Abstract
Serotonergic antidepressant drugs have been commonly used to treat mood and anxiety disorders, and increasing evidence suggests potential use of these drugs beyond current antidepressant therapeutics. Facilitation of adult neurogenesis in the hippocampal dentate gyrus has been suggested to be a candidate mechanism of action of antidepressant drugs, but this mechanism may be only one of the broad effects of antidepressants. Here we show a distinct unique action of the serotonergic antidepressant fluoxetine in transforming the phenotype of mature dentate granule cells. Chronic treatments of adult mice with fluoxetine strongly reduced expression of the mature granule cell marker calbindin. The fluoxetine treatment induced active somatic membrane properties resembling immature granule cells and markedly reduced synaptic facilitation that characterizes the mature dentate-to-CA3 signal transmission. These changes cannot be explained simply by an increase in newly generated immature neurons, but best characterized as “dematuration” of mature granule cells. This granule cell dematuration developed along with increases in the efficacy of serotonin in 5-HT4 receptor-dependent neuromodulation and was attenuated in mice lacking the 5-HT4 receptor. Our results suggest that serotonergic antidepressants can reverse the established state of neuronal maturation in the adult hippocampus, and up-regulation of 5-HT4 receptor-mediated signaling may play a critical role in this distinct action of antidepressants. Such reversal of neuronal maturation could affect proper functioning of the mature hippocampal circuit, but may also cause some beneficial effects by reinstating neuronal functions that are lost during development.
Keywords: dentate gyrus, development, mossy fiber, serotonin receptor, serotonin reuptake inhibitor
Serotonergic antidepressant drugs have been widely used to treat mood and anxiety disorders (1). Although some adverse effects have been reported (1, 2), there is accumulating evidence for potential use of these drugs beyond current therapeutic application (3, 4). However, cellular mechanisms underlying broad effects of antidepressants remain largely unknown. In experimental animals, chronic antidepressant treatments can facilitate neurogenesis in the dentate gyrus (DG) of the adult hippocampus (5), a process that has been implicated in some of the behavioral effects of antidepressants (6–8). The dentate gyrus is positioned at the entrance of the hippocampal excitatory trisynaptic circuit, and the mossy fiber (MF) from the DG plays a critical role in regulating associative synaptic plasticity in the hippocampal CA3 region (9, 10). Because physiological properties of granule cells (GCs), the principal neurons of the DG, greatly change with development or neuronal maturation (11–16), an increase in a proportion of relatively young GCs by facilitated neurogenesis may alter functional roles of the DG in the hippocampal circuit. However, the majority of the neuronal population in adult DG is composed of mature GCs. Considering the rate of neurogenesis in adult DG reported previously (17–19), the number of additional new neurons that can be produced by facilitated neurogenesis in typical animal studies would be only a few percent or less of the total GC number. It remains unclear whether such a small population of neurons can solely make a significant impact on brain functions.
Because behavioral effects of antidepressants are not always accompanied by increased neurogenesis (20–22), modifications of functions of existing neurons would also be important for antidepressant action. In mice, fluoxetine, a widely used selective serotonin reuptake inhibitor (SSRI), has been reported to have no significant effects on the neuronal precursor cell proliferation in adult C57BL/6J (23) and BALB/c mice (20, 21). We have recently examined effects of chronic fluoxetine treatments on serotonergic modulation at the synapse between MF and CA3 pyramidal cells in C57BL/6J mice and found that chronic fluoxetine can stabilize the potentiating effect of serotonin (5-hydroxytriptamine, 5-HT) at this synapse (24). In the present study, we further characterized cellular and physiological changes in DG and MF synapses caused by chronic fluoxetine. We found that fluoxetine can substantially modify GC functions by transforming the phenotype of normally matured GCs into immature-like one. Our results also suggest that the serotonin 5-HT4 receptor plays a crucial role in this distinct action of fluoxetine.
Results
Chronic Fluoxetine Down-Regulates Expression of Mature Granule Cell Markers.
Adult male C57BL/6J mice were treated with fluoxetine at a dose of 22 mg/kg/day for 4–5 weeks. As described above, the number of additional new neurons produced by the facilitated neurogenesis can be estimated to be a few percent or less of the total GC number. Thus, mature GCs would constitute the majority of DG neurons even after the fluoxetine treatment. To confirm this prediction, we examined expression of calbindin, a marker for mature GCs (17, 25, 26). In fluoxetine-treated mice, however, calbindin-like immunoreactivity in the GC layer was greatly reduced (Fig. 1A). In contrast, the number of cells positive for calretinin, a marker for immature GCs at the early postmitotic stage (26), appeared to be increased (Fig. 1A). The reduced calbindin-like immunoreactivity in DG was not due to a loss of GCs, because immunostaining for the neuronal marker NeuN and nuclear staining with DAPI were not appreciably affected (Fig. 1A). Immunoblot and PCR analyses confirmed the marked reduction of protein and mRNA levels of calbindin in DG (Fig. 1 B and C). We also examined the effect of fluoxetine on expression of potential GC-specific maturation markers, desmoplakin, tryptophan-2,3-dioxygenase, and interleukin-1 receptor type I, whose expression is restricted to dentate GCs (27) and increases with postnatal development similarly to calbindin (Fig. S1). Consistent with the reduced calbindin level, the expression of all of these genes was also strongly reduced in the fluoxetine-treated mice (Fig. 1C). These results suggest that most dentate GCs were not properly expressing genes of the mature state after the chronic fluoxetine treatment.
Fig. 1.
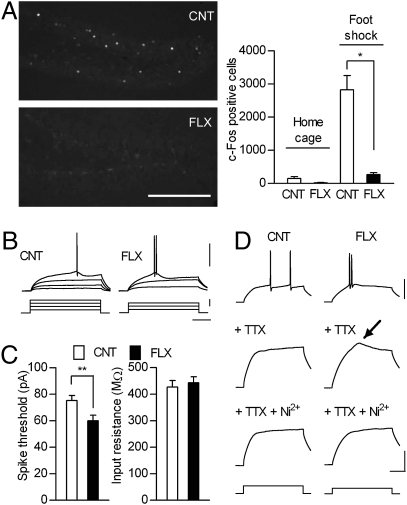
Chronic fluoxetine down-regulates markers for mature granule cells. (A) Reduction in calbindin-like immunoreactivity, increase in calretinin-positive cells, but no appreciable change in NeuN-like immunoreactivity or DAPI staining in DG of fluoxetine-treated mice (FLX) compared with control mice (CNT). (Scale bar, 100 μm.) (B) Reduced calbindin protein levels in fluoxetine-treated DG revealed by immunoblot analyses (n = 5 each, P = 0.0079). (C) Reduced expression levels of mRNAs for calbindin, desmoplakin (Dsp), tryptophan-2,3-dioxygenase (TDO), and type I interleukin 1 receptor (IL1R) in fluoxetine-treated DG (n = 6 each). **, P < 0.01; ***, P < 0.005. (D) Confocal images showing immunostaining for BrdU (green) and calbindin (red) in GC layer. Images were from 9-week-old mice before fluoxetine treatment (Left), 13-week-old control (Center), and 13-week-old fluoxetine-treated mice (Right). Arrow: BrdU-positive cells without calbindin-like immunoreactivity. Arrow head: cells with calbindin-like immunoreactivity. (Scale bar, 10 μm.) Data are presented as mean ± SEM.
One might imagine that mature GCs were largely replaced by immature neurons generated during the fluoxetine treatment. To address this issue, we labeled neurons generated in neonates with the S-phase marker BrdU and started the fluoxetine treatment after a delay of 9 weeks to allow BrdU-labeled neurons to mature. Double immunostaining analyses just before the fluoxetine treatment showed that BrdU-positive cells in the GC layer were embedded in strong calbindin-like immunoreactivity (Fig. 1D) and not colocalized with calretinin or doublecortin, a marker for progenitor cells and newborn neurons less than a few weeks old (25) (Fig. S2A). The fluoxetine treatment strongly reduced calbindin-like immunoreactivity (Fig. 1D), but had no significant effects on the number of BrdU-positive cells (Fig. S2B), which argues against the above possibility. Almost all of these BrdU-positive cells were negative for calbindin (Fig. 1D), but positive for NeuN (Fig. S2C). Thus, in addition to newborn cells, such immature-like neurons appeared to constitute a substantial part of the calbindin-negative cells in fluoxetine-treated DG. These results suggest that fluoxetine changed the phenotype of GCs that had been generated early in development and had already maturated before the treatment was started.
Fluoxetine-Treated Granule Cells Exhibit Immature-Like Functional Characteristics.
If the reduced calbindin expression indeed reflects a change in the state of GC maturation, functions of these cells would also be altered accordingly. To address this issue, we analyzed stimulus-induced expression of the immediate early gene c-Fos, a marker for the maturity of in vivo activity-dependent responsiveness of GCs (25, 28). In mice placed in home cages, c-Fos protein was expressed at low levels in the GC layer (Fig. 2A). After electrical foot shocks, robust c-Fos protein expression was seen in the control mice. However, this c-Fos expression was strongly suppressed in the fluoxetine-treated mice (Fig. 2A). This result can hardly be explained by lack of synaptic activation of GCs, because synaptic inputs to GCs were not impaired (Fig. S3A), and GCs were more excitable (Fig. 2 B and C) and could generate repetitive action potentials after the fluoxetine treatment (Fig. S3B). In addition, there was no significant difference in the sensitivity to the foot shocks or behavioral immobility during shock intervals between control and fluoxetine-treated mice (SI Methods). These results suggest that the activity-dependent gene expression characterizing the GC maturity was not properly functioning in the fluoxetine-treated mice.
Fig. 2.
Dentate granule cells in fluoxetine-treated mice show immature-like natures. (A) Left, c-Fos-like immunoreactivity in GC layer after foot shocks. Right, quantitative data showing the number of c-Fos-positive cells in mice in home cages and after foot shocks (n = 4 each). Fluoxetine strongly reduced foot shock-induced c-Fos expression (P = 0.0286). (Scale bar, 200 μm.) (B) Sample recordings of GC spikes (Upper) evoked by depolarizing currents (Lower). (Scale bars: 100 ms, 40 pA, and 50 mV.) (C) Left, the minimal current intensity required to evoke a single spike is smaller in fluoxetine-treated GCs (CNT, n = 31 cells; FLX, n = 35 cells; P = 0.0099). Right, no significant difference in input resistance. (D) Left, TTX (1 μM) completely blocked spikes in control cells. Right, TTX-resistant component (arrow) in fluoxetine-treated cells was blocked by Ni2+ (50 μM). The magnitude of injected currents was 140 pA (CNT) and 100 pA (FLX). (Scale bars: 50 ms, 50 mV (Top), and 20 mV (Middle and Bottom). Data are presented as mean ± SEM.
In comparison with mature cells, young GCs can be more easily excited by somatic current injection, because they have higher input resistance and can generate tetrodotoxin (TTX)-resistant, but Ni2+-senstive, low-threshold spikes that can boost sodium spikes (12, 14). Fluoxetine-treated GCs were more excitable (Fig. 2 B and C), but there was no significant change in the input resistance (Fig. 2C), membrane time constant, or resting membrane potential (SI Methods). On the other hand, although action potentials in control GCs were completely blocked by TTX in all cases examined (n = 18 cells), a TTX-resistant component was observed in 14 out of 18 fluoxetine-treated GCs (Fig. 2D). This component was suppressed by Ni2+ (Fig. 2D), similarly to the TTX-resistant spike in young GCs. Thus, GCs in the fluoxetine-treated mice exhibited some active membrane properties resembling those in young GCs. Taken together, our results suggest that the fluoxetine treatment changed the functional phenotype of GCs to immature-like one.
We also examined synaptic plasticity in DG. It has been reported that long-term depression (LTD) at the medial perforant path-GC synapse is larger in magnitude in juvenile animals as compared with young adults (29, 30). In newborn GCs generated in adults, the magnitude of long-term potentiation (LTP) initially appears to be small, but is greatly enhanced during a critical period between 1 and 1.5 months of the cell age (15). In fluoxetine-treated DG, LTD at the perforant path synapse was enhanced (Fig. S4A), whereas LTP was reduced (Fig. S4B). Although the possibility of presynaptic effects of fluoxetine cannot be excluded, these results are in line with the idea that the fluoxetine-treated GCs exhibit immature-like functional properties.
Chronic Fluoxetine Reduces Mossy Fiber Synaptic Facilitation to Juvenile Level.
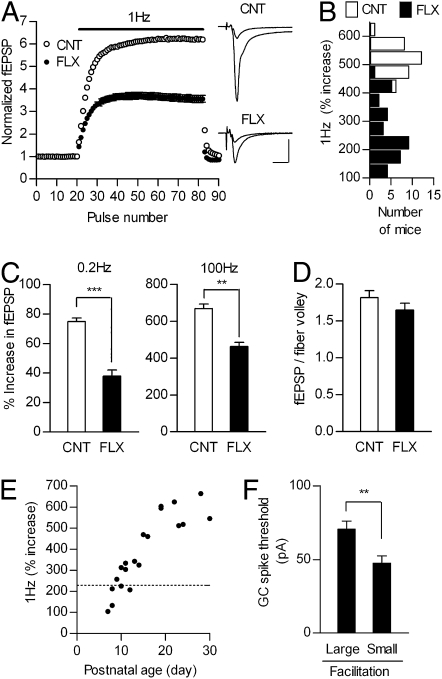
We then examined properties of dentate-to-CA3 signal transmission mediated by MF, the axon of GC. The mature MF-CA3 pyramidal cell synapse is characterized by exceptionally strong frequency facilitation (13, 27) (see Fig. 3A), a presynaptic enhancement of synaptic strength during higher-frequency transmission (31). In young mice less than 3 weeks old, the magnitude of the frequency facilitation is much smaller (13) (see Fig. 3E). Thus, MF synapses, formed by immature-like GCs in the fluoxetine-treated mice, would exhibit smaller frequency facilitation. As predicted, chronic fluoxetine clearly reduced frequency facilitation at the MF synapse at all frequencies examined (Fig. 3 A–C), but had no detectable effects on the basal synaptic efficacy (Fig. 3D). This reduced frequency facilitation is unlikely to be due to a decrease in the number of activated GCs during higher frequency stimulation (SI Methods). The magnitude of facilitation in the fluoxetine-treated mice was comparable to that of control mice at postnatal day 10 (Fig. 3E), the age around which the putative GC-specific maturation markers are beginning to be expressed (Fig. S1). In the fluoxetine-treated group, GCs from mice with smaller frequency facilitation showed higher excitability (Fig. 3F), and all GCs with the TTX-resistant component (see Fig. 2D) were from mice with greatly reduced 1 Hz facilitation (<300% increase). Therefore, the apparent change in the state of synaptic maturation was in good agreement with that of somatic functional maturation. The effects of fluoxetine on the facilitation depended on the duration (Fig. S5A) and dose of the treatment (Fig. S5 A and B). Importantly, the reduction in the calbindin protein level induced by fluoxetine exhibited a similar dose and duration dependence (Fig. 1B and Fig. S5 C and D). These results further support the idea that the fluoxetine-treated GCs were in the immature-like state. The magnitude of frequency facilitation could be a good measure to assess the change in the state of functional maturation of GCs caused by fluoxetine. Although frequency facilitation at the MF synapse has been reported to decrease with postnatal development from 3 to 9 weeks in CD-1 mice (32), this late developmental change was less clear in the present study, probably due to a difference in the strain of mice used.
Fig. 3.
Chronic fluoxetine reduces mossy fiber synaptic facilitation to juvenile level. (A) Pooled data showing marked reduction of 1 Hz frequency facilitation at the MF synapse in fluoxetine-treated mice (CNT, n = 30; FLX, n = 35; see Fig. S5A for statistics). Sample recordings are averages of 15 consecutive fEPSPs during baseline and 1 Hz stimulation. (Scale bars: 10 ms and 0.5 mV.) (B) A histogram of magnitude of facilitation at 1 Hz. (C) Reduced frequency facilitation at 0.2 Hz (n = 14 each, P < 0.0001) and 100 Hz (n = 8 each, P = 0.0022). (D) No significant difference in ratios of fEPSP to fiber volley amplitude (CNT, n = 18; FLX, n = 20). (E) Developmental increases in magnitude of 1 Hz facilitation. Each symbol represents a single mouse. The dotted line shows the median value in adult fluoxetine-treated mice. (F) Higher GC excitability in mice with smaller synaptic facilitation. Fluoxetine-treated mice were divided into two groups (five mice each) by order of magnitude of 1 Hz facilitation, and the threshold current for GC spike generation was compared (large, n = 19 cells; small, n = 16 cells; P = 0.0093). Data are presented as mean ± SEM.
We examined the possibility that reduced Ca2+ buffering in the MF terminals due to the loss of calbindin caused the reduction in frequency facilitation. In control mice, an exogenous membrane-permeable fast Ca2+ buffer reduced the basal synaptic transmission and increased the steady-state level of 1 Hz facilitation (Fig. S6). However, in fluoxetine-treated mice, although the exogenous Ca2+ buffer similarly reduced the basal synaptic transmission, it did not restore the large facilitation of mature GCs (Fig. S6). Therefore, the reduced frequency facilitation cannot be simply explained by a decrease in concentrations of fast Ca2+ buffers in the MF terminals.
Involvement of 5-HT4 Receptor in Effects of Fluoxetine.
We then examined the role of the serotonergic system in changing the apparent state of GC maturation. Another SSRI paroxetine similarly reduced the frequency facilitation (Fig. S5A) and calbindin protein level (Fig. S5C), and the effects of fluoxetine on the frequency facilitation and calbindin level were blocked by a lesion of central serotonergic neurons with 5,7-dihydroxytriptamine (Fig. S7). These results confirmed the importance of the serotonergic system in the modification of the GC phenotype. We have recently shown that 5-HT induces MF synaptic potentiation that is exclusively mediated by the 5-HT4 receptor (24) (also see below), most likely in GCs, and chronic fluoxetine at 10 mg/kg/day can reduce the magnitude of 5-HT-induced synaptic potentiation (24). We found that the effect of chronic fluoxetine on 5-HT-induced synaptic potentiation switched from reduction to augmentation with increasing doses of fluoxetine (Fig. 4 A and B). This augmentation of potentiation significantly correlated with the reduction of the frequency facilitation (Fig. 4C). To directly address the involvement of the 5-HT4 receptor in effects of fluoxetine, we examined responsiveness of mice lacking the 5-HT4 receptor to fluoxetine. In the 5-HT4 mutant mice, 5-HT had no potentiating effects on MF synaptic transmission (Fig. 4D). The effect of the gene knockout appeared specific to the serotonergic modulation, because dopamine-induced synaptic potentiation that shares the intracellular pathway with 5-HT at the MF synapse (24, 33) was preserved (Fig. S8A), and other properties of synaptic transmission were generally normal (Fig. S8 B and C). In the wild-type mice, fluoxetine significantly reduced the calbindin level in DG and frequency facilitation at the MF synapse as in normal C57BL/6J mice (Fig. 4 E and F). However, fluoxetine had no significant effects on either calbindin level or frequency facilitation in the 5-HT4-deficient mice (Fig. 4 E and F). These results suggest that the 5-HT4 receptor plays a critical role in the induction of the immature-like GC phenotype by the chronic fluoxetine treatment.
Fig. 4.
Involvement of 5-HT4 receptor in effects of chronic fluoxetine. (A) Fluoxetine at 22 mg/kg/day augmented MF synaptic potentiation induced by bath application of 5-HT for 5 min (CNT, n = 9; FLX, n = 9; P = 0.004). Sample recordings are averages of nine consecutive fEPSPs during baseline and 5-HT application. (Scale bars: 10 ms and 0.2 mV.) (B) Dose-dependent effects of fluoxetine on 5-HT-induced potentiation. Each symbol represents a single mouse. In mice shown by triangles, fluoxetine concentrations were calculated on the basis of averaged water consumption, as in Fig. S5B. The dotted line shows the control level. (C) Correlation between frequency facilitation and 5-HT-induced potentiation. Data from mice treated at 18–22 mg/kg/day were included. P < 0.0001, r2 = 0.6657. (D) Lack of 5-HT-induced synaptic potentiation in 5-HT4 receptor-deficient mice (+/+, n = 6; −/−, n = 9). (E) Significant effects of chronic fluoxetine on calbindin levels in wild-type (n = 5 each, P = 0.0159), but not in mutant mice (n = 6 each). (F) Significant effects of fluoxetine on frequency facilitation in wild-type (CNT, n = 6; FLX, n = 5; P = 0.0173), but not in mutant mice (CNT, n = 9; FLX, n = 7). Data are presented as mean ± SEM.
Discussion
Dentate GCs in the fluoxetine-treated mice exhibited some of characteristics resembling those of immature or developing GCs. The neonatal BrdU-labeling analysis suggested that fluoxetine changed the phenotype of mature GCs. The input resistance and membrane time constant of the fluoxetine-treated GCs were almost the same as those of control cells, suggesting lack of substantial changes in the cell size or gross morphology. In addition, the intact basal synaptic efficacy at both input and output synapses of the fluoxetine-treated GCs implies that the formation of synaptic connection itself was preserved. Young GCs generated during the fluoxetine treatment would be smaller and have higher input resistance than mature cells (12, 14, 16), and would have less synaptic contacts (16). Thus, these results are consistent with the idea that the immature-like GCs in fluoxetine-treated mice mostly originated from mature cells. Taken together, our results suggest that chronic fluoxetine reversed phenotypic maturation of adult dentate GCs, primarily in the functional aspect of the maturation. This fluoxetine-induced GC “dematuration” is distinct from any of previously reported effects of antidepressants on DG. However, it is unlikely to be specific to our experimental condition, because the effect of fluoxetine on the MF synaptic facilitation was evident in some of the mice treated at 18 mg/kg/day, the dose used in recent related studies (8, 34), and the hippocampal expression of calbindin has been reported to be reduced after chronic SSRI treatments (34, 35).
The GC dematuration induced by fluoxetine was characterized by the marked suppression of the expression of calbindin and c-Fos protein. In developing DG, calbindin-like immunoreactivity in the entire GC layer begins to be detected around postnatal day 9 (36), and kainate-induced expression of c-Fos protein in GCs can be detected at postnatal day 13, but not at postnatal day 7 in rats (37). These previous results are consistent with our finding that the magnitude of frequency facilitation at the MF synapse formed by “dematurated” GCs was comparable to that of control mice at postnatal day 10 (Fig. S9A). At this developmental stage, the age of GCs would be about 3 weeks or less in mice, because the cytogenesis in DG starts around embryonic day 12 (38). In adult-generated GCs, maturational changes in protein expression and somatic electrophysiological properties have been investigated in detail (25). Our results suggest that the state of fluoxetine-treated GCs is comparable to that of 3- to 4-week-old adult-generated neurons (Fig. S9B), because (i) the calbindin expression and stimulus-induced c-Fos expression are established in 3- to 5-week-old GCs (17, 25, 28); (ii) whereas the number of calretinin-positive GCs was increased by fluoxetine, most cells were negative for this early postmitotic marker; and (iii) GCs less than 3 weeks old hardly generate repetitive action potentials (16), but fluoxetine-treated GCs did. In other words, fluoxetine reversed the late step of GC maturation. We have recently shown that dentate GCs stay “immature” in adults in mice heterozygous for alpha-calcium/calmodulin-dependent protein kinase II (alpha-CaMKII) (27). The phenotype of these GCs is similar to that of dematurated GCs in fluoxetine-treated mice: Both alpha-CaMKII-deficient and fluoxetine-treated GCs exhibit down-regulation of mature molecular markers, suppression of c-Fos expression, increases in GC excitability, and greatly reduced frequency facilitation at the MF synapse. Since GCs in the alpha-CaMKII mutants showed impairment in repetitive spiking and a further trend toward immaturity in general, they are supposed to be in a slightly younger state than the fluoxetine-treated GCs. Thus, on the basis of these properties as a whole, we would be able to define an apparent stage of GC maturation, irrespective of the GCs’ exact state of being immature or dematurated. Chronic fluoxetine treatments have also been shown to accelerate early maturational processes of adult-generated GCs expressing doublecortin (8). Therefore, chronic fluoxetine seems to have bidirectional effects on the GC maturation, depending on the maturational stage of the cells.
In mice lacking the 5-HT4 receptor, chronic fluoxetine had no significant effects on the calbindin level or frequency facilitation. Because the 5-HT4 receptor is abundantly expressed in dentate GCs (39), it may play a cell-automonous role in the fluoxetine-induced GC dematuration, although it is also possible that the 5-HT4 receptor indirectly contributed to the effects of fluoxetine by increasing the activity of serotonergic neurons (40). We have recently shown that chronic fluoxetine at 10 mg/kg/day causes reduction of 5-HT4 receptor-mediated MF synaptic potentiation induced by a relatively high concentration of 5-HT (24). With increasing doses, the effect of fluoxetine switched from suppression to marked augmentation (Fig. 4B). The 5-HT4 receptor is coupled to Gs-cAMP cascades, and 5-HT-induced potentiation at the MF synapse has been shown to be mediated by cAMP (24). The augmentation of 5-HT-induced synaptic potentiation by fluoxetine could be due to up-regulation of Gs-cAMP signaling cascades (41). The reduction of frequency facilitation that represents the GC dematuration significantly correlated with the augmentation of 5-HT-induced synaptic potentiation (Fig. 4C). Taken together, our results suggest that up-regulation of the cAMP cascades downstream from the 5-HT4 receptor may play an instructive role in the induction of the GC dematuration. It remains to be elucidated whether the 5-HT4 receptor itself is involved in the up-regulation of its own signaling pathway.
Relatively low excitability of GCs is likely essential for gating of cortical excitation by DG (42). The prominent synaptic facilitation plays a critical role in regulation of the CA3 neuronal activity and plasticity by MF (9, 10). The dematuration affected both of these important physiological properties of mature GCs, thereby potentially impeding proper functioning of the adult hippocampus (43). Similar changes in GC functions and/or in the state of GC maturation have been demonstrated in mice disrupted for the gene encoding alpha-CaMKII (27), brain-derived neurotrophic factor (44), or the glutamate receptor subunit GluK2/GluR6 (10, 45). The deficiency in either of these genes has been shown to cause substantial behavioral abnormalities (27, 46, 47), which supports the idea that the GC dematuration can potentially cause dysfunction of the adult hippocampus. On the other hand, the increased GC excitability caused by the dematuration may improve some pathological conditions, such as reduced GC activity in animals exposed to chronic stress (7). The enhancement of LTD at the perforant path-GC synapse in fluoxetine-treated DG suggests that the dematuration can reinstate synaptic plasticity that is reduced with development (29, 30) or facilitated only during a critical period (15), thereby potentially causing beneficial effects on the adult brain. Chronic fluoxetine can indeed restore neuronal plasticity in the visual cortex of adult rats (3). Although we showed reduced perforant path LTP in fluoxetine-treated DG, Wang et al. have shown an enhancement of LTP induced in the presence of intact synaptic inhibition in slices prepared from SvEv129 mice (8). The discrepancy in the results may be due to the difference in the strain of mice and/or use of disinhibited slices in our study (SI Methods). Perforant path LTP in slices with intact synaptic inhibition can be blocked by ablation of adult neurogenesis (8) and could be distinct from that in disinhibited slices (11).
In conclusion, our results demonstrate that the established state of hippocampal neuronal maturation can be relatively easily changed by antidepressant treatments. This finding raises the possibility that the state of neuronal maturation, including aberrant maturation, can be controlled or corrected in adults, which suggests a unique approach to treat neuronal dysfunctions associated with neurodevelopmental disorders.
Methods
Drug Treatments.
Male C57BL/6J mice, 5-HT4 receptor homozygous mutant mice (−/−) and their wild-type (+/+) littermates were singly housed from the age of 8 weeks. Following 1 week of acclimation, fluoxetine was orally applied in drinking water at a dose of 22 mg/kg/day for 4–5 weeks unless otherwise specified. Concentrations of fluoxetine were determined for individual mice. All procedures were approved by the institutional animal care and use committee. See SI Methods for more details.
Electrophysiology.
Transverse hippocampal slices were prepared and electrophysiological recordings were made as described (27, 33). Electrical stimulation was delivered in DG at 0.05 Hz unless otherwise specified and field excitatory postsynaptic potentials (fEPSPs) at the MF synapse were recorded in CA3. Whole-cell current-clamp recordings were made from GCs in DG. See SI Methods for more details.
Histochemical Analysis.
Brain sections were incubated with anti-calbindin-D-28K, anti-calretinin, anti-NeuN, or anti-c-Fos antibody, and subsequently incubated with secondary antibody labeled with Alexa Fluor 488 or Alexa Fluor 594. For labeling nuclei, sections were mounted with VECTASHIELD mounting medium with 4',6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured using a microscope equipped with a digital camera. See SI Methods for more details.
Immunohistochemistry for BrdU.
Neonatal mice were s.c. injected with 100 mg/kg BrdU. After the fluoxetine treatment in adults, the brain sections were prepared for immunostaining with anti-BrdU antibody. Images were captured using a confocal microscope. See SI Methods for more details.
Immunoblot Analysis.
Isolated DG was sonicated, and homogenates were centrifuged. Supernatants were electrophoresed on SDS-polyacrylamide gel and electroblotted onto PVDF membrane. The membrane was incubated with anti-calbindin-D28-K or anti-GAPDH antibody and detected using HRP-conjugated secondary antibody and chemiluminescence. See SI Methods for more details.
Quantitative RT-PCR.
RT-PCR was performed essentially as described (27). Total RNA was isolated from the hippocampi of P6, P8, P14, P18, P21, P28, P35, and P240 control mice and from DG of the fluoxetine-treated mice and their control mice. See SI Methods for more details.
Analysis of c-Fos Expression After Foot Shock.
Mice received electrical foot shocks and were processed for c-Fos immunostaining 2 h later. See SI Methods for more details.
Statistics.
The number of data (n) represents the number of mice unless otherwise specified. Because some data from drug-treated mice did not distribute normally, a nonparametric two-tailed Mann-Whitney test was used to evaluate statistical significance, with the significance level P < 0.05, unless otherwise specified.
Supplementary Material
Acknowledgments
We thank Drs. Hiroyuki Katagiri and Sayaka Sugiyama for discussion and Yasunori Mikahara, Ayaka Matsuda, and Kumiko Takasu for technical assistance. This work was supported by the Human Frontier Science Program Organization (K.K.), and a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, (no. 1659028 to H.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912690107/DCSupplemental.
References
- 1.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 2.Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: Children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16:159–169. doi: 10.1089/cap.2006.16.159. [DOI] [PubMed] [Google Scholar]
- 3.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 4.Mostert JP, Koch MW, Heerings M, Heersema DJ, De Keyser J. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci Ther. 2008;14:153–164. doi: 10.1111/j.1527-3458.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 7.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 8.Wang J-W, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi K, Poo MM. Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron. 2004;41:445–454. doi: 10.1016/s0896-6273(03)00873-0. [DOI] [PubMed] [Google Scholar]
- 10.Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH, Mulle C. Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J Neurosci. 2009;29:5000–5008. doi: 10.1523/JNEUROSCI.5807-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 12.Ambrogini P, et al. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Marchal C, Mulle C. Postnatal maturation of mossy fibre excitatory transmission in mouse CA3 pyramidal cells: A potential role for kainate receptors. J Physiol. 2004;561:27–37. doi: 10.1113/jphysiol.2004.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 15.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mongiat LA, Espósito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 19.Ninkovic J, Mori T, Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 21.Huang G-J, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiatry. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- 22.Petersén A, Wörtwein G, Gruber SHM, El-Khoury A, Mathé AA. Nortriptyline mediates behavioral effects without affecting hippocampal cytogenesis in a genetic rat depression model. Neurosci Lett. 2009;451:148–151. doi: 10.1016/j.neulet.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 23.Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Ikeda Y, Haneda E, Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci. 2008;28:6272–6280. doi: 10.1523/JNEUROSCI.1656-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Brandt MD, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki N, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1:6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 29.Trommer BL, Liu Y-B, Pasternak JF. Long-term depression at the medial perforant path-granule cell synapse in developing rat dentate gyrus. Brain Res Dev Brain Res. 1996;96:97–108. doi: 10.1016/0165-3806(96)00104-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Chang L, Rowan MJ, Anwyl R. Developmental dependence, the role of the kinases p38 MAPK and PKC, and the involvement of tumor necrosis factor-R1 in the induction of mGlu-5 LTD in the dentate gyrus. Neuroscience. 2007;144:110–118. doi: 10.1016/j.neuroscience.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 32.Mori-Kawakami F, Kobayashi K, Takahashi T. Developmental decrease in synaptic facilitation at the mouse hippocampal mossy fibre synapse. J Physiol. 2003;553:37–48. doi: 10.1113/jphysiol.2003.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K, Suzuki H. Dopamine selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. Neuropharmacology. 2007;52:552–561. doi: 10.1016/j.neuropharm.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Miller BH, Schultz LE, Gulati A, Cameron MD, Pletcher MT. Genetic regulation of behavioral and neuronal responses to fluoxetine. Neuropsychopharmacology. 2008;33:1312–1322. doi: 10.1038/sj.npp.1301497. [DOI] [PubMed] [Google Scholar]
- 35.Sillaber I, et al. Profiling of behavioral changes and hippocampal gene expression in mice chronically treated with the SSRI paroxetine. Psychopharmacology (Berl) 2008;200:557–572. doi: 10.1007/s00213-008-1232-6. [DOI] [PubMed] [Google Scholar]
- 36.Abrahám H, Orsi G, Seress L. Ontogeny of cocaine- and amphetamine-regulated transcript (CART) peptide and calbindin immunoreactivity in granule cells of the dentate gyrus in the rat. Int J Dev Neurosci. 2007;25:265–274. doi: 10.1016/j.ijdevneu.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Silveira DC, Sogawa Y, Holmes GL. The expression of Fos following kainic acid-induced seizures is age-dependent. Eur J Neurosci. 2002;15:329–344. doi: 10.1046/j.0953-816x.2001.01849.x. [DOI] [PubMed] [Google Scholar]
- 38.Stanfield BB, Cowan WM. The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:423–459. doi: 10.1002/cne.901850303. [DOI] [PubMed] [Google Scholar]
- 39.Vilaró MT, Cortés R, Mengod G. Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: Distribution and effects of neurotoxic lesions. J Comp Neurol. 2005;484:418–439. doi: 10.1002/cne.20447. [DOI] [PubMed] [Google Scholar]
- 40.Conductier G, et al. Adaptive changes in serotonin neurons of the raphe nuclei in 5-HT(4) receptor knock-out mouse. Eur J Neurosci. 2006;24:1053–1062. doi: 10.1111/j.1460-9568.2006.04943.x. [DOI] [PubMed] [Google Scholar]
- 41.Donati RJ, Rasenick MM. G protein signaling and the molecular basis of antidepressant action. Life Sci. 2003;73:1–17. doi: 10.1016/s0024-3205(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 42.Hsu D. The dentate gyrus as a filter or gate: A look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- 44.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 46.Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Shaltiel G, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.