Abstract
Mammalian sweet, bitter, and umami taste is mediated by a single transduction pathway that includes a phospholipase C (PLC)β and one cation channel, TRPM5. However, in insects such as the fruit fly, Drosophila melanogaster, it is unclear whether different tastants, such as bitter compounds, are sensed in gustatory receptor neurons (GRNs) through one or multiple ion channels, as the cation channels required in insect GRNs are unknown. Here, we set out to explore additional sensory roles for the Drosophila TRPA1 channel, which was known to function in thermosensation. We found that TRPA1 was expressed in GRNs that respond to aversive compounds. Elimination of TRPA1 had no impact on the responses to nearly all bitter compounds tested, including caffeine, quinine, and strychnine. Rather, we found that TRPA1 was required in a subset of avoidance GRNs for the behavioral and electrophysiological responses to aristolochic acid. TRPA1 did not appear to be activated or inhibited directly by aristolochic acid. We found that elimination of the same PLC that leads to activation of TRPA1 in thermosensory neurons was also required in the TRPA1-expressing GRNs for avoiding aristolochic acid. Given that mammalian TRPA1 is required for responding to noxious chemicals, many of which cause pain and injury, our analysis underscores the evolutionarily conserved role for TRPA1 channels in chemical avoidance.
Keywords: transient receptor potential, chemosensation, taste, phospholipase C, aristolochic acid
TRPA1 is an evolutionarily conserved cation channel (1–4), which in vertebrates is expressed in nociceptive neurons and functions in the detection of noxious or pungent chemicals ranging from mustard oil to environmental irritants (4–11). Mammalian TRPA1 may also function in the detection of thermal cold (1, 7, 12), although this issue is controversial (6). Nevertheless, Drosophila TRPA1 is a known mediator of temperature sensation, as it is activated directly by temperatures in the warm range (2) and is necessary for thermotaxis in response to excessively warm temperatures (13). In addition, this channel also functions in the discrimination of the preferred temperature over suboptimal temperatures in the comfortable range (14). In this latter case, TRPA1 is activated downstream of a phospholipase C (PLC)-dependent signaling cascade, demonstrating that it can be activated by intracellular chemical signals.
Given the broad roles of PLC signaling in sensory physiology in many organisms, we wondered whether TRPA1 might also function in other sensory modalities, such as contact chemosensation or taste. Mammalian sweet, bitter, and umami taste is universally dependent on PLC signaling and the TRPM5 channel (15, 16). In insects, such as the fruit fly Drosophila melanogaster, it is unclear if the detection of tastants in gustatory receptor neurons (GRNs) operates through PLC signaling or TRP channels. It has been reported that the wasabi response requires the fly TRP channel, Painless (17). However, it is not known if Painless functions in GRNs or in the central nervous system or whether it responds directly to wasabi or through a signaling cascade.
Our understanding of the molecular components required for contact chemosensation in insects is limited primarily to the identification of a series of gustatory receptors (18–25). Currently, the channels required for contact chemosensation in insects are not known. Contact chemosensation is critically important in insects as it allows the animals to detect nonvolatile pheromones and differentiate attractive foods from noxious tastants (antifeedants) produced by plants (26–28). Whether insects use a single set of signaling molecules, such as channels, to sense all bitter chemicals or whether there are distinct channels employed for sensing different tastants in GRNs is unclear. On the basis of electrophysiological analyses in insects, such as Manduca sexta, it has been proposed that there are multiple transduction pathways that function in response to bitter compounds. Specifically, it has been suggested that the plant antifeedant, aristolochic acid, is detected through a transduction mechanism distinct from what acts in response to caffeine and other bitter compounds (29, 30).
In the current study, we found that Drosophila trpA1 was expressed in a subset of avoidance GRNs. Rather than displaying a broad role in the sensation of bitter compounds, we found that trpA1 was required in GRNs for avoiding aristolochic acid, but not any other bitter compound tested. Thus, in contrast to mammals, Drosophila does not rely on a single channel to mediate the deterrent response to noxious chemicals. TRPA1 was not modulated directly by aristolochic acid, suggesting that it is activated downstream of a signaling cascade. We found that the same PLC that functioned in concert with TRPA1 for larval thermosensation (14) was also required in the trpA1-expressing GRNs for sensing aristolochic acid.
Many mammalian thermoTRPs serve as molecular integrators of sensory input (31). An example of this phenomenon is the hyperalgesia that results from activation of TRP channels in nociceptive neurons by thermal heat or cold and by chemicals produced during inflammation (5, 6, 9, 32–34). The mammalian taste transduction channel, TRPM5 (15, 16), also integrates chemical and thermal input, as the response to certain tastants such as sugars is enhanced at higher temperatures (35). However, Drosophila TRPA1 represents the first TRP that is required in separate chemosensory and thermosensory receptor cells, to function in taste and temperature discrimination. Because mammalian TRPA1 functions in the avoidance of pungent or dangerous compounds, our analysis underscores that avoidance to noxious chemicals is a phylogenetically conserved feature of TRPA1 channels.
Results
Expression of trpA1 in Aversive Gustatory Receptor Neurons.
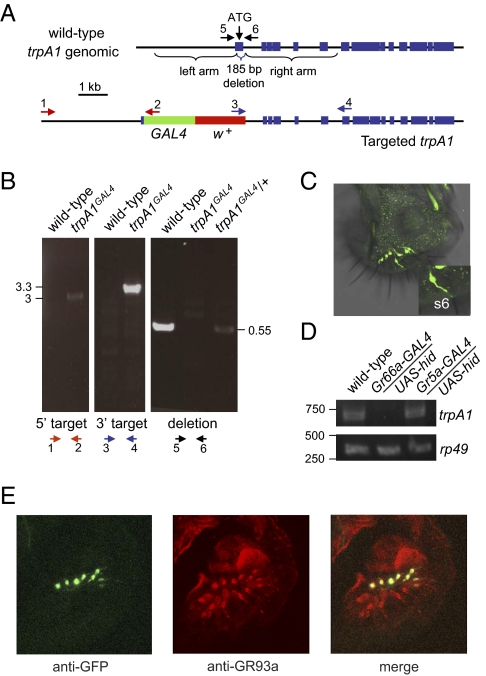
Currently, the only known role for Drosophila TRPA1 is in temperature discrimination (13, 14). To identify additional functions for TRPA1, we set out to examine the presumptive expression pattern using a GAL4 reporter. We used homologous recombination to introduce the GAL4 gene at the site of the normal translation initiation codon for TRPA1 (trpA1GAL4), so that the reporter would be expressed under control of the transcriptional regulatory region of trpA1. In addition, we deleted a 185-bp region encoding the N-terminal 61 residues, thereby generating a new trpA1 allele (Fig. 1 A and B).
Fig. 1.
Generation and expression of a trpA1GAL4 reporter and mutant. (A) Schematic of the trpA1 genomic locus. The trpA1 exons are indicated by blue rectangles. The targeted trpA1GAL4 was generated by ends-out homologous recombination (49), resulting in deletion of 185 nucleotides spanning the trpA1 translation initiation codon (ATG). The green and red rectangles indicate the GAL4 and miniwhite genes, respectively. The arrows indicate the primers used for PCR confirmation of the targeting and deletion in trpA1. (B) PCR analyses of genomic DNA to verify the targeting of the left and right arms and the deletion in trpA1. The size markers indicate kilobases. (C) Spatial distribution of the trpA1 reporter in a dissected labellum. A UAS-mCD8::GFP transgene was crossed into a trpA1GAL4/+ background and the tissue was stained with anti-GFP. The Inset (lower right) shows a magnified view of a GRN from an s6 sensillum. (D) RT-PCR analyses performed using RNA prepared from the indicated flies. (E) Coexpression of the trpA1 reporter with a subset of GR93a-expressing GRNs. The labellum was dissected from UAS-GFP;trpA1/+ flies and stained with anti-GFP and anti-GR93a.
To examine the spatial distribution of the trpA1-GAL4 reporter, we introduced a UAS-mCD8::GFP transgene into the trpA1GAL4/+ background. We found that expression of GFP was pronounced in the adult labellum, which houses gustatory sensilla and serves as the functional equivalent of the mammalian tongue (Fig. 1C). The labellum includes three types of sensilla, which are categorized on the basis of their length (s-type, short; i-type, intermediate; and l-type, long) (26, 27). Aversive compounds are detected principally by GRNs in the s- and i-type sensilla, whereas sugars are most effective in stimulating GRNs in the l-type sensilla. We found that the GFP labeled 11–13 GRNs that were housed in both i-type (i1–i9) and s-type (s6, s9, and s12) sensilla, but not in l-type sensilla (Fig. 1 C and E).
Nearly all GRNs that respond to avoidance chemicals (∼22) express the gustatory receptors (GRs), GR66a and GR93a (18, 21, 36, 37). Neither of these GRs is detected in GRNs that express GR5a and respond to sugars (18, 21, 23, 36–38). We found that all of the 11–13 GRNs that were labeled with the trpA1 reporter, using anti-GFP, stained a subset of anti-GR93a positive GRNs (Fig. 1E). However, we were unable to detect anti-TRPA1 staining, possibly due to low levels of the TRPA1 protein in the labellum.
To verify that the trpA1 reporter reflected the bona fide expression pattern in the labellum, we examined trpA1 RNA levels after genetically ablating either Gr66a- or Gr5a-expressing GRNs. This was accomplished by expressing the cell death gene (UAS-hid) under control of either the Gr66a-GAL4 or the Gr5a-GAL4. In support of the conclusion that the trpA1 reporter was expressed in aversive GRNs, we detected the trpA1 RT-PCR product using RNA prepared from labella dissected from Gr5a-GAL4/UAS-hid flies but not from Gr66a-GAL4/UAS-hid animals (Fig. 1D).
trpA1 Was Required in Gustatory Receptor Neurons for Avoiding Aristolochic Acid.
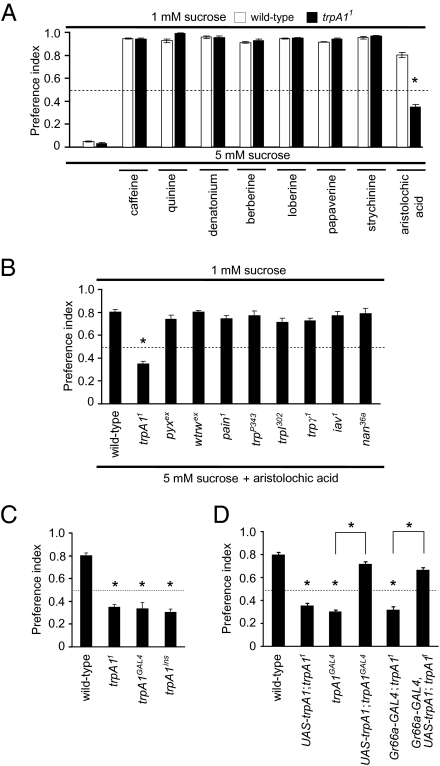
To address whether trpA1 was required for the behavioral responses to aversive tastants, we conducted a binary food-choice assay. We placed flies in 72-well microtiter dishes that contained wells alternating between 1 mM sucrose and 5 mM sucrose laced with an aversive tastant. We mixed the two different tastants with red or blue food coloring and scored food preferences by examining whether the abdomens were red, blue, or purple. A preference index (P.I.) of 1 or 0 results if there is a complete preference for one or the other tastant, whereas a P.I. of 0.5 indicates a lack of bias for the two alternatives. If wild-type flies are given a choice between 1 mM and 5 mM sucrose, they display a strong preference for the higher concentration of sugar (Fig. 2A). However, if the 5 mM sugar is combined with an aversive tastant, they consume the 1 mM sucrose. As expected, because trpA1 is expressed in avoidance GRNs, the trpA11 mutant flies exhibited a normal preference for 5 mM over 1 mM sucrose (Fig. 2A). However, the mutant animals also avoided most bitter chemicals tested. These included caffeine, quinine, strychnine, and four others (Fig. 2A).
Fig. 2.
trpA1 mutation caused impaired behavioral avoidance of aristolochic acid. (A) Survey of behavioral response to bitter compounds in trpA11 flies. Binary food-choice assays were performed using 1 mM sucrose alone versus 5 mM sucrose alone or 5 mM sucrose with 10 mM caffeine, 1 mM quinine, 0.3 mM denatonium, 0.1 mM berberine, 0.3 mM lobeline, 2 mM papaverine, 0.5 mM strychnine, or 10 mM aristolochic acid (n ≥ 4). (B–D) Flies were allowed to select 1 mM sucrose alone or 5 mM sucrose plus 10 mM aristolochic acid. (B) Survey of trp mutants for avoidance to aristolochic acid. (C) Verification of aristolochic acid behavioral avoidance in three trpA1 mutant alleles: trpA11, trpA1ins, and trpA1GAL4. (D) Rescue of trpA1 impairment by expressing UAS-trpA1 under control of the trpA-GAL4 (trpA1GAL4) or the Gr66a-GAL4. Asterisks denote statistical significance (P < 0.05) using unpaired Student's t test. The error bars represent SEMs. See Tables S1–S4 for detailed statistics.
In contrast to the wild-type avoidance to most bitter compounds, we found that the trpA11 flies displayed a reduced aversion to aristolochic acid (Fig. 2A). In addition to TRPA1, there are 10 additional group 1 TRPs encoded in the Drosophila genome (39), and with the exception of trpm and nompC (trpn), mutations affecting the remaining 8 have no obvious effect on viability. All 8 of these other viable trp mutants displayed a normal preference for 1 mM sucrose over 5 mM sucrose plus aristolochic acid (Fig. 2B).
To confirm that trpA1 was required for avoiding aristolochic acid, we examined additional alleles and tested for rescue of the mutant phenotype with a UAS-trpA1 transgene (13). We found that the behavioral phenotype was indistinguishable between trpA11 and two other alleles: trpA1ins and trpA1GAL4 (Fig. 2C). In addition, we rescued the loss of avoidance to aristolochic acid by expressing UAS-trpA1 under control of the trpA1-GAL4 (trpA1GAL4; Fig. 2D). This chemosensory impairment was caused by a defect in GRNs rather than cells in the central nervous system because we also rescued the trpA11 phenotype by expressing UAS-trpA1 under control of the Gr66a-GAL4 (Fig. 2D).
Because TRPA1 is activated with a thermal threshold between 24 °C and 29 °C (2), we considered whether the response to aristocholic acid was temperature dependent. The aversion to caffeine, which is independent of TRPA1, was virtually identical at 18 °C and 29 °C. Avoidance to aristocholic acid was slightly increased at 29 °C relative to 18 °C (Fig. S1). However, this difference was not statistically significant (P = 0.10).
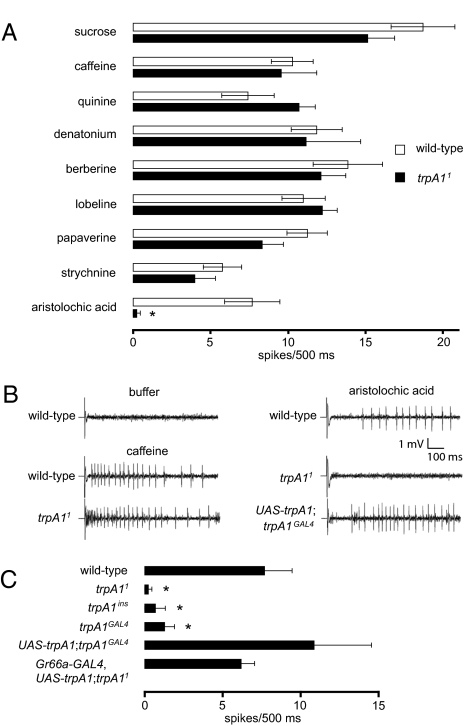
Requirement for trpA1 for Aristolochic Acid-Induced Action Potentials.
To verify that trpA1 was required in GRNs for sensing aristolochic acid, we performed tip recordings. To conduct this analysis we focused on a sensillum (s6), which is a readily accessible sensillum that expresses trpA1 (Fig. 1C) and responds to a wide diversity of aversive compounds (40). Consistent with the behavioral analyses, application of sucrose, caffeine, and most other bitter compounds induced a similar frequency of action potentials in wild-type or trpA11 flies (Fig. 3 A and B). However, loss of trpA1 virtually eliminated aristolochic acid-induced action potentials (Fig. 3 A and B). Because these same s6 sensilla still responded to other bitter compounds, the defect in the aristolochic acid response did not appear to reflect a requirement for trpA1 for development or maintenance of the GRNs in the s6 sensilla. The impairment in aristolochic acid-induced action potentials was due to loss of trpA1 because we rescued this phenotype by expressing UAS-trpA1 under control of either the trpA1-GAL4 or the Gr66a-GAL4 (Fig. 3 B and C).
Fig. 3.
trpA1 is required for aristolochic acid-induced action potentials. (A) Tip recordings were performed on s6 bristles except for the sucrose recordings, which were performed on l4 and l6. Shown are the average frequencies of action potentials after application of 50 mM sucrose, 10 mM caffeine, 1 mM quinine, 1 mM denatonium, 0.1 mM berberine, 1 mM lobeline, 1 mM papaverine, 1 mM strychnine, or 1 mM aristolochic acid (n ≥ 7). (B) Sample tip recordings after application of recording pipettes with buffer alone, caffeine, or aristolochic acid. (C) Impairment of aristolochic acid-induced action potentials in multiple trpA1 alleles and rescue of the defect by expressing UAS-trpA1 under control of either trpA1-GAL4 (trpA1GAL4) or Gr66a-GAL4 (n ≥ 7). The error bars represent SEMs. The asterisks indicate significant differences from wild type (P < 0.05). Detailed statistics are presented in Tables S5 and S6.
Requirement for Phospholipase C in GRNs for Sensing Aristolochic Acid.
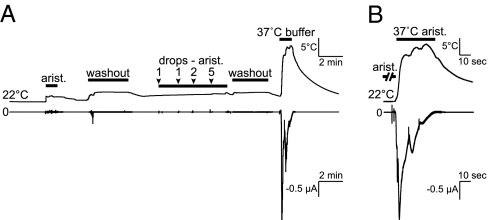
Because TRPA1 is essential for aristolochic acid avoidance, it could be a direct sensor for aristolochic acid or function downstream of a signaling cascade. To address whether TRPA1 was directly activated by aristolochic acid, we expressed the same trpA1 DNA construct in Xenopus oocytes that rescued the trpA1 phenotype, and measured membrane current following application of aristolochic acid. Addition of aristolochic acid to the bath solution did not activate TRPA1 (Fig. 4A). This result was not due to an absence of functional TRPA1 channels because TRPA1 was thermally activated by warm temperatures (Fig. 4A), as previously reported (2). Thermal activation of TRPA1 was not inhibited by aristolochic acid (Fig. 4B). Thus, aristolochic acid neither activated nor inhibited TRPA1 directly.
Fig. 4.
Aristolochic acid did not activate or inhibit Drosophila TRPA1 in vitro. (A) Two-electrode voltage clamp recordings from Xenopus oocytes expressing TRPA1. Aristolochic acid (0.1 mM) (23 °C) did not activate TRPA1 (ΔI ± SEM = −0.0004 ± 0.0002 μA, n = 3). Lower concentrations of aristolochic acid (dropwise addition of 0.1 mM solution) to the bath did not induce channel activation (ΔI = −0.0002 ± 0.0002 μA, n = 4). Heated (37 °C) ND96 buffer resulted in the strong activation of TRPA1 (ΔI = −2.662 ± 0.418 μA, n = 6). (B) Aristolochic acid did not inhibit TRPA1. Voltage-clamped oocytes were preincubated in 0.25 mM aristolochic acid for 5 min and then warmed in the presence of aristolochic acid (ΔI = −2.190 ± 0.591 μA, n = 4).
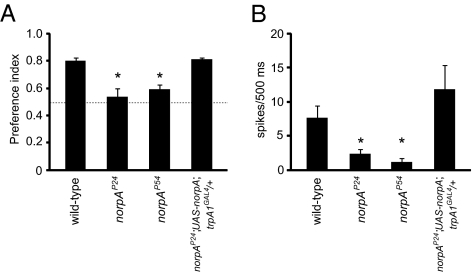
The activity of many TRP channels is linked to PLC signaling cascades in vivo, and mammalian taste sensation requires PLCβ. However, a role for a PLC in Drosophila taste has not been reported. We tested whether PLC was required for sensing aristolochic acid by examining two norpA alleles (norpAP24 and norpAP54), which did not express detectable levels of the same PLC (41, 42) that was required for activation of TRPA1 in a subset of thermosensory neurons in larvae (14). Avoidance of aristolochic acid was significantly reduced in norpAP24 and norpAP54 flies (Fig. 5A), although the P.I.’s were higher than in the trpA1 mutant. We rescued the impairment in norpA mutant flies by expressing UAS-norpA under control of the trpA1-GAL4 (trpA1GAL4/+). In addition, the frequencies of aristolochic acid-induced action potentials were reduced greatly in norpAP24 and norpAP54 flies (Fig. 5B), and this deficit was rescued by expression of UAS-norpA in trpA1-expressing GRNs. These results indicated that TRPA1 and PLC functioned in the same cells for sensing aristolochic acid.
Fig. 5.
Requirement for PLC for sensing aristolochic acid. (A) Binary food-choice assays were performed using 1 mM sucrose alone vs. 5 mM sucrose plus 10 mM aristolochic acid. Two independent norpA alleles, norpAP24 and norpAP54 showed reduced avoidance to aristolochic acid. Expression of UAS-norpA using the trpA1-GAL4 (trpA1GAL4/+) rescued the phenotype. (B) Tip recording analyses showed that both norpA allele mutants had reduced frequencies of aristolochic acid-induced action potentials. This deficit was fully restored by the introduction of UAS-norpA and trpA1GAL4. The error bars represent SEMs. The asterisks indicate significant differences from wild type (P < 0.05). Detailed statistics are presented in Tables S7 and S8.
Discussion
In mammals, distinct TRP channels function in thermosensation and in taste. These include the taste transduction channel, TRPM5, and several other TRPs that respond to temperatures ranging from noxious cold to noxious heat (31). Nevertheless, it is clear that mammalian TRPM5, which is activated in taste receptor cells downstream of a PLC cascade (15, 16), is also modulated by temperature in taste receptor cells (35). Moreover, the activities of multiple thermoTRPs in thermosensory neurons are regulated either directly or indirectly by chemicals (31).
In the current work we demonstrate that the same Drosophila TRP channel (TRPA1), which is known to be required in thermosensory neurons for temperature sensation (13, 14, 43), is also required in GRNs for the taste response. These results demonstrate that a TRP channel functions in insect GRNs and is required in both thermosensory and gustatory receptor neurons, rather than serving exclusively as a molecular integrator of thermal and chemical input in the same neurons.
In mammals, a single transduction pathway that includes a PLC and TRPM5 is required broadly for the responses to all sugars, bitter compounds, and L-glutamate (15, 16). The specificity for different bitter and sweet tastants is mediated exclusively by distinct G protein-coupled receptors.
In insects, such as Manduca, it has been proposed that multiple signaling pathways are involved in the response to different bitter compounds, such as aristolochic acid and caffeine (29, 30). Currently, there are no reports providing molecular or genetic evidence in favor of this proposal. In the present study, we present findings supporting this hypothesis. TRPA1 was expressed in a subset of aversive but not attractive GRNs and was dispensable for sensing sucrose and most bitter chemicals tested, such as caffeine. Among the aversive chemicals tested, TRPA1 was required exclusively for avoiding the naturally occurring plant antifeedant, aristolochic acid. Thus, in contrast to mammalian taste, there is not a single set of signaling proteins, such as one taste transduction channel, which functions in the sensation of a diversity of tastants.
It appears that TRPA1 was not activated directly by aristolochic acid, but functioned in vivo through a transduction pathway that employs a PLC. Consistent with this proposal, we found that mutation of the PLC, encoded by the norpA locus, greatly reduced aristolochic acid-induced action potentials and this impairment was rescued by expressing norpA under control of the trpA1 promoter. The small remaining response in the norpA mutant flies raises the possibility that an additional PLC may also contribute to response to aristocholic acid. Consistent with the link between TRPA1 and PLC in Drosophila taste, we have shown recently that TRPA1 functioned in larvae in the selection of the preferred temperature (18 °C) over other temperatures in the comfortable range (19–24 °C) downstream of a PLC-dependent signaling cascade (14).
Finally, it appears that Drosophila TRPA1 has a common role in avoiding noxious stimuli. In thermosensation, TRPA1 functions in sensory cells involved in selecting the preferred temperature over noxious heat and over slightly suboptimal temperatures, whereas in the gustatory system TRPA1 is required for avoiding aristolochic acid. Given that vertebrate TRPA1 channels participate in the sensation of excessively cold temperatures and noxious chemicals, it appears that TRPA1 is an ancient evolutionarily conserved detector of aversive stimuli.
Methods
Fly Stocks.
The norpAP24 and norpAP54 alleles were from W. Pak. The following fly lines were described previously: (i) trpA11 (14), (ii) trpA1ins (43), (iii) pain1 (44), (iv) trpP343 (originally isolated in the W. Pak lab) (45), (v) trpl302 (46), (vi) nan36a (47), (vii) Gr66a-GAL4 (48), and (viii) Gr5a-GAL4 (36). Additional trp mutants were deletions generated by P-element excisions: (i) pyxex and (ii) wtrwex. The following flies were from the Bloomington Stock Center: (i) iav1, (ii) w1118, and (iii) UAS-mCD8::GFP. The w1118 strain was used as the “wild-type” control. The trpA11 mutation was recombined into a w1118 background for five generations. The UAS-trpA1 transgene (13) contained a single residue change (H408R) (43).
Homologous Recombination.
We generated the trpA1GAL4 allele by ends-out homologous recombination (49). We subcloned 2.9- and 3.2-kb trpA1 genomic DNA fragments into pw35GAL4 (Fig. 1A) (25). We generated nontargeted transgenic flies by germ-line transformation (BestGene) and generated the targeted allele as described (49). We confirmed targeting of PCR analyses and outcrossed the trpA1GAL4 flies to w1118 for five generations.
Immunostaining of Labella.
Antibody stainings of whole labella (Fig. 1C) were performed as described (18). Briefly, we dissected labella from 3- to 7-day-old flies and placed them in 24-well cell culture cluster plates (Costar) containing 940 μL of fix buffer [0.1 M Pipes (pH 6.9), 1 mM EGTA, 1% Triton X-100, 2 mM MgSO4, 150 mM NaCl] and 60 μL of 37% formaldehyde on ice for 45 min. The tissues were washed three times (1× PBS, 0.2% saponin) and blocked for 4–8 h at 4 °C with 1 mL of blocking buffer (1× PBS, 0.2% saponin, 5 mg/mL BSA). The tissues were incubated with the primary antibodies overnight at 4 °C, washed three times, blocked for 15 min, and incubated with the secondary antibodies (Alexa 488 and Alexa 568, 1:200; Invitrogen–Molecular Probes) for 4 h at 4 °C and washed three times. To obtain improved antibody penetration for detecting endogeneous GR93a (Fig. 1E), we fixed the tissue in 2% paraformaldehyde in PBS at room temperature for 15 min, washed it three times, and bisected the tissue as described (25). The tissue was blocked in 5% goat serum, transferred into 1.25× PDA solution [37.5% glycerol, 187.5 mM NaCl, 62.5 mM (Tris pH 8.8)], and viewed by confocal microscopy (Carl Zeiss LSM510). Primary antibodies were used at the following dilutions: mouse anti-GFP, 1:1,000 (Invitrogen–Molecular Probes); and rabbit anti-GR93a, 1:1,000.
RT-PCR Analyses of Gr66a-GAL4/UAS-hid Flies.
Adult labella from wild-type, Gr5a-GAL4/UAS-hid, and Gr66a-GAL4/UAS-hid flies were dissected and RNA was extracted (Stratagene) as described (18). AMV reverse transcriptase was used for generating cDNAs (Promega). For RT-PCR analyses, we used the following primers for trpA1: 5′-GACTTCGGGCGACAAGGAGA-3′ and 5′- CTCGCCCCACTGGAAGAAGA-3′ and rp49 primers as a control. The trpA1 and rp49 products were produced after 35 and 25 cycles, respectively.
Chemicals.
Sucrose, aristolochic acid, caffeine, denatonium benzoate, papaverine hydrochloride, quinine hydrochloride, and strychnine nitrate salt were obtained from Sigma-Aldrich. Lobeline hydrochloride was purchased from Fluka, and berberine sulfate trihydrate was purchased from Wako Chemical. All compounds were dissolved in H2O.
Behavioral Assays.
Two-way choice assays were performed essentially as described (50). Briefly, 3- to 6-day-old flies (50 ± 10 flies per experiment) were starved on 1% agarose for ∼18 h and placed into 72-well microtiter dishes with 1% agarose. Alternating wells contained either red (sulforhodamine B, 0.2 mg/mL; Sigma-Aldrich) or blue dye (Brilliant blue FCF, 0.125 mg/mL; Wako Chemical) plus 1 mM sucrose or 5 mM sucrose alone or 5 mM sucrose laced with 1 mM quinine, 0.3 mM denatonium, 0.1 mM berberine, 0.3 mM lobeline, 2 mM papaverine, 0.5 mM strychnine, 10 mM caffeine, or 10 mM aristolochic acid. The flies were allowed to feed for 90 min at room temperature. The numbers of flies with blue (NB), red (NR), or purple (NMIX) abdomens were tabulated, and the P.I. was determined: (NB + 0.5Nmix)/(NR + NB + Nmix).
Tastant-Induced Action Potential Recordings (Also Referred to as Tip Recordings).
Tastant-induced action potentials were determined as described (21). Briefly, 3- to 7-day-old flies were immobilized with a reference electrode filled with Drosophila Ringer's solution by inserting the electrode from the thorax all the way through to the labellum. We stimulated bristles with a recording electrode (10- to 20-μm tip diameter), which included the indicated concentration of each of the bitter compounds and 1 mM KCl or 50 mM sucrose with 30 mM tricholine citrate as the electrolyte. We performed tip recordings on s6 (bitter compounds), l4, or l6 sensilla (sucrose). The recording electrode was connected to a preamplifier (TASTE PROBE; Syntech), which amplified the signals 10-fold, in conjunction with a 100- to 3,000-Hz band pass filter. The action potentials were acquired at a 12-kHz sampling rate. The number of action potentials was based on the activity recorded 50–550 msec after application of the chemicals. We performed all recordings 6–21 times.
Functional Expression of TRPA1 in Xenopus Oocytes.
Xenopus laevis (Xenopus 1) ovaries were removed and treated with 2 mg/mL collagenase A (Roche) in OR2 ringers. Oocytes were cultured in OR3 medium at 15–19 °C. The pOX-ER trpA1 vector (H408R; gift of A. Patapoutian), or a wild-type (H408) trpA1 construct provided by P. Garrty (43), was linearized, and capped mRNA was transcribed using a T3 mMessage mMachine (Ambion). Stage V–VI oocytes were injected (Nanoject II; Drummond Scientific) with 50 nL TRPA1 mRNA or H2O.
Two-electrode voltage clamp recordings were conducted 3–4 days postinjection. Membrane potentials were maintained at −40 mV. Currents and bath temperature were recorded in pClamp 8 (Molecular Devices), using a Warner Instruments oocyte clamp (OC-725A) and an Axon DigiData 1320 digital acquisition system (Molecular Devices) with 500 Hz filtering. The bath chamber was perfused with ∼22–24 °C ND96 buffer (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM Hepes, 1.8 mM CaCl2, pH 7.5). To activate TRPA1 by heat, ND96 was prewarmed to 37 °C and perfused into the recording chamber three times. Aristolochic acid I (Sigma A9451)-containing solutions (0.1 and 0.25 mM in ND96 buffer) were sonicated for 30 min at 37 °C before use. Aristolochic acid did not directly activate either the H408 or the H408R TRPA1 derivatives.
Supplementary Material
Acknowledgments
S.H.K. is a recipient of a predoctoral fellowship from the Samsung Foundation and Y.L. was supported partially by a postdoctoral fellowship from the Korea Research Foundation (2006-352-C00065). This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC007864 (to C.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001425107/DCSupplemental.
References
- 1.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 2.Viswanath V, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 3.Kindt KS, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 4.Prober DA, et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28:10102–10110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 6.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 10.Talavera K, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 11.Macpherson LJ, et al. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Karashima Y, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenzweig M, et al. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 16.Pérez CA, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 17.Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 24.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 27.Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- 29.Glendinning JI, Davis A, Ramaswamy S. Contribution of different taste cells and signaling pathways to the discrimination of “bitter” taste stimuli by an insect. J Neurosci. 2002;22:7281–7287. doi: 10.1523/JNEUROSCI.22-16-07281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glendinning JI, Hills TT. Electrophysiological evidence for two transduction pathways within a bitter-sensitive taste receptor. J Neurophysiol. 1997;78:734–745. doi: 10.1152/jn.1997.78.2.734. [DOI] [PubMed] [Google Scholar]
- 31.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 33.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 34.Obata K, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talavera K, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 36.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Ueno K, et al. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- 39.Montell C. Drosophila TRP channels. Pflugers Arch. 2005;451:19–28. doi: 10.1007/s00424-005-1426-2. [DOI] [PubMed] [Google Scholar]
- 40.Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- 41.Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J Biol Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- 42.Yoon J, et al. Specific molecular alterations in the norpA-encoded phospholipase C of Drosophila and their effects on electrophysiological responses in vivo. J Neurochem. 2004;89:998–1008. doi: 10.1111/j.1471-4159.2004.02384.x. [DOI] [PubMed] [Google Scholar]
- 43.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Jiao Y, Montell C. Dissecting independent channel and scaffolding roles of the Drosophila transient receptor potential channel. J Cell Biol. 2005;171:685–694. doi: 10.1083/jcb.200508030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 48.Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 49.Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci USA. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.