Abstract
Aging is broadly defined as a progressive decline of tissue and organ functions due to deregulation of various cell intrinsic and extrinsic factors. In the immune system, aging preferentially affects lymphopoiesis and thus results in the reduced competence of the adaptive immune system in the elderly. Despite recent discoveries that shed light on the molecular basis of aging, pathways that lead to diminished lymphoid development in aging individuals remain largely unknown. In the present study, we document that a deficiency of the E3 ubiquitin ligase c-Cbl in lymphocytes results in an age-dependent lymphopenia. c-Cbl-deficient mice show normal frequencies of lymphocytes at 12 weeks of age; however, their development and functions were remarkably diminished at 24 weeks after birth. Intriguingly, c-Cbl mutant lymphocytes displayed increased responses to IL7 in vitro and failed to down-regulate surface levels of IL7Rα. Further, our biochemical studies have identified an interaction of c-Cbl with IL7Rα and have unraveled the involvement of c-Cbl in the ubiquitylation of IL7Rα. In essence, our studies demonstrate that a lack of signaling events mediated by c-Cbl might result in diminished lymphocyte development and functions, particularly, at the later stages of life.
Keywords: aging, E3 ligase, IL7 receptor, immunosenescence
Hematopoietic stem cells (HSCs) have the ability to strike a balance between self-renewal and lineage commitment (1). Lymphocyte development begins with the differentiation of HSCs into lymphoid primed multipotent progenitors (LMPPs) in the bone marrow (BM) (2). Subsequent lineage differentiation steps result in the differentiation of LMPPs into earliest lymphocyte progenitors (ELPs) (3). Further ELPs differentiate into common lymphoid progenitors (CLPs) and early thymic progenitors (ETPs) from which B and T cell development occurs in the BM and thymus, respectively.
Whereas there is an increasing wealth of knowledge available on the importance of transcription factors and cytokines in the differentiation of HSCs into lymphoid lineage cells (4–6), the roles of posttranslational modifications of proteins, such as ubiquitylation and glycosylation, in hematopoiesis remain elusive.
Casitas B lineage lymphoma (Cbl) family of proteins functions as E3 ubiquitin ligases that participate in the ubiquitylation of various cellular proteins (7–14). Despite much available knowledge on the biochemistry of c-Cbl, mainly based on in vitro studies, the physiological significance and in vivo functions of c-Cbl protein are largely unknown. In particular, the importance of c-Cbl in the development of the immune system has not been studied. Earlier analysis of c-Cbl deficient mice did not reveal any major developmental abnormalities, although c-Cbl deficient thymocytes show marked activation of ZAP70 in response to TCR stimulation (15, 16). Recently, we have identified c-Cbl as a unique factor restricting the self-renewal of HSCs (17, 18).
In the present study, we establish an unexpected role for c-Cbl in development of lymphocytes. Cbl-deficient mice show accelerated thymic involution and reduced B cell development in the bone marrow starting at 6 months of age. Intriguingly, our data identify a unique function of c-Cbl in the ubiquitylation of IL7Rα protein in lymphocytes.
Results
Reduced B Cell Differentiation in Older c-Cbl-Deficient Mice.
In an attempt to understand the specific role of c-Cbl in hematopoiesis, we made use of the c-Cbl−/− mice that were generated and reported earlier (15). In accordance with the earlier report (15), a thorough analysis of various hematopoietic lineages revealed no significant difference between c-Cbl−/− and wild type (WT) littermates at 4 weeks of age. However, an augmented HSC pool size was observed in c-Cbl−/− mice (17).
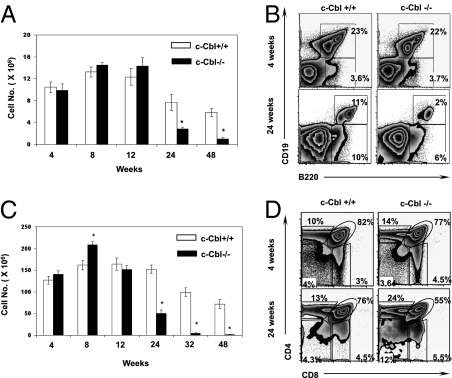
Next, we assessed whether lymphopoiesis is affected in older c-Cbl−/− mice. First, we quantified the absolute numbers of CD19+B220+ B lineage cells of the BM at 4, 8, 12, 24, and 48 weeks of age. Although both the absolute and relative numbers of CD19+B220+ B cells were comparable between c-Cbl−/− and WT animals at younger ages (4, 8, and 12 weeks), their numbers were significantly reduced in the older (24 and 48 weeks) c-Cbl−/− mice (Fig. 1 A and B). Nevertheless, the frequencies of B220+CD19− progenitor B cells were comparable, between c-Cbl−/− and WT mice, at all indicated time points (Fig. 1B and Fig. S1A). To identify whether the reduction of B cell numbers in older c-Cbl−/− mice is due to perturbed B cell development, BM B220+ cells of 24-week-old mice were subjected to “Hardy fractions” analysis (19). Interestingly, this revealed an abnormal developmental pattern of B cells in c-Cbl−/− mice when compared to WT animals (Fig. S1 B and C). In essence, a significant reduction of fractions B, D, E, and F and an increase of fraction C were observed in c-Cbl−/− mice. Interestingly, the frequency of B lineage cells in fraction A was comparable between c-Cbl−/− and WT mice (Fig. S1 B–E). As expected, Hardy fractions analysis of 4-week-old animals did not reveal any significant differences between c-Cbl−/− and WT. Further, we quantified the numbers of pro B (B220+CD43+AA4.1+CD19+) cells in 4- and 24-week-old c-Cbl-deficient mice. Interestingly, the frequencies of pro B cells were significantly reduced in 24-week-old c-Cbl-deficient mice, although their numbers were normal at 4 weeks of age (Fig. S1FFig. S1F).
Fig. 1.
Diminished lymphocyte development in older c-Cbl−/− mice. (A) Absolute numbers of CD19+ B cells of the BM were determined as average per animal (two tibia and fibula) at indicated weeks of age. Each group contains n = 5 mice. Data are representative of five independent experiments. Data are presented as mean ± SEM. *, P values <0.05. (B) FACS plots indicating relative frequencies of B cells at 4 (Upper) and 24 weeks (Lower) of age. Data are representative of 10 independent experiments. (C) Absolute numbers of thymocytes at indicated weeks of age. Each group contains n = 5 mice. Data are representative of 10 independent experiments. Data are presented as mean ± SEM. *, P values <0.05. (D) Analysis of thymic T cell development at 4 (Upper) and 24 weeks of age (Lower) in c-Cbl−/− mice.
Augmented Thymic Involution in the Absence of c-Cbl.
To check whether the T cell development is also perturbed in older c-Cbl-deficient mice, absolute numbers of thymocytes from 4-, 8-, 12-, 24-, 32-, and 48-week-old c-Cbl−/− and WT littermates were enumerated. Strikingly, the total numbers of thymocytes in c-Cbl−/− mice were significantly reduced at 24, 32, and 48 weeks of age, even though their numbers were comparable between c-Cbl−/− and WT mice at 4, 8, and 12 weeks of age (Fig. 1C). Next, we checked whether thymic T cell development is intact in older c-Cbl-deficient mice. T cell development in the thymus has been identified to pass through defined stages of development (20). Even though all developmental stages of T cells were represented in older c-Cbl-deficient thymus, the numbers and distribution of CD4+CD8+ (DP), CD4+CD8− (SP4), and CD4−CD8+ (SP8) subsets were significantly altered (Fig. 1D and Fig. S2 A and B). Surprisingly, the absolute numbers of CD4-CD8- (DN) progenitor T cells were normal in older c-Cbl−/− thymus. However, further dissection of DN cells into distinct subsets (DN1–DN4), based on the expression of CD44 and CD25, revealed an increased frequency of the DN1 fraction and decreased frequencies of DN2, DN3, and DN4 subsets in older c-Cbl−/− mice. Of note, the transition between DN1 and DN2 was significantly affected. Intriguingly, an altered distribution of DN1 and DN2 cells was also observed in younger c-Cbl−/− mice (Fig. S2 C–E and Fig. S3 A and B). Together, these data suggest that the B cell development in the BM and T cell development in the thymus are selectively affected in older c-Cbl mutant mice.
Perturbed Secondary Lymphocyte Development in Older c-Cbl-Deficient Mice.
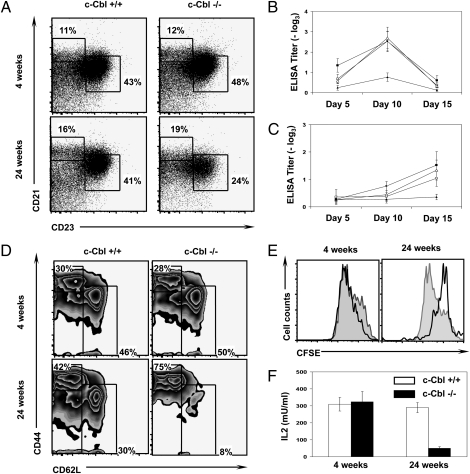
To evaluate whether the secondary B cell development is intact in older c-Cbl−/− mice, first the total numbers of CD19+ B cells of the spleen were calculated. Interestingly, our analyses revealed normal relative numbers of B cells at 4 weeks of age and a reduced relative frequency of B cells in 24-week-old mice (Fig. S3C). However, the absolute numbers of splenic CD19+ cells were significantly increased in 24-week-old mice (Fig. S3C). However, the absolute numbers of splenic CD19+ cells were significantly increased in 24-week-old mice (Fig. S3D). Next, we determined the frequencies of marginal zone (B220+CD19+CD21+CD23−) B cells and follicular (B220+CD19+CD21+/lowCD23+) B cells in the spleens of older c-Cbl−/− mice. A detailed analysis of the peripheral B cells indicated a decline in follicular B cell production and undisturbed frequencies of marginal zone B cell subset in the spleens of older c-Cbl-deficient mice (Fig. 2A and Fig. S3E). Moreover, the capacity to generate high affinity antibodies by peripheral B cells was compromised in older c-Cbl-deficient animals (Fig. 2 B and C).
Fig. 2.
Reduced B and T cell responses in older c-Cbl−/− mice. (A) FACS plots indicating frequencies of marginal zone B cells (MZB) and follicular B cells (FCB) at 4 (Upper) and 24 weeks (Lower) of age. Analysis was performed on pregated CD19+B220+ cells. Data are representative of three independent experiments. (B and C) Reduced IgM and IgG antibody responses in older c-Cbl−/− mice. Five mice per group were injected s.c. with 10 μg of TNP KLH in CFA. At indicated time points after immunization, mice were bled, and the IgM (B) and IgG (C) antibody titers in the serum were determined by ELISA. Error bars indicate the standard errors of the mean. (D) FACS plots indicating frequencies of naÏve (CD62LhighCD44low/−) and memory (CD44highCD62Llow/−) CD4+ T cells of the spleen at 4 (Upper) and 24 weeks (Lower) of age. Analysis was performed on pregated CD4+ cells. Data are representative of five independent experiments. (E) T cell proliferation assays. Sorted naïve CD4 T cells of 4- and 24-week-old c-Cbl+/+ (filled) and c-Cbl−/− (open) were CFSE labeled and stimulated with plate bound CD3e (5 μg/mL) and soluble CD28 (2 μg/mL). Three days after stimulation (Left), cells were analyzed by flow cytometry. (F) IL2 secretion assay. Sorted naïve CD4 T cells of 4- and 24-week-old c-Cbl+/+ and c-Cbl−/− were stimulated with plate bound CD3e (5 μg/mL) and soluble CD28 (2 μg/mL). Supernatants were collected at 48 hours after stimulation, and IL2 cytokine levels were determined by ELISA. Data are representative of two independent experiments. Shown are the mean values of the triplicate samples. Data are presented as mean ± SEM. *, P values <0.05.
Next, we focused on the T cell compartment in the periphery of c-Cbl−/− mice. Analysis of splenic CD4 and CD8 T cells revealed comparable frequencies between c-Cbl−/− and WT animals at 4 weeks of age (Fig. S3 F and G). Even though the relative frequencies of the CD4 subset were comparable, and of the CD8 subset were slightly reduced in c-Cbl-deficient mice at 24 weeks of age, their absolute numbers were significantly increased in c-Cbl−/− mice due to overall increase of splenic cellularity. Nevertheless, the frequencies of naïve T cells of both CD4 (Fig. 2D) and CD8 (Fig. S3H) subsets were significantly decreased in c-Cbl−/− mice at 24 weeks of age, although their frequencies were comparable at 4 weeks of age. Moreover, naive T cells of 24-week-old c-Cbl−/− mice exhibited a severe defect in proliferation upon stimulation with αCD3 and αCD28 (Fig. 2E) and showed diminished responses to secondary stimulation (Fig. S3I). In addition, the viability of CD4 T cells from 24-week-old c-Cbl−/− mice was significantly reduced when stimulated with αCD3 and αCD28 (Fig. S3J). In line with these observations, the capacity to secrete IL2 upon activation by 24-week-old c-Cbl−/− T cells was significantly reduced when compared to the T cells of WT mice (Fig. 2F).
Cell Intrinsic Role of c-Cbl in Lymphopoiesis.
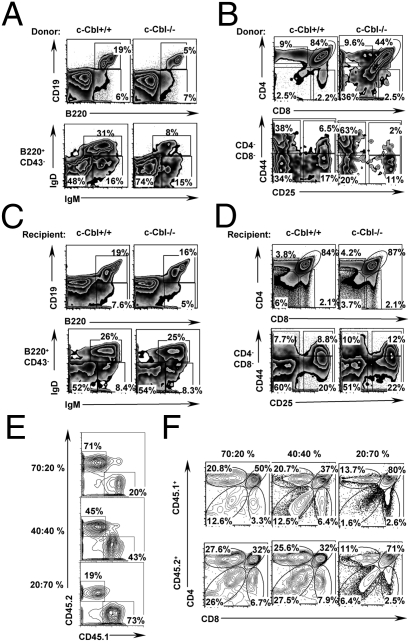
To assess whether the reduced B and T cell development in older c-Cbl−/− mice is due to a cell intrinsic phenomenon, HSC transplantation experiments were performed. In the first set of experiments, purified HSCs (Lin−c-kit+Sca1+CD34−) of 24-week-old c-Cbl−/− and WT mice (CD45.2) were transplanted into lethally (11 Gy) irradiated congenic (CD45.1) WT recipients. Twelve weeks after transplantation, donor-derived hematopoiesis was confirmed in the peripheral blood (Fig. S4A) and the recipients were euthanized to analyze B and T cell development. As observed in c-Cbl-deficient BM, the frequencies of CD19+B220+ and B220+CD43−IgD+IgM+ (fraction F) cells were significantly reduced in recipients that received c-Cbl-deficient HSCs (Fig. 3A and Fig. S4B). Similarly, analysis of thymus revealed a severe reduction of total thymocytes, DP, SP4, and SP8 T cells in recipients transplanted with c-Cbl−/− HSCs. On the other hand, the absolute numbers of DN1, DN2, DN3, and DN4 T cell progenitor subsets were increased in mice that received c-Cbl−/− HSCs (Fig. 3B and Fig. S4 C and D). Nevertheless, as we observed in c-Cbl-deficient mice, the relative frequency of DN2 cells is affected in WT recipients that were transplanted with c-Cbl−/− cells. Conversely, sorted WT (CD45.1) HSCs were transplanted into lethally irradiated 24-week-old either c-Cbl−/− or WT recipients (CD45.2). Interestingly, analysis of donor-derived B and T cell development, 12 weeks after transplantation, did not show any significant differences between c-Cbl−/− and WT recipients (Fig. 3 C and D and Fig. S5).
Fig. 3.
Perturbed lymphocyte development in older c-Cbl−/− mice is cell intrinsic. (A and B) Sorted HSCs (2 × 104) from 24-week-old c-Cbl−/− and WT mice were injected into lethally irradiated CD45.1 WT recipients. Twelve weeks after transplantation, analysis of recipient BM (A) and thymus (B) was performed by flow cytometry. (A) Donor derived (CD45.2+) cells were pregated and analyzed for B220 and CD19 expression (Upper). CD45.2+B220+CD43− cells were pregated and analyzed for IgD and IgM expression (Lower). (B) FACS plots indicating CD4 and CD8 expression in pregated CD45.2+ thymocytes (Upper). CD44 and CD25 expression in donor derived DN thymocytes (Lower). Cells were pregated on CD45.2+CD4−CD8− fraction. (C and D) Sorted HSCs (2 × 104) of 24-week-old (CD45.1) WT mice were injected into lethally irradiated either c-Cbl−/− or WT (CD45.2) recipients. Twelve weeks after transplantation, BM (C) and thymi (D) of recipients were analyzed by flow cytometry. (C) B220 and CD19 expression in pregated CD45.1+ BM cells (Upper). IgD and IgM expression in CD45.1+B220+CD43− cells (Lower). (D) CD4 and CD8 expression in pregated CD45.1+ thymocytes (Upper). CD44 and CD25 expression in pregated CD45.1+CD4−CD8− thymocytes (Lower). (E and F) Mixed BM chimera experiments. Varying proportions of WT (CD45.1) and c-Cbl (CD45.2) deficient BM cells were mixed and injected into lethally irradiated CD45.1 congenic recipients. After 16 weeks of transplantation, recipients (n = 5) were sacrificed, thymi were harvested, single cell suspensions were made, and WT vs. KO chimera was assessed by flow cytometry based on differential expression of CD45.1 vs. CD45.2. Analysis of chimera revealed recipients with three different ratios of WT vs. KO cells as indicated (E). WT (CD45.1; Upper) and KO (CD45.2; Lower) derived thymocytes were pregated and further discriminated based on the CD4 and CD8 expression (F).
To further substantiate the cell intrinsic role of c-Cbl and to exclude the possible involvement of other hematopoietic cells in the observed phenotype, WT mice with mixed (WT and KO) BM chimera were generated as described earlier (17). Overall, three groups of mice with three different ratios (∼20:70%, ∼40:40%, and ∼70:20%) of WT (CD45.1): c-Cbl−/− (CD45.2) cells were obtained (Fig. 3E). After 16 weeks of transplantation, analysis of thymocytes of recipient mice with a chimera of ∼20:70% (WT: c-Cbl−/− cells, respectively), revealed an impairment of both WT (CD45.1) and c-Cbl−/− (CD45.2) T cell development. Similarly, analysis of chimera with ∼40:40%, (WT: c-Cbl−/−, respectively), suggested a decline in T cell development of both WT (CD45.1) and c-Cbl−/− (CD45.2) origin. Intriguingly, analysis of chimera with ∼70:20%, (WT: c-Cbl−/−, respectively), revealed an intact T cell development of both WT (CD45.1) and c-Cbl−/− (CD45.2) origin, although the ratio between DN and DP cells was altered in c-Cbl−/− thymocytes (Fig. 3F).
Taken together, the data of HSC transplantation and mixed BM chimera experiments, essentially, rule out the possible involvement of cell extrinsic factors that might contribute to diminished lymphopoiesis in older c-Cbl-deficient mice.
Normal Progenitor Numbers and Reduced Expression of Transcription Factors.
A possible explanation for the reduced lymphocyte development in older c-Cbl mutant animals might be due to reduced numbers of lymphocyte progenitors at 24 weeks of age. Thus, analysis of common lymphoid progenitors (CLP; Lin−IL7Ra+Sca1+c-Kitlow) of the BM and early thymic progenitors (ETP; Lin−CD25−CD44+NK1.1−IL7Rα−c-Kit+) of the thymus was performed, as CLPs and ETPs are the precursors of B and T cells, respectively (21). Interestingly, an increased numbers of CLPs (Fig. S6 A and B) and normal numbers of ETPs (Fig. S6 C and D) were observed in older c-Cbl−/− mice. The increase of CLPs in the BM of c-Cbl-deficient mice could be a consequence of an increased frequency of HSCs as reported earlier (17). These data suggest that the defective lymphocyte differentiation in c-Cbl-deficient mice might not be due to reduced lymphoid progenitors.
Development of B cells in the BM critically depends on transcription factors (TF) such as E2A, EBF, and PAX5 (4) and the early development of T cells in the thymus is instructed by TFs such as TCF1, LEF1, and RORγT (22, 23). Of note, earlier studies have documented an age-related decline in the expression of E2A in B cells (24). To check whether the diminished lymphocyte development in older c-Cbl−/− mice is due to reduced expression levels of key transcription factors, real-time PCR analysis was performed. Surprisingly, expression levels of both B cell lineage (E2A, EBF, and Pax5) and T lineage (TCF1, LEF1, and RORγT) transcription factors were significantly reduced in lymphocytes of older c-Cbl-deficient mice (Fig. S7 A and B).
IL7-Mediated Signals in c-Cbl−/− Lymphocytes.
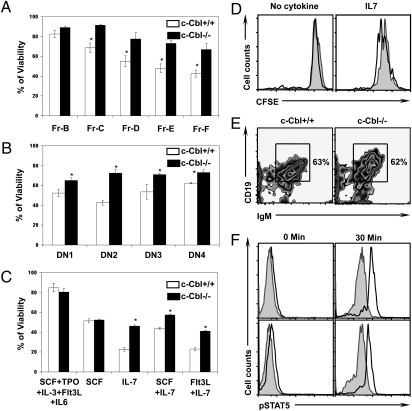
Interleukin 7 (IL7) is a nonredundant and crucial cytokine for the development of both B and T cells (25, 26). IL7 signals through IL7R, which consists of two chains, IL7Rα and common cytokine receptor γ-chain (27, 28). Cross linking of IL7R with IL7 results in the phosphorylation of STAT5 through JAK1 and JAK3. Phosphorylated STAT5 then dimerizes and translocates to the nucleus, where it induces transcription of target genes (29). In view of our recent finding that c-Cbl deficiency leads to augmented STAT5 signaling in response to thrombopoietin in HSCs (17), we hypothesized that IL7 signaling events might be augmented in c-Cbl-deficient lymphocytes. To assess the importance of c-Cbl in orchestrating IL7 signal cascades, we analyzed trophic and proliferative effects of IL7 signaling. Interestingly both B and T cell progenitors of c-Cbl-deficient mice exhibited increased viability in response to IL7 (Fig. 4 A and B and Fig. S7 C and D). Similar results were obtained using HSCs (Fig. 4C). Next, we measured the proliferative responses of c-Cbl-deficient HSCs in response to IL7. CFSE dilution analysis indicated an augmented proliferation associated with c-Cbl deficiency (Fig. 4D). Furthermore, we tested whether the HSCs of older c-Cbl−/− mice have the potential to differentiate into Ig positive B cells in vitro. Interestingly, flow cytometric analysis revealed a comparable in vitro B cell differentiation potential between older c-Cbl-deficient and WT HSCs (Fig. 4E). To understand the hyperresponsiveness of c-Cbl-deficient cells to IL7 at a molecular level, STAT5 phosphorylation levels in B and T cells were quantified by the phosflow technique. In line with our previous observations in HSCs (17), p-STAT5 levels were higher in c-Cbl-deficient lymphocytes (Fig. 4F). Collectively, these data suggest that, in the presence of unlimited amounts of IL7, the lymphocyte development potential of c-Cbl-deficient HSCs is not affected.
Fig. 4.
c-Cbl deficient lymphocytes exhibit increased responses to IL7. (A and B) Hardy Fractions of BM B cells (A) and DN subsets (DN1-DN4) of thymocytes (B) were sorted from 24-week-old mice and cultured for 48 hours in the presence of IL7. Percentage of viability was measured by flow cytometry based on propidium iodide (PI) exclusion. Data are representative of three independent experiments. Data are presented as mean ± SEM. *, P values <0.05. (C–E) Lin-Sca1+c-Kit+CD34− of 24-week-old c-Cbl−/− and c-Cbl+/+ mice were sorted and initially cultured in the presence of SCF+ IL3+ IL6+ Flt3L and TPO for 36 hours. (C) Cells were washed and cultured in the presence of indicated cytokines for 48 hours. Viability was measured by flow cytometry based on PI exclusion. Data are representative of two independent experiments. Shown are the mean values of the triplicate samples. Data are presented as mean ± SEM. *, P values <0.05. (D) Cells were washed, labelled with CFSE, and cultured in the presence of IL7 for 48 hours. CFSE dilution in proliferating cells was measured by flow cytometry. Filled histograms represent c-Cbl+/+ cells and open histograms represent c-Cbl−/− cells. (E) Cells were washed and cultured in the presence of IL7 for 14 days. Cells were washed and stained with CD19 and IgM antibodies and measured by flow cytometry. (F) Sorted B220+CD43+CD24+BP1− (Fr.B) cells of BM (Upper) and CD44+CD25+CD4-CD8− (DN2) cells of thymus (Lower) of 24-week-old mice were stimulated with IL7 for 30 minutes. Cells were fixed and permeabilized, and phospho-STAT5 levels were measured by flow cytometry. Filled histograms represent WT cells and open histograms represent c-Cbl deficient cells. Cells at time point 0 served as controls. Data are presented as mean ± SEM and *, P < 0.05.
Deficiency of IL7Rα Down-Regulation in c-Cbl−/− Lymphocytes.
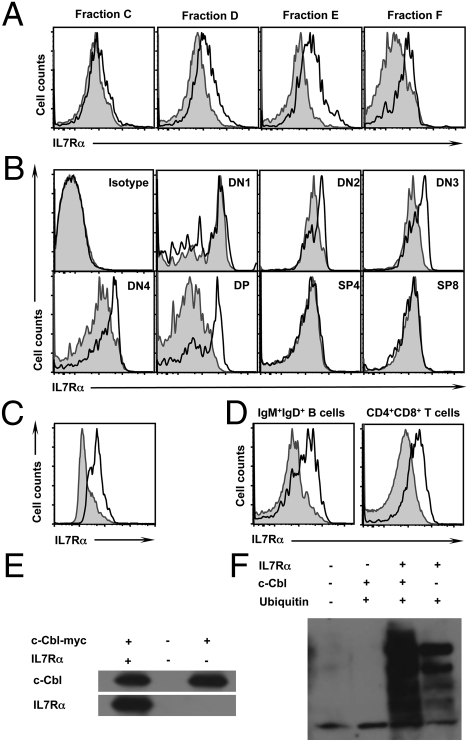
Finally, we sought to understand the mechanism behind diminished lymphocyte development in older c-Cbl−/− mice. In light of our observation that lymphopoiesis in general, both B and T cell development, is perturbed in older c-Cbl−/− mice, we hypothesized that a deregulated IL7 signal cascade might be responsible for the phenotype. Previous studies have demonstrated that IL7 production by stromal cells and lymphopoietic support potential of hematopoietic microenvironment decrease with age (21, 30, 31). However, the data of our transplantation experiments rule out the possibility that diminished IL7 production might be responsible for the defective lymphocyte development in older c-Cbl-deficient mice. Moreover, previous studies have reported earlier that coordinated lymphocyte development is not only dependent on IL7-mediated signals but also on dynamic and developmental-stage-specific regulation of the IL7Rα chain expression (5, 32–34). We therefore hypothesized that the defective lymphoid development in older c-Cbl-deficient mice might be due to deregulated IL7Rα expression. We therefore checked IL7Rα expression levels in Hardy fractions of the BM. Although the expression levels were comparable between c-Cbl−/− and WT at earlier stages of B cell development (Fr. B and Fr. C), IL7Rα expression levels in Fr. D, Fr. E, and Fr. F of c-Cbl−/− mice were higher (Fig. 5A). Similarly, the expression levels of IL7Rα were quantified at various stages of T cell development. Interestingly, the surface expression levels of IL7Rα were significantly higher at DN2, DN3, DN4, and DP stages of T cell differentiation in c-Cbl-deficient mice (Fig. 5B). Next, we analyzed whether the augmented expression of IL7R is specific to the α-subunit. Intriguingly, expression levels of γc (CD132) subunit were comparable, between c-Cbl−/− and WT mice, at all stages of B and T cell development (Fig. S8).
Fig. 5.
Defective IL7R down-regulation in c-Cbl−/− cells. (A and B) Surface expression of IL7R in Hardy Fractions of the BM (A) and T cell subsets of the thymus (B) of 24-week-old mice. Filled histograms represent c-Cbl+/+ cells and open histograms represent c-Cbl−/− cells. (C) Surface expression of IL7R in in vitro differentiated B cells from HSCs. Filled histograms represent c-Cbl+/+ cells and open histograms represent c-Cbl−/− cells. (D) Surface expression of IL7R in pregated B220+CD43−IgM+IgD+ (Fraction F) cells of the BM (Left) and CD4+CD8+ (DP) cells of the thymus (Right) of 24-week-old mice. Filled histograms represent c-Cbl+/+ cells and open histograms represent c-CblA/− cells. (E) Coimmunoprecipitation studies. 293T cells were cotransfected with plasmids expressing c-Cbl and IL7R proteins. Cell lysates were coimmunoprecipitated using myc antibodies. Precipitates were subjected to immunoblotting and detected using c-Cbl (Upper) and IL7R (Lower) antibodies. (F) Ubiquitylation assays. 293T cells were cotransfected with plasmids expressing c-Cbl, IL7R, and ubiquitin proteins. Cell lysates were immunoprecipitated using IL7R antibodies. Precipitates were subjected to immunoblotting and detected using ubiquitin antibodies.
To investigate whether the increased surface level of IL7Rα is due to their increased transcription, mRNA levels of IL7Rα were measured by real-time PCR. At all stages of B and T cell development, the IL7Rα mRNA levels were comparable between c-Cbl−/− and WT mice (Fig. S9 A and B). To rule out any possible impact of the “niche” in this phenomenon, sorted HSCs were differentiated in vitro into B cells and surface levels of IL7Rα were measured. A direct comparison revealed an increase of IL7Rα surface levels in c-Cbl-deficient cells (Fig. 5C).
Involvement of c-Cbl in the Ubiquitylation of IL7Rα Protein.
As c-Cbl mainly functions as E3 ubiquitin ligase and targets various receptors for degradation (11, 12, 14), we speculated that c-Cbl might be involved in the ubiquitylation of IL7Rα. Recently, transgenic mice (referred as c-CblA/−) with a loss-of-function mutation in the c-Cbl RING finger domain have been generated and reported (35). Although the E3 ligase functions of c-Cbl protein are perturbed, the expression levels and functions of tyrosine kinase binding domain of c-Cbl protein are normal in c-CblA/− mice. Thus, the cells of c-CblA/− mice, only, lack c-Cbl-mediated ubiquitylation of target proteins. Next, we quantified the surface levels of IL7Rα in lymphocytes of c-CblA/− mice. As observed in c-Cbl−/− cells, B and T cells of c-CblA/− mice exhibited increased surface levels of IL7Rα (Fig. 5D), suggesting that the augmented IL7Rα levels is due to deficient c-Cbl-mediated ubiquitylation. Of note, both age-dependent thymic loss (35) and diminished B cell development in the BM have been observed in c-CblA/− mice. To exclude the possible involvement of Cbl-b, a homolog of c-Cbl, in this phenomenon, B and T cell development of 24- and 42-week-old Cbl-b−/− mice was analyzed. As expected, both the lymphocyte numbers and IL7Rα expression levels were comparable between Cbl-b−/− and WT animals (Fig. S9 C and D).
Finally, using coimmunoprecipitation and ubiquitylation experiments, we formally demonstrated an interaction between c-Cbl and IL7Rα proteins (Fig. 5E) and the involvement of c-Cbl in the ubiquitylation of IL7Rα (Fig. 5F). Taken together, these experiments suggest that c-Cbl might be involved in the ubiquitylation of IL7Rα.
Discussion
In keeping with our findings, it has been recently proposed (29) that IL7 is regulated by consumption rather than production. In contrast to constitutive production of IL7, mounting evidence documents that IL7Rα expression is strictly modulated, either increased or decreased, during lymphocyte development (5, 29, 32–34, 36, 37). Although, IL7Rα is expressed as early as DN1 stage of thymocyte development, IL7Rα signaling is critically required at the DN2 and DN3 stages, where most thymocyte division occurs (38). Massive expansion of DN4 and early DP cells results in the complete loss of IL7Rα expression. Once thymic selection is completed, IL7Rα is reexpressed in thymocytes that undergo positive selection. A profound expression of IL7Rα is documented at CD4 and CD8 single positive cells, as IL7 signals are required for their survival (39, 40). Mounting evidence underlines the importance of transient down-regulation of IL7Rα between DN3 and DP stages of thymocyte development (29). A pioneering study by Munitic et al. has demonstrated that transgenic expression of IL7Rα, under the control of CD2 promoter, throughout thymocyte development results in overall reduction in the cellularity of the thymus and accelerated thymic involution (34).
Similarly, IL7Rα expression is crucial for murine B cell devel-opment in the bone marrow. IL7Rα is expressed in B cells from the earliest CLP stage to the early surface IgM+ stage; however, it is absent in mature B cells (41, 42). Consistent with T cell development, retroviral-mediated overexpression of IL7Rα in hematopoietic progenitor cells leads to perturbed B cell development (37). Earlier, we have reported that deregulated expression of IL7Rα in B lymphocytes leads to incomplete blockade of B cell development (5). Taken together, it has become evident that perturbed down-regulation of IL7Rα during lymphocyte development results in reduced differentiation of B and T cells.
Aging is broadly defined as a progressive decline of tissue and organ functions due to deregulation of various cell intrinsic and extrinsic factors. One of the hallmark features of the aging immune system is reduced B cell development in the bone marrow and T cell development in the thymus (21, 31). The reduction of lymphocyte development in aging animals results in fewer naïve cells available to replenish the peripheral lymphocyte pool and ultimately leading to constriction of B and T cell repertoire. This results in deterioration of immune functions and contributes to the increased incidence of morbidity and mortality from infectious diseases, autoimmunity and cancer (21). Studies over many decades have suggested a number of critical factors involved in the aging process. These include cell intrinsic factors such as oxidative stress, telomere shortening, accumulation of mutations, mitochondrial dysfunction, altered composition of lipid bilayer and changes in receptor binding affinities, and cell extrinsic factors such as failed generation of one or more stromal derived cytokines (31). Recently, it has been proposed that a more likely cause for the defective lymphoid differentiation of aging HSCs is the age dependent acquisition of defects in the genomic DNA (31).
Intriguingly, our analysis suggested that the perturbed lymphocyte development in older c-Cbl mutants might be due to impaired down regulation of IL7Rα in lymphocytes. Of note, c-Cbl deficiency results in lymphopenia and reduced lymphocyte functions, exclusively, in older animals. In view of this fact, it is tempting to hypothesize that c-Cbl deficiency might result in accelerated aging of the immune system. Although c-Cbl deficiency results in a phenotype that is very similar to the “immune aging” phenotype seen in wild type mice, it should be emphasized here that, the “immune aging” phenomenon of wild type mice might be caused by mechanisms other than c-Cbl deficiency, since c-Cbl expression in lymphocytes is comparable between young and aging wild type mice. This is not surprising, as aging is complex and, most likely, is the result of defects in multiple cell intrinsic and extrinsic pathways. Recently, several human patients have been identified to harbor mutations in the c-CBL gene, which abolish its E3 ligase functions. Even though there is no information available yet on the lymphocyte development and functions in these patients, our study suggests consideration of this possibility.
We believe that the insights obtained from the current study might open a new perspective in the understanding of aging biology. In addition, it provides evidence that the modulation of cytokine receptor expression through post-translational modifications, such as ubiquitylation, is one of the key determinants of cytokine signaling in hematopoiesis.
Methods
Mice.
c-Cbl−/− mice and c-CblA/− (c-Cbl Ring finger mutant) mice were generated and reported earlier (15, 35). c-Cbl−/−, c-Cbl+/+, c-CblA/−, and CD45.1 mice were kept under specific pathogen-free conditions in the animal care facility at Yale University. All mouse experiments were approved by the Institutional Animal Care and Use Committee of Yale University.
Immunization Studies.
Four- and 24-week-old c-Cbl−/− and WT mice (n = 5) were injected s.c. with 10 μg of TNP KLH in CFA. Five, 10, and 15 days after immunization, mice were bled and the IgM and IgG antibody titers in the serum were determined by ELISA.
Cell Culture.
In vitro cultures were performed using purified B and T cells. For viability assays, sorted cells were cultured for 48 h in the presence of 10 ng/mL IL7 (PeproTech) in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine, 1% penicillin-streptomycin, and 1 mM nonessential amino acids.
T Cell Proliferation Studies.
Naïve (CD62Lhigh CD44low/−) CD4 T cells were sorted and labeled with CFSE (3 μM) at 37 °C for 10 min. Cells were stimulated with plate-bound αCD3e (5 μg/mL) and soluble αCD28 (2 μg/mL).
Supplementary Material
Acknowledgments
We appreciate Hua Gu and Wallace Langdon for their willingness to share the c-Cbl−/− and c-CblA/+ mice with us. We are very thankful to Frances Manzo for the assistance with manuscript submission. We acknowledge the extended support of the Yale Cell Sorter Facility. R.A.F. is an investigator of Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914496107/DCSupplemental.
References
- 1.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 2.Cobaleda C, Busslinger M. Developmental plasticity of lymphocytes. Curr Opin Immunol. 2008;20:139–148. doi: 10.1016/j.coi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nat Rev Immunol. 2008;8:95–106. doi: 10.1038/nri2234. [DOI] [PubMed] [Google Scholar]
- 4.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 5.Rathinam C, Klein C. Transcriptional repressor Gfi1 integrates cytokine-receptor signals controlling B-cell differentiation. PLoS One. 2007;2:e306. doi: 10.1371/journal.pone.0000306. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Dias S, Xu W, McGregor S, Kee B. Transcriptional regulation of lymphocyte development. Curr Opin Genet Dev. 2008;18:441–448. doi: 10.1016/j.gde.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs SY. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol Ther. 2002;1:337–341. [PubMed] [Google Scholar]
- 8.Joazeiro CA, Weissman AM. RING finger proteins: Mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 9.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 10.Joazeiro CA, et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 11.Ryan PE, Davies GC, Nau MM, Lipkowitz S. Regulating the regulator: Negative regulation of Cbl ubiquitin ligases. Trends Biochem Sci. 2006;31:79–88. doi: 10.1016/j.tibs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Thien CB, Langdon WY. Cbl: Many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 13.Duan L, Reddi AL, Ghosh A, Dimri M, Band H. The Cbl family and other ubiquitin ligases: Destructive forces in control of antigen receptor signaling. Immunity. 2004;21:7–17. doi: 10.1016/j.immuni.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt MH, Dikic I. The Cbl interactome and its functions. Nat Rev Mol Cell Biol. 2005;6:907–918. doi: 10.1038/nrm1762. [DOI] [PubMed] [Google Scholar]
- 15.Naramura M, Kole HK, Hu RJ, Gu H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci USA. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thien CB, Bowtell DD, Langdon WY. Perturbed regulation of ZAP-70 and sustained tyrosine phosphorylation of LAT and SLP-76 in c-Cbl-deficient thymocytes. J Immunol. 1999;162:7133–7139. [PubMed] [Google Scholar]
- 17.Rathinam C, Thien CB, Langdon WY, Gu H, Flavell RA. The E3 ubiquitin ligase c-Cbl restricts development and functions of hematopoietic stem cells. Genes Dev. 2008;22:992–997. doi: 10.1101/gad.1651408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokomizo T, Dzierzak E. Fine-tuning of hematopoietic stem cell homeostasis: Novel role for ubiquitin ligase. Genes Dev. 2008;22:960–963. doi: 10.1101/gad.1669908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 20.Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nat Immunol. 2007;8:137–144. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- 21.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 22.Clevers H, Ferrier P. Transcriptional control during T-cell development. Curr Opin Immunol. 1998;10:166–171. doi: 10.1016/s0952-7915(98)80245-8. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 24.Frasca D, Van Der Put E, Riley RL, Blomberg BB. Age-related differences in the E2A-encoded transcription factor E47 in bone marrow-derived B cell precursors and in splenic B cells. Exp Gerontol. 2004;39:481–489. doi: 10.1016/j.exger.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin RG, et al. Cloning of the human and murine interleukin-7 receptors: Demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- 28.Page TH, Willcocks JL, Taylor-Fishwick DA, Foxwell BM. Characterization of a novel high affinity human IL-7 receptor. Expression on T cells and association with IL-7 driven proliferation. J Immunol. 1993;151:4753–4763. [PubMed] [Google Scholar]
- 29.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: Intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 30.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37:455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 31.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/s1074-7613(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 33.Laouar Y, Crispe IN, Flavell RA. Overexpression of IL-7R alpha provides a competitive advantage during early T-cell development. Blood. 2004;103:1985–1994. doi: 10.1182/blood-2003-06-2126. [DOI] [PubMed] [Google Scholar]
- 34.Munitic I, et al. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood. 2004;104:4165–4172. doi: 10.1182/blood-2004-06-2484. [DOI] [PubMed] [Google Scholar]
- 35.Thien CB, et al. Loss of c-Cbl RING finger function results in high-intensity TCR signaling and thymic deletion. EMBO J. 2005;24:3807–3819. doi: 10.1038/sj.emboj.7600841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 37.Purohit SJ, et al. Determination of lymphoid cell fate is dependent on the expression status of the IL-7 receptor. EMBO J. 2003;22:5511–5521. doi: 10.1093/emboj/cdg522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim K, Lee CK, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol. 1998;160:5735–5741. [PubMed] [Google Scholar]
- 39.Fry TJ, Mackall CL. Interleukin-7: From bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 40.Yu Q, Erman B, Bhandoola A, Sharrow SO, Singer A. In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J Exp Med. 2003;197:475–487. doi: 10.1084/jem.20021765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudo T, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei C, Zeff R, Goldschneider I. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. J Immunol. 2000;164:1961–1970. doi: 10.4049/jimmunol.164.4.1961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.