- 14.Lee J, Isobe T, Senna M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J Colloid Interface Sci. 1996;177:490–494. [Google Scholar]
- 15.Verrecchia T, et al. Non-stealth (poly(lactic acid/albumin)) and stealth (poly(lactic acid-polyethylene glycol)) nanoparticles as injectable drug carriers. J Control Release. 1995;36:49–61. [Google Scholar]
- 16.Verrecchia T, et al. Adsorption/desorption of human serum albumin at the surface of poly(lactic acid) nanoparticles prepared by a solvent evaporation process. J Biomed Mater Res. 1993;27:1019–1028. doi: 10.1002/jbm.820270807. [DOI] [PubMed] [Google Scholar]
- 17.Bazile DV, et al. Body distribution of fully biodegradable [14C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials. 1992;13:1093–1102. doi: 10.1016/0142-9612(92)90142-b. [DOI] [PubMed] [Google Scholar]
- 18.Colombo A, et al. TAXUS II Study Group. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–794. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 19.van Beusekom HM, Schoemaker R, Roks AJ, Zijlstra F, van der Giessen WJ. Coronary stent healing, endothelialisation and the role of co-medication. Neth Heart J. 2007;15:395–396. doi: 10.1007/BF03086022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yellen BB, et al. Targeted drug delivery to magnetic implants for therapeutic applications. J Magn Magn Mater. 2005;293:647–654. [Google Scholar]
- 21.Chorny M, et al. In: Tissue Engineering and Novel Delivery Systems. Yaszemsky MJ, et al., editors. New York: Marcel Dekker; 2004. pp. 393–422. [Google Scholar]
- 22.Moghimi SM, Porter CJ, Muir IS, Illum L, Davis SS. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: Influence of particle size and hydrophilic coating. Biochem Biophys Res Commun. 1991;177:861–866. doi: 10.1016/0006-291x(91)91869-e. [DOI] [PubMed] [Google Scholar]
- 23.Moghimi SM, Hedeman H, Muir IS, Illum L, Davis SS. An investigation of the filtration capacity and the fate of large filtered sterically-stabilized microspheres in rat spleen. Biochim Biophys Acta. 1993;1157:233–240. doi: 10.1016/0304-4165(93)90105-h. [DOI] [PubMed] [Google Scholar]
- 24.Vassileva V, Grant J, De Souza R, Allen C, Piquette-Miller M. Novel biocompatible intraperitoneal drug delivery system increases tolerability and therapeutic efficacy of paclitaxel in a human ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;60:907–914. doi: 10.1007/s00280-007-0449-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim SC, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J Control Release. 2001;72:191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 26.Yonemoto H, Ogino S, Nakashima MN, Wada M, Nakashima K. Determination of paclitaxel in human and rat blood samples after administration of low dose paclitaxel by HPLC-UV detection. Biomed Chromatogr. 2007;21:310–317. doi: 10.1002/bmc.759. [DOI] [PubMed] [Google Scholar]
- 27.Liggins RT, Hunter WL, Burt HM. Solid-state characterization of paclitaxel. J Pharm Sci. 1997;86:1458–1463. doi: 10.1021/js9605226. [DOI] [PubMed] [Google Scholar]
- 28.Fishbein I, et al. Bisphosphonate-mediated gene vector delivery from the metal surfaces of stents. Proc Natl Acad Sci USA. 2006;103:159–164. doi: 10.1073/pnas.0502945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.