Mitotic recombination is required to repair DNA double-strand breaks (DSBs), to restart stalled/collapsed replication forks, and functions as an alternative mechanism to elongate telomeres in cells lacking telomerase. Although these functions preserve genome integrity, mitotic recombination can also cause loss of heterozygosity in cells with polymorphic chromosomes and lead to gross chromosome rearrangements if it occurs between dispersed repeats. Much of our understanding of recombination mechanisms is based on studies in fungi in which all of the products of an individual meiotic recombination event can be recovered. Two types of recombination events have been identified during meiosis: crossing over and gene conversion. A crossover between linked heterozygous markers results in new linkage arrangements, but the markers still display 2:2 segregation. By contrast, gene conversion represents the nonreciprocal transfer of information between two homologous sequences where one allele is duplicated and the other is lost, resulting in a 3:1 segregation for heterozygous markers in the spore colonies. About 50% of meiotic conversions are associated with crossing over, suggesting that these processes are mechanistically linked. Because mitotic recombination is much less frequent than during meiosis, events are usually selected and typically only one of the two daughter cells produced following recombination is recovered. Thus, little information exists on the mechanism, the time in the cell cycle when recombination occurs, and the nature of the initiating lesion(s). Using a clever genetic assay that enables recovery of both products of a reciprocal crossover event (RCO), Lee and Petes (1) provide compelling evidence that spontaneous mitotic RCOs in G2 cells result from a DSB present on one chromosome before DNA synthesis.
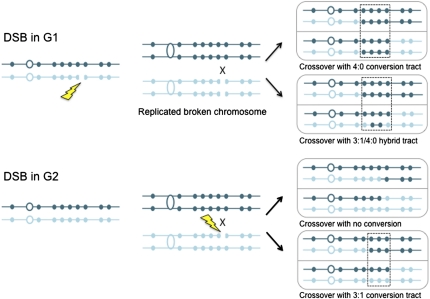
In previous studies Barbera and Petes (2) described a genetic assay that allows selection of both products of a rare G2 mitotic RCO in the form of red/white sectored colonies. In a refinement of this assay using haploid parents with 0.5% sequence divergence, Lee et al. (3) were able to map the position of RCOs within a 120-kb region of chromosome V and to detect marker conversions associated with the crossover (Fig. 1). Thus, this system is analogous to meiotic tetrad analysis in that all of the chromatids engaged in the recombination event are recovered and, by scoring the presence of the heterozygous markers in the two halves of the sectored colony, knowledge of the mechanism can be derived. Two surprising results emerged from these studies. First, conversion tracts associated with spontaneous mitotic crossovers were much longer than meiotic conversion tracts. Second, some conversion tracts showed a 4:0 segregation of the markers or showed hybrid tracts with some markers segregating 4:0 adjacent to markers segregating 3:1. Although gene conversion in a G1 cell could give rise to 4:0 segregations, a crossover in G1 would not generate a sectored colony. Thus, one possible explanation for the 4:0 and hybrid tracts is that a DSB present on one chromosome in a G1 cell is replicated, resulting in two broken sister chromatids that are repaired from the intact homolog nonsisters in G2. One of these repair events would have to be associated with a crossover to generate the sectored colony diagnostic of an RCO.
Fig. 1.
Gene conversion events associated with crossing over following G1- or G2-induced DSBs. A DSB in G1 is replicated, and both sister chromatids are repaired using the homolog nonsisters as templates; alternatively, one broken chromatid might repair first and then be used to template repair of the other broken sister chromatid. Repair of the breaks is accompanied by transfer of polymorphic markers from the undamaged to the broken chromatid, resulting in gene conversion (boxed area). Only one of the two repair events is associated with an RCO. If the same polymorphic markers are converted during both repair events, then a 4:0 tract will result; if one repair event involves more markers, a hybrid 4:0/3:1 tract will be formed. A G2 DSB would be expected to give rise to a RCO with no detectable gene conversion if the conversion tract is very short or to a 3:1 conversion. The homologs are shown in dark and light blue, respectively. The polymorphic sites are indicated by small solid circles, and the open circles represent the centromeres.
To test this hypothesis, Lee and Petes (1) treated diploid cells synchronized in the G1 or G2 stages of the cell cycle with ionizing radiation (IR) and then analyzed the spectrum of recombination events in the red/white sectored colonies. The conversion events in the G1-irradiated cells were remarkably similar to the events observed spontaneously, with 4:0 and 3:1/4:0 hybrid conversion tracts representing about 40% of the total events. In contrast, only simple crossovers (no associated conversion) and 3:1 conversion tracts were recovered from the G2-irradiated cells; no 4:0 or 3:1/4:0 hybrid tracts were detected. Furthermore, the median conversion tract lengths associated with RCOs from G1-irradiated cells was 7.3 kb, not significantly different from the spontaneous conversion tract lengths (6.5 kb), but significantly longer than the G2 conversion tracts (2.7 kb). Consistent with the decrease in conversion tract length, more of the G2 RCOs have no associated conversion, compared with the G1-irradiated and spontaneous events.
These findings support the idea that a DSB present in G1 persists through S-phase and that the duplicated sister chromatids, both harboring a DSB at the same position, are repaired in G2 (Fig. 1). A number of questions arise from these observations. First, what is the source of spontaneous DSBs in G1 cells? Second, why do cells fail to repair the DSB in G1 and progress through S-phase with a broken chromosome? Third, do gene conversion events derive from a heteroduplex DNA (hDNA) precursor?
Spontaneous DSBs in G1 could result from closely spaced excision repair intermediates or from the activity of topo-isomerases. Most spontaneous DSBs are thought to occur during S-phase, for example, when the replication fork encounters a transient single-strand break on one of the template strands resulting in replication fork collapse. Collapsed forks are repaired by homologous recombination using the partially replicated sister chromatid. DSBs made by IR in G2 cells are also preferentially repaired using the sister chromatid (4). These events would go undetected in the Lee and Petes (1) assay, which requires recombination between homologs. It is possible that a broken chromatid present in G2/M that fails to repair using the sister or that is generated during mitosis might be segregated and progress to the next cell cycle. Consistent with this idea, rare spontaneous Rad52 foci that fail to resolve in G2 are sometimes observed in G1 cells, suggesting that a cell with a broken chromosome sometimes adapts and divides (5).
These findings support the idea that a DSB present in G1 persists through S-phase.
In principle, a G1 DSB in diploid cells can be repaired by nonhomologous end joining (NHEJ) or by homologous recombination. Although DSBs made by rare-cutting endonucleases are substrates for NHEJ, this pathway functions poorly to repair IR-induced lesions in Saccharomyces cerevisiae (6). However, NHEJ is the primary mechanism to repair IR-induced DSBs in G1-phase mammalian cells (7). Thus, replication of a G1 DSB and subsequent repair in G2 might be less frequent in mammalian cells than budding yeast. Several lines of evidence suggest that HR is suppressed in G1 cells. First, the resection of DSBs in haploid G1 cells is less extensive than observed in cycling or G2-arrested cells and is activated by cyclin-dependent kinase as cells progress through S-phase (8, 9). The resection of DNA ends to generate 3′ single-strand DNA tails is necessary for Rad51 binding to initiate homologous pairing and strand exchange (10). Second, Rad52, which is essential for HR in budding yeast, does not associate with G1 DSBs to form detectable foci (11). Third, several recombination genes are transcriptionally regulated and not expressed during the G1 phase of the cell cycle (12). G1 DSBs do not activate the DNA damage checkpoint in yeast, cells initiate S-phase, and replication forks progress with normal kinetics in the presence of a DSB (13). The two broken chromatids resulting from replication through the DSB then engage one or both nonsister chromatids to template repair (Fig. 1). These two repair events could result in differing conversion tract lengths, giving rise to the hybrid 3:1/4:0 tracts observed for both spontaneous and G1-irradiated diploids.
The mitotic conversion tracts associated with spontaneous and G1 DSB-induced RCOs are long and continuous (1, 3). These could result from a long excision tract by mismatch repair of an hDNA intermediate or by double-strand gap repair. Studies of DNA end resection have shown the preferential degradation of the 5′ strand and have demonstrated that the 3′ end remains intact for several hours. However, in the absence of repair, the 3′ end is lost as well (14, 15). In the time between the induction of a DSB in G1 and repair in G2, the ends could be resected more than 5 kb, resulting in long hDNA tracts, or both 5′ and 3′ ends could be degraded, resulting in large gaps that would give rise to gene conversion without an hDNA intermediate. Analysis of recombination events in mismatch repair mutants using this genetic assay should address the question of whether an hDNA intermediate is involved.
Acknowledgments
Work in my laboratory on recombination mechanisms is supported by National Institutes of Health Grant GM041784.
Footnotes
The author declares no conflict of interest.
See companion article on page 7383 in issue 16 of volume 107.
References
- 1.Lee PS, Petes TD. Mitotic gene conversion events induced in G1-synchronized yeast cells by gamma rays are similar to spontaneous conversion events. Proc Natl Acad Sci USA. 2010;107:7383–7388. doi: 10.1073/pnas.1001940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbera MA, Petes TD. Selection and analysis of spontaneous reciprocal mitotic cross-overs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2006;103:12819–12824. doi: 10.1073/pnas.0605778103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee PS, et al. A fine-structure map of spontaneous mitotic crossovers in the yeast Saccharomyces cerevisiae. PLoS Genet. 2009;5:e1000410. doi: 10.1371/journal.pgen.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadyk LC, Hartwell LH. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvaro D, Lisby M, Rothstein R. Genome-wide analysis of Rad52 foci reveals diverse mechanisms impacting recombination. PLoS Genet. 2007;3:e228. doi: 10.1371/journal.pgen.0030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daley JM, Palmbos PL, Wu D, Wilson TE. Nonhomologous end joining in yeast. Annu Rev Genet. 2005;39:431–451. doi: 10.1146/annurev.genet.39.073003.113340. [DOI] [PubMed] [Google Scholar]
- 7.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ira G, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- 11.Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci USA. 2001;98:8276–8282. doi: 10.1073/pnas.121006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basile G, Aker M, Mortimer RK. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol Cell Biol. 1992;12:3235–3246. doi: 10.1128/mcb.12.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doksani Y, Bermejo R, Fiorani S, Haber JE, Foiani M. Replicon dynamics, dormant origin firing, and terminal fork integrity after double-strand break formation. Cell. 2009;137:247–258. doi: 10.1016/j.cell.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mimitou EP, Symington LS. DNA end resection: Many nucleases make light work. DNA Repair (Amst) 2009;8:983–995. doi: 10.1016/j.dnarep.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27:1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]