Abstract
Cerebellar neurons are generated from two germinal neuroepithelia: the ventricular zone (VZ) and rhombic lip. Signaling mechanisms that maintain the proliferative capacity of VZ resident progenitors remain elusive. We reveal that Sonic hedgehog (Shh) signaling is active in the cerebellar VZ and essential to radial glial cell proliferation and expansion of GABAergic interneurons. We demonstrate that the cerebellum is not the source of Shh that signals to the early VZ, and suggest a transventricular path for Shh ligand delivery. In agreement, we detected the presence of Shh protein in the circulating embryonic cerebrospinal fluid. This study identifies Shh as an essential proliferative signal for the cerebellar ventricular germinal zone, underscoring the potential contribution of VZ progenitors in the pathogenesis of cerebellar diseases associated with deregulated Shh signaling, and reveals a transventricular source of Shh in regulating neural development.
Keywords: cerebellum, cerebrospinal fluid, choroid plexus, radial glia, Shh
The cerebellum plays important roles in sensory perception and motor coordination. These functions are critically dependent on diverse neurons and glia originating from two distinct germinal neuroepithelia: the rhombic lip (RL) and ventricular zone (VZ).
As in the cerebrum, neurons of the cerebellum are either inhibitory or excitatory, depending on the expression of neurotransmitter GABA or glutamate, respectively (1). The principal glutamatergic neurons residing in the cerebellar granule layer are granule cells. During development, granule precursor cells generated from the RL migrate tangentially to the external granule layer (2), where they proliferate in response to Purkinje-derived Sonic hedgehog (Shh) during late embryogenesis and the early postnatal period (3–5). Aberrant activation of the Shh pathway in Patched1 heterozygous mice results in medulloblastoma (MB), a malignant cerebellar tumor that is thought to stem from massive deregulated granule neuron precursor proliferation (6). Similarly, mutations in PATCHED1 significantly increase the occurrence of sporadic MB in humans (7).
In contrast to glutamatergic neurons, the GABAergic neurons are generated from the VZ and consist of at least five different neuronal subtypes, including Purkinje cells and GABAergic interneurons (8). Birthdating studies have revealed that neurons of the cerebellar VZ are generated in three sequential but overlapping waves (8, 9). The first-born are small DCN neurons [embryonic day (E)10 to E12 in mice], which eventually settle in the white matter beneath internal granule neurons. Purkinje cell progenitors are second to be generated from the VZ, and they become postmitotic from E11 to E13 (8). Purkinje cells then migrate dorsally along guiding radial glial processes to their final destination beneath the external granule layer. A third population of neurons, which consists of GABAergic interneurons of the DCN, stellate, basket, Lugaro, and Golgi cells, is generated during late embryonic (from E14) and postnatal development. In addition to GABAergic neurons, astrocytes including Bergmann glia and oligodendrocytes are also derived from the VZ (9). By fate-mapping studies, radial glial cells are recognized as VZ multipotent progenitors that give rise to all of the aforementioned cerebellar cell types (10, 11). Although the cellular origin and final fate of many cerebellar neurons are known, the signaling pathways that maintain the proliferative capacity of resident progenitors in the ventricular germinal neuroepithelium remain elusive.
The role of Shh signaling in regulating adult forebrain subventricular zone neural stem cells has been studied extensively (reviewed in ref. 12). Although these studies focused primarily on the role of Shh signaling in postnatal and adult stages, its definitive function during embryonic forebrain radial glia cell development has not been demonstrated. Moreover, prototypic midline signaling centers, such as the prechordal plate and floor plate, that are continuous with the embryonic neural epithelium and normally associated with neural progenitor specification/maintenance are absent during cerebellar VZ development. Here, we reveal an essential and previously unappreciated function of Shh signaling in embryonic cerebellar VZ development, providing evidence that Shh signaling regulates proliferation and expansion of cerebellar radial glia and neural progenitors derived from the VZ. We further determined that the cerebellum itself is not a source of Shh that targets the early cerebellar VZ and strongly implicate a transventricular route for delivery of Shh ligand to the cerebellar VZ. Because current pathologic studies associated with deregulation of Shh signaling are mainly focused on external granule neurons, our findings may provide additional insights into the diverse cellular origin of cerebellar diseases.
Results
Cerebellar Ventricular Zone Progenitors Display Shh Signaling.
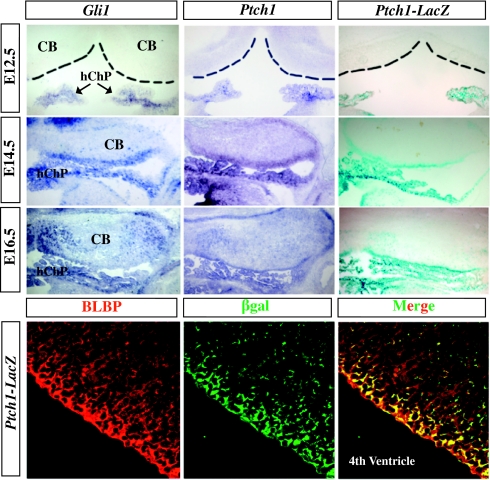
We have recently reported that the developing hindbrain choroid plexus epithelium (hChPe) robustly expresses Shh, which targets a discrete epithelial tissue situated between the hChP and lower RL (13). This distinct epithelial domain functions as the hChPe progenitor domain, which directly contributes to hChP epithelial growth by a proliferative process that depends on active Shh signaling activity (13). To our surprise, during our examination of the expression of Gli1 and Ptch1 by RNA in situ hybridization for Shh pathway activation in the hChP and surrounding tissue, the adjacent cerebellum, we also detected Shh signaling pathway activity in the cerebellar VZ along the medial–lateral axis at E14.5 (Fig. 1). We observed similar expression pattern in sections from Ptch1-LacZ mice containing a LacZ reporter knock-in at the endogenous Ptch1 locus (Fig. 1). Shh signaling continued to be detected at E16.5 (Fig. 1) in the cerebellar medial VZ and vermal region. Although Gli1 expression was relatively higher in the medial VZ, Ptch1 and Ptch1-lacZ staining seemed to span the VZ medial–lateral axis. Shh signaling activity was not detectable in the cerebellum at E12.5, although evident in the developing hChP at all stages presented (Fig. 1) (13). Because radial glial cells are localized to the apical surface of the brain ventricular zone and recognized as the major source of neural progenitors in the cerebellum (10), we examined whether Shh signaling is activated in these cells. Indeed, we observed colocalization of brain lipid binding protein (BLBP), a radial glial cell marker, and Ptch1-lacZ expression in E13.5 cerebellar VZ (Fig. 1).
Fig. 1.
Shh signaling in the embryonic cerebellum. Cerebellar VZ displays Shh signaling activity by E14.5. At E16.5, Shh signaling continues to be apparent along cerebellar VZ, with higher expression in the medial portion. Note that at this stage, there are many positive cells beyond VZ within the cerebellar tissue. β-gal+ cells overlap with BLBP+ cells in E13.5 Ptch1LacZ/+ embryos.
Shh Signaling Regulates Cerebellar Ventricular Zone Progenitor Proliferation and GABAergic Neuronal Progenitor Expansion.
Next we determined the role of Shh pathway activation in the cerebellar VZ at E16.5, before the stages when Purkinje neurons strongly express Shh. We first analyzed the cerebellar phenotype of Nestin-cre;Smof/- mutants, in which Shh signaling is conditionally ablated in all neural cells throughout the developing CNS after E11.5 (14) (Fig. 2A). At E16.5, Nestin-cre;Smof/- cerebellar VZ was remarkably thin, particularly in the medial region, and VZ cells were loosely arranged along the medial–lateral axis. We found significant reduction in VZ proliferation, by 1-h BrdU pulse labeling, in Nestin-cre;Smof/- cerebella compared with WT (14% ± 1.9% vs. 100% ± 3.4% in vermis region, P < 0.001; 22% ± 4% vs. 100% ± 7.2% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C). CyclinD1 expression was prominent in E16.5 WT VZ cells and numerous migrating cells beyond the VZ, but its expression was dramatically down-regulated in Nestin-cre;Smof/- cerebella (12% ± 2.5% vs. 100% ± 8.8% in vermis region, P < 0.001; 14% ± 3.7% vs. 100% ± 6.1% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C). Thus, CyclinD1 is also an essential downstream mediator of Shh signaling in the VZ, as in the external granule neuron layer (EGL) (15). Expression of Pax2, a paired box transcription factor, has been shown to identify GABAergic interneuron progenitors in the cerebellar cortex (including Golgi, basket, and stellate cells) and in the DCN (16). Significantly reduced Pax2+ cell number was found in Nestin-cre;Smof/- compared with WT (52% ± 3.8% vs. 100% ± 7.5% in vermal region, P < 0.001; 48% ± 4.9% vs. 100% ± 6% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C), demonstrating a dramatic reduction of GABAergic interneuron population. Similarly, the expression of homeodomain protein Lhx1/5, which marks the immature Purkinje cell (8) and Pax2+ GABAergic interneurons (Fig. S1A), were also significantly reduced in Nestin-cre;Smof/- mutants (66% ± 2.9% vs. 100% ± 1.6% in vermal region, P < 0.001; 68% ± 2.9% vs. 100% ± 5% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C). Together, we conclude that lack of sufficient Shh signaling in the embryonic cerebellar VZ leads to significant reduction in VZ proliferation and GABAergic interneuron progenitor cell expansion.
Fig. 2.
Nestin-cre;Smof/- or Nestin-cre;SmoM2 mutants exhibit, respectively, reduced or augmented VZ progenitor proliferation and GABAergic interneuron expansion. (A) Nestin-cre activity is detected in almost all neural cells throughout the cerebellum, as indicated by whole-mount and sectioned view of X-gal staining in E13.5 Nestin-cre;R26R embryo; however, Nestin-cre;SmoM2 mutants preferentially display enhanced Shh signaling in the cerebellar VZ. (B) BrdU proliferation, CyclinD1, Pax2, and Lhx1/5 immunohistochemical analyses on WT, Nestin-cre;Smof/-, and Nestin-cre;SmoM2 mutants. Red boxes represent higher magnifications of medial and lateral cerebellar regions in adjacent panels. (C) Quantifications of marker stainings in WT, Nestin-cre;Smof/-, and Nestin-cre;SmoM2 mutants. CB, cerebellum.
To further support the critical role of Shh in promoting embryonic cerebellar VZ proliferation, we crossed the Nestin-cre driver strain with SmoM2 conditional mutant, which harbors a constitutively activated form of Smo (17), to generate mice with ectopic Shh signaling. At E14.5, we observed enhanced Gli1 expression in Nestin-cre;SmoM2 cerebella (Fig. 2A). Interestingly, ectopic Shh signaling was largely confined to the VZ, where Shh signaling normally occurs, despite widespread Nestin-cre;R26R reporter expression in almost all neural cells (Fig. 2A). Nestin-cre;SmoM2 VZ displayed augmented proliferation and enhanced BrdU incorporation compared with WT (231% ± 8% vs. 100% ± 3.4% in the vermal region, P < 0.001; 345% ± 17.5% vs. 100% ± 7.2% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C). CyclinD1 was robustly expressed in a larger percentage of cells in Nestin-cre;SmoM2 compared with WT (221% ± 3.5% vs. 100% ± 8.8% in the vermal region, P < 0.001; 158% ± 6% vs. 100% ± 6.1% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C). In agreement, a larger number of GABAergic neuronal progenitors was present in the ventral cerebellum of Nestin-cre;SmoM2 compared with WT, such as Pax2+ (237% ± 2.2% vs. 100% ± 7.4%, in vermal region, P < 0.001; 276% ± 18.1% vs. 100% ± 6% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C) and Lhx1/5+ (135% ± 3% vs. 100% ± 1.6% in vermal region, P < 0.001; 133% ± 3.5% vs. 100% ± 5% in lateral VZ, P < 0.001, n = 3; Fig. 2 B and C). Our observation that the cerebellar VZ neural progenitor population is expanded in Nestin-cre;SmoM2 mutants is consistent with the recently reported phenotype in another gain-of-function mutant, in which Patched1 was conditionally removed from radial glial cells (18). Together, we provide compelling evidence for the essential role of Shh signaling in driving proliferation of cerebellar VZ progenitors and expansion of GABAergic-lineage cells.
Cerebellar Radial Glial Cell Proliferation Is Defective in Mutants Deficient in Shh Signaling.
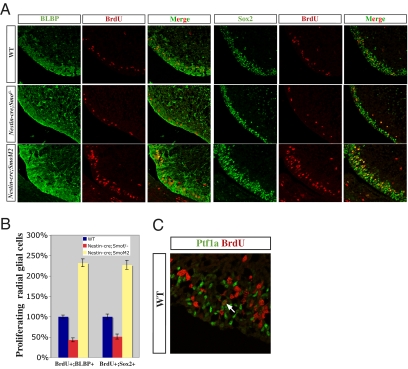
In line with our observation that Shh signaling directly targets radial glial progenitors in the cerebellar VZ, we detected strong reduction in radial glial cell proliferation in E14.5 Nestin-cre;Smof/- mutants (43.2% ± 4.9% vs. 100% ± 3.9% for BLBP+ cells, P < 0.001; 51.3% ± 6.2% vs. 100% ± 6.2% for Sox2+ cells, P < 0.001, n = 5; Fig. 3 A and B), whereas Nestin-cre;SmoM2 mutants displayed enhanced radial glial proliferation (232.2% ± 9.5% vs. 100% ± 8.2% for BLBP+ cells, P < 0.001; 227.4% ± 11.4% vs. 100% ± 7.3% for Sox2+ cells, P < 0.001, n = 5; Fig. 3 A and B). Interestingly, the majority of proliferating cells in WT as indicated by 1-h BrdU pulse are radial glial cells (94.4%, n = 284 of 301) (Fig. 3). Previous studies have shown that Ptf1a-expressing VZ progenitors give rise to mature Purkinje neurons and GABAergic interneurons (20, 21). Surprisingly, we found that Ptf1a-expressing VZ progenitors are largely nonmitotic (Fig. 3C). Similarly, Ptf1acre/+;Smof/- mutants did not show an apparent VZ proliferation phe-notype (Fig. S2). Taken together, these data demonstrate that cerebellar VZ neurogenesis and progenitor expansion are fulfilled by the proliferation of radial glial cells but not committed Ptf1a-expressing progenitor cells, and that this process is essentially regulated by Shh signaling.
Fig. 3.
Radial glia cell proliferation is defective in Nestin-cre;Smof/- and Nestin-cre;SmoM2 mutants. (A) Nestin-cre;Smof/- or Nestin-cre;SmoM2 mutants exhibit impaired or augmented BrdU incorporation in BLBP+ or Sox2+ radial glial cells, respectively. (B) Quantification of proliferating radial glial cell number in WT, Nestin-cre;Smof/-, and Nestin-cre;SmoM2 mutants. (C) Ptf1a-expressing cells are essentially not mitotic, indicated by largely nonoverlapping staining after 1-h BrdU pulse in E14.5 WT embryos. Arrow indicates one Ptf1a-expressing cell with weak, punctate BrdU signal that is rarely observed.
The Cerebellum Does Not Express Shh Endogenously Before E15.5.
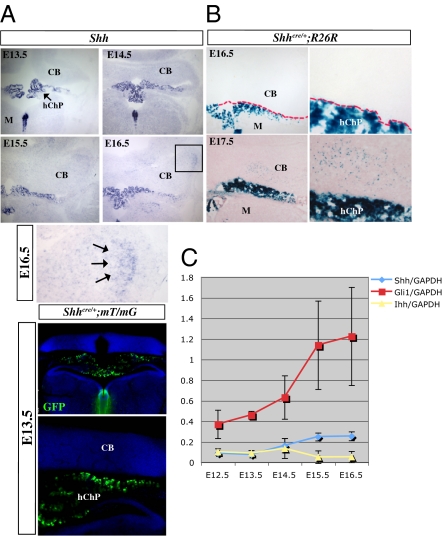
Previous studies showed that Shh expression in the cerebellum begins at E17.5 in the Purkinje cell layer but not in the ventral VZ (4, 21, 22). Our study revealing early Shh signaling in the cerebellar VZ prompted us to reexamine Shh expression in the developing cerebellum. Whereas Shh is prominently expressed in the developing hChPe starting at E12.5 (Fig. 4A), we found that Shh transcript only began to be detectable in the cerebellum at E16.5, particularly in the putative Purkinje cell domain beneath the EGL (arrows in Fig. 4A). We also performed genetic fate mapping in E13.5–E18.5 embryos by mating Shhcre/+ mice with mT/mG reporter, in which membrane-tethered GFP indelibly marks cells expressing, or once expressed Shh. We did not observe any GFP signal within the E13.5 cerebellum, a stage when Shh pathway activity in the cerebellar VZ was evident (Fig. 1B). Because the rostral cerebellum adjoins the developing midbrain midline tissue, which strongly expresses Shh, we asked whether this adjacent neural source of Shh could signal to the cerebellum, potentially by sending neuronal processes into the cerebellar tissue. However, we found that these neuronal processes do not extend anywhere close to the dorsal cerebellum (Fig. S1B). In addition, we mated Shhcre/+ mice with R26R reporter and again only detected significant lacZ-positive cells in the cerebellum at E17.5 (Fig. 4B), which is in agreement with in situ analysis considering that Cre recombinase begins to be functional ≈12–24 h after its promoter activation. We further performed quantitative real-time RT-PCR using dissected whole cerebella at E12.5–E17.5, using Indian hedgehog (Ihh) as a negative control. We found that cerebellar Shh expression was only significantly different from that of Ihh starting from E15.5 (Fig. 4C). In contrast, significant Gli1 expression, which increased with developmental time, was detected at all stages analyzed starting from E12.5 (Fig. 4C). Together, these findings suggest that the cerebellum is unlikely the source of Shh that regulates early VZ proliferation and strongly argue for a transventricular route in delivering Shh ligand to the cerebellar VZ.
Fig. 4.
Shh expression is not detected in the cerebellum before E15.5. (A) Shh mRNA expression in developing cerebellum; a faint signal could be detected at E16.5 in the putative Purkinje domain beneath EGL, as indicated by arrows. (B) Indelible marking and fate-mapping of Shh-expressing cells in the developing cerebellum. Note lack of any GFP signal in the E13.5 Shhcre/+;mT/mG cerebella. Shhcre/+;R26R mice begin to show positive staining within the cerebellar tissue starting at E17.5. (C) Quantitative real-time RT-PCR analysis of Shh, Gli1, and Ihh expression in the developing cerebellum. Of note, Shh expression significantly differs from that of Ihh control starting at E15.5, whereas a significant level of Gli1 expression is already detected as early as E12.5.
Shh Protein Is Present in the Circulating Embryonic CSF.
CSF, primarily generated from choroid plexi, is a source of chemicals and polypeptides with neuroprotective, surveillance, and repair functions (23). Recent studies also suggest that embryonic CSF harbors signaling molecules, such as Slit2, Fgf2, and ciliary neurotrophic factors, that may influence neuronal migration and development (23, 24). Because the cerebellar VZ is exposed to circulating CSF filling the fourth brain ventricle, we reason that Shh might be present in the CSF to regulate cerebellar VZ development. In line with this notion, by ELISA, we detected Shh protein at a concentration range of 100–300 pg/mL in the pooled CSF retrieved from hindbrain ventricles of E12.5–E15.5 WT embryos (2 or 4 μL CSF per embryo; see Materials and Methods) (Fig. 5A). One limitation is that it is not possible to retrieve only hindbrain CSF; therefore, we had to perform ELISA on pooled CSF circulating in all brain ventricles, and we always observed collapsed forebrain, midbrain, and hindbrain ventricles after retrieval of the CSF. Thus, the measured Shh concentration may be significantly lower than the local concentration close to the cerebellar VZ. We did not observe an age-dependent increase in Shh protein in the CSF, perhaps owing to the increase in CSF volume of the older embryos, thereby diluting overall protein concentration. Increasing evidence suggests that primary cilia function as the essential sensor of Shh ligand and regulator for transducing pathway activity (reviewed in refs. 25, 26). The primary cilia can be visualized by immunostaining with acetylated tubulin or Arl13b, a small GTPase of the Arf/Arl family (27). In agreement, we detected widespread presence of primary cilia in proliferating VZ radial glial progenitors (Fig. 5B).
Fig. 5.
Shh protein is present in embryonic CSF. (A) Embryonic CSF harbors Shh protein, as assayed by ELISA. The two white lines in A depict capillary tube inserted into the fourth ventricle to retrieve fresh CSF. ShhNp (starting at 0.063 nM) was used as positive control, whereas NIH 3T3 cell-conditioned media served as negative control in the analyses. (B) Primary cilia are present in proliferative radial glial VZ cells. The white frames in acetylated tubulin/Sox2 staining represent zoomed-in areas shown in the adjacent panels. Note that the white arrows indicate acetylated tubulin–labeled cilia protruding into the fourth ventricle.
Wnt1-cre;Shhf/- Mutants Display Similar Cerebellar VZ Defects.
Throughout the developing hindbrain system, we observed two prominent regions that express Shh: the ventral midline of the medulla and the hChPe (Fig. 4) (13). We found that the medulla is unlikely the source of Shh ligand in activating Shh signaling in the cerebellar VZ, because targeted deletion of Shh using Wnt1-cre leads to strong defects in VZ proliferation and GABAergic neuronal expansion without affecting Shh expression in the hindbrain medulla (Fig. 6A). The cerebellar VZ defects in Wnt1-cre;Shhf/- mutants were observed starting at E13.5 (Fig. 6 B and C) and became progressively more severe at E16.5 (Fig. S3). Consistent with our observation that Shh signaling is activated in the radial glial population, BLBP+ and Sox2+ expressing cells are all reduced in Wnt1-cre;Shhf/- mutants (Fig. 6B). Moreover, the proliferative defect as indicated by reduced number of BLBP+, BrdU+ or Sox9+, BrdU+ cells was restricted to radial glial cells (Fig. S4). Previous studies showed that Sox9 labels BLBP+, S100β+ cerebellar VZ radial glial cells (28). The expressions of Ptf1a, Ngn2 and Mash1 mark cerebellar VZ GABAergic progenitor cells (19, 29, 30), and their expressions are also largely reduced in the Wnt1-cre;Shhf/- mutants (Fig. S5). In contrast to the medulla, Shh expression in the hChPe is completely abolished in Wnt1-cre;Shhf/- mutants, suggesting that the hChPe is likely a source of Shh for cerebellar VZ progenitor proliferation. Consistent with this notion, we observed more than 3-fold induction in luciferase activity when E16.5 hChPs were cocultured with Shh-responsive LIGHT2 cells (Fig. S6).
Fig. 6.
Genetic ablation of Shh results in marked cerebellar VZ phenotype. (A) Wnt1-cre deleter strain drives Cre activity in hChPe, effectively ablating Shh expression selectively in hChPe but not the ventral midline of hindbrain medulla. (B) E13.5 Wnt1-cre;Shhf/- mutant cerebellar VZ shows severely impaired proliferative capacity and GABAergic and radial glia population expansion compared with WT. BrdU, Ki67, and CyclinD1 stainings indicate proliferative activity. BLBP+ and Sox2+ cells represent radial glial population. (C) Statistical comparisons between Wnt1-cre;Shhf/- mutant and WT cerebella for BrdU+ (41.5% ± 10% vs. 100% ± 12.6%, P < 0.001, n = 5), Ki67+ (37.9% ± 3.1% vs. 100% ± 6%, P < 0.001, n = 5), CyclinD1+ (35.2% ± 4.2% vs. 100% ± 9%, P < 0.001, n = 5) and Sox2+ (71.3% ± 2.9% vs. 100% ± 9.1%, P < 0.005, n = 5) cells.
Discussion
We provide genetic evidence that activation of Shh signaling is necessary to regulate cerebellar VZ progenitor proliferation and GABAergic progenitor expansion. We show that the source of Shh ligand to activate signaling in the early cerebellar VZ is not the cerebellum, therefore implicating a transventricular system as a means to deliver Shh signal to the VZ (Fig. S7).
It has been shown that Shh promotes GABAergic neuronal lineage restriction of forebrain stem cells, in part, by activation of the basic helix–loop–helix transcription factors Olig2 and Mash1 (31). In addition, recombinant Shh protein similarly increased the number of tyrosine hydroxylase–positive GABAergic neurons in the midbrain (32). Therefore, the action of Shh on RL-derived glutamatergic granule neurons in the cerebellum is in contrast with its well-established role in specifying and promoting proliferation of GABAergic interneurons in other regions of the CNS. It is not known whether a parallel situation exists in which Shh signaling plays a similar role in the developing cerebellar VZ, which gives rise to GABAergic interneurons. In this study, we identify Shh as the key signal in regulating cerebellar VZ radial glial cell proliferation and GABAergic interneuron population expansion, implicating VZ progenitors as a potential source for the cellular origin of cerebellar disorders associated with Shh deregulation. This finding is significant because current developmental or disease-related studies pertaining to Shh function in the cerebellum have been largely focused on granule neuron precursor cells at very late embryonic to postnatal stages, since it is thought that Shh signaling plays a physiologically relevant role from E17.5 onward in mice.
Our data indicated that Shh is present in the embryonic CSF during critical stages of cerebellar VZ development. CSF is generated from choroid plexi situated at different sites in the brain ventricles (23). Notably, the hindbrain choroid plexus is in close apposition with the cerebellar VZ throughout development (Fig. 4), and it is the only choroid plexus that expresses Shh (13). The observation that the hindbrain medulla, the only other noncerebellar source of Shh within the developing hindbrain system, is not required for expansion of VZ progenitors supports the argument that the hChP contributes to cerebellar VZ development. Additional support is the finding that the hChP is capable of eliciting Shh pathway activation when cultured with a Shh reporter cell line and that this effect can be blocked by Shh-blocking antibody or by a Smo antagonist (Fig. S6). However, Wnt1-cre is capable of removing Shh in other regions of the CNS, including the ventral midbrain, suggesting that other neural tissues may also secrete Shh into the CSF. It remains to be determined whether the hChP is the major source of Shh promoting cerebellar VZ development. Previous studies have shown that embryonic CSF contains a heterogeneous population of lipoprotein-rich microparticles, which can interact with the surface of the neuroepithelium (33). Interestingly, lipoprotein particles have been proposed to carry lipid-modified ligands such as Hedgehog from the cell surface, acting as vehicles for long-range transport in Drosophila (34). Although lipoproteins have not yet been implicated in transporting Shh, the low-density lipoprotein receptor (Lrp2, Megalin) can function as an endocytic receptor for Shh in cultured cells (35). Future studies are required to determine the presence of Shh in CSF microparticles.
Extensive effort has been made to understand the molecular basis of genetic disorders involving cerebellar defects, such as the neurodevelopmental Joubert syndrome and Bardet-Biedl syndrome, both being characterized by aplasia or severe hypoplasia of the cerebellar vermis (36). Recent progress in genetic mapping of human patients suggested a strong link to mutations in protein components of the primary cilia, such as the intraflagellar transport proteins (36). The correlation between defective ciliogenesis and the etiology of cerebellar genetic disorders is particularly interesting, because many core components of the Shh pathway are localized to, and function in, cilia during Shh signal transduction (reviewed in refs. 25 and 26). In light of our finding that Shh is required to stimulate VZ radial glial proliferation, and the fact that abundant primary cilia were detected along the developing cerebellar VZ (Fig. 5), it would be important to fully assess the cerebellar defects of conditional cilia mutants and to establish a requirement of the primary cilia in VZ radial glial progenitor proliferation in response to Shh signal.
Emerging lines of evidence suggest that there are two subtypes of MB with distinct oncogenic location and molecular characteristics; MBs are detected in most afflicted children in the vermal cerebellar region showing no MATH1 expression and in many adult patients, in the cerebellar hemispheres with high level of MATH1 expression (37). In addition, whereas a subset of human MB samples displayed up-regulation of MATH1, others selectively exhibit expression of NGN1, a VZ-specific transcription factor, with no MATH1 expression (38). It is therefore likely that current animal models of MB only mimic one subtype of MB that is generated from transformed EGL as the mice develop to adulthood. Because our study demonstrated an essential role of Shh signaling in the regulation of embryonic cerebellar VZ proliferation, it remains possible that aberrant Shh signaling at embryonic or perinatal stages could promote vermal MB oncogenesis originating from the cerebellar VZ.
Materials and Methods
Defined areas selected for cerebella cell counting are shown in Fig. S8. Details about mice, retrieval of embryonic CSF and Shh ELISA, immunohistochemistry, cell counting and statistics, X-gal staining and transcript detection, and real-time RT-PCR are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Chris Wright (Vanderbilt Medical Center) for providing the Ptf1a antibody and the Ptf1acre/+ line; Tamara Caspary (Emory University) for the Arl13b antibody; Michael Wegner (Universität Erlangen, Germany) for the Sox9 antibody; and Vanderbilt University Cell Imaging Shared Resource for use of the confocal microscope (supported by Grant CA068485). This study was supported by the National Institutes of Health Grant NS042205 (to C.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911838107/DCSupplemental.
References
- 1.Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- 2.Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- 3.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 4.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 5.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 7.Raffel C, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 8.Morales D, Hatten ME. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J Neurosci. 2006;26:12226–12236. doi: 10.1523/JNEUROSCI.3493-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman J, Bayer S. Development of the Cerebellar System in Relation to Its Evolution, Structures and Functions. New York: CRC Press; 1997. [Google Scholar]
- 10.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 11.Mori T, et al. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 12.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: The multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, et al. Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development. 2009;136:2535–2543. doi: 10.1242/dev.033795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graus-Porta D, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 15.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 16.Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999;41:281–294. doi: 10.1002/(sici)1097-4695(19991105)41:2<281::aid-neu10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang ZJ, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino M, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Pascual M, et al. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci USA. 2007;104:5193–5198. doi: 10.1073/pnas.0605699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development. 2004;131:5581–5590. doi: 10.1242/dev.01438. [DOI] [PubMed] [Google Scholar]
- 22.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol. 2005;71:1–52. doi: 10.1016/S0070-2153(05)71001-2. [DOI] [PubMed] [Google Scholar]
- 24.Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 25.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Hachem S, Laurenson AS, Hugnot JP, Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC Dev Biol. 2007;7:17. doi: 10.1186/1471-213X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zordan P, Croci L, Hawkes R, Consalez GG. Comparative analysis of proneural gene expression in the embryonic cerebellum. Dev Dyn. 2008;237:1726–1735. doi: 10.1002/dvdy.21571. [DOI] [PubMed] [Google Scholar]
- 30.Grimaldi P, Parras C, Guillemot F, Rossi F, Wassef M. Origins and control of the differentiation of inhibitory interneurons and glia in the cerebellum. Dev Biol. 2009;328:422–433. doi: 10.1016/j.ydbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Yung SY, et al. Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci USA. 2002;99:16273–16278. doi: 10.1073/pnas.232586699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpicelli F, et al. Enhancement of dopaminergic differentiation in proliferating midbrain neuroblasts by sonic hedgehog and ascorbic acid. Neural Plast. 2004;11:45–57. doi: 10.1155/NP.2004.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachy I, Kozyraki R, Wassef M. The particles of the embryonic cerebrospinal fluid: How could they influence brain development? Brain Res Bull. 2008;75:289–294. doi: 10.1016/j.brainresbull.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Panáková D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem. 2002;277:25660–25667. doi: 10.1074/jbc.M201933200. [DOI] [PubMed] [Google Scholar]
- 36.Louie CM, Gleeson JG. Genetic basis of Joubert syndrome and related disorders of cerebellar development. Hum Mol Genet. 2005:R235–R242. doi: 10.1093/hmg/ddi264. 14(Spec No 2) [DOI] [PubMed] [Google Scholar]
- 37.Salsano E, Pollo B, Eoli M, Giordana MT, Finocchiaro G. Expression of MATH1, a marker of cerebellar granule cell progenitors, identifies different medulloblastoma sub-types. Neurosci Lett. 2004;370:180–185. doi: 10.1016/j.neulet.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Salsano E, et al. Expression of the neurogenic basic helix-loop-helix transcription factor NEUROG1 identifies a subgroup of medulloblastomas not expressing ATOH1. Neuro-oncol. 2007;9:298–307. doi: 10.1215/15228517-2007-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.