Abstract
S-palmitoylation is a conserved feature in many G protein–coupled receptors (GPCRs) involved in a broad array of signaling processes. The prototypical GPCR, rhodopsin, is S-palmitoylated on two adjacent C-terminal Cys residues at its cytoplasmic surface. Surprisingly, absence of palmitoylation has only a modest effect on in vitro or in vivo signaling. Here, we report that palmitoylation-deficient (Palm−/−) mice carrying two Cys to Thr and Ser mutations in the opsin gene displayed profound light-induced retinal degeneration that first involved rod and then cone cells. After brief bright light exposure, their retinas exhibited two types of deposits containing nucleic acid and invasive phagocytic macrophages. When Palm−/− mice were crossed with Lrat−/− mice lacking lecithin:retinol acyl transferase to eliminate retinoid binding to opsin and thereby rendering the eye insensitive to light, rapid retinal degeneration occurred even in 3- to 4-week-old animals. This rapid degeneration suggests that nonpalmitoylated rod opsin is unstable. Treatment of 2-week-old Palm−/−Lrat−/− mice with an artificial chromophore precursor prevented this retinopathy. In contrast, elimination of signaling to G protein in Palm−/−Gnat1−/− mice had no effect, indicating that instability of unpalmitoylated opsin lacking chromophore rather than aberrant signal transduction resulted in retinal pathology. Together, these observations provide evidence for a structural role of rhodopsin S-palmitoylation that may apply to other GPCRs as well.
Keywords: G protein–coupled receptor, light damage, rhodopsin, S-palmitoylation, vision
Class A receptors of the membrane-bound G protein–coupled receptor (GPCR) superfamily contain either a single or tandem Cys residues following the putative helix 8 on their cytoplasmic surface (1). In a few cases, including rhodopsin (PDB:F88) and the β1-adrenergic receptor (PDB: 2RH1), biochemical approaches and more recent structural data (2) provide evidence that these Cys residues are S-palmitoylated. The effect of this modification on GPCR function in experimental cell lines varies and palmitoylation/depalmitoylation appears to be a dynamic process that can be ligand-dependent (reviewed in ref. 3). However, to our knowledge, no in vivo data are available for any of these receptors except rhodopsin.
A prototypical member of the GPCR family (4), rhodopsin has two palmitoylated Cys residues next to helix 8 that are highly conserved among different species (Fig. S1 A and B). One of the cone opsins which is sensitive to short wavelength light also carries a possible palmitoylated Cys residue (Fig. S1B), but this residue is not palmitoylated in mice (5).
Effects of palmitoylation have been studied either by chemically removing the fatty acid with DTT or hydroxylamine treatment or by mutating the Cys residues forming the covalent fatty acid linkage and expressing the mutant receptor in a heterologous expression system (6–10). These approaches revealed palmitoylation-related changes under certain conditions. For instance, lack of palmitoylation was reported to affect both transducin activation and rhodopsin regeneration (8, 10). However, these changes were not consistently observed in other studies (9), emphasizing the difficulty in distinguishing between effects of harsh chemical treatment, expression in a heterologous system, and the fatty acid linkage itself.
To circumvent secondary effects on receptor structure and function, a knock-in mouse model expressing palmitoylation-deficient rhodopsin was generated wherein Cys residues at positions 322 and 323 were mutated to Thr and Ser, respectively (11). Initial characterization of these Palm−/− mice showed that the mutant rhodopsin became hyperphosphorylated upon light activation, thereby enhancing “shut-off” of rhodopsin (11). Palm−/− mice manifested differences in the rate of transducin activation by rhodopsin and single-molecule force microscopy revealed changes in the force needed to unfold the last stable structural segment of rhodopsin at its carboxyl terminal end (12). However, these differences, although significant, were relatively minor, suggesting that the principal role of these conserved palmitoylation sites remains undefined. By using the Palm−/− model mouse to further clarify the role of palmitoylation on rhodopsin structure and function, we now show under physiological conditions that rhodopsin palmitoylation is critical for the stability and proper function of opsin in rod photoreceptor cells.
Results
Palmitoylation Does Not Affect Retinal Morphology and Visual Retinoid Kinetics in Mice with Defects in Enzymes of the Visual Cycle Kept Under Normal Laboratory Lighting Conditions.
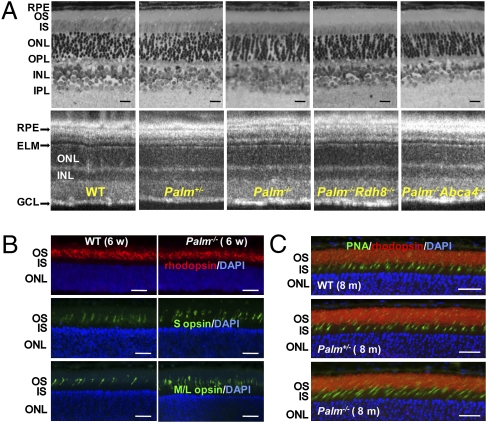
Palmitoylation of rhodopsin was reported to be associated with proper transport of the visual chromophore, 11-cis-retinal, and its photoisomerized counterpart, all-trans-retinal (13). Because delayed all-trans-retinal clearance directly leads to retinal degeneration (14, 15), we established mice with a deletion of the palmitoylation sites (Palm−/−) combined with a knockout of either ATP-binding cassette transporter 4 (Abca4−/−) or retinol dehydrogenase 8 (Rdh8−/−), two important enzymes for all-trans-retinal clearance from the retina. All these mutant animals, namely Palm+/−, Palm−/−, Palm−/−Rdh8−/−, and Palm−/−Abca4−/− mice, showed retinal morphologies similar to WT mice at 6 weeks of age (Fig. 1A). Expression of rhodopsin, S cone opsin, and M/L cone opsin in Palm+/− and Palm−/− mice was also similar to that of WT mice at 6 weeks and 8 months of age (Fig. 1 B and C). No retinal degeneration was observed in mutant mice kept under normal laboratory lighting conditions. Palmitoylation deficiency did not change the kinetics of all-trans-retinal clearance and 11-cis-retinal production in these mice after illumination with 500 cd/m2 for 3 min (decay rates: WT, 2.49e+2; Palm+/−, 2.48e+2; Palm−/−, 2.44e+2; Palm−/−Rdh8−/−, 2.28e+2; Palm−/−Abca4−/−, 2.46 e+2; and Rdh8−/−, 2.29e+2; Fig. S1C).
Fig. 1.
Palmitoylation does not affect retinal morphology of mice with different genetic backgrounds maintained under normal laboratory lighting conditions. (A) Representative retinal histology (Upper) and B-scanned averaged SD-OCT images (Lower) are shown for WT, Palm+/−, Palm−/−, Palm−/−Rdh8−/−, and Palm−/−Abca4−/− mice at 6 weeks of age (n > 3). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ELM, external limiting membrane; IS, inner segment; OS, outer segment; RPE, retinal pigmented epithelium. (Scale bars: 10 μm.) (B) Immunohistochemistry of 6-week-old WT and Palm−/− retinas stained with antirhodopsin (1D4), anti–S opsin and anti–M/L opsin antibodies. No degeneration was observed. Abbreviations are the same as in A. (Scale bars: 10 μm). (C) Immunohistochemistry of 8-month-old WT, Palm+/−, and Palm−/− retinas stained with peanut agglutinin lectin (PNA) and antirhodopsin (1D4) antibody. No degeneration was observed. (Scale bars: 10 μm.)
Bright Light Induces Severe Retinal Degeneration in Palmitoylation-Deficient Mice.
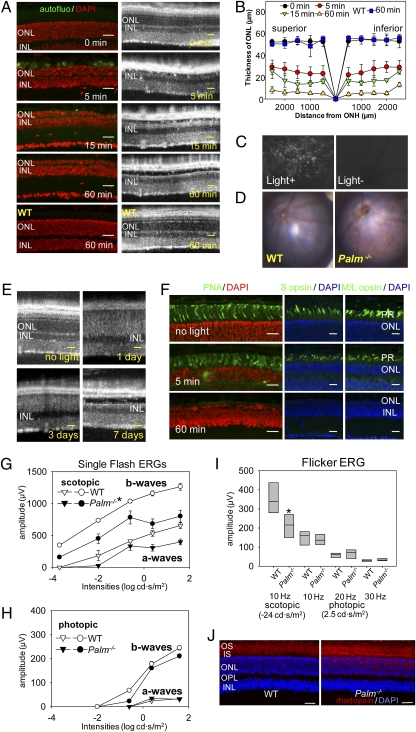
Surprisingly, 6-week-old Palm−/− mice illuminated with 10,000 lx light for only 5 min exhibited a 50% loss of photoreceptors, whereas WT control mice exhibited no significant retinal degeneration after the same light exposure for 60 min (Fig. 2 A and B). More than 80% of 11-cis-retinal was photoisomerized in Palm−/− mice after this 5-min exposure (Fig. S2), which also caused autofluorescent deposits between the outer nuclear layer (ONL) and the retinal pigmented epithelium (RPE) (Fig. 2 A and C). Retinas of Palm−/− mice showed major atrophic changes after illumination for 60 min (Fig. 2D). Photoreceptor death was observed 1 and 3 d after 60 min light exposure and retinas exhibited atrophic changes 7 d thereafter (Fig. 2E). Importantly, cone photoreceptors were preserved after illumination with 10,000 lx for 5 min (Fig. 2F). Less intense light exposure (12 h at 1,000 lx/12 h darkness daily for 4 weeks) induced rod photoreceptor dysfunction with mislocalization of rhodopsin in the ONL and photoreceptor inner segments, but did not impair cone photoreceptor function (Fig. 2 G–J).
Fig. 2.
Bright light induces severe retinal degeneration in Palm−/− mice. (A) Palmitoylation-deficient (Palm−/−) and WT mice at 6 weeks of age were exposed to 10,000 lx light for periods indicated and then kept in the dark for 7 d, at which time SD-OCT and histological examinations were performed. Severe retinal degeneration was observed in Palm−/− mice whereas no degeneration was detected in WT retinas exposed to bright light for 60 min. INL, inner nuclear layer. (Scale bars: 20 μm.) (B) The thickness of the outer nuclear layer was measured 7 d after illumination with 10,000 lx for the indicated periods. ONH, optic nerve head. Error bars indicate SD of the means (n > 3). (C) Shown are representative outer retinal images obtained by SLO from 6-week-old Palm−/− mice exposed to 10,000 lx light for 5 min and then kept in the dark until examined 7 d later (Left) or from unexposed control mice kept in the dark (Right). Numerous autofluorescent retinal deposits are observed in illuminated Palm−/− mice. (D) Representative fundus images are shown from 6-week-old WT and Palm−/− mice taken 7 d after retinal illumination with 10,000 lx light for 60 min. Palm−/− mice manifest widespread atrophic changes. (E) SD-OCT B-scan imaging of the same eye performed at 1, 3, and 7 d after retinal exposure of 6-week-old Palm−/− mice to 10,000 lx light for 30 min. Hazy changes of photoreceptor layers 1 and 3 d after illumination indicate ongoing photoreceptor cell death. (Scale bars: 10 μm.) (F) Immunohistochemistry of cone photoreceptors performed with PNA, anti–S cone opsin, and anti–M/L cone opsin antibodies 7 d after retinal illumination of 6-week-old Palm−/− mice with 10,000 lx light for 5 and 60 min. (Scale bars: 10 μm.) Palm−/− and WT mice at 3 months of age were kept under 12-h light (1,000 lx)/12-h dark conditions for 4 weeks, and then ERG and histological examinations were done 7 d after dark adaptation. Full-field ERG responses were recorded under scotopic (G) and photopic (H) conditions. Both a- and b-wave amplitudes under scotopic conditions were attenuated in Palm−/− mice compared with WT animals. (I) Flicker ERGs recorded at 10, 20, and 30 Hz showed significant decreases for Palm−/− compared with WT mice under scotopic conditions, whereas no differences were observed under photopic conditions. Error bars indicate SE of the means (n > 3; *P < 0.03) vs. WT animals. (J) Retinal structures were assessed with outer segment (red, antirhodopsin 1D4), and nuclear (blue, DAPI) staining. Whereas retinal structure was preserved in both WT and Palm−/− mice, mislocalization of some rhodopsin in the ONL was observed in Palm−/− mice. Representative images are presented (n > 3). (Scale bars: 10 μm.)
Heterozygous Palm+/− Retinas Are More Resistant to Light-Induced Retinal Degeneration Than Retinas from Palm−/− Mice.
Although light-induced severe retinal degeneration was observed in 6-week-old Palm−/−, Palm−/−Rdh8−/−, and Palm−/−Abca4−/− mice, Palm+/− retinas showed much milder changes (Fig. S3 A and B). Only a few Palm+/− retinas (three of eight eyes) showed rosette-like structures at the central part of the retina after 60 min light exposure (Fig. S3B). Palm−/− mice lost 70% and 90% of their photoreceptors after 15 min and 60 min light exposure at 10,000 lx, respectively, whereas Palm+/− photoreceptors were completely preserved after 15 min light exposure and less than 20% loss was seen after 60 min exposure (Fig. S3 C and D). These data suggest that having only half the rhodopsin unpalmitoylated is not sufficient to induce the extreme light sensitivity observed when all of the rhodopsin lacks palmitate. Among palmitoylation-deficient mice, Palm−/−Rdh8−/− animals showed the most severe retinal degeneration, and 6-month-old Palm−/−Rdh8−/− mice exhibited rosette-like retinal degeneration after routine maintenance in a 12 h light (10 lx)/12 h dark cycle (Fig. S4).
Deposits and Macrophage Infiltration in Degenerating Retinas of Bright Light–Exposed Palm−/− mice.
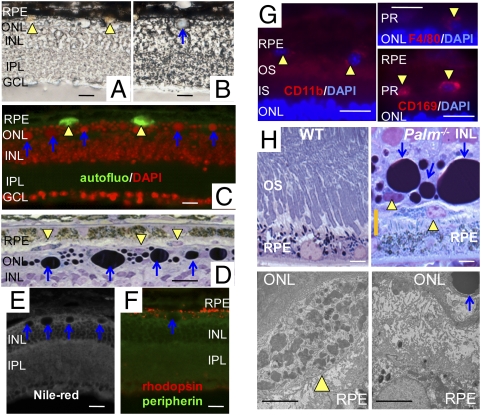
Severe light-induced retinal degeneration in Palm−/− mice revealed two morphologically distinct deposits, colored yellowish and blue, between the ONL and the RPE (Fig. 3 A and B). Yellow deposits exhibited autofluorescence (Fig. 3C), and the largest number of them were detected at 5 min after exposure to 10,000 lx light (Fig. S5A). These deposits were also identified by toluidine blue staining in cells recognized by antimacrophage markers including CD11b, F4/80, and CD169 (Fig. 3 D and G). Blue deposits were recognized by either DNA-staining DAPI and Hoechst 33342 or 33258 dyes, as well as by nucleic acid-staining SYTOX (Invitrogen; Fig. S5B). They also stained dark purple with toluidine blue, but not with Nile red dye (lipid detection) or retina-specific antirhodopsin and antiperipherin antibodies (Fig. 3 C–F), suggesting that they contained nucleic acid–related material. Together these findings are consistent with EM images that showed macrophages containing retinal debris between the ONL and the RPE but no photoreceptors between the RPE and neural retina (Fig. 3H). Importantly, strong light illumination did not increase amounts of all-trans-retinal condensation products including di-retinoid-pyridinium-ethanolamine (A2E) in the retinas of Palm−/− mice (Fig. S6).
Fig. 3.
Retinal deposits and macrophage infiltration in Palm−/− mice. Six-week-old Palm−/− mice with pupils dilated with 1% tropicamide were illuminated with 10,000 lx light for 60 min and then dark-adapted for 7 d before analyses. Bright field cryosection images revealed yellowish (yellow arrowheads) (A) and light blue (blue arrow) (B) retinal deposits between the ONL and the RPE. INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. (Scale bars: 10 μm.) (C) Yellowish retinal deposits in A were autofluorescent (green) and blue deposits in B stained with DAPI (red). (Scale bar: 10 μm.) (D) Retinal deposits with yellow color in A were located in invasive cells at the interface of the RPE and the ONL in epon-embedded sections. Toluidine blue staining shows dark purple coloring of the blue deposits in B. (Scale bar: 10 μm.) (E) Retinal deposits with blue color in B were not stained by Nile red dye, which recognizes lipids. (Scale bars: 10 μm.) (F) The retinal deposit with blue color in B was not recognized by antirhodopsin or antiperipherin antibodies. (Scale bar: 10 μm.) (G) Yellow deposits in A were found in cells located between the outer segment (OS) and RPE that stained with macrophage markers, CD11b (Left), F4/80 (Upper Right), and CD169 (Lower Right). PR, photoreceptors. (Scale bar: 10 μm.) (H) Higher magnified images of a photoreceptor/RPE junction in WT and Palm−/− retina are shown (Upper). Yellow arrowheads and blue arrows indicate yellow and blue deposits in A and B. EM images of the area indicated as an orange bar in Palm−/− retina (Upper Right) indicate that infiltrated macrophages contained photoreceptor debris (Lower Left), and there were no photoreceptors between the RPE and the neural retina (Lower Right). OS, outer segment; INL, inner nuclear layer. (Scale bars: 3 μm.)
Chromophore Production Is Required for Rod Outer Segment Formation in Palm−/− Mice.
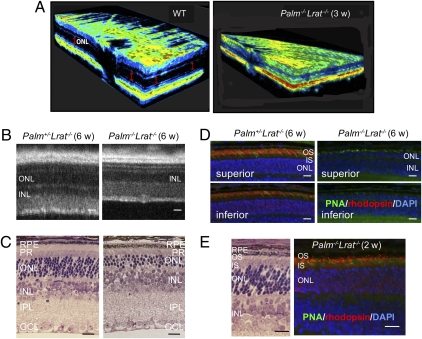
We used Palm−/−Lrat−/− mice to examine the role of retinoids in light-induced retinal degeneration of palmitoylation-deficient mice. Lrat−/− mice lack retinoids, including 11-cis-retinal, in the retina because LRAT is essential for retinoid storage in the eyes (16). By 3 weeks of age, Palm−/−Lrat−/− mice exhibited a striking loss of the ONL in a 3D spectral domain (SD) optical coherence tomography (OCT) image (Fig. 4A). Even more intriguing, 6-week-old Palm+/−Lrat−/− retinas had a normal structure with nearly normal rhodopsin expression, whereas 75% of the photoreceptors had degenerated and rhodopsin was not detectable by our antibody to its C terminus in retinas of similarly aged Palm−/−Lrat−/− mice (Fig. 4 B–D). Fewer PNA-positive cells, indicating cone photoreceptor sheaths, were observed in the inferior retinas of both Palm+/−Lrat−/− and Palm−/−Lrat−/− mice as a result of the loss of Lrat (17) (Fig. 4D and Fig. S7A). However, these PNA-positive cells did not stain with cone pigment antibodies (Fig. S7B). EM imaging showed only inner segments of cone photoreceptors, and outer segment–like structures were completely disrupted in 6-week-old Palm−/−Lrat−/− mice (Fig. S7C). Notably, developing retinas of Palm−/−Lrat−/− mice at the age of 2 weeks did express rhodopsin (Fig. 4E), indicating that defects in rhodopsin expression in 6-week-old Palm−/−Lrat−/− mice occurred after initiation of retinal outer segment development. By the age of 3 months, Palm−/−Lrat−/− mice had lost all photoreceptors in the inferior retina, and only a few remained in the superior retina (Fig. S7D). The severity of retinal degeneration was much less in Palm−/−Gnat−/− than in Palm−/−Lrat−/− mice but similar to that observed in Palm−/− animals. The fact that light-induced retinal degeneration in Palm−/−Gnat1−/− mice (mice lacking both palmitoylation and the photoreceptor G protein, transducin) was similar to that found in Palm−/− mice (Fig. S8) suggests that phototransduction signaling plays a minor role in light-induced retinal degeneration in Palm−/− mice. These data also imply that unliganded rhodopsin lacking palmitoylation can cause rod photoreceptor degeneration in Palm−/− mice.
Fig. 4.
Absence of retinal ligand causes rapid rod photoreceptor degeneration in young Palm−/−Lrat−/− mice. (A) Three-dimensional OCT images of WT and Palm−/−Lrat−/− 3-week-old mouse retinas show reduced thickness of Palm−/− ONL (Right). (B) B-scan SD-OCT images reveal reduced thickness of ONL in Palm−/−Lrat−/− retinas, whereas Palm+/−Lrat−/− retinas exhibit a healthy structure in 6-week-old mice. (Scale bars: 10 μm.) (C) Retinal histology displays a nearly normal Palm+/−Lrat−/− retina but obvious photoreceptor degeneration in a Palm−/−Lrat−/− retina from 6-week-old mice. (Scale bars: 10 μm.) (D) Immunohistochemistry with PNA and antirhodopsin antibody reveals lack of rhodopsin expression in Palm−/−Lrat−/− retinas from 6-week-old mice. Fewer cone photoreceptors are observed in both Palm+/−Lrat−/− and Palm−/−Lrat−/− retinas, especially in the inferior retina. (Scale bars: 10 μm.) (E) Morphology and immunohistochemistry with PNA and antirhodopsin antibody staining of developing retinas from 2-week-old Palm−/−Lrat−/− mice are presented. Rhodopsin is clearly visible in the OS. (Scale bars: 10 μm.)
To test whether liganding rhodopsin with retinoid chromophore would prevent retinal degeneration in Palm−/−Lrat−/− mice, we supplemented 2-week-old Palm−/−Lrat−/− mice with 150 μg of 9-cis-retinyl acetate, a precursor of the artificial chromophore 9-cis-retinal. This compound was selected because rhodopsin can use the chemically more stable 9-cis-retinal just as efficiently as the naturally unstable 11-cis-retinal for its chromophore ligand (18, 19). 9-cis-Retinyl acetate in DMSO was given intraperitoneally on d 14, 16, and 18 after birth and retinas were examined when mice were 3 weeks old. Indeed, 9-cis-retinyl acetate did succeed in preserving rod and cone photoreceptors as well as rhodopsin and cone opsins in the retinas of Palm−/−Lrat−/− mice (Fig. S9). Collectively, these data strongly indicate that palmitoylation plays an important role in preventing retinal degeneration in mice, presumably because unliganded depalmitoylated opsin is less stable than the native opsin.
Discussion
Aside from classical biochemical, biophysical, and histopathological studies, the visual system is amenable to exquisite in vivo evaluation of molecular events. Retinal rod photoreceptors contain rhodopsin, a prototypical GPCR signaling system. Palmitoylation of rhodopsin was first characterized by O'Brien and Zatz (20) and the sites of palmitoylation were determined by Ovchinnikov and colleagues (21). Originally, it was speculated that palmitoylation could be a nonenzymatic event (22, 23), but with the discovery of a family of fatty acid CoA transferases (24), this assumption remains an open question.
Depalmitoylation of rhodopsin by chemical treatment with hydroxylamine was extensively used to elucidate the role of palmitoylation (6, 10, 25–27), but the results should be interpreted with caution, especially because high concentrations of this strong nucleophile can produce off-target effects on membrane structures. Expressed in COS cells, bovine opsin mutants containing a serine substitution at Cys-323 showed reduced light-dependent phosphorylation by rhodopsin kinase (9), but subsequent studies employing Cys-322-Thr and Cys-323-Ser knock-in mice revealed that, after exposure to light, rhodopsin became phosphorylated at a faster rate in mutant than in WT retinas (11). Conformational changes in the C terminus were observed upon depalmitoylation in biochemical assays (25). Ensuing structural analysis of rhodopsin by single-molecule force spectroscopy revealed that depalmitoylation was associated with a 2.1-fold reduction in the normalized force required to unfold its carboxyl terminus. Accordingly, lack of palmitoylation in light-activated rhodopsin probably destabilizes molecular interactions in the carboxyl terminal end of this receptor that appear to be required for proper activation of transducin (12). The region containing helix 8 and palmitoylation sites was also considered to be involved in retinoid-binding that activates opsin without light (13). Removal of the palmitoyl groups from Cys-322 and Cys-323 of the rhodopsin COOH terminus impaired all-trans-retinal–stimulated activity, suggesting that palmitoylation mediated hydrophobic interactions with retinoids that could activate a large part of the catalytic receptor/G protein interface (6). However, all these reported effects were relatively modest and did not address the central question of why these sites are evolutionarily conserved. To clarify these findings, we investigated the role of palmitoylation of rhodopsin under natural conditions of in vivo bright light stress to more precisely reveal its function.
Lack of Palmitoylation Failed to Affect Rhodopsin's Function and Biochemistry in Mice Maintained Under Normal Laboratory Lighting.
The rod-and-cone visual system of mice lacking palmitoylated rhodopsin was not affected if animals were maintained in the dark or under mild cyclic lighting conditions; retinoid flow and retinal structure were only minimally compromised (Fig. 1). To stress the visual system, we crossed Palm−/− mice with mice having gene deletions for two enzymes involved in all-trans-retinal clearance, thereby generating double-knockout Palm−/−Rdh8−/− and Palm−/−Abca4−/− animals. Standard laboratory cyclic lighting conditions (12 h in 10 lx light/12 h darkness) apparently had little effect on rods or cones of either model. All these mice showed retinal morphology similar to WT mice at 6 weeks of age and the kinetics of all-trans-retinal clearance and 11-cis-retinal production were unchanged (Fig. S1). The next question was whether stronger light, known to enhance retinoid flow through the visual cycle, might selectively damage the retinas of these knockout mice and, if so, whether ablation of the visual cycle might protect their retinas from such damage.
Exposure of Palmitoylation-Deficient Mice to Bright Light Causes Severe Retinal Degeneration.
Palm−/− mice, but not Palm+/− or WT mice, displayed severe retinal degeneration when exposed to 10,000 lx light, comparable to that of bright daylight (Fig. 2). Most striking was that only 5 min of such light exposure was needed to produce severe retinal degeneration. This suggests an acute mechanism of injury rather than involvement of slower cellular processes such as formation of retinoid conjugates that were elevated but unchanged by bright light exposure in both Palm−/−Abca4−/− and Abca4−/− mice (Fig. S6). The short time course also implies that this retinal pathology resulted from either depalmitoylated opsin, a burst of released all-trans-retinal, or both. Involvement of all-trans-retinal is supported by the finding that retinal damage after bright light exposure was more severe in Palm−/−Rdh8−/− mice than in either Palm−/− or Palm−/−Abca4−/− mice (Fig. S3) (15), which also suggests that cytoplasmic released all-trans-retinal is more toxic than the fraction retained within rod disk membranes. Additionally, Palm−/−Rdh8−/− mice showed the most severe retinal degeneration among all tested Palm−/− mice, displaying characteristic rosette-like retinal degeneration after routine laboratory light exposure that typifies all-trans-retinal toxicity (Fig. S4). The last issue was further addressed by removing the visual cycle in subsequent experiments discussed in subsequent sections.
Along with degeneration, rod cell function also diminished and numerous autofluorescent retinal deposits were observed in brightly illuminated Palm−/− mice. Predictably, cone function was initially well preserved, and the number of cones were substantially reduced only after a large fraction of rod photoreceptors had degenerated.
Retinal Pathology and Macrophage Infiltration.
The earliest signs of retinal pathology in light exposed Palm−/− mice were two types of deposits originating from photoreceptor cell degeneration (Fig. 3). These could originate from membrane and nuclear proteins and from condensation of chromophore. The yellow deposits were autofluorescent and appeared to be engulfed by phagocytes recognized by antimacrophage markers including CD11b, F4/80, and CD169 (Fig. 3G). These deposits probably represent condensation products of rod cell membrane proteins and retinoids, but they are not condensation products of the A2E type (Fig. S6) (28). A2E would require more time for its formation, phagocytosis, and deposition in discrete compartments of the RPE. Indeed, EM revealed macrophages containing retinal debris between the ONL and the RPE. In contrast, the so-called blue deposits primarily consist of nucleic acid–related molecules because they stained with nuclear DNA- and RNA-recognizing reagents (Fig. S5), but not with Nile red dye or anti–retina-specific antibodies such as antirhodopsin and antiperipherin antibodies (Fig. 3). The rapid onset of severe bright light–induced retinal pathology then raises the question of whether aberrant signaling of depalmitoylated rhodopsin, its phosphorylation, or removal of retinoids as the main light absorbing material in the retina is the major determinant of acute light-induced retinal degeneration in Palm−/− mice.
Palmitoylation-Deficient Opsin Is Unstable, Leading to Light-Induced Photoreceptor Degeneration.
First we investigated whether possible aberrant signaling by palmitoylation-deficient rhodopsin might contribute to the observed acute retinal degeneration (29). Palm−/−Gnat1−/− mice were generated to eliminate the phototransduction cascade because the rod photoreceptor–specific G protein is genetically removed (Fig. S8). Collectively, our experimental results suggest that light-induced retinal damage in these double-knockout mice is no different than in Palm−/− mice, eliminating the possibility that signaling plays a major role in the observed retinal degeneration. Next, we removed retinoids and inhibited the visual cycle that produces chromophore (16) by producing Palm−/−Lrat−/− mice that cannot store retinoids in the eye (30). Here we found that Palm−/−Lrat−/− mice exhibited a striking loss of rod photoreceptor cells by the age of 6 weeks. Cones, when they had been formed, disappeared more rapidly and were largely absent in the inferior retina, probably because Lrat−/− mice lack chromophore required for cone cell maintenance (Fig. S7). Importantly, this severe retinopathy in Palm−/−Lrat−/− mice could be prevented by pharmacological pretreatment with the artificial chromophore precursor, 9-cis-retinyl acetate, indicating that lack of chromophore contributes to this type of retinal degeneration. Thus, processes leading to massive removal of chromophore, either by light stimulation or elimination of the visual cycle, markedly destabilize palmitoylation-deficient opsin, resulting in preferential destruction of rod photoreceptors.
Implications for Human Retinal Diseases.
To our knowledge, no human retinal degeneration associated with mutations at palmitoylation sites of rhodopsin has yet been reported, most likely because recessive removal of both palmitoylation sites at positions 322 and 323 would be required (Fig. S1B). Palm−/− mice are highly sensitive to light, whereas Palm+/− mice are far less susceptible to light-induced retinal degeneration (Fig. S3). This suggests that partial palmitoylation is sufficient to protect rhodopsin's structure, perhaps because it exists as oligomers within native rod outer segment membranes, which also helps stabilize its structure (31). Our results also unveil a possible mechanism for early cone degeneration in Lrat−/− and Rpe65−/− (lacking retinoid isomerase RPE65) mice lacking functional chromophore. This phenotype is also observed in certain patients with early-onset severe retinal degeneration, namely Leber congenital amaurosis with RPE65 mutations (17). Based on our observations and because cone opsins structurally are closely related to rhodopsin but could be unpalmitoylated (5), lack of chromophore may selectively destabilize cone opsins, leading to subsequent cone degeneration.
In summary, rod and cone photoreceptor death is induced by a lack of chromophore and palmitoylation of opsins. Palmitoylation was shown to be important for stabilizing unliganded opsins and tandem palmitoylation of rhodopsin helps protect against light-induced retinal degeneration.
Methods
Animals.
All animal procedures and experiments were approved by the Case Western Reserve University Institutional Animal Care and Use Committees and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology.
Induction and Analysis of Light Damage.
Six-week-old mice were dark-adapted for 48 h before exposure to light. Light damage was induced by eye exposure to 10,000 lx of diffuse white fluorescent light (150 W spiral lamp; Commercial Electric) for the indicated time periods. Before such light exposure, pupils of mice were dilated with 1% tropicamide and after exposure animals were kept in the dark until evaluation.
Full descriptions of analytical methods employed such as SD-OCT, scanning laser ophthalmoscopy (SLO), electroretinography (ERG), and retinoid and histological analyses can be found in SI Methods.
Supplementary Material
Acknowledgments
We thank Drs. L. T. Webster, Jr., M. Golczak, S. Shiose, Y. Chen, T. Orban, M. Hitomi, S. Roos, M. S. Matosky, and H. Matsuyama (Case Western Reserve University) for their comments and technical support. This work was supported by National Institutes of Health Grants K08 EY019031, K08 K08EY019880, R01 EY009339, EY004939, EY019478, EY008061, EY008123, EY018085, EY012008, and P30 EY011373; an unrestricted grant from Research to Prevent Blindness Foundation to the Department of Ophthalmology and Visual Sciences at Case Western Reserve University (A.M., T.M., and P.S.-H.P.) and a Research to Prevent Blindness Career Development Award (P.S.-H.P.); Career Development Award to Medical University of South Carolina and Challenge Grant to Tufts Medical Center; Foundation Fighting Blindness; and the Ohio and Massachusetts Lions Eye Research Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000640107/DCSupplemental.
References
- 1.Mirzadegan T, Benkö G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42:2759–2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustafi D, Palczewski K. Topology of class A G protein-coupled receptors: insights gained from crystal structures of rhodopsins, adrenergic and adenosine receptors. Mol Pharmacol. 2009;75:1–12. doi: 10.1124/mol.108.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrecilla I, Tobin AB. Co-ordinated covalent modification of G-protein coupled receptors. Curr Pharm Des. 2006;12:1797–1808. doi: 10.2174/138161206776873716. [DOI] [PubMed] [Google Scholar]
- 4.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ablonczy Z, Kono M, Knapp DR, Crouch RK. Palmitylation of cone opsins. Vision Res. 2006;46:4493–4501. doi: 10.1016/j.visres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachs K, Maretzki D, Meyer CK, Hofmann KP. Diffusible ligand all-trans-retinal activates opsin via a palmitoylation-dependent mechanism. J Biol Chem. 2000;275:6189–6194. doi: 10.1074/jbc.275.9.6189. [DOI] [PubMed] [Google Scholar]
- 7.Traxler KW, Dewey TG. Effects of depalmitoylation on physicochemical properties of rhodopsin. Biochemistry. 1994;33:1718–1723. doi: 10.1021/bi00173a014. [DOI] [PubMed] [Google Scholar]
- 8.Marin EP, et al. The amino terminus of the fourth cytoplasmic loop of rhodopsin modulates rhodopsin-transducin interaction. J Biol Chem. 2000;275:1930–1936. doi: 10.1074/jbc.275.3.1930. [DOI] [PubMed] [Google Scholar]
- 9.Karnik SS, Ridge KD, Bhattacharya S, Khorana HG. Palmitoylation of bovine opsin and its cysteine mutants in COS cells. Proc Natl Acad Sci USA. 1993;90:40–44. doi: 10.1073/pnas.90.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison DF, O'Brien PJ, Pepperberg DR. Depalmitylation with hydroxylamine alters the functional properties of rhodopsin. J Biol Chem. 1991;266:20118–20123. [PubMed] [Google Scholar]
- 11.Wang Z, et al. Enhanced shutoff of phototransduction in transgenic mice expressing palmitoylation-deficient rhodopsin. J Biol Chem. 2005;280:24293–24300. doi: 10.1074/jbc.M502588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park PS, et al. Modulation of molecular interactions and function by rhodopsin palmitylation. Biochemistry. 2009;48:4294–4304. doi: 10.1021/bi900417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schadel SA, et al. Ligand channeling within a G-protein-coupled receptor. The entry and exit of retinals in native opsin. J Biol Chem. 2003;278:24896–24903. doi: 10.1074/jbc.M302115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda A, et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batten ML, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda T, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009;18:2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Hooser JP, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci USA. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batten ML, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien PJ, Zatz M. Acylation of bovine rhodopsin by [3H]palmitic acid. J Biol Chem. 1984;259:5054–5057. [PubMed] [Google Scholar]
- 21.Ovchinnikov YuA, Abdulaev NG, Bogachuk AS. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988;230:1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien PJ, St Jules RS, Reddy TS, Bazan NG, Zatz M. Acylation of disc membrane rhodopsin may be nonenzymatic. J Biol Chem. 1987;262:5210–5215. [PubMed] [Google Scholar]
- 23.St Jules RS, O'Brien PJ. The acylation of rat rhodopsin in vitro and in vivo. Exp Eye Res. 1986;43:929–940. doi: 10.1016/0014-4835(86)90071-0. [DOI] [PubMed] [Google Scholar]
- 24.Planey SL, Zacharias DA. Palmitoyl acyltransferases, their substrates, and novel assays to connect them (Review) Mol Membr Biol. 2009;26:14–31. doi: 10.1080/09687680802646703. [DOI] [PubMed] [Google Scholar]
- 25.Ohguro H, et al. Structural and enzymatic aspects of rhodopsin phosphorylation. J Biol Chem. 1996;271:5215–5224. doi: 10.1074/jbc.271.9.5215. [DOI] [PubMed] [Google Scholar]
- 26.Pepperberg DR, Morrison DF, O'Brien PJ. Depalmitoylation of rhodopsin with hydroxylamine. Methods Enzymol. 1995;250:348–361. doi: 10.1016/0076-6879(95)50084-7. [DOI] [PubMed] [Google Scholar]
- 27.Young JE, Albert AD. Rhodopsin palmitoylation in bovine rod outer segment disk membranes of different age/spatial location. Exp Eye Res. 2001;73:735–737. doi: 10.1006/exer.2001.1081. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow JR, et al. A2E, a byproduct of the visual cycle. Vision Res. 2003;43:2983–2990. doi: 10.1016/s0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 29.Hao W, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- 30.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fotiadis D, et al. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.