Abstract
Astroglial reactivity associated with increased production of NFκB-dependent proinflammatory molecules is an important component of the pathophysiology of chronic neurological disorders such as multiple sclerosis (MS). The use of estrogens as potential anti-inflammatory and neuroprotective drugs is a matter of debate. Using mouse experimental allergic encephalomyelitis (EAE) as a model of chronic neuroinflammation, we report that implants reproducing pregnancy levels of 17β-estradiol (E2) alleviate ongoing disease and decrease astrocytic production of CCL2, a proinflammatory chemokine that drives the local recruitment of inflammatory myeloid cells. Immunohistochemistry and confocal imaging reveal that, in spinal cord white matter EAE lesions, reactive astrocytes express estrogen receptor (ER)α (and to a lesser extent ERβ) with a preferential nuclear localization, whereas other cells including infiltrated leukocytes express ERs only in their membranes or cytosol. In cultured rodent astrocytes, E2 or an ERα agonist, but not an ERβ agonist, inhibits TNFα-induced CCL2 expression at nanomolar concentrations, and the ER antagonist ICI 182,170 blocks this effect. We show that this anti-inflammatory action is not associated with inhibition of NFκB nuclear translocation but rather involves direct repression of NFκB-dependent transcription. Chromatin immunoprecipitation assays further indicate that estrogen suppresses TNFα-induced NFκB recruitment to the CCL2 enhancer. These data uncover reactive astrocytes as an important target for nuclear ERα inhibitory action on chemokine expression and suggest that targeting astrocytic nuclear NFκB activation with estrogen receptor α modulators may improve therapies of chronic neurodegenerative disorders involving astroglial neuroinflammation.
Keywords: multiple sclerosis, glia, sex steroids, spinal cord, chemokine
Inflammation plays a central role in numerous central nervous system (CNS) diseases. Multiple sclerosis (MS) is characterized by autoimmune neuroinflammation and axonal and oligodendrocyte pathology, with ensuing demyelination and neurological dysfunction (1). Although astrocyte reactivity has been considered as a phenomenon secondary to demyelination or microglial responses, astrogliosis occurs early during experimental autoimmune encephalomyelitis (EAE), a model of MS (2). Reactive astrocytes may serve to protect the CNS from injury via release of growth factors (3). However, their harmful role during chronic neuroinflammation has been demonstrated using mice with astrocyte-restricted knockout of upstream activators of NFκB (4), which binds promoters of proinflammatory cytokine genes (5). Among those, CCL2 (MCP-1) is a prototypic highly regulated inflammatory chemokine that, via its receptor CCR2, drives myeloid cell recruitment to sites of CNS injury (6, 7). In EAE and MS lesions, CCL2 is expressed mostly by reactive astrocytes, whereas CCR2 is associated with macrophages/activated microglia and some lymphocytes (8–10). CCR2 knockout mice are resistant to EAE induction (11) whereas transgenic overexpression of CCL2 in astrocytes increases blood brain barrier permeability and leads to encephalopathy in mice challenged with adjuvants (12). Taken together, these data indicate an active role of astrocytes in the amplification of the immune response through CCL2 signaling on blood-derived inflammatory cells.
Preclinical data using estriol at pregnancy levels support estrogens as potential therapeutic drugs for improving MS symptoms (13, 14). In mice, although the effectiveness of estrogen treatment after EAE onset is ill defined, estrogens from estrus to pregnancy levels are known to drastically reduce EAE disease activity when the treatment starts before disease induction (15, 16). Estrogen receptor (ER)α is crucial for this protective effect, whereas ERβ plays a neuroprotective role only in the late phase of the disease (16, 17). An anti-inflammatory action of estrogen is partly explained by mechanisms involving activation of ERα and/or GPR30 on immune cells, leading to cytokine changes consistent with a reduced Th1/Th17 response and moderate Th2 shift, a decreased expression of matrix metalloproteinases by inflammatory cells, and expansion or increased activity of the T regulatory (Treg) cell population (14–21). Yet, ERα signaling in leukocytes is dispensable for mediating protection from EAE and bone marrow chimera experiments further suggest the potential involvement of ERα-expressing CNS parenchymal cells (22, 23). Estrogens may play a beneficial role through their pleiotropic actions in the CNS, notably via glial ERs. Microglial ERα mediates anti-neuroinflammatory effects (24, 25) but its weak expression in vivo, which is down-regulated after an inflammatory challenge, has raised the notion that responsiveness of microglia to ERα activation may be negligible during persistent inflammation (26). In contrast, astrocytic ERα is up-regulated in in vivo models of brain injury (27, 28). The CNS expression of ERs in MS or EAE has not been described. We here examined whether E2 treatment after chronic EAE onset was able to reduce disease and astrocyte reactivity and where ERα and ERβ localize in the CNS during EAE. On the basis of our observations, in vitro experiments were done to provide evidence for a direct anti-inflammatory mechanism of estradiol on cultured astrocytes via nuclear inhibition of NFκB-dependent CCL2 transcription.
Results
Estradiol Treatment Initiated After Disease Onset Suppresses Chronic EAE Clinical Signs.
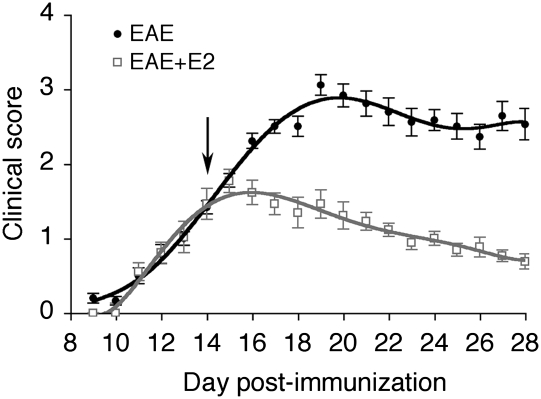
Administration of E2 pellets after EAE onset in C57BL/6 mice immunized with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide decreased clinical score at days postimmunization (dpi) 16–18 (Mann–Whitney, P < 0.02) and further declined at dpi 19–21 (P < 0.001) and dpi 22–28 (P < 0.0001), leading to a mean score <1 at the day of sacrifice (Fig. 1). The uterine weights of placebo-treated EAE mice were decreased by 2-fold compared to placebo-treated control mice, but increased by 2-fold in E2-treated EAE mice (Table S1). Measurement of plasma E2 confirmed that pregnancy levels (4–8 nM) were achieved in EAE animals treated with E2 pellets (Table S1).
Fig. 1.
Estradiol treatment after disease onset suppresses the clinical symptoms of experimental autoimmune encephalomyelitis. Clinical score is shown of placebo- (EAE, solid circles, n = 14) and estradiol (EAE + E2, open squares, n = 13) -treated EAE mice. Implants (placebo or E2, 5 mg) were performed at day 14 postimmunization (arrow).
Estradiol Treatment Decreases Astrocytic CCL2 Expression and Leukocyte Infiltration in the Spinal Cord White Matter.
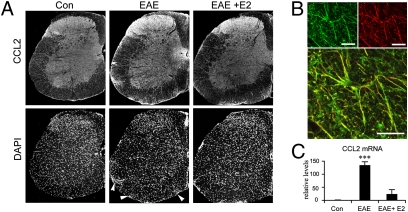
In control spinal cords, CCL2 immunoreactivity (IR) was mostly detected in the gray matter, with a diffuse staining pattern in the superficial laminae (neuronal afferents) and variable intensity in perikarya of deeper layers of the dorsal horn. In the white matter, only faint labeling was observed on astrocytic fibers. In EAE, CCL2-IR increased drastically in the white matter, correlating with areas of dense cell infiltration as determined by DAPI staining (Fig. 2A). We then determined the cell types expressing increased levels of CCL2 by dual immunohistofluorescence with the astrocytic marker GFAP or the leukocytic marker CD45, labeling all cells from the hematopoietic lineage including lymphocytes, macrophages, and dendritic and mast cells (29). In EAE mice, astrocytes with swollen processes and increased GFAP-IR were found in the gray matter and in the white matter, notably in its ventral and lateral parts where cellular infiltrates were prominent (Fig. S1 and Table S2). In the white matter, both GFAP- and CCL2-IR were reduced in estrogen-treated EAE mice compared to EAE mice (Fig. 2A and Fig. S1). CCL2-IR was clearly associated with GFAP-IR fibers as confirmed by confocal imaging (Fig. 2B), whereas CD45+ cells did not exhibit significant CCL2-IR (Fig. S2). Measurements of immunofluorescence from the different regions of the spinal cord indicate that E2 treatment decreases EAE-induced CCL2 and GFAP expression in the ventrolateral white matter (Table S2). Real-time quantitative (q)PCR confirmed that EAE-induced increase in CCL2 expression was reduced by the estrogen treatment (F2,17 = 38.8, P < 0.0001; Fig. 2C).
Fig. 2.
Treatment with estradiol in vivo reduces CCL2 expression in the spinal cord of EAE mice. (A) CCL2 immunoreactivity (IR) and corresponding DAPI staining on hemisections of control, EAE, and E2-treated EAE spinal cords. Increased CCL2-IR in EAE mice correlated with multifocal areas of increased infiltrated cells disseminated in the white matter (as revealed by DAPI staining, arrowheads). In E2-treated EAE mice, CCL2-IR was decreased in the white matter, remaining in smaller areas of infiltrating cells restricted around leptomeninges. For pictures of whole hemisections from ×5 objective, corner areas outside the spinal cord have been filled because of rotation of the initial pictures and presence of occasional nerve remainings. (B) Confocal imaging showing colocalization of CCL2-IR (green) in astrocytic fibers (GFAP-IR, red). Each panel is a z-stack of eight consecutive confocal sections with 1-μm increments. (Scale bars, 10 μm.) (C) qPCR of CCL2 mRNA from spinal cord extracts. Difference between placebo- and E2-treated EAE mice (post hoc analysis): ***, P < 0.001.
We further assessed the anti-inflammatory effects of E2 by examining immunoreactivities for CD45 with osteopontin (OPN), a pathogenic cytokine secreted by macrophages and dendritic cells during EAE and MS (30). OPN expression in controls was mostly confined to the gray matter, with high levels in some motoneurons, whereas very low OPN-IR was detected in the white matter (Figs. S3A and S4). In EAE, additional OPN-IR in the white matter was found in infiltrating cells and in a diffuse pattern within the extracellular compartment of the lesion (Fig. S4); the neuronal pattern of OPN expression in the gray matter was similar to that in control mice. The increase in OPN expression during EAE was confirmed at the RNA level (Fig. S3B). E2 treatment of EAE mice resulted in decreased OPN expression in the white matter and reduced immune cell infiltration in CNS parenchyma as indicated by the counts of CD45+ cells in the white matter (Table S3); the proportion of OPN+ cells in the CD45+ population did not differ between placebo- and E2-treated EAE mice (52 ± 7% and 48 ± 11%, respectively).
Nuclear ERα Immunoreactivity Is Induced in EAE White Matter Astrocytes and Reduced by Estrogen.
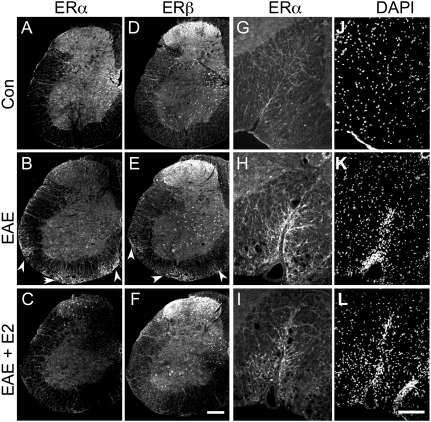
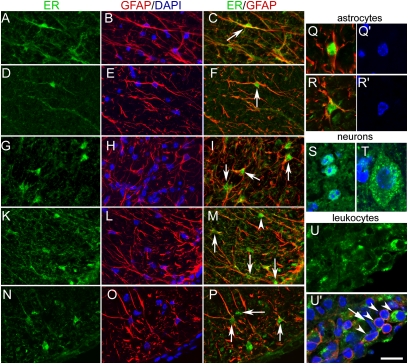
In the gray matter of the spinal cord, the expression pattern of ERα and ERβ by immunocytochemistry was in agreement with the reported neuronal distribution of these receptors (31) and did not differ between control and EAE mice (Fig. 3 A–F). We confirmed the high level of nuclear ER expression in neurons of the dorsal horn and the intermediate gray area (Figs. 3 and 4S) and the low ERα and moderate ERβ immunoreactivities in motoneurons, with a clear extranuclear localization (Fig. 4T). GFAP-IR stellate astrocytes in the gray matter of control or EAE mice were not labeled with ERα antibodies and were only weakly labeled with ERβ antibody, with light punctuate staining over the nucleus. In contrast, in the white matter of control and EAE mice, ERα was detected in GFAP-IR radial glia (Fig. 4 A–F). In EAE mice, GFAP-IR multipolar astrocytes also exhibited intense ERα immunoreactivities, especially in or close to areas of infiltration (Figs. 3 and 4 G–I). The two ERα antibodies stained both the nuclear and the extranuclear compartments of astrocytes, with a preferential nuclear labeling for clone 60C. ERβ antibody also stained radial glia with a preferential nuclear localization (Fig. 4 K–M, arrowhead). In EAE white matter, ERβ antibody additionally stained hypertrophic multipolar astrocytes in the cytoplasm and nucleus (Fig. 4 K–P). In the white matter of EAE mice, weak to moderate ERα and ERβ staining on infiltrating CD45-IR small cells (7–8 μm) was clearly associated with the plasma membrane after confocal analysis, although cytoplasmic staining was also evidenced in larger (11–12 μm) CD45-IR cells (Fig. 4U). Only faint cytoplasmic/membrane ERα or ERβ staining was detected in other GFAP− or CD45− elements such as endothelial cells (Fig. 4U′, arrow). Thus, in the white matter, nuclear ERα as well as nuclear ERβ was associated only with astroglia. No specific nuclear marker exists for astrocytes. Thus, the number of ER-IR nuclei per section in the white matter was used to evaluate the number of ER-expressing astroglia after the different treatments. The number of ERα-IR nuclei doubled in EAE mice compared to controls (C1355 antibody, controls, 42 ± 7; EAE, 90 ± 4; EAE + E2, 28 ± 7; and 60C antibody, controls, 40 ± 9; EAE, 70 ± 12; EAE + E2, 33 ± 2; Kruskal–Wallis test, P = 0.006), whereas the number of ERβ-IR nuclei decreased slightly in EAE and EAE + E2 mice (controls, 41 ± 4; EAE, 28 ± 2; EAE + E2, 23 ± 2; Kruskal–Wallis test, P = 0.04). These data support that nuclear ERα is preferentially expressed (over ERβ) by reactive astrocytes in EAE.
Fig. 3.
Increased estrogen receptor ERα and ERβ immunoreactivities in EAE white matter. (A, D, G, and J) Control mouse; (B, E, H, and K) EAE mouse; (C, F, I, and L) E2-treated EAE mouse. (A–F) ERα immunoreactivity (C1335 antibody, A–C) or ERβ immunoreactivity (D–F) in hemisections of the spinal cord. Arrowheads point to increased immunoreactivities (IR) in areas of white matter infiltrates in EAE mice. (Scale bar, 200 μm.) (G–I) Higher magnification of ERα labeling (C1355 antibody) in the ventral funiculus with (J–L) corresponding DAPI staining. (Scale bar, 80 μm.)
Fig. 4.
Confocal imaging of estrogen receptors in the spinal cord of control and EAE mice. (A–C) Radial glia (arrowhead) stained with anti-ERα C1355 (A), anti-GFAP and DAPI (B), or anti-ERα C1355 and anti-GFAP (C) in the white matter of a control mouse. (D–F) Radial glia (arrowhead) stained with anti-ERα clone 60C (D), anti-GFAP and DAPI (E), or anti-ERα and anti-GFAP (F) in the white matter of a control mouse. (G–I) Example of reactive astrocytes stained with anti-ERα clone 60C (G), anti-GFAP and DAPI (H), or anti-ERα and anti-GFAP (I) in EAE white matter. (K–P) Radial glia (arrowhead) and reactive astrocytes (arrows) stained with anti-ERβ (K and N), anti-GFAP and DAPI (L and O), or anti-ERβ and anti-GFAP (M and P) in EAE white matter. (Q and R) Higher magnification showing nuclear localization of ERα (Q, clone 60C) or ERβ (R) in GFAP-immunoreactive cells and corresponding DAPI staining (Q′ and R′). (S) Dorsal horn neurons stained with anti-ERα (clone 60C, green) and DAPI. (T) Motoneuron stained with anti-ERβ and DAPI (the contrasts for the green and blue channels have been enhanced by ×2 and ×3, respectively, for better visualization). (U and U′) CD45-IR cells in a perivascular infiltrate with ERβ localization restricted to the membrane or cytosol compartment (arrowheads); the arrow points to an endothelial cell identified by its long fusiform nucleus and constituting part of a blood vessel when observed in consecutive sections. In A–P, each image is a z-stack of eight consecutive confocal sections with 1-μm increments. (Scale bar, 25 μm.) In Q–U, single confocal sections are shown. (Scale bar, 13 μm.)
Estradiol Inhibits CCL2 Expression in Astrocyte Cultures.
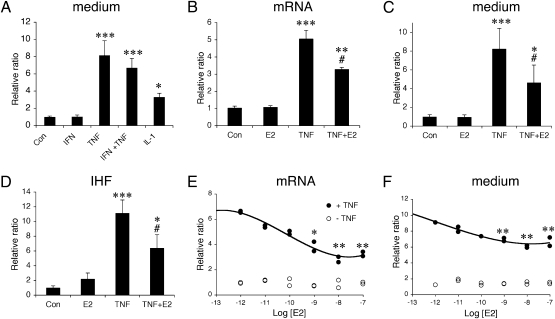
As our data indicate reactive astrocytes as primary targets for nuclear estrogen action in the white matter likely via ERα, we examined the effects of estrogen on the expression of the proinflammatory chemokine CCL2 in astrocyte cultures. Initially, to determine the effects of different proinflammatory inducers on astrocytic production of CCL2, we used the classic model of astrocyte cultures from rat neonatal cortex. TNFα and to a lesser extent IL-1β induced CCL2 production, in contrast to IFNγ (Fig. 5A). We thus used TNFα as an inducer to further investigate the inhibitory effect of E2 on CCL2 mRNA and content in the medium (Fig. 5 B and C). The TNFα-induced CCL2 mRNA and CCL2 levels were significantly decreased by 10 nM E2. This was confirmed semiquantitatively on fixed astrocyte cultures by immunofluorescence, which also ascertained that all astrocytes exhibited strong CCL2-IR upon TNFα exposure (Fig. 5D). Moreover, the estradiol dose effect indicated a significant decrease in TNFα-induced CCL2 mRNA and CCL2 levels at 1–100 nM (Fig. 5 E and F). In additional experiments, neither 17α-estradiol nor the membrane impermeant E2-BSA conjugate (100 nM), two estrogen compounds acting on membrane ER, affected significantly basal or TNFα-induced CCL2 content. Next, we checked for possible species, regional, and age differences in this effect; mouse astrocytes derived from neonatal cortex or spinal cord as well as from adult spinal cord were also tested. The mRNA levels were below detection in control and E2-treated mouse cultures. The levels of CCL2 mRNA observed after TNFα treatment were reduced by E2 in astrocytes derived from neonatal cortex (−56 ± 17%, n = 4 dishes/group, two experiments) and neonatal spinal cord (−49 ± 10%, n = 4 dishes/group, two experiments) as well as from adult spinal cord (−68 ± 12%, n = 9 dishes/group, three experiments). Measurements of CCL2 levels in the medium of treated mouse spinal cord astrocyte cultures corroborated these findings (Fig. S5).
Fig. 5.
Effects of cytokines and estradiol on CCL2 expression in astrocyte cultures from neonatal rat cortex. (A) Effect of proinflammatory cytokines (50 ng/mL) on CCL2 content in medium. Data are expressed as content ratio (n = 6–9/group) relative to control (3.8 ng/mL). Con, control; E2, 17β-estradiol; IFN, IFNγ; IL-1, interleukin-1β; TNF, TNFα. ANOVA: F4,26 = 20.8, P < 0.001. (B and C) Effect of E2 (10 nM) and TNFα (10 ng/mL) on (B) CCL2 mRNA expression (F3,16 = 10.34, P < 0.0001, n = 4–6/group, two experiments), (C) CCL2 content in the medium (n = 5–9/group; ANOVA, F3,27 = 10.34, P < 0.0001; control levels = 3.9 ng/mL). (D) Semiquantitative analysis of CCL2 immunohistofluorescence (IHF) in astrocytes treated with E2 and TNFα. Data are expressed as ratio of immunofluorescence intensity relative to control (n = 3/group). (E and F) Dose-dependent effect of E2 on (E) CCL2 mRNA or (F) CCL2 levels in the medium. Data are expressed as relative ratio compared to controls (two dishes/dose/group). Post hoc analysis: (A–D) Difference vs. control, *, P < 0.05; **, P < 0.01; ***, P < 0.001; difference vs. TNFα, #, P < 0.05; (E and F) difference vs. TNFα without estradiol, *, P < 0.05; **, P < 0.01.
ERα Localizes in the Nucleus of Cultured Astrocytes and Mediates the Inhibitory Effects on CCL2 Production.
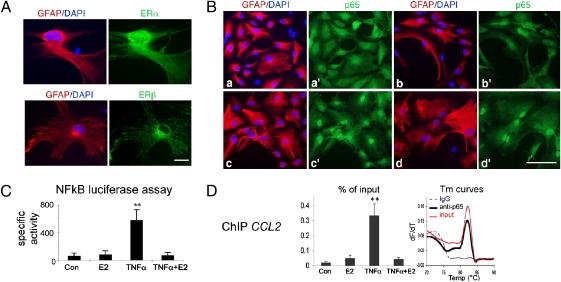
ER expression was examined by immunofluorescence on cultured astrocytes from rat and mouse neonatal cortical as well as adult mouse spinal cord. In these cultures, ERα localized to the cytoplasm and the nucleus whereas ERβ localized mainly to the cytoplasm (with a mitochondrial pattern) (Fig. 6A). Furthermore, propyl pyrazole triol (PPT, 100 nM), a specific ERα agonist, reduced TNFα-induced CCL2 levels in rat neonatal cortical astrocyte cultures by 55 ± 7% (n = 3/group; t test, P < 0.01 vs. TNFα) whereas the ERβ agonist, 2,3-bis(4-hydroxyphenyl) proprionitrile (DPN, 30 nM) had no effect (two experiments). Similar results were obtained from neonatal mouse spinal cord astrocytes and the effect of E2 or PPT was blocked by the ER antagonist ICI 182,170 (Fig. S5).
Fig. 6.
Estradiol does not impede TNFα-induced p65 nuclear translocation but suppresses NFκB-dependent transcription in cultured astrocytes. (A) ERα and ERβ immunolocalization in mouse spinal cord astrocyte cultures. ERα is detected in the nucleus as well as in the cytoplasm/membrane compartments whereas ERβ is detected mainly in the cytoplasm. GFAP (red) and DAPI (blue) stainings and corresponding ERα and ERβ immunoreactivities (green) are shown. (scale bar, 25 μm.) (B) p65 immunoreactivity (green) in astrocytes stained for GFAP (red) and DAPI (blue). (scale bar, 100 μm.) a and a′, controls; b and b′, E2, 10 nM; c and c′, TNFα, 10 ng/mL; and d and d′, TNFα + E2. (C) NFκB-dependent transcription assay. Mouse neonatal spinal cord astrocytes (NSCA) transfected with a luciferase NFκB reporter plasmid were treated with vehicle (Con) or 10 nM E2 for 30 min, followed by 2 h incubation in the presence or absence of 10 ng/mL TNFα, before luciferase assay. Data are expressed as relative light units (n = 3/group). Post hoc analysis: **, P < 0.01 vs. control. (D) Recruitment of p65 to the NFκB-dependent CCL2 enhancer in NSCA shown by ChIP assay. Cells were treated with E2 or TNFα as in C. Fragmented chromatin was subjected to ChIP analysis and real-time PCR. Left, percentage of CCL2 enhancer precipitated with anti-p65 relative to corresponding input (n = 3/group). Post hoc analysis: **, P < 0.01 vs. control. Right, representative melting temperature (Tm) curves showing recovery of CCL2 amplicon from anti-p65 ChIP and 0.5% input samples (Tm 82 °C) but not from mock sample (immunoprecipitation with a rabbit IgG).
Estradiol Does Not Impede p65 Nuclear Translocation in Astrocytes but Suppresses NFκB-Mediated Transcription.
We next examined whether the translocation of the NFκB subunit p65, which is part of the complex inducing TNFα-dependent CCL2 expression, was affected by the estrogen treatment. In control and E2-treated neonatal cortical astrocytes, p65 was localized in the cytoplasm. TNFα (10 ng/mL) induced p65 nuclear translocation in all astrocytes treated with E2 or not (Fig. 6B). Moreover, with lower concentrations of TNFα (1 ng/mL) that induced p65 nuclear translocation in only 56 ± 7% of astrocytes, pretreatment with E2 did not affect this ratio (50 ± 7%). The inability of E2 to prevent p65 nuclear translocation induced by TNFα was also confirmed in mouse neonatal and adult spinal cord cultures. In contrast, TNFα-induced nuclear translocation of p65 was prevented by E2 in microglia cultures (Fig. S6), in agreement with others (32). To delineate the mechanism underlying the inhibitory effects of E2, downstream to p65 nuclear translocation, we next examined NFκB-mediated transcription by using a sensitive NFκB reporter assay in transfected astrocytes. TNFα induced a 10-fold increase in NFκB-dependent luciferase activity, which was suppressed by E2 (F3,11 = 8.0, P < 0.01; Fig. 6C). Chromatin immunoprecipitation (ChIP) assays further indicate that estrogen inhibits TNFα-induced p65 recruitment to the NFκB-dependent CCL2 enhancer (F3,11 = 13.3, P < 0.02; Fig. 6D).
Discussion
In this study, we examined the therapeutic effect of E2 on chronic EAE by administrating s.c. implants of the hormone after the onset of symptoms in C57BL/6 mice with special focus on astrocyte reactivity in vivo and in vitro. The protective effect of various doses of estrogen given from time of immunization has been clearly evidenced (15–17, 33). Fewer studies have addressed whether estrogen treatment after disease onset may afford protection. Thus, one study reported that pregnancy levels of estrogen do not affect significantly active EAE progression in two mouse strains (33), although the number of animals tested may have been too low to reach statistical difference. Another study indicated that treatment with high doses of estriol was only slightly effective (34), which may be due to its 3- to 4-fold higher affinity for ERβ than for ERα or the model used (passive EAE). In contrast, high doses of the orally active estrogen, 17α-ethynyl estradiol, reduced severity of chronic EAE in an SJL strain (35). Our data show a drastic reduction in EAE clinical scores following E2 supplementation provided that sufficient dosage is achieved. Interestingly, we observed a pronounced decrease in the normal cycling uterine weight of EAE animals, indicating a depressed hypothalamo-pituitary–gonadotropic activity. Women with MS have 2-fold lower estradiol levels than controls during the luteal phase (36). This may be an important issue as a long period of hypoestrogenicity in rodents disrupts the anti-inflammatory and neuroprotective actions of estradiol (37). Taken together, these data suggest that chronic disease affects sex steroid plasma levels in both rodents and humans and support the notion that estrogenic compounds can alleviate ongoing chronic neuroinflammation.
Determining where and how sex steroids act is of paramount importance to delineate the best sex steroid therapeutics for MS and neuroinflammatory disorders. As stressed in the Introduction, previous reports have shown how estrogen can shape the immune system and inhibit the development of autoimmune encephalomyelitis, with the involvement of both ERα and GPR30 signaling. However, bone marrow chimera experiments have indicated that ERα in nonhematopoietic cells may be required for estrogen protection (22). A consistent body of literature also indicates that estrogen is protective in a variety of neurodegenerative models and that astrocytes may play a role in the estrogen-mediated attenuation of CNS damage (38). Yet, in EAE, their potential contribution to estrogen-mediated protection was not considered and the CNS expression pattern of ERα or ERβ was not reported. Our data reveal that, in the white matter lesions during chronic EAE, reactive astrocytes represent primary targets for a direct nuclear estrogenic action, as they express ERα (and to a lesser extent ERβ) preferentially in the nucleus. Considering the increasing involvement of astrocytes in neuroinflammatory disorders, this observation strongly supports these parenchymal cells as key estrogen targets via ERα during white matter inflammation. We focused on the regulation of CCL2 expression as this chemokine is produced mainly by astrocytes in EAE as well as in MS lesions and is a key player in CNS infiltration of inflammatory leukocytes. Estrogen treatment in EAE mice was indeed associated with a decreased astrocytic CCL2 expression, as well as reduced white matter infiltrates including leukocytes expressing the Th1/Th17 proinflammatory cytokine, osteopontin. The anti-inflammatory action of E2 on CCL2 astrocytic production that we reproduced in vitro with E2 or an ERα agonist could result from two different molecular mechanisms involving modulation of NFκB signaling. In cells such as microglia/macrophages, the anti-inflammatory effects of E2 involve ERα-mediated activation of phosphatidylinositol 3-kinase, preventing nuclear translocation of NFκB (32). In cells such as MCF-7, NFκB translocation is not affected by E2 but repression of chemokine gene expression is mediated through nuclear action of liganded ERα inhibiting an NFκB-containing transcription factor complex on the CCL2 gene promoter (39). This is relevant to MS treatment given that nuclear p65 immunostaining is increased in astroglial cells within chronic active MS lesions (40). The present in vitro findings indicate that, in astrocytes, the anti-inflammatory action of E2 does not result from a membrane action of estrogen, but rather involves nuclear liganded ER and suppression of NFκB-dependent transcriptional activity via inhibition of p65 recruitment to the CCL2 enhancer. This action represents one mechanism among potential pleiotropic actions of estrogens in protecting the brain from insult, including actions via extranuclear ER such as enhancement of glial release of neurotrophic factors (38) and antioxidant effects in mitochondria (41). Moreover, a direct action on glia does not exclude the contribution of other players, such as an anti-inflammatory action of estrogen on immune cells via the receptor GPR30. The lack of specific CNS restricted ERα/β knockout mice limits further studies that would address the contribution of ERs in neurons or glia. Nevertheless, the estrogen-mediated inhibition of NFκB-dependent expression of a proinflammatory chemokine in astrocytes is of interest in light of the deleterious effect of astroglial NFκB signaling in neurodegenerative disorders (3, 4, 42). It should be stressed that pregnancy levels of E2 were required to reduce the symptoms of established EAE as well as CCL2 production by astrocytes in vitro. Because estrogen acting via ERα is associated with detrimental effects in women, notably uterotropic effects and increased cancer risk, selective estrogen receptor modulators such as tamoxifen or raloxifene have been developed as alternative therapeutic tools. However, these compounds are still much less efficient than natural estrogens in protecting against EAE (43), and they do not inhibit and rather increase the DNA binding and transcriptional activity of the nuclear NFκB complex (44). Thus, the development of selective estrogen receptor ligands that suppress NFκB-dependent transcriptional activity with impaired activation of uterotropic/estrogen response element (ERE)-dependent transcriptional activity (45, 46) may improve the therapeutics of chronic CNS disorders involving astrocytic NFκB-dependent inflammation.
Materials and Methods
For induction of active EAE and hormone pellet treatment, C57BL/6 female mice were immunized with MOG35–55, complete Freund's adjuvant, and pertussis toxin, and clinical score was assessed daily as described in SI Text. On dpi 14, EAE mice were implanted with 17β-estradiol (E2, 5 mg) or placebo as detailed in SI Text. Spinal cords for mRNA studies, uteri for assessment of estrogen body impregnation, and atrial blood for E2 measurements were taken from animals at 28–30 dpi after lethal anesthesia. For immunohistochemistry, spinal cord sections (three perfused mice/group) were prepared and processed for fluorecent microscopy and/or confocal imaging. All animal procedures were performed according to approved institutional guidelines (agreement 75-1161 from the Services Vétérinaires de Paris). For astrocyte cultures, cortices or spinal cords from Wistar rat or C57BL/6 mouse pups as well as spinal cords from C57BL/6 female adult mice were used as described in SI Text. Cell treatments, measurements of CCL2 content in the medium, CCL2 mRNA levels, immunocytochemical analysis, and luciferase assay are detailed in SI Text. For ChIP assay, treated astrocytes were subjected to ChIP with rabbit anti-p65 or control IgG (mock), and amplification of the fragment containing the kB1 and kB2 binding sites in the TNFα-dependent enhancer region of the CCL2 gene was as described in SI Text.
All data shown are mean ± SEM. Nonparametric tests were used to analyze clinical score (Mann–Whitney), cell counts, and intensity of immunofluorescence (Kruskal–Wallis followed by Dunn's post test). Otherwise, ANOVA followed by Sheffe's post hoc analysis was employed throughout the study unless specified differently.
Supplementary Material
Acknowledgments
We thank Dr. D. Chabas for initial discussions and Dr. M. Sabbah for advice on the ChIP assay, S. Galier for technical assistance, the Plate-forme d'Imagerie Cellulaire Pitié-Salpêtrière for help in confocal microscopy, and I. Renault and P. Casanovas for animal care. This work was supported by Université Pierre et Marie Curie (Bonus Qualité Recherche to A.B.N.) and Institut National de la Santé et de la Recherche Médicale. S.N.G. was the recipient of a thesis fellowship from the French Ministry of Research.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910627107/DCSupplemental.
References
- 1.Zamvil SS, Steinman L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron. 2003;38:685–688. doi: 10.1016/s0896-6273(03)00326-x. [DOI] [PubMed] [Google Scholar]
- 2.Luo J, Ho P, Steinman L, Wyss-Coray T. Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J Neuroinflammation. 2008;5:6. doi: 10.1186/1742-2094-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Brambilla R, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 6.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 7.Mildner A, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff RM, et al. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Voorn P, et al. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson J, et al. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- 11.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang D, et al. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci. 2002;22:10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicotte NL, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 14.Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171:6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- 15.Offner H. Neuroimmunoprotective effects of estrogen and derivatives in experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. J Neurosci Res. 2004;78:603–624. doi: 10.1002/jnr.20330. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci USA. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu HB, et al. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J Immunol. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 18.Gold SM, et al. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha) Lab Invest. 2009;89:1076–1083. doi: 10.1038/labinvest.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai P, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, et al. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasko E, et al. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garidou L, et al. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2004;173:2435–2442. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- 23.Polanczyk MJ, et al. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. Am J Pathol. 2004;165:2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vegeto E, et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapia-Gonzalez S, Carrero P, Pernia O, Garcia-Segura LM, Diz-Chaves Y. Selective oestrogen receptor (ER) modulators reduce microglia reactivity in vivo after peripheral inflammation: Potential role of microglial ERs. J Endocrinol. 2008;198:219–230. doi: 10.1677/JOE-07-0294. [DOI] [PubMed] [Google Scholar]
- 26.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 27.Blurton-Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol. 2001;433:115–123. doi: 10.1002/cne.1129. [DOI] [PubMed] [Google Scholar]
- 28.García-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 29.Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chabas D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 31.Papka RE, et al. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 32.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bebo BF, Jr, et al. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: Implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 36.Tomassini V, et al. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. 2005;76:272–275. doi: 10.1136/jnnp.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki S, et al. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Nettles KW, et al. CBP is a dosage-dependent regulator of nuclear factor-kappaB suppression by the estrogen receptor. Mol Endocrinol. 2008;22:263–272. doi: 10.1210/me.2007-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonetti B, et al. Activation of NF-kappaB and c-jun transcription factors in multiple sclerosis lesions. Implications for oligodendrocyte pathology. Am J Pathol. 1999;155:1433–1438. doi: 10.1016/s0002-9440(10)65456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang SH, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bensinger SJ, Tontonoz P. A Nurr1 pathway for neuroprotection. Cell. 2009;137:26–28. doi: 10.1016/j.cell.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Bebo BF, Jr, et al. Treatment with selective estrogen receptor modulators regulates myelin specific T-cells and suppresses experimental autoimmune encephalomyelitis. Glia. 2009;57:777–790. doi: 10.1002/glia.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daosukho C, Kiningham K, Kasarskis EJ, Ittarat W, St Clair DK. Tamoxifen enhancement of TNF-alpha induced MnSOD expression: Modulation of NF-kappaB dimerization. Oncogene. 2002;21:3603–3610. doi: 10.1038/sj.onc.1205448. [DOI] [PubMed] [Google Scholar]
- 45.Chadwick CC, et al. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci USA. 2005;102:2543–2548. doi: 10.1073/pnas.0405841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nettles KW, et al. NFkappaB selectivity of estrogen receptor ligands revealed by comparative crystallographic analyses. Nat Chem Biol. 2008;4:241–247. doi: 10.1038/nchembio.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.