Abstract
Mice reproduce interesting effects in auditory discrimination learning and knowledge transfer discussed in human studies: (i) the advantage in the transfer from a hard to an easy task by benefits from transfer of procedural knowledge and information-integration learning, and (ii) the disadvantage in the transfer from easy to hard tasks by inability to generalize across perceptually different classes of stimuli together with initially unsuccessful attempts to transfer cognitive skills from one task to the other. House mice (NMRI strain) were trained in a shuttle-box stimulus discrimination task. They had to discriminate either between two pure tones of different frequencies (PT) or between two different modulation frequencies of an amplitude-modulated tone (AM). Then transfer of knowledge between these two tasks was tested. Mice rapidly learned PT discrimination within two to three training sessions (easy task). AM discrimination learning took longer and did not reach the high performance level of PT discrimination (hard task). No knowledge transfer was detected in animals first trained with the easy (PT) followed by the hard (AM) discrimination task. Mice benefited, however, from knowledge transfer when the AM discrimination was followed by the PT discrimination. When the task changed, confusion of conditioned stimuli occurred if the carrier frequency of the AM was the same as one of the frequencies in the PT task. These results show a hard-to-easy effect when possible knowledge transfer is tested between qualitatively different stimulus classes. The data establish mice as promising animal model for research on genetics of auditory perception and learning.
Keywords: cognitive skill learning, go/no-go paradigm, hard-to-easy effect, shuttle-box, stimulus generalization
Complex forms of auditory learning, such as generalization of stimuli within and across physical categories or transfer of knowledge from one stimulus discrimination task to another, are known from humans (1–6) but rarely have been investigated in other mammals (5, 7, 8). Research in mammalian models is important, however, for an in-depth understanding of neural mechanisms and the genetics of learning abilities and performances. The mouse (Mus musculus), with its great potential for studying genetic effects on auditory perception, learning, and motor performance, is an ideal candidate species for investigations of auditory learning, because in normal-hearing mice many hearing abilities and functions of processing sounds in the auditory system are already known (9–11). What is missing is an automated and reliable learning paradigm for mice that can be adapted to a variety of learning tasks (e.g., detection, discrimination, generalization, transfer learning), showing that learning rules derived from human studies apply to mice. The shuttle-box seems to be the appropriate learning apparatus because it has been used successfully in simple learning and stimulus detection tasks in mice (12–15) and in complex auditory discrimination and memory tasks in another rodent species, the Mongolian gerbil (7, 8, 16–19). Hence, we designed our present study (i) to test the usefulness of the shuttle-box discrimination learning for simple and complex auditory learning tasks in mice; (ii) to investigate complex auditory learning and discrimination performance in mice to see whether mouse data may be generalized, at least to gerbils; and (iii) to test for stimulus generalization and knowledge transfer across different stimulus classes, experiments that have not been done in the auditory domain in mammals other than humans (3, 20).
A foot shock-motivated go/no-go shuttle-box paradigm was used to train mice for discrimination either of two pure tones of different frequencies (PT) or two different modulation frequencies of an amplitude-modulated tone (AM). The carrier frequency of the AM was either the same as one of the frequencies or different from both of the frequencies used in the PT discrimination task. After a stable discrimination performance was reached in both tasks, the tasks were changed to test possible knowledge transfer from the PT discrimination to the AM discrimination and vice versa. Knowledge transfer may be understood as profit from previous learning of procedures, stimuli, cognitive skills, and/or tasks in the subsequent task (1, 21–24). Thus, our results from mice illuminate phenomena and hypo-theses about auditory learning, discrimination, and knowledge transfer previously found in humans.
Results
A total of 73 female laboratory mice (Mus musculus, outbred strain NMRI) with normal hearing (25) were trained successfully in a shuttle-box go/no-go sound discrimination paradigm. The mice were divided into 10 groups according to the paradigms to be learned (Table S1). Mice in groups A1–A4 were trained in the PT or in one of the three AM discrimination tasks. Then, mice from group A1 were divided to form the groups B1 to B3, which subsequently were trained on one of the three AM discrimination tasks. The mice in groups A2–A4 went in groups C1–C3, respectively, and were further trained on PT discrimination (for details see SI Text and Table S1).
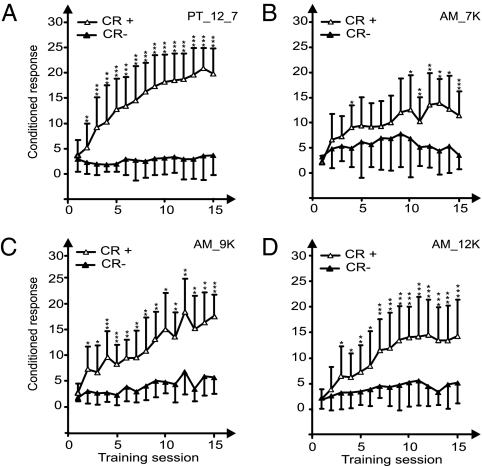
Groups A1–A4 (Fig. 1) showed that PT discrimination was significantly easier to learn than the AM discrimination. The number of conditioned responses [“hits” (CR+) or “false alarms” (CR−)] diverged faster as a function of training sessions (1–15); i.e., statistically significant differences between CR+ and CR− in the sequence of training sessions developed faster and reached a higher significance level for PT (Fig. 1A) than for AM discrimination learning (Fig. 1 B–D).
Fig. 1.
Learning curves for the PT discrimination group A1 (A) and the three AM discrimination groups A2 (B), A3 (C), and A4 (D). (A) PT 12 kHz vs. 7 kHz. (B–D) AM with modulation frequencies of 20 vs. 40 Hz and carrier frequency of 7 kHz (B), 9 kHz (C), or 12 kHz (D). Plotted is the number of hits (CR+) and false alarms (CR−) as a function of number of training sessions. SDs deviations are shown only unilaterally for clarity. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Mann–Whitney U test).
The differences between PT and AM discrimination learning also are obvious in Fig. S1, in which the mean maximal response difference (CR+ − CR−) (SI Materials and Methods) as a measure of discrimination performance is plotted over the first training session with significant discrimination as a measure of learning speed.
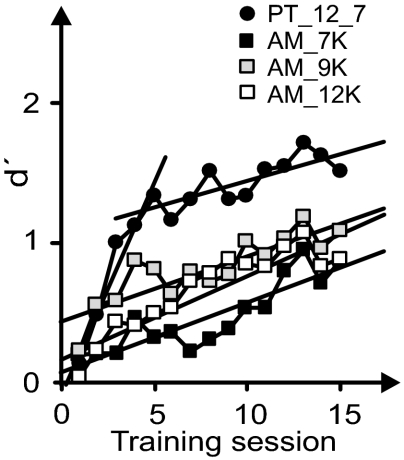
The dynamics of learning of groups A1–A4 are shown in Fig. 2 in which the discrimination index d′ (SI Materials and Methods) is plotted over the training sessions. In the PT group, d′ increased in two separable phases. The slope values of the linear regression lines before and after the fifth training session differ significantly (P < 0.001). The d′ functions of the AM groups can be approximated by a single linear regression line showing a shallow improvement of d′ over all training sessions. There are no statistically significant differences between the slopes of the d′ functions of the three AM groups and the slope characterizing the later learning phase of the PT group (Fig. 2).
Fig. 2.
The dynamics of learning are expressed by the development of average d′ values as a function of the training session. The d′ functions are approximated by linear regression lines. For the PT group (group A1, black circle), fast improvement in discrimination over the first five training sessions with slope b = 0.302 (r = 0.975, P < 0.01) was followed by a slower improvement with slope b = 0.038 (r = 0.775, P < 0.01) during later training sessions. For AM groups there was shallow improvement of discrimination over all training sessions with slope b = 0.050 (r = 0.878, P < 0.001) for AM 7 kHz (group A2, black square), b = 0.046 (r = 0.856, P < 0.01) for AM 9 kHz (group A3, gray square), and b = 0.059 (r = 0.913, P < 0.001) for AM 12 kHz (group A4, open square).
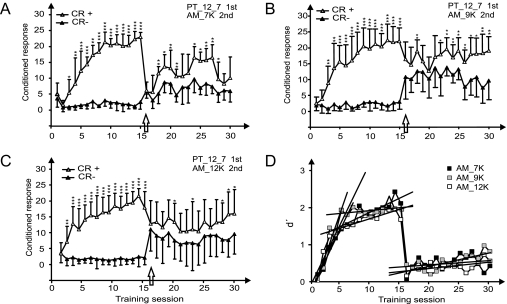
Fig. 3 shows the averaged learning curves of the animals first trained in the PT discrimination task and then in one of the three AM discrimination tasks (groups B1–B3). The PT discrimination performance of these groups was not significantly different from that of group A1 (statistics are given in SI Text). The change from the comparably easy PT discrimination to the more difficult AM discrimination resulted in a strong decrease in discrimination performance to a nonsignificant level at the first day of change (training session 16, indicated by arrows in Fig. 3 A–C). Relearning of discrimination between the two different AM rates (20 or 40 kHz) occurred in all B groups, although at different average speeds: The animals in group B1 needed 3 days, those in group B2 needed 7 days, and those in group B3 needed 13 days to reach a stable, significant discrimination performance again (Fig. 3 A–C). Taking the mean maximal response difference and the first day with a significant discrimination performance as criteria for the learning success, the AM discrimination performances among groups B1–B3 were similar (Fig. S2 A–C) but were significantly worse than PT discrimination in the maximal response difference (Fig. S2D). Clearly, in the AM discrimination task the animals did not profit from their previous experience with the PT discrimination task.
Fig. 3.
Learning transfer from an easy to a hard task. (A–C) Learning curves for the groups B1–B3 which were trained for PT discrimination in the first training period (sessions 1–15) and then for AM discrimination with the carrier frequency (A) 7 kHz (group B1), (B) 9 kHz (group B2), or (C) 12 kHz (group B3) in the second training period starting with training session 16 (arrow). (For further information, see Fig. 1.) (D) The dynamics of learning are expressed by the development of average d′ values as a function of the training session. The d′ functions are approximated by linear regression lines with the following parameters. PT discrimination showed an initial fast increase [group B1 (7 kHz, black square): slope b = 0.298 (r = 0.977); group B2 (9 kHz, gray square): slope b = 0.462 (r = 0.945); group B3 (12 kHz, open square): slope b = 0.330 (r = 0.917)] followed by a later slow increase [group B1 (7 kHz, black square): slope b = 0.054 (r = 0.803); group B2 (9 kHz, gray square): slope b = 0.015 (r = 0.334); group B3 (12 kHz, open square): slope b = 0.043 (r = 0.762)]. The slow improvements during AM discrimination learning from training session 16 onwards are characterized by the slope b = 0.009 (r = 0.246) for group B1 (7 kHz), b = 0.035 (r = 0.752) for group B2 (9 kHz), and b = 0.017 (r = 0.565) for group B3 (12 kHz). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Mann–Whitney U test).
Furthermore, the task required for successful PT discrimination influenced the performance in the subsequent AM discrimination task in a specific way, depending on the AM carrier frequency (Fig. 3). The animals in group B1 had learned in the PT discrimination task to respond to the negative conditioned stimulus (CS−) of 7 kHz with a no-go response and continued to do so in the AM task with the 7-kHz carrier frequency, resulting in an initially low hit rate to the positive conditioned stimulus (go, CS+) and an equally low false-alarm rate to the CS− (Fig. 3A). The animals in group B3 had learned in the PT discrimination to respond to the CS+ of 12 kHz with a go response (i.e., to jump across the hurdle) and continued to do so in the AM task with the 12-kHz carrier frequency, resulting in an initially high false-alarm rate to the CS− and an equally high hit rate to the CS+ (Fig. 3C). Thus, the animals tended to continue with the learned association between a specific sound and a certain motor performance although the perceptual discrimination task had changed qualitatively from PT to AM so that the previously learned PT lost its information in the AM task. In the case of 9 kHz as carrier frequency (Fig. 3B), the animals responded in a way similar to the change to 12-kHz carrier frequency. It seems that they generalized over 9 and 12 kHz, although the AM task required the discrimination in a new stimulus dimension.
The development of d′ as expression of the average learning speed over the course of training of the B groups is shown in Fig. 3D. As in the PT group A1, in all B groups PT discrimination learning improved in two phases. The slope values of these improvements did not differ significantly from the slopes obtained in the PT discrimination shown in Fig. 2. The AM discrimination learning improved slowly for all three AM tasks (Fig. 3D). The slope of the learning function in the AM task with 9 kHz as carrier frequency did not differ from the slope reached in the corresponding AM task without previous PT discrimination learning (Fig. 2). However, the slopes in the AM tasks with 7 kHz and 12 kHz as carrier frequencies were smaller (significantly so for 12 kHz) than the respective slopes reached in the corresponding AM task without previous PT discrimination learning (Fig. 2). That is, previous experience in the PT discrimination task slowed down the learning speed in the AM task specifically in the cases in which the AM carrier frequency was identical with one of the frequencies to be discriminated in the previous PT task. In summary, these data indicate that training with an easy PT discrimination task has negative effects, if any, on later learning the more difficult AM discrimination task.
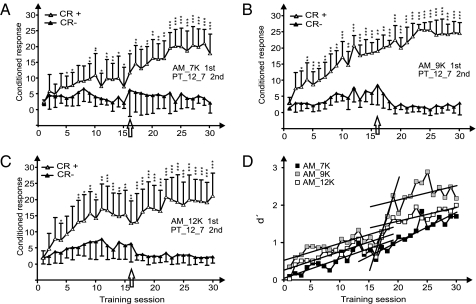
Finally, we investigated the potential effect of previous experience with the difficult AM discrimination task on the following, easier PT discrimination task (Fig. 4). Learning dynamics were significantly different in these C groups than in the B groups. At the change from the AM discrimination to the PT discrimination (training session 16, indicated by arrows in Fig. 4 A–C), no drop of discrimination performance could be detected in any of the C groups. The animals immediately learned the new task in the 16th training session and improved in discrimination performance in the following sessions so that the PT discrimination performance became significantly better than the previous AM discrimination performances (Fig. 4 A–C and Fig. S3 A–C).
Fig. 4.
Learning transfer from a hard to an easy task. (A–C) Learning curves for the groups C1–C3, which were trained for AM discrimination with the carrier frequency 7 kHz (group C1) (A), 9 kHz (group C2) (B), or 12 kHz (group C3) (C) in training sessions 1–15 and for PT discrimination (12 vs. 7 kHz) in the second training period starting with training session 16 (arrow). (For further information, see Fig. 1.) (D) The dynamics of learning are expressed by the development of average d′ values as a function of the training session. The parameters of the linear regression lines for training sessions 1–15 (AM training) are slope b = 0.054 (r = 0.887) for group C1 (7 kHz); slope b = 0.047 (r = 0.857) for group C2 (9 kHz); and slope b = 0.051 (r = 0.860) for group C3 (12 kHz). The parameters of the linear regression lines for training sessions 16–30 (PT training) are slope b = 0.078 (r = 0.907) for group C1 (previously trained in the AM 7-kHz task); an initial steep slope b = 0.443 (r = 0.995) and a later shallow slope b = 0.038 (r = 0.450) for group C2 (previously trained in the AM 9-kHz task); and slope b = 0.036 (r = 0.787) for group C3 (previously trained in the AM 12-kHz task). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (Mann–Whitney U test)
By comparing the PT discrimination performances among the C groups and comparing the performances of the C groups with the performance of the naively trained PT group A1, we found interesting effects. Group C2 trained with AM with a carrier frequency of 9 kHz showed significantly better average (Fig. 4D) and maximal (Fig. S3D) PT discrimination performance than the other C groups (groups C1 and C3) and the naïve PT group A1 (compare d′ values in Fig. 2 with d′ values in Fig. 4D and see Fig. S3D). This significant advantage in PT discrimination of group C2 compared with the other C groups and group A1 resulted from the rapid increase in discrimination performance after training session 16 (Fig. 4D). The slope of d′ of this improvement in performance did not differ from the slope of the initial improvement in the PT discrimination task (Fig. 2). In groups C1 and C3, the improvement in performance during the PT learning phase was not different from that during the previous AM task or from the slope of the late phase of the C2 group during PT learning. In summary, these results point to a beneficial transfer of knowledge from the AM discrimination training to the PT discrimination training with an especially strong performance gain in the group C2.
Discussion
The results of auditory discrimination training and knowledge transfer in mice are discussed in the context of studies in humans showing procedural and perceptual learning, discrimination and nongeneralization between perceptual classes of sounds, transfer of cognitive skills from one task to another, increase of attention to stimuli over training sessions, and hard-to-easy effects in the transfer of knowledge by information integration.
The shuttle-box paradigm allowed us to measure learning speed and discrimination performance and was sensitive enough to differentiate between perceptually easy and hard tasks (Figs. 1 and 2). In this respect, mice reproduced learning and discrimination performance previously found via shuttle-box training in Mongolian gerbils (7, 8, 16–19, 26). In general, NMRI mice were not as fast as gerbils in their initial learning speeds for perceptually easy and hard tasks and did not reach the high levels of discrimination performance of gerbils in a hard task (17, 19, 26). They were, however, similar to gerbils in showing an initially fast improvement followed by a slow improvement in discrimination performance for an easy task and a continuously slow improvement for a hard task (17, 18). In humans, an initial rapid improvement in auditory learning is interpreted as rapid procedural learning (learning the response demands of the task), whereas the subsequent slower improvement is taken as evidence for perceptual learning [improvement of perceptual judgment (21, 22)]. This interpretation means that procedural and perceptual learning effects can be differentiated in auditory discrimination learning in humans and mice. Further, we can infer that the perceptual dimension for the discrimination in the easy PT task is clear from the beginning of the training, so a rapid procedural learning effect can become obvious in the initial improvement of the performance. This procedural learning effect is masked in the hard AM task in which the mice initially seem unable to identify perceptually what has to be discriminated, so that the acquired procedural knowledge does not help to do the task. As in the hard discrimination tasks in gerbils (17, 18), mice show a slow improvement in the AM task (Fig. 1 B–D), presumably because of the difficulties in identifying the perceptual task, i. e, their difficulties in directing their attention to the stimulus features to be discriminated. Because the slopes of improvement in the discrimination performance in the AM tasks are very similar to the slope of the late improvement in the PT task (Fig. 2), we may assume a constant average increase in attention to the stimuli to be discriminated in the mice of all groups over the 15 training sessions. Based on this assumption, the average level of attention is the limiting factor for the level of the discrimination performance reached in a training session in the perceptually hard task. In the perceptually easy task, the amount of attention to the stimuli would be a limiting factor for the performance only after the initial improvement in performance resulting from procedural knowledge.
The results of the tests of knowledge transfer between the PT and AM discrimination tasks lead to the following interpretation. Data from humans indicate no perceptual generalization and knowledge transfer across sounds from different perceptual classes and, therefore, no performance benefit in discrimination when switching the sounds between the classes in a discrimination task (3, 5, 20, 27). This interpretation explains why we did not see a beneficial effect when switching from the easy PT to the hard AM discrimination task (Fig. 3 and Fig. S2). In fact, the discrimination of frequencies of pure tones, on the one hand, and of low modulation rates of an amplitude-modulated pure tone, on the other, involves different neural and perceptual processes, namely frequency coding by place in tonotopic maps in the former and temporal coding in the latter case (28, 29).
Fig. 3 A–C indicates, apart from the missing performance benefit when switching from the easy to the hard task, a specific carry-over effect in behavior at the day of the switching. Mice trained to stay in the compartment of the shuttle-box in response to the 7-kHz tone in the PT task continued this behavior in the AM task with a carrier frequency of 7 kHz, resulting in a very low false-alarm rate (Fig. 3A). Similarly, mice trained to jump over the hurdle in response to the 12-kHz tone in the PT task continued this behavior in the AM task with a carrier frequency of 12 kHz, resulting in a high false-alarm rate (Fig. 3C). This transfer of a learned behavioral strategy between tasks, regardless of the effectiveness of the strategy in the new task, is found in humans as transfer of a cognitive skill (23). Accordingly, the mice of groups B1 and B3 tried the transfer of a cognitive skill when switching from the PT to the AM discrimination task. Because this transfer was unproductive for doing the task, the mice had actively to adapt the cognitive skill to the new requirements. This unlearning and relearning of a cognitive skill in addition to learning the discrimination of new stimuli after switching of tasks obviously led to slower learning in the AM task (smaller slope values in Fig. 3D) compared with the cases in which the mice started with AM learning at session 1 (Fig. 2). Unlike groups B1 and B3, the mice of group B2 did not have to adapt their cognitive skills in the AM task, because the AM carrier frequency of 9 kHz was different from the 7-kHz and 12-kHz frequencies previously learned to be associated with a certain behavior. Therefore, no unlearning and relearning of a cognitive skill, but only new perceptual learning, was required, leading to the same improvement in performance as in the 9-kHz AM group in Fig. 2.
There was a clear hard-to-easy effect in switching from the AM to the PT task. In the PT task, the mice benefited from knowledge acquired in the previous AM task, because PT discrimination already was statistically significant at the day of switching (training session 16, Fig. 4 A–C), and performance was significantly better than the initial performance in the PT discrimination task without pretraining (Fig. 1A). Because the stimulus classes in the two tasks were different, human studies suggest that the benefit (hard-to-easy effect) was not based on knowledge of or generalization across stimuli (3, 5, 20, 27) but rather was based on implicitly learned information integration (30, 31). Integration of information about the stimuli, the procedural context, and the acquired cognitive skills in the shuttle-box seems to be the key for understanding the curves of discrimination performance of our mice in Fig. 4. When the task changed in training session 16, the new stimulus situation was clear immediately, and the learned cognitive skill of either staying in or changing the compartment of the shuttle-box when hearing one of two different sounds could be adapted rapidly to the new sounds because of the perceptually easy discrimination of the tones. This adaptation was especially effective when the tones in the AM and PT discrimination tasks were different in frequency (Fig. 4B) so that no unlearning of cognitive skills was required. In that case, the discrimination performance improved rapidly after training session 16, similar to the initial improvement of the PT group in Fig. 2. In the cases in which one of the AM carrier frequencies (7 or 12 kHz) was the same as one of the frequencies in the PT discrimination task, the need to unlearn and relearn cognitive skills may have impeded a rapid improvement in performance in groups C1 and C3.
After 15 training sessions the mice no longer were naïve with respect to general procedures and the given general stimulus context, so the rapid improvement in performance of group C2 after training session 16 (Fig. 4D) may not be based on procedural learning but rather on a rapid improvement in directing attention to the new and clearly perceptible stimuli. The d′ values larger than 2.0 reached by the C2 group in the PT task (Fig. 4D) can be expected by linearly extrapolating the d′ values from the PT task in Fig. 2 through the assumed continuously increasing attention to the stimuli up to a virtual 30th training session.
In summary, our data are comparable with those from the Mongolian gerbil and are in harmony with human data on auditory discrimination learning and transfer of knowledge and cognitive skills between tasks, suggesting that shuttle-box discrimination learning is an adequate means to perform complex auditory learning tasks in small rodents. This experience suggests a promising use of mice of different genetic backgrounds to test genetic influences on auditory perception and/or learning abilities.
Materials and Methods
Animals.
We successfully trained and tested 73 female mice (Mus musculus, outbred strain NMRI). For details see SI Materials and Methods. The experiments were approved by the Regierungspräsidium Tübingen, Germany.
Apparatus and Training Procedure.
Mice were trained in a two-compartment shuttle-box for small rodents (Coulbourn Instruments) using go/no-go avoidance discrimination learning. The sound stimuli were digitally synthesized pure tones (PT) and amplitude-modulated (AM) pure tones used as CS+ (go) and CS− (no-go). Each daily training session consisted of 60 trials with 30 randomized presentations of each of the conditioned stimuli (CS+ and CS−). Training for a given task lasted 15 days, because in preliminary tests this period proved to be optimal for the mice to reach, on average, a constant, maximum performance level (SI Materials and Methods and Table S1).
Data Analysis.
For every training session and experimental group, group means with SD were calculated from individual CR+ and CR− response rates (hits and false alarms) as well as d’ = z(CR+ rate) − z(CR− rate) (z is the normal distribution) according to signal detection theory (32). In addition, the significance of discrimination performance on each single training session was calculated for each individual animal by testing the response rates to the CS+ and CS− stimuli (hits, misses, false alarms, correct rejections) in a χ2 test. Finally, differences between the rates of CR+ and CR− responses were tested for each training session in all training groups by the Mann–Whitney U test. In all these tests, significance levels of P < 0.05, P < 0.01, and P < 0.001 were applied and are indicated in the figures. The development of the d′ function over the training sessions was approximated by linear regressions which are meant simply as low-parameter estimates to quantify and to compare present data from different learning tasks. The statistical significance of the regression lines is expressed by the correlation coefficient r. The slopes of the regression lines were tested for significant differences.
Supplementary Material
Acknowledgments
We thank Anja Dorrn, Elisabeth Picca, and Sabine Schmidt for taking part in animal training and for skillful technical assistance. We also thank the Leibniz Institute for Neurobiology, Magdeburg, Germany, for providing the shuttle-box and Dr. Frank Ohl for comments on a previous version of the manuscript.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912357107/DCSupplemental.
References
- 1.Delhommeau K, Micheyl C, Jouvet R, Collet L. Transfer of learning across durations and ears in auditory frequency discrimination. Percept Psychophys. 2002;64:426–436. doi: 10.3758/bf03194715. [DOI] [PubMed] [Google Scholar]
- 2.Demany L, Semal C. Learning to perceive pitch differences. J Acoust Soc Am. 2002;111:1377–1388. doi: 10.1121/1.1445791. [DOI] [PubMed] [Google Scholar]
- 3.Grimault N, Micheyl C, Carlyon RP, Bacon SP, Collet L. Learning in discrimination of frequency or modulation rate: Generalization to fundamental frequency discrimination. Hear Res. 2003;184:41–50. doi: 10.1016/s0378-5955(03)00214-4. [DOI] [PubMed] [Google Scholar]
- 4.Karmarkar UR, Buonomano DV. Temporal specificity of perceptual learning in an auditory discrimination task. Learn Mem. 2003;10:141–147. doi: 10.1101/lm.55503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu EH, Mercado E, III, Church BA, Orduña I. The easy-to-hard effect in human (Homo sapiens) and rat (Rattus norvegicus) auditory identification. J Comp Psychol. 2008;122:132–145. doi: 10.1037/0735-7036.122.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright BA, Zhang Y. A review of the generalization of auditory learning. Philos Trans R Soc Lond B Biol Sci. 2009;364:301–311. doi: 10.1098/rstb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wetzel W, Wagner T, Ohl FW, Scheich H. Categorical discrimination of direction in frequency-modulated tones by Mongolian gerbils. Behav Brain Res. 1998;91:29–39. doi: 10.1016/s0166-4328(97)00099-5. [DOI] [PubMed] [Google Scholar]
- 8.Ohl FW, Scheich H, Freeman WJ. Change in pattern of ongoing cortical activity with auditory category learning. Nature. 2001;412:733–736. doi: 10.1038/35089076. [DOI] [PubMed] [Google Scholar]
- 9.Ehret G. Psychophysics. In: Willott JF, editor. The Auditory Psychobiology of the Mouse. Springfield. IL: CC Thomas; 1983. pp. 13–56. [Google Scholar]
- 10.Ehret G. Hearing in the mouse. In: Dooling RJ, Hulse SH, editors. The Comparative Psychology of Audition: Perceiving Complex Sounds. Hillsdale: Lawrence Erlbaum; 1989. pp. 3–32. [Google Scholar]
- 11.Willott JF, editor. Handbook of Mouse Auditory Research. From Behavior to Molecular Biology. FL: CRC, Boca Raton; 2001. [Google Scholar]
- 12.Bovet D, Bovet-Nitti F, Oliverio A. Memory and consolidation mechanisms in avoidance learning of inbred mice. Brain Res. 1968;10:168–182. doi: 10.1016/0006-8993(68)90120-0. [DOI] [PubMed] [Google Scholar]
- 13.Bovet D, Bovet-Nitti F, Oliverio A. Genetic aspects of learning and memory in mice. Science. 1969;163:139–149. doi: 10.1126/science.163.3863.139. [DOI] [PubMed] [Google Scholar]
- 14.Schleidt WM, Kickert-Magg M. Hearing thresholds of albino house mouse between 1 and 80 kHz by shuttle-box training. J Aud Res. 1979;19:37–40. [PubMed] [Google Scholar]
- 15.Alleva E, de Acetis L, Amorico L, Bignami G. Amphetamine, conditioned stimulus, and nondebilitating preshock effects on activity and avoidance: Further evidence for interactions between associative and nonassociative changes. Behav Neural Biol. 1983;39:78–104. doi: 10.1016/s0163-1047(83)90654-4. [DOI] [PubMed] [Google Scholar]
- 16.Wetzel W, Ohl FW, Scheich H. Global versus local processing of frequency-modulated tones in gerbils: An animal model of lateralized auditory cortex functions. Proc Natl Acad Sci USA. 2008;105:6753–6758. doi: 10.1073/pnas.0707844105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze H, Scheich H. Discrimination learning of amplitude modulated tones in Mongolian gerbils. Neurosci Lett. 1999;261:13–16. doi: 10.1016/s0304-3940(98)00991-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaernbach C, Schulze H. Auditory sensory memory for random waveforms in the Mongolian gerbil. Neurosci Lett. 2002;329:37–40. doi: 10.1016/s0304-3940(02)00570-0. [DOI] [PubMed] [Google Scholar]
- 19.Deutscher A, Kurt S, Scheich H, Schulze H. Cortical and subcortical sides of auditory rhythms and pitches. Neuroreport. 2006;17:853–856. doi: 10.1097/01.wnr.0000221837.20255.62. [DOI] [PubMed] [Google Scholar]
- 20.Van Wassenhove V, Nagarajan SS. Auditory cortical plasticity in learning to discriminate modulation rate. J Neurosci. 2007;27:2663–2672. doi: 10.1523/JNEUROSCI.4844-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson K, Summerfield AQ. Adult auditory learning and training. Ear Hear. 1996;17:51S–65S. doi: 10.1097/00003446-199617031-00006. [DOI] [PubMed] [Google Scholar]
- 22.Amitay S, Hawkey DJC, Moore DR. Auditory frequency discrimination learning is affected by stimulus variability. Percept Psychophys. 2005;67:691–698. doi: 10.3758/bf03193525. [DOI] [PubMed] [Google Scholar]
- 23.Doane SM, Sohn YW, Schreiber B. The role of processing strategies in the acquisition and transfer of a cognitive skill. J Exp Psychol Hum Percept Perform. 1999;25:1390–1410. [Google Scholar]
- 24.Karni A, Bertini G. Learning perceptual skills: Behavioral probes into adult cortical plasticiry. Curr Opin Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 25.Ehret G. Age-dependent hearing loss in normal hearing mice. Naturwissenschaften. 1974;61:506–507. doi: 10.1007/BF00622976. [DOI] [PubMed] [Google Scholar]
- 26.Ohl FW, Wetzel W, Wagner T, Rech A, Scheich H. Bilateral ablation of auditory cortex in Mongolian gerbil affects discrimination of frequency modulated tones but not of pure tones. Learn Mem. 1999;6:347–362. [PMC free article] [PubMed] [Google Scholar]
- 27.Demany L. Perceptual learning in frequency discrimination. J Acoust Soc Am. 1985;78:1118–1120. doi: 10.1121/1.393034. [DOI] [PubMed] [Google Scholar]
- 28.Langner G. Periodicity coding in the auditory system. Hear Res. 1992;60:115–145. doi: 10.1016/0378-5955(92)90015-f. [DOI] [PubMed] [Google Scholar]
- 29.Langner G. Topographic representation of periodicity information: The 2nd neural axis of the auditory system. In: Syka J, Merzenich MM, editors. Plasticity and Signal Representation in the Auditory System. New York: Springer; 2004. pp. 37–51. [Google Scholar]
- 30.Ashby FG, Alfonoso-Reese LA, Turken U, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychol Rev. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- 31.Spiering BJ, Ashby FG. Initial training with difficult items facilitates information-integration but not rule-based category learning. Psychol Sci. 2008;19:1169–1177. doi: 10.1111/j.1467-9280.2008.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macmillan NA, Creelman CD. Detection Theory: A User's Guide. New York: Cambridge University Press; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.