Abstract
Efficient integration of functional genes is an essential prerequisite for successful gene delivery such as cell transfection, animal transgenesis, and gene therapy. Gene delivery strategies based on viral vectors are currently the most efficient. However, limited cargo capacity, host immune response, and the risk of insertional mutagenesis are limiting factors and of concern. Recently, several groups have used transposon-based approaches to deliver genes to a variety of cells. The piggyBac (pB) transposase in particular has been shown to be well suited for cell transfection and gene therapy approaches because of its flexibility for molecular modification, large cargo capacity, and high transposition activity. However, safety considerations regarding transposase gene insertions into host genomes have rarely been addressed. Here we report our results on engineering helper-independent pB plasmids. The single-plasmid gene delivery system carries both the piggyBac transposase (pBt) expression cassette as well as the transposon cargo flanked by terminal repeat element sequences. Improvements to the helper-independent structure were achieved by developing new plasmids in which the pBt gene is rendered inactive after excision of the transposon from the plasmid. As a consequence, potentially negative effects that may develop by the persistence of an active pBt gene posttransposition are eliminated. The results presented herein demonstrate that our helper-independent plasmids represent an important step in the development of safe and efficient gene delivery methods that should prove valuable in gene therapy and transgenic approaches.
Keywords: gene therapy, transfection, transgenesis, transposase, transposon
The ability to safely and efficiently integrate genes into a host genome is essential for successful genetic modification strategies in the context of functional genomic studies, transgenesis, and gene therapy. Currently, the most commonly used vectors for permanent or transient transfer of genes in preclinical gene therapy trials are virus-based; however, nonviral vectors are also being developed. Whereas it is possible to achieve stable genomic integration with high efficiency using viral vectors, multiple studies have pointed out serious disadvantages. Adenoviruses, for example, have been shown to evoke host immune responses (1), whereas retroviruses preferentially integrate transgenes into euchromatin thereby increasing the risk of insertional mutagenesis. Viral systems are also limited in cargo size (2, 3), restricting the size and number of transgenes and their regulatory elements. Finally, biosafety considerations and production costs are additional factors to consider when using viral vectors (3, 4).
To avoid some of the potential drawbacks of viral systems, transposons have been tested and successfully used as an alternative in a diverse field of applications. DNA transposons are mobile elements that generally use a “cut and paste” mechanism in which the DNA is excised by double strand cleavage from the donor molecule and consecutive integration into the acceptor molecule. Many of the initial experiments were conducted using Sleeping Beauty (SB) (5). More recently however, pBt from the moth Trichoplusia ni has been widely used as a means for gene delivery in a variety of applications such as cell line transformation (6–9), mutational analysis (10), and gene therapy (11). Additionally, the pB system displays several highly desirable features that are of great advantage for transgene integration; pBt is very efficient and has a higher transposition activity than any of the Sleeping Beauty variants (9, 12, 13); the pB system allows for remobilization of transposons as they can be removed without leaving a “footprint” (14) and under certain conditions, pBt does not show any overproduction inhibition as do some members of the Tc1/mariner transposon superfamily (13, 15). Finally pBt has been shown to deliver transposons up to 18 kb (8), the largest cargo size described to date in any of the transposon or integrating viral systems.
Most transposition attempts for cell line transfections or gene therapy experiments with pB have used the two-plasmid donor-helper system. The donor plasmid contains inverted terminal repeats flanking the transgene while the helper plasmid transiently expresses the pBt enzyme, which catalyzes the insertion event from the donor plasmid to the host genome (16). Similarly, two-component systems have been used for pBt-initiated transgenesis where a donor plasmid is coinjected with transposase RNA (17, 18). However, this approach is problematic because of issues with RNA stability. The two-plasmid approach works well with cell line transfections but in our experience, this approach did not support transgenesis using Intracytoplasmic Sperm Injection (ICSI) (19).
To develop a pB system that is more suitable for transgenesis and gene therapy experiments, helper-independent single plasmids were constructed, containing both the donor and helper elements of the pB transposition system in the same circular construct, similar to plasmids previously described (20, 21). This cis-acting plasmid configuration ensures that both components, transposon, and transposase, are delivered simultaneously to the nucleus and hence should result in an improved transposition efficiency. Here, we describe the structure and function of three such plasmids (piGENIE, pmGENIE-2, and pmGENIE-3): The plasmids were designed to contain the transposase in an arrangement that prevents the activation of the enzyme in case of random, nontranspositional integration of the plasmid backbone that originates from the plasmid after transgene excision. Such a feature will be invaluable in clinical relevant settings as it increases the safety of plasmid-based research and gene therapy approaches as it prevents some of the potential genotoxic effects intrinsic in these systems.
Results
Helper-Independent Insect pB Vectors (piGENIE) are Active in HEK293T Cells and Support Transgenesis.
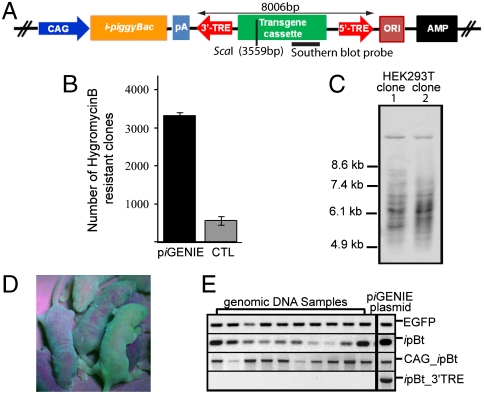
As a first step, a helper-independent plasmid was constructed. This plasmid contained a CMV-early-enhancer/chicken β-actin and β-globin intron (CAG) promoter-driven insect pB transposase gene (ipBt), where both the CAG promoter and the pBt gene are located on the backbone, outside the terminal repeat elements (TREs) of the transposon (piGENIE). The transposon for piGENIE was engineered to contain the enhanced green fluorescent protein gene as a reporter, as well as a cassette with Hygromycin resistance and Kanamycin resistance genes to facilitate selection in eukaryotes and prokaryotes, respectively (Fig. 1A and Fig. S1).
Fig. 1.
piGENIE supports cell transfections and animal transgenesis. (A) Schematic representation of piGENIE. The transposon cassette is delimited by the 3′- and 5′-TREs. Transposon size, restriction sites within the transposon, and Southern blot probe location are indicated. (B) 0.5 × 105 HEK293Tcells were transfected with 400 ng of piGENIE plasmid. As control for random nontranspositional integration of the plasmid HEK293T cells were transfected with 400 ng of a construct (piGENIE/ΔpiggyBac) lacking a functional pBt gene. Transposition activity was measured by counting methylene blue stained, hygromycin-resistant colonies after a three-week selection period. Data are shown as mean values with SD (N = 3). (C) Southern blot analysis of gDNA from a clonal expansion of transfected cells to analyze insertion events. Samples were digested with ScaI and BamHI and probed with a DIG-labeled DNA fragment corresponding to the EGFP gene. (D) Depiction of mosaic piGENIE transgenic mice. (E) Assessment of plasmid backbone insertions into the genome of ten piGENIE mice by PCR analysis (piGENIE plasmid served as a control). The analysis revealed that whereas all animals are transgenic for EGFP (top panel), they also displayed ipBt gene insertions (second panel from the top). Moreover, amplification products for the ipBt gene with its CAG promoter were obtained (second panel from the bottom) but not from a region spanning the ipBt gene and the 3′-TRE (bottom panel), indicating nontranspositional insertion of the plasmid backbone only but not of the entire plasmid.
To assess the transposition activity in human cells, embryonic kidney cells (HEK293T) were transfected with 400 ng of piGENIE plasmids. The plasmid was administered in a circular form to cells by lipofection. To contrast the efficiency of piGENIE-mediated transgene integration with nontranspositional (i.e., endogenous) integration of the plasmid, cells were also transfected with 400 ng of a construct lacking a functional pBt gene (piGENIE/ΔpiggyBac). Resistant clones larger than 1 mm were counted after 3 weeks of selection on Hygromycin (Fig. 1B). These experiments demonstrated that piGENIE was active in mammalian cells: 5.8% of all cells used for the experiment displayed stable integration as exemplified by EGFP expression and Hygromycin resistance. Furthermore, piGENIE was able to mediate transposition at levels significantly above those of random, nontranspositional integrations (Table S1).
Genomic DNA (gDNA) from two clones derived from two individual, stably transfected single HEK293T cells was analyzed for the number of transgene integration sites by Southern blot with a transposon-specific probe for EGFP. The gDNA was digested with restriction enzymes ScaI and BamHI. Only ScaI cuts once inside the transposon to leave a 4,447 bp fragment containing the EGFP gene. BamHI is expected to cut human gDNA about every 7,000 bp. Depending on genomic environment of the transposon insertion site, each of the individual insertions facilitated by piGENIE resulted in an individual band of at least 4,447 bp in Southern blots. At the used concentration, piGENIE proved to be extremely competent as both clones tested carried multiple transposons (Fig. 1C).
Only a few laboratories have employed transposases as a tool for animal transgenesis (2, 17, 18, 22, 23). These experiments have been based on supplying the transposase either as protein, mRNA, or in the helper plasmid of the donor-helper system. To avoid difficulties with proteins or mRNA and because, in our hands, the helper-donor approach did not yield any transgenic animals when employing ICSI, we examined whether piGENIE could be used as an effective tool for transgenesis. ICSI transgenesis performed with the circular form of the plasmid and fresh sperm resulted in high rates of transgenic animals. For example using 0.663 pg of piGENIE resulted in 18 transgenic mice, representing 69.2% of all animals born and 22.8% of oocytes injected (Table S2). Surprisingly, all of the transgenic animals exhibited mosaic EGFP signals by epifluorescence (Fig. 1D). When F0 animals were mated to wild-type mates of the same strain, none of the resulting transgenic F1 pups displayed mosaicism. All of the F1 animals that survived to adulthood were sacrificed at 2 years of age and examination of their tissues showed no signs of tumor formation. The phenomenon of mosaicism in F0 transgenic animals is thought to be due to transgene integration after the first chromosomal replication. However, it is also possible that nontranspositional integration of the plasmid as a whole or of the backbone only (posttransposition) resulted in the integration of an active ipBt, which ultimately led to the observed mosaicism. From cell transfection and transgenesis studies with transposons, it is evident that, in virtually every instance reported, there is some nontranspositional integration of plasmids (2, 5, 9, 13, 18, 24, 25). Such nontranspositional integrations were shown to apply to both helper and donor plasmids (2). To distinguish backbone insertions from whole plasmid insertions, several PCR assays were performed on gDNA from 10 founder mice, and the piGENIE plasmid as a control, priming for several sites within the plasmid: (i) EGFP, (ii) ipBt, (iii) a region spanning the 3′-end of the CAG promoter to ipBt, and finally (iv) a sequence spanning ipBt to the 3′-TRE (Fig. 1E). Whereas, as expected, all of the mice were positive for EGFP, this analysis also yielded amplification products in all of the samples for the ipBt gene and the promoter, indicating nontranspositional insertion of the plasmid. More specifically, as no amplicons were obtained for the region spanning the ipBt gene and the 3′-TRE, we assumed that all of these observed nontranspositional insertions were derived from backbone segments only. pBt has been shown to excise transposons without leaving a footprint in the donor genome (14). It is therefore probable that these nontranspositionally inserted plasmid backbones originated from the recircularized plasmid after excision of the transposon and its TREs as previously reported (9). Because insertion of an active pBt with its promoter into the genome of a host may result in hopping of the transgene—a feature advantageous for gene function studies, but undesirable for gene therapy or transgenesis experiments—a unique plasmid was engineered to ameliorate this issue.
pmGENIE-2: A Mouse Codon-Biased Self- Inactivating Vector.
pmGENIE-2 contained the same transposon as piGENIE, but differed from it in two components: (i) a mouse-codon-optimized pBt gene proven more effective in cell transfection experiments (26) replaced the ipBt gene, and (ii) the 3′-TRE was located between the CAG promoter and pBt gene (Fig. 2A and Fig. S1). Therefore, enzymatic excision of the transposon from the plasmid during transposition would result in the separation of the CAG promoter from the mpBt gene. The promoterless mpBt gene, residing in the remaining plasmid backbone should therefore be inactive if inserted nontranspositionally into the genome of the host by the DNA repair mechanism, reducing the possibility of potential genotoxic effects.
Fig. 2.
Analysis of pmGENIE-2. (A) Schematic representation of pmGENIE-2. The transposon cassette is delimited by the 3′- and 5′-TREs. Transposon size, restriction sites within the transposon, and Southern blot probe location are indicated. (B) The mpBt is separated from its CAG promoter during transposition: 0.5 × 105 HEK293Tcells were transfected with 400 ng of pmGENIE-2 plasmid. As a control, HEK293T cells were transfected with 400 ng pmGENIE-2/ΔpiggyBac lacking a functional pBt gene. Genomic DNA, extracted from a mixed population of HEK293T cells transfected with piGENIE or pmGENIE-2 were used for PCR analysis, where piGENIE or pmGENIE-2 plasmid DNA served as control. (i) Amplification of a region spanning the 3′-end of the CAG promoter and the ipB gene resulted (schematic depiction of primers are indicated as arrows) in products from the gDNA and from the piGENIE plasmid control. (ii) Long-range PCR of the complete transposon, priming within the mpBt gene and downstream of the 5′′-TRE resulted in full-length 11.2 kb products from the pmGENIE-2 plasmid control but not from the gDNA of pmGENIE-2 transfected cells. (iii) In contrast, the same amplification reaction gave rise to a smaller 1.0 kb amplicon from gDNA, but not from the plasmid. (C) Transposition activities of pmGENIE-2 (400 ng) and the helper-donor (200 ng donor and 100 ng helper) plasmids (and equal amounts of their respective controls) as measured by counting hygromycin-resistant colonies. Data are shown as mean values with SD (N = 3). (D) Representative samples of a Southern blot analysis of HEK293T cells transfected with different amounts of pmGENIE-2 (E) Depiction of full transgenic, mosaic and nontransgenic pmGENIE-2 transgenic littermates. (F) Southern blot analysis of gDNA from pmGENIE-2 transgenic founder and F1 mice.
HEK293T cells were transfected with 400 ng of circular pmGENIE-2 plasmid or the respective control (pmGENIE-2/ΔpiggyBac). To compare our helper-independent system to the more commonly used two-component donor-helper system (Fig. S1), HEK293T cells were also transfected with 100 ng of helper plasmid containing the mpBt gene driven by the CAG promoter (pCX-mpB) and 200 ng of donor plasmid carrying the EGFP, Hygromycin resistance, and Kanamycin resistance genes. Cells were maintained under Hygromycin selection for 3 weeks to exclude the possibility of detecting persistent episomal plasmid. To validate our approach of inactivating nontranspositionally inserted pBt genes, several PCR screens were performed. Genomic DNA, extracted from mixed populations of HEK293T cells transfected with pmGENIE-2 were used for these reactions. For comparison, gDNA from piGENIE transfected cells was used. The respective plasmids (piGENIE and pmGENIE-2) served as positive controls for successful amplification. The obtained amplicons were subsequently analyzed by sequencing. Amplification of a region spanning the 3′-end of the CAG promoter and the ipBt gene resulted in products from the gDNA as well as the piGENIE plasmid control [Fig. 2B (i)] indicating some nontranspositional insertion of the piGENIE plasmid backbone as also demonstrated in Fig. 1E. Long-range PCR using primers that anneal within the mpBt gene and downstream of the 5′-TRE result in 11.2 kb products from the pmGENIE-2 plasmid only but not from the gDNA of pmGENIE-2 transfected cells [Fig. 2B (ii)], verifying the absence of persistent episomal plasmids in the sample. It further suggests the lack of nontranspositional insertion of full-length plasmid. In contrast, the same amplification reaction gave rise to a 1.0 kb PCR product from gDNA, an amplicon absent in the plasmid control reaction (Fig. 2B (iii)], representing amplification from the promoterless backbone that remains after transposon excision. Taken together, these findings validate our strategy of inactivating mpBt in pmGENIE-2 by separating the gene from its promoter during transposition. Thus, nontranspositional insertion of the plasmid backbone can result only in the incorporation of a promoterless transposase gene. The importance of this feature of pmGENIE-2 is further emphasized by the detection of PCR amplicons for pBt from nontranspositionally inserted helper plasmid in donor-helper experiments (Fig. S2).
As a next step, pmGENIE-2 was evaluated in regards to its transposition activity. Colony-forming assays showed that the donor-helper system, which demonstrated efficiencies similar to those of previous experiments (9), resulted in half as many stably transfected colonies when compared to the helper-independent approach (Fig. 2C). Furthermore, pmGENIE-2 was able to mediate transfection rates at levels considerably above those resulting from piGENIE-mediated integration (Table S1).
To assess the influence of plasmid concentration on the number of simultaneous transgene integrations, HEK 293T cells were transfected with different concentrations of pmGENIE-2 (Fig. 2D). Genomic DNA was extracted from clonal expansions of single transfected HEK293T cells after they had reached confluence in a T75 flask at approximately four months. Southern blot analysis of this gDNA revealed that lower plasmid amounts result in fewer transgene insertions, with the highest number of insertions at 400 ng. These preliminary data suggest that pmGENIE-2, in the highest amount tested, is a powerful tool for in vitro transposition and may be better for applications, such as mutagenesis, where large numbers of inserts are desirable.
Subsequently, ICSI was performed to investigate pmGENIE-2’s ability to support animal transgenesis, using the same experimental setup as described for piGENIE. Visual assessment of the animals by epifluorescence revealed that pmGENIE-2 is indeed amenable to in vivo experiments and, contrary to piGENIE, results in both mosaic and full transgenic animals (Fig. 2E and Table S2). Similarly to piGENIE ICSI experiments, all transgenic F1 animals obtained from mating F0 to wild-type animals were true transgenics and survived to 2 years of age without any detectable tumorigenesis.
To assess transposon integration frequency for these transgenesis experiments, gDNA obtained from five founder mice (F0) was analyzed by Southern blots with an EGFP-specific probe. All of the mice displayed only a few transgene insertions, with one mouse having seven insertions whereas two other founders only showed the presence of one transgene (Fig. 2F). In comparison to cell transfections, it appears that pmGENIE-2 is less efficient in inserting transposons into oocytes. However, the significant differences in the two experimental systems do not allow for such a conclusion. Transgenic founders (F0) were mated with wild-type B6D2F1 mice to evaluate transposon transmission through the germline, again by subjecting gDNA from these F1 animals to Southern blot analysis. Two animals (F1-6 and F1-8) inherited one out of two transgenes, whereas the other animals inherited all copies present in the respective founders. Moreover, a comparison of the banding patterns for F0 and F1 animals indicated that the transgenes were not subject to relocation by local hopping, demonstrating the validity of our approach for inactivating the transposase gene.
The Southern blot analyses for both pmGENIE-2 HEK293T cell transfection and ICSI-mediated mouse transgenesis indicated the presence of bands smaller than 5 kb. However, HindIII restriction digestion of the gDNA used in these experiments should result in fragments of at least 5004 bp or larger (Fig. 2 and Fig. S1). We assumed that these bands originate from nontranspositional insertions of plasmid fragments that contain the EGFP transgene.
Genomic location of transposon integrations were then characterized in several individual HEK293T clones and F1 transgenic mice by either inverse or vectorette PCR. A summary of the identified insertion sites are listed in Table S3. Our results were similar to other studies describing insertion patterns for pBt (12, 27). Approximately half of the cell line integrations were found in intergenic regions. However, we also identified three intronic locations and one insertion into an exon of the cadherin-10 gene. In contrary, all of the transposon-gDNA junctions in the transgenic animals could be assigned to intergenic regions. While it is possible that pBt displays specific integration patterns depending on cell type, species, or experimental setup, the limited number of integration sites analyzed precludes such a conclusion.
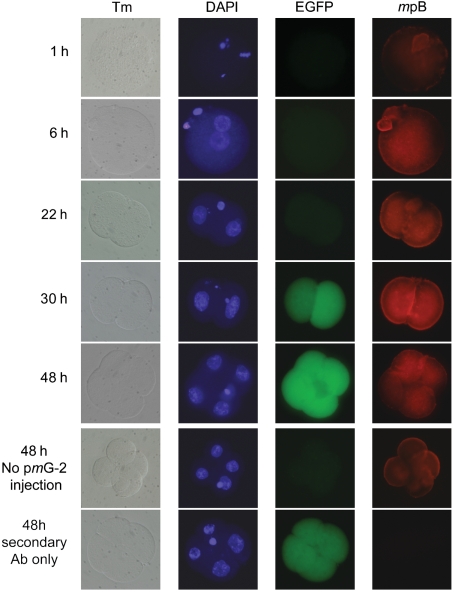
Additional evidence for the functional competence of the plasmid was obtained by antibody studies with oocytes injected with pmGENIE-2. The time course demonstrated pBt expression as early as 6 hours after sperm injection with peak production at 30 hours (Fig. 3). At this time point the transgenic embryos were at the two-cell stage and showed early EGFP expression, as previously reported (21). At 48 hours, the embryos were at the four-cell stage: the signal for the pBt decreased, presumably because of inactivation of the pBt gene during transposition. EGFP expression however, remained constant because of insertion of the transposon into the oocyte genome.
Fig. 3.
Time course of piggyBac and EGFP protein expression in transgenic embryos generated by ICSI. pBt expression was visualized by immunofluorescence using a polyclonal antibody for the pBt protein and is detectable above background levels as early as 6 hours. Peak expression was observed at about 30 hours corresponding with early detection of EGFP transgene expression. Pictures in the Tm column represent transmission images of the developing embryos. DAPI was used to visualize nuclei.
pmGENIE-3: A Helper-Independent piggyBac Plasmid Optimized For Gene Therapy Experiments.
During pmGENIE-2 mediated transposition the CAG promoter and pBt are separated, rendering the transposase inactive. Moreover, should pBt be inserted into the genome by nontranspositional integration of the backbone, it would remain inactive unless the activity of a promoter adjacent to the site of insertion would drive its transcription in the correct orientation. However, as the CAG promoter is part of the inserted transposon, it is possible that it could potentially influence the transcription of genes downstream of the insertion site.
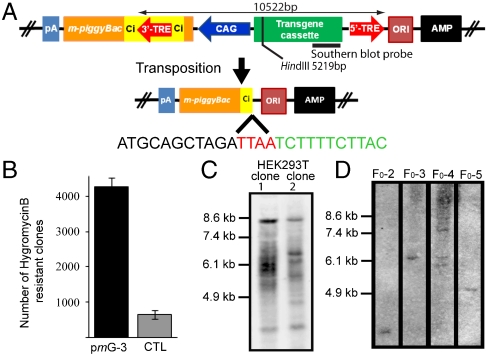
In the pmGENIE-3, the 3′-TRE of the transposon resides within an intron engineered into the pBt gene near its 5′-end (Fig. 4A and Fig. S1). Such a design allows transcription of pBt only from pretransposition plasmids but not from recircularized plasmid backbones that remain after the transposition event. During transposition, pBt binds to the 3′-TRE inside the chimeric intron as well as to the 5′-TRE, forming the synaptic complex. Hence, only a truncated, inactive pBt gene would remain on the plasmid backbone after excision. This truncated pBt cannot contribute to any hopping of inserted transposons, even if integrated into the host genome by nontranspositional insertion and driven by a promoter from an adjoining gene. Additionally, the CAG promoter left in the transposon cannot contribute to aberrant gene activation, as the small 5′-end fragment of the pBt gene and the remaining intron terminate at a stop codon (UAA) engineered into the construct. This design should, in effect, mitigate any influence on downstream host DNA genes.
Fig. 4.
Analysis of pmGENIE-3 transposition. (A) Schematic representation of pmGENIE-3 and the truncation of the pBt gene during transposition. The transposon cassette for genomic integration is delimited by the 3′- and 5′-TREs. Ci represents the chimeric intron. Transposon size, restriction sites within the transposon, and Southern blot probe location are indicated. (B) 0.5 × 105 HEK293Tcells were transfected with 400 ng of pmGENIE-3 or pmGENIE-3/ΔpiggyBac. Transposition activity was measured by counting methylene blue stained, hygromycin-resistant colonies after a 3 week selection period. Data are shown as mean values with SD (N = 3). (C) Southern blot analysis of HindIII digested gDNA from clonal expansions of pmGENIE-3 transfected cells. Samples were probed with a DIG-labeled DNA fragment corresponding to the EGFP gene. (D) Southern blot analysis of HindIII digested gDNA from pmGENIE-3 transgenic mice.
As a first step, we evaluated the presence of an integrated pBt backbone by genomic PCR of pmGENIE-3 transfected HEK293T cells following methods described for piGENIE transfections. Sequence analysis of the obtained amplicon indicated that the insertions derived from recircularized pmGENIE-3 plasmids, as they contained reconstituted TTAA sites generated by transposon excision and consecutive recircularization of the backbone plasmid. The data also confirmed that nontranspositional insertion of the pmGENIE-3 backbone leaves only an inactive pBt behind (Fig. 4A and Fig. S3). Again, we found no evidence of full-length pmGENIE-3, indicating the absence of episomal pretranspositional plasmid. More importantly, the data also indicates the lack of nontranspositional insertion of full-length plasmid.
We investigated if the design of the plasmid would negatively influence its transposition efficiency in in vitro and mouse transgenesis experiments. Our results from the colony-forming assay demonstrated that pmGENIE-3 displayed the highest gene-delivery efficiency as compared to the less intricate piGENIE and pmGENIE-2 plasmids (Fig. 4B, Fig. S4, and Table S1). A Southern blot analysis of HEK293T clones derived from transfected, single HEK293T cells showed a similar number of transgene insertions as observed for the other two plasmids, indicating that pmGENIE-3 retained transposition competence (Fig. 4C). Insertion site data obtained for pmGENIE-3 provided evidence that the modified pBt gene retains its enzymatic transposition activity (Table S3).
ICSI transgenesis experiments were performed to assess the potential of pmGENIE-3 as a tool for generating transgenic animals. At the most efficient concentration of circular pmGENIE-3, 11 transgenic mice were obtained, five of which were true transgenics (Table S2). Similarly to piGENIE and pmGENIE-2 ICSI experiments, all pmGENIE-3 transgenic F1 animals obtained by mating the F0 animals to wild-type mates were full transgenics and are surviving to date without any detectable tumorigenesis.
Discussion
In this study, we developed unique helper-independent piggyBac gene delivery plasmids. These plasmids are more effective for HEK293T cell transfections and ICSI transgenesis than the two-plasmid donor-helper approach. In piGENIE mediated transgenesis, all of the transgenic animals were mosaic. A PCR analysis of gDNA from piGENIE mice revealed random, nontranspositional insertion of the plasmid backbone, presumably in the linearized form. pBt-mediated transposition from chromosomal DNA does not alter the donor site but instead reconstitutes the original TTAA tetranucleotide after excision of the transposon (14). In plasmids, reconstitution of the TTAA site posttransposition should result in the recircularization of the remaining linear backbones. Similar recircularization mechanisms have been described in systems other than pB (28, 29). Recircularized plasmids remain in the nucleus and are thought to be degraded over time. However, our data suggests that some of these plasmids are inserted into the host genome, possibly by the host DNA repair mechanism, as reported elsewhere (30). As the piGENIE plasmid backbone contains an active piggyBac gene, such insertions may result in pBt transcription in the host cell and can thus cause transgene relocation. This may contribute to the observed transgenic mosaicism and potentially to deleterious genotoxic modifications, resulting in decreased fitness of the organism.
As an alternative approach, transpositions can be catalyzed by supplying the transposase as mRNA (17). Instability of mRNA restricts its duration of activity and therefore the risk of introducing genotoxic effects. However, it is possible for the mRNA to undergo reverse transcription, potentially resulting in insertion of the pBt cDNA into the host genome by nonhomologous recombination (31). We modified our helper-independent piGENIE plasmid in two steps, thereby significantly improving its safety. The latest plasmid, pmGENIE-3 features an intron spanning the 3′-TRE that allows the transposase to be transcribed from unmodified plasmids only. After transposition, the remaining recircularized plasmid backbone contains a truncated transposase gene. Even if the recircularized plasmid is then integrated into the host genome near an active promoter, the nonfunctional pBt gene cannot be expressed due to lack of an initiation codon. Additionally, after transposition, the pmGENIE-3 transposon retains the CAG promoter, which now can only drive the expression of a nonfunctional short transcript with a preengineered stop codon. As a consequence, the transposed promoter cannot activate neighboring host genes upon integration. These safety features integrated into pmGENIE-3 are of great importance for any gene therapy approach using transposase-based systems, as we and others (2, 5, 9, 13, 18) have reported a certain amount of nontranspositional plasmid integration when using piggyBac- or Sleeping Beauty-based transposition, independent of the specific system or application. The lack of full-length GENIE plasmid insertions in all of our transfection experiments, further emphasizes the importance of self-inactivating plasmids for gene delivery experiments.
To further reduce the risk of unwanted, potentially oncogenic modifications of the host genome, additional elements can be integrated into the vector design. For example, the use of a chimeric transposase consisting of the transposase itself and a DNA binding domain such as zinc finger motifs, could potentially target the transposon integration to specific DNA regions. pBt was previously shown to be amenable to such molecular modification while retaining its full transpositional activity, whereas other transposases lost their activity (9, 32). Furthermore, Chen et al. (33) reported using plasmids modified to carry chicken-beta-globin insulators flanking the transgene in their pBt-mediated transposition system in human embryonic stem cells. These insulators have the potential not only to shield transcribed regions of the host genome from outside regulatory influences but also to act as barriers against position-dependent transgene silencing.
In our initial experiments with HEK293T cell transfection, a large number of transposition events per transfection were observed at the highest plasmid concentration. This result suggests that our CAG promoter-driven pmGENIE plasmid is a powerful tool for in vitro transposition. However, it may be better suited for mutagenesis experiments or other applications where large numbers of inserts are desirable. For example, a high number of transgene insertions may be of advantage during tumor reduction approaches where plasmids are used to introduce suicide genes. Here, multiple insertions of transgenes can potentially amplify the toxic effect by increasing expression of the suicide gene. Transfections with lower amounts of plasmids yielded cells with fewer transgene integrations. Hence, for transfection experiments requiring fewer inserts, using lower plasmid concentrations, or modifying the plasmids to contain a less potent pBt promoter may prove beneficial.
Southern blots analyses of gDNA from transgenic animals did not indicate any concatemerized insertion of the transgenes. Such concatemerized insertions are commonly reported for transgenesis performed with pronuclear microinjections and ICSI. This phenomenon is believed to be due to ligation of the linear transgenes in a head to tail orientation and subsequent integration into the host genome by homologous recombination (2, 30, 34–36). Transgene insertions mediated by the pBt seem to avoid such concatemers. During pB transposition a single transposon is excised from the plasmid to form a synaptic complex that appears to prevent the ligation of the transgenes into concatemers. Therefore, the cut and paste mechanism of transposases appears to ensure that only individual transgenes excised from the plasmid participate in transposition.
We have noted nontranspositional insertions of single plasmid constructs as well as donor and helper plasmids during our transfection and transgenesis experiments. These observations are shared by many others (2, 5, 9, 13, 18, 24, 25); however, there are reports demonstrating a complete removal of the plasmids by degradation, posttransposition (37). We are presently unable to consolidate these contradictory findings; however, it is possible that the extend of this random, nontranspositional insertion of plasmids is cell and tissue specific or dependent on plasmid architecture and methods used in plasmid preparation. In in vitro experiments, multitransgene insertion or local hopping of the transposon may be a lesser concern. Here, such events may potentially be genotoxic, but can result only in an increased number of dead cells, thereby reducing transfection efficiencies. In gene therapy approaches, avoiding deleterious events introduced by the plasmid or plasmid fragments is critical. Therefore, the safety features incorporated into pmGENIE-3 represent an important step toward using transposon-based systems for gene therapy where reporter genes will be replaced by therapeutic genes. The architecture described herein should also be applicable to transposase systems such as Sleeping Beauty or Tol2, improving their safety in gene therapy experiments.
Materials and Methods.
Here, we provide a brief summary of the applied methods. Please see SI Text for a detailed description.
Plasmid Development.
The piGENIE and pmGENIE plasmids by basic molecular biology methods as described previously (9). The GATEWAY recombineering method of Invitrogen was employed for the construction of the final transposon bearing pGENIE plasmids by the use of LR Clonase enzyme.
Cell Transfections.
Cell culture and transfections were performed as previously (9).
Southern Blot Analysis.
Southern blot analysis was performed as described previously (22).
Transposon Insertion Sites.
Insertion sites were analyzed, either by Vectorette (Sigma) or inverse PCR.
Detection of NonEnzymatic Plasmid Insertion.
PCR was performed to analyze nontranspositional insertion.
Immunohistochemistry.
After fixation and removal of the zona pelucida, oocytes were incubated with primary piggyBac antibody followed by secondary antibody staining.
Transgenesis.
The procedures were performed as previously described with only minor modifications (19).
Supplementary Material
Acknowledgments.
We thank Dr. Malcolm J. Fraser for providing us with the insect piggyBac construct and piggyBac antibody, Dr. Junichi Miyazaki for the CAG promoter, Dr. Allen Bradley for the mouse codon-optimized piggyBac plasmid and Dr. Ming-Li Wang for help with the GATEWAY recombineering system. This work was initially supported by the National Center for Research Resources and the National Institutes of Health Grant 5P20RR016467-07 and is currently supported by National Institutes of Health Grants PAR-07-229 and R01 GM083158-01A1. Additional support came from National Institutes of Health Grant U54RR014607-09 (J.U.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003674107/-/DCSupplemental.
References
- 1.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10(6):965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 2.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122(3):473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski J, Summers JB. Delivering zinc fingers. Nat Biotechnol. 2003;21(5):492–493. doi: 10.1038/nbt0503-492b. [DOI] [PubMed] [Google Scholar]
- 4.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 5.Ivics Z, et al. Transposon-mediated genome manipulation in vertebrates. Nat Methods. 2009;6(6):415–422. doi: 10.1038/nmeth.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saridey SK, et al. piggyBac transposon-based inducible gene expression in vivo after somatic cell gene transfer. Mol Ther. 2009;17(12):2115–2120. doi: 10.1038/mt.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacoste A, Berenshteyn F, Brivanlou AH. An efficient and reversible transposable system for gene delivery and lineage-specific differentiation in human embryonic stem cells. Cell Stem Cell. 2009;5(3):332–342. doi: 10.1016/j.stem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Wu SC, et al. piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci USA. 2006;103(41):15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Bradley A, Huang Y. A piggyBac transposon-based genome-wide library of insertionally mutated Blm-deficient murine ES cells. Genome Res. 2009;19(4):667–673. doi: 10.1101/gr.085621.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang Y, et al. The piggyBac transposon is an integrating non-viral gene transfer vector that enhances the efficiency of GDEPT. Cell Biol Int. 2009;33(4):509–515. doi: 10.1016/j.cellbi.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Liang Q, Kong J, Stalker J, Bradley A. Chromosomal mobilization and reintegration of Sleeping Beauty and PiggyBac transposons. Genesis. 2009;47(6):404–408. doi: 10.1002/dvg.20508. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MH, Coates CJ, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15(1):139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 14.Mitra R, Fain-Thornton J, Craig NL. piggyBac can bypass DNA synthesis during cut and paste transposition. EMBO J. 2008;27(7):1097–1109. doi: 10.1038/emboj.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampe DJ, Grant TE, Robertson HM. Factors affecting transposition of the Himar1 mariner transposon in vitro. Genetics. 1998;149(1):179–187. doi: 10.1093/genetics/149.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser MJ, Ciszczon T, Elick T, Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol. 1996;5(2):141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 17.Mates L, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;41(6):753–761. doi: 10.1038/ng.343. [DOI] [PubMed] [Google Scholar]
- 18.Dupuy AJ, et al. Mammalian germ-line transgenesis by transposition. Proc Natl Acad Sci USA. 2002;99(7):4495–4499. doi: 10.1073/pnas.062630599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52(4):709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski JM, Huber MR, Summers JB, Ward MB. Design of a nonviral vector for site-selective, efficient integration into the human genome. Faseb J. 2002;16(10):1242–1247. doi: 10.1096/fj.02-0127hyp. [DOI] [PubMed] [Google Scholar]
- 21.Shinohara ET, et al. Active integration: New strategies for transgenesis. Transgenic Res. 2007;16(3):333–339. doi: 10.1007/s11248-007-9077-z. [DOI] [PubMed] [Google Scholar]
- 22.Suganuma R, et al. Tn5 transposase-mediated mouse transgenesis. Biol Reprod. 2005;73(6):1157–1163. doi: 10.1095/biolreprod.105.044669. [DOI] [PubMed] [Google Scholar]
- 23.Coussens M, et al. Regulation and effects of modulation of telomerase reverse transcriptase expression in primordial germ cells during development. Biol Reprod. 2006;75(5):785–791. doi: 10.1095/biolreprod.106.052167. [DOI] [PubMed] [Google Scholar]
- 24.Dalsgaard T, et al. Shielding of sleeping beauty DNA transposon-delivered transgene cassettes by heterologous insulators in early embryonal cells. Mol Ther. 2009;17(1):121–130. doi: 10.1038/mt.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronovich EL, et al. Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: Implications for non-viral gene therapy of mucopolysaccharidoses. J Gene Med. 2007;9(5):403–415. doi: 10.1002/jgm.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadinanos J, Bradley A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 2007;35(12):e87. doi: 10.1093/nar/gkm446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvan DL, et al. Genome-wide mapping of PiggyBac transposon integrations in primary human T cells. J Immunother. 2009;32(8):837–844. doi: 10.1097/CJI.0b013e3181b2914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell JB, et al. Duration of Sleeping Beauty transposase in mouse liver following hydrodynamic delivery. Mol Ther. 2006;13(Supplement 1):S150. doi: 10.1038/mt.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68(8):2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith K. Theoretical mechanisms in targeted and random integration of transgene DNA. Reprod Nutr Dev. 2001;41(6):465–485. doi: 10.1051/rnd:2001102. [DOI] [PubMed] [Google Scholar]
- 31.Wilber A, et al. RNA as a source of transposase for Sleeping Beauty-mediated gene insertion and expression in somatic cells and tissues. Mol Ther. 2006;13(3):625–630. doi: 10.1016/j.ymthe.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Maragathavally KJ, Kaminski JM, Coates CJ. Chimeric Mos1 and piggyBac transposases result in site-directed integration. Faseb J. 2006;20(11):1880–1882. doi: 10.1096/fj.05-5485fje. [DOI] [PubMed] [Google Scholar]
- 33.Chen YT, et al. PiggyBac transposon-mediated, reversible gene transfer in human embryonic stem cells. Stem Cells Dev. 2009 doi: 10.1089/scd.2009.0118. doi:10.1089/scd.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishop JO. Chromosomal insertion of foreign DNA. Reprod Nutr Dev. 1996;36(6):607–618. [PubMed] [Google Scholar]
- 35.Bishop JO, Smith P. Mechanism of chromosomal integration of microinjected DNA. Mol Biol Med. 1989;6(4):283–298. [PubMed] [Google Scholar]
- 36.Brinster RL, Chen HY, Trumbauer ME, Yagle MK, Palmiter RD. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci USA. 1985;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. Long-term reduction of jaundice in Gunn rats by nonviral liver-targeted delivery of Sleeping Beauty transposon. Hepatology. 2009;50(3):815–824. doi: 10.1002/hep.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.