Abstract
Sm and Sm-like proteins are RNA-binding factors found in all three domains of life. Eukaryotic Sm proteins play essential roles in pre-mRNA splicing, forming the cores of spliceosomal small nuclear ribonucleoproteins (snRNPs). Recently, Sm proteins have been implicated in the specification of germ cells. However, a mechanistic understanding of their involvement in germline specification is lacking and a germline-specific RNA target has not been identified. We demonstrate that Drosophila SmB and SmD3 are specific components of the oskar messenger ribonucleoprotein (mRNP), proper localization of which is required for establishing germline fate and embryonic patterning. Importantly, oskar mRNA is delocalized in females harboring a hypomorphic mutation in SmD3, and embryos from mutant mothers are defective in germline specification. We conclude that Sm proteins function to establish the germline in Drosophila, at least in part by mediating oskar mRNA localization.
Keywords: snRNP biogenesis, RNA localization, Dart5 (Capsuleen; PRMT5), Arginine methyltransferase, Drosophila
INTRODUCTION
Primordial germ cells are formed by one of two general mechanisms: cytoplasmic inheritance of specificity factors or de novo induction of germline fate (Extavour and Akam, 2003). Drosophila represents the best-characterized model of the former, whereas mice represent the best-studied example of the latter (Hayashi et al., 2007; Mahowald, 2001; Santos and Lehmann, 2004). In Drosophila, formation of the germline-specific cytoplasm (referred to as pole plasm) begins during oogenesis and involves restriction of Oskar protein to the posterior of the oocyte (Ephrussi and Lehmann, 1992). Oskar recruits the downstream factors for pole plasm assembly (Breitwieser et al., 1996; Ephrussi et al., 1991; Smith et al., 1992).
Although the precise mechanism of pole plasm formation is unknown, Oskar plays a central role, and its mislocalization can result in the ectopic formation of pole plasm (Ephrussi et al., 1991; Smith et al., 1992). The Drosophila pole plasm is also required for embryonic patterning (Ephrussi et al., 1991; Wang and Lehmann, 1991). Therefore, embryonic development requires precise spatial and temporal restriction of Oskar to the posterior of the oocyte. This restriction involves multiple levels of regulation. oskar mRNA, which is transcribed by nurse cells, is actively transported to the posterior of the oocyte (Ephrussi et al., 1991; Kim-Ha et al., 1991; Martin and Ephrussi, 2009; St Johnston, 2005). During transport, oskar mRNA is maintained in a translationally inactive state (Kim-Ha et al., 1995; Wilhelm and Smibert, 2005). Once delivered to the posterior, the translational repression of the message is relieved and the mRNA is converted to a translationally active form (Gunkel et al., 1998; Micklem et al., 2000; Wilhelm and Smibert, 2005). Oskar protein subsequently anchors its own message at the oocyte posterior (Vanzo and Ephrussi, 2002). Finally, recent data suggest that additional features, such as long F-actin projections and a polarized endocytic pathway, also restrict Oskar activity to the posterior pole of the oocyte (Tanaka and Nakamura, 2008; Vanzo et al., 2007).
Numerous trans-acting factors function at the various stages of oskar mRNA localization. In fact, oskar mRNA appears to be marked from the time of splicing for its unique cytoplasmic fate (Hachet and Ephrussi, 2004). Consistent with a function for splicing in the localization of oskar mRNA, exon junction complex (EJC) proteins are components of oskar messenger ribonucleoproteins (mRNPs) and are involved in mediating proper localization of the message (Hachet and Ephrussi, 2001; Micklem et al., 1997; Mohr et al., 2001; Newmark et al., 1997; Palacios et al., 2004; van Eeden et al., 2001).
The core machinery of the spliceosome consists of small nuclear ribonucleoproteins (snRNPs) (Matera et al., 2007). Each snRNP contains a small non-coding RNA, several snRNP-specific proteins and a heptameric core of Sm or Sm-like (Lsm) proteins (see Fig. 1A). Although best known for their essential roles in splicing, recent findings suggest that Sm proteins have a novel, non-splicing function in the specification of germ cells. In C. elegans, depletion of Sm proteins results in breakdown and mislocalization of P-granules, structures that are functionally similar to the Drosophila pole plasm (Barbee and Evans, 2006; Barbee et al., 2002). Importantly, Sm proteins, but not core splicing factors, are present within P-granules (Barbee et al., 2002). In addition, Sm proteins have been shown to localize to the mitochondrial cement in Xenopus oocytes and to the chromatoid body in mouse spermatocytes, structures that are equivalent to those present within the fruit fly pole plasm (Bilinski et al., 2004; Chuma et al., 2003). Finally, Drosophila mutants in dart5 (capsuleen), an arginine methyltransferase responsible for post-translational modification of Sm proteins, are unable to specify germ cells (Anne et al., 2007; Gonsalvez et al., 2006). The functional and mechanistic significance of these findings, however, is largely unknown.
Fig. 1.
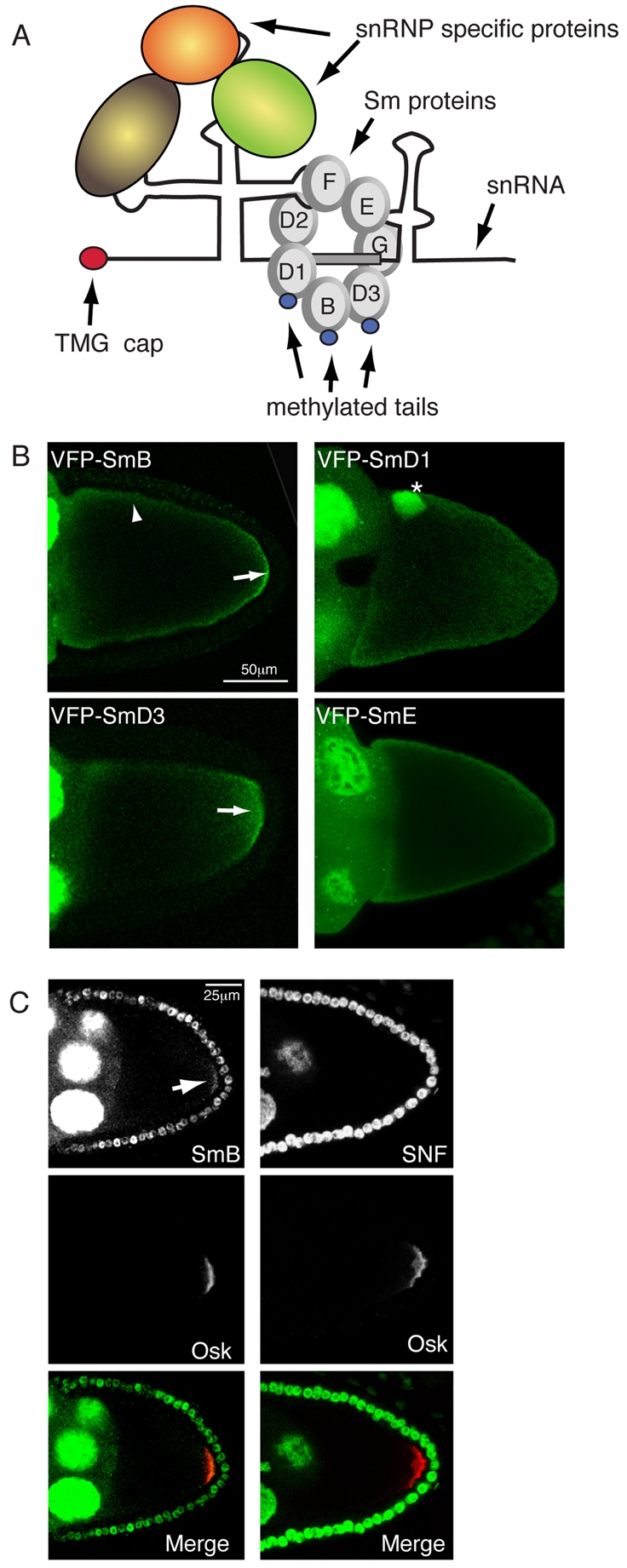
Germline localization of Drosophila Sm proteins. (A) Schematic of a spliceosomal snRNP. Each of the five spliceosomal snRNPs contains a non-coding RNA (an snRNA) and several snRNP-specific proteins. In addition, U1, U2, U4 and U5 contain trimethylguanosine (TMG) caps and the same set of seven Sm proteins (B, D1-3, E, F, G). The C-terminal tails of SmB, SmD1 and SmD3 contain symmetric dimethylarginine-modified residues. (B) Localization of UAS-VFP-tagged SmB, SmD1, SmD3 and SmE was examined in the female germline using the nanos-Gal4 driver. The arrow indicates the posterior enrichment of VFP-SmB or VFP-SmD3. The arrowhead indicates cortical localization and the asterisk indicates accumulation of Sm proteins within the oocyte nucleus. (C) Females expressing UAS-oskar driven by nanos-Gal4 were processed for immunofluorescence using Y12 (SmB, left), Snf (right) and Oskar (Osk) antibodies. The arrow indicates posterior-enriched endogenous SmB. Scale bars: 50 μm in B; 25 μm in C.
In order to elucidate the mechanism whereby Sm proteins function to establish germ cell identity, we used Drosophila as a model system. We identified a viable mutant allele of the SmD3 gene. In contrast to wild-type protein, the mutant protein fails to localize to the pole plasm, and embryos derived from mutant mothers are defective in germ cell specification. Molecular characterization revealed that oskar mRNA was delocalized in mutant oocytes. Importantly, we demonstrate that SmB and SmD3, but not other core splicing factors, are associated with oskar mRNA. Collectively, our results indicate that Sm proteins are required at numerous points in the oskar mRNA life cycle. In the context of the spliceosome, Sm proteins are involved in splicing oskar pre-mRNA. Subsequently, a novel Sm complex associates with spliced oskar mRNA and ensures its proper localization to the posterior pole. We therefore conclude that Sm proteins function in the establishment of germline fate in Drosophila.
MATERIALS AND METHODS
Transgenic constructs
The UAS-driven VFP-Sm constructs were generated by cloning the respective cDNAs into the Gateway transgenesis vector pPVW (Drosophila Genome Research Center; contributed by T. Murphy, Carnegie Institution, Baltimore, MD, USA). The GFP-SmD3 (wild-type and R-K mutant) constructs were generated by cloning the following fragments into pattB (obtained from K. Basler, University of Zurich, Zurich, Switzerland): SmB promoter, gfp, and the SmD3 open reading frame and 3′UTR.
Fly strains
Oregon-R was used as the wild-type strain. Unless otherwise noted, all stocks were cultured at 25°C. The transgenic fly strains were injected by BestGene. The driver strains were obtained from the Bloomington Stock Center (nos-Gal4 stock number 4937; da-Gal4 stock number 8641). Other fly strains used were: UAS-driven oskar overexpression (Zimyanin et al., 2007), stauD3 and Df(2R)PC4 (St Johnston et al., 1991), tud1 (Boswell and Mahowald, 1985), dart5-1 (Gonsalvez et al., 2006), GFP-Stau (Schuldt et al., 1998), par-1w3 (Shulman et al., 2000), par-16323 (Shulman et al., 2000; Tomancak et al., 2000) and UAS-driven osk-bcd (Tanaka and Nakamura, 2008). The SmD3pt allele was generated by Quinones-Coello et al. (Quinones-Coello et al., 2007), and was obtained from L. Cooley (Yale, New Haven, CT, USA).
Antibodies
The following antibodies were used for immunofluorescence: rabbit anti-Oskar (A. Ephrussi, EMBL, Heidelberg, Germany; 1:2000), rabbit anti-Stau (D. St Johnston, University of Cambridge, Cambridge, UK; 1:2500), rabbit anti-Tud (P. Lasko, McGill University, Montreal, Canada; 1:250), mouse anti-Grk (Developmental Studies Hybridoma Bank, contributed by T. Schüpbach, Princeton University, Princeton, NJ, USA; 1:100), rabbit anti-Khc (Cytoskeleton; 1:150) and rabbit anti-Vasa (P. Lasko; 1:2000). The secondary antibodies used were goat anti-rabbit Alexa 594 and Alexa 488 (Invitrogen; 1:400 and 1:200, respectively) and goat anti-mouse Alexa 594 (Invitrogen; 1:400). The following were used for immunoprecipitation: Y12 (J. Steitz, Yale, New Haven, CT, USA), rabbit anti-Yps (J. Wilhelm, University of California, San Diego, CA, USA), rabbit anti-GFP (Abcam) and mouse anti-Snf (H. Salz, Case Western Reserve University, Cleveland, OH, USA). For western blot analysis, we used the following: SYM10 (Upstate), mouse anti-GFP (Roche), rabbit anti-GFP (Abcam) and Y12.
Fixation of oocytes and embryos
Females fattened on yeast paste were dissected as described (Findley et al., 2003). The oocytes were fixed for 20 minutes in PBS containing 4% formaldehyde. After fixation, the ovaries were washed in PBST (PBS containing 0.1% Triton X-100) before being used in downstream procedures. Embryos were collected from females of the indicated genotypes mated to wild-type males. The embryos were fixed as described by Findley et al. (Findley et al., 2003). Embryos that were not immediately used for downstream procedures were stored in methanol at −20°C.
Immunofluorescence and in situ hybridization
For immunofluorescence, ovaries and embryos were treated identically. The samples were blocked in PBST containing 2% BSA (blocking solution). The primary antibody, diluted in blocking solution, was incubated with the samples overnight at 4°C. Next, the samples were washed with PBST. The secondary antibody, also diluted in blocking solution, was incubated with the samples overnight at 4°C. The samples were washed with PBST as before, stained with DAPI and mounted in antifade solution (0.233 g DABCO, 800 μl water, 200 μl 1 M Tris-HCl pH 8.0, 9 ml glycerol). For in situ hybridization, the fixed ovaries were incubated for 5 minutes at room temperature in a 1:1 PBST:hybridization buffer (50% deionized formamide, 5× SSC, 0.1% Tween 20) solution. This solution was removed and pre-hybridization buffer [hybridization buffer plus 50 μg/ml salmon sperm DNA (Invitrogen)], warmed to 85°C for 5 minutes, was added. Pre-hybridization was performed at 65°C for 90 minutes. The DIG-labeled antisense RNA probe was diluted in fresh hybridization buffer containing 50 μg/ml salmon sperm DNA, warmed to 85°C for 5 minutes and then chilled on ice for 2 minutes. Next, the pre-hybridization solution was removed, the probe was added and hybridization was performed overnight at 65°C. The next day, the samples were washed in pre-warmed hybridization buffer for 30 minutes at 65°C. This solution was replaced by a 1:1 mix of PBST:hybridization buffer and incubated at 65°C for 30 minutes. The samples were then washed with several changes of PBST and blocked in blocking solution. Sheep anti-DIG peroxidase Fab fragments (Roche) diluted 1:50 in blocking solution were added to the samples and incubated overnight at 4°C. The next day, the samples were washed with PBST and incubated with Cy3 tyramide (Perkin Elmer) diluted 1:50 in the provided amplification buffer. The amplification was performed at room temperature for 2 hours. The samples were DAPI stained, washed with PBST and mounted in antifade. For the combined in situ hybridization immunofluorescence procedure, the samples were first processed for in situ hybridization before being processed for immunofluorescence.
SnRNP immunoprecipitation
Ovaries from well-fed females were dissected as described above. The ovaries were homogenized using a pestle in lysis buffer A [50 mM Tris pH 7.5, 200 mM NaCl, 0.2 mM EDTA, 0.05% NP40 and Halt Protease Inhibitor Cocktail (Pierce)]. The lysates were cleared by centrifugation at 10,000 g at 4°C for 10 minutes. SnRNPs were immunoprecipitated from 500 μg of total lysate using Y12 antibody coupled to protein A-agarose beads (Pierce). For RNA isolation, total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's instructions.
Immunoprecipitation followed by RT-PCR
Ovaries from well-fed females were dissected in Express Five SFM medium (Invitrogen). The ovaries were homogenized using a pestle in lysis buffer B (25 mM Hepes pH 6.8, 50 mM KCl, 1 mM MgCl2, 1 mM DTT, 125 mM sucrose, 0.1% NP40, 50 μg/ml yeast tRNA, 50 μg/ml salmon sperm DNA and a protease inhibitor cocktail). The lysates were cleared by centrifugation at 10,000 g at 4°C for 10 minutes. For each immunoprecipitation, 600 μg of lysate was incubated with the respective antibodies at 4°C for 1.5 hours. The complexes were isolated with protein A-coupled Dyna magnetic beads (Invitrogen). The beads were washed four times for 10 minutes each with gentle shaking at 4°C using wash buffer A (25 mM Hepes pH 6.8, 200 mM KCl, 1 mM MgCl2, 125 mM sucrose, 0.1% NP40). The bound complexes were eluted in 100 μl of elution buffer (100 mM Hepes pH 6.8, 150 mM NaCl, 12.5 mM EDTA, 1% SDS) at 68°C for 10 minutes. RNA was extracted from the eluate by phenol:chloroform (25:24; Sigma Aldrich) extraction. The RNA pellet was resuspended in 20 μl RNAsecure (Ambion). One microliter of each sample was reverse-transcribed using Superscript III (Invitrogen) and random hexamers. The following PCR conditions were used: oskar, vasa and SmB mRNAs, 35 cycles; U1 snRNA, 25 cycles. A Biorad iCycler was used for the quantitative (q) PCR analysis.
RESULTS
SmB and SmD3 are specific components of the Drosophila pole plasm
Drosophila mutants in dart5 are defective in germline formation (Anne et al., 2007; Gonsalvez et al., 2006). Dart5 is an arginine methyltransferase that is responsible for methylating residues in the C-terminal tails of the spliceosomal proteins SmB, SmD1 and SmD3. Tudor, an essential pole plasm component, is delocalized within dart5 mutant oocytes (Anne et al., 2007; Gonsalvez et al., 2006). Tudor is the eponymous member of a family of proteins that contain a structural motif known as the tudor domain. Importantly, tudor domains function as methyl-binding modules (Cote and Richard, 2005). Loss of Dart5 activity results in Sm proteins that are unmethylated (Gonsalvez et al., 2006) and presumably unable to bind Tudor (Anne et al., 2007). Based on these findings, we initially hypothesized that methylated Sm proteins function to anchor Tudor within the pole plasm.
To test this hypothesis, we examined the localization of Venus fluorescent protein (VFP)-tagged Sm constructs within Drosophila egg chambers. Similar to the situation in mammals, Drosophila SmB, SmD1 and SmD3 contain symmetric dimethylarginine (sDMA)-modified residues, whereas SmD2, SmE, SmF and SmG do not (Fig. 1A). All four tagged Sm proteins localized around the cortex of the oocyte and also occasionally within the oocyte nucleus (Fig. 1B, arrowhead and asterisk). Interestingly, VFP-SmB and VFP-SmD3 were convincingly enriched at the posterior of stage 10 oocytes, but VFP-SmD1 and VFP-SmE were not (Fig. 1B, arrow). As a consequence of this differential localization, we focused the remainder of our studies on SmB and SmD3. Posterior enrichment of VFP-SmB and VFP-SmD3 persists after fertilization, as they can be detected at the posterior of pre-blastoderm stage embryos (see Fig. S1B in the supplementary material).
In order to validate these findings, egg chambers were analyzed with the anti-Sm antibody Y12, which in Drosophila primarily recognizes SmB. Under native conditions, Y12 immunostaining did not display posterior SmB enrichment (see Fig. S1A in the supplementary material). However, mild proteinase K digestion of egg chambers (see Fig. S1A in the supplementary material) or overexpression of oskar (Fig. 1C, arrow) revealed posteriorly enriched SmB. Under similar conditions, Sans fille (Snf), a core spliceosomal protein, was not detected at the posterior (Fig. 1C). A likely explanation for this finding is that the Y12 epitope is masked when SmB is localized at the posterior. Proteinase K digestion and overproduction of pole plasm via oskar overexpression partially exposes this epitope. Similar results have been observed for other pole plasm components (Micklem et al., 1997; Newmark et al., 1997; Webster et al., 1997).
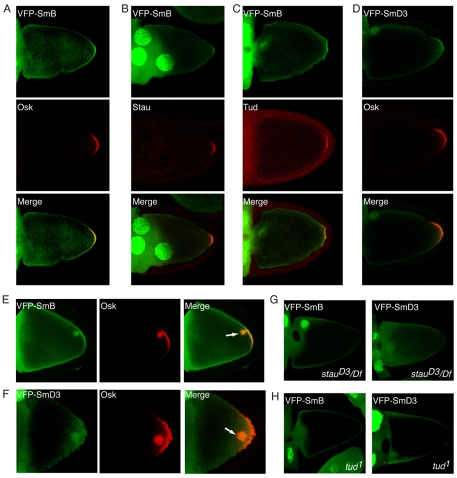
We next examined whether VFP-SmB and VFP-SmD3 colocalized with known components of the pole plasm. As shown in Fig. 2A-D, VFP-SmB and VFP-SmD3 colocalized with Oskar, Staufen (Stau) and Tudor (Tud) at the posterior. As an additional demonstration of specificity, we analyzed the recruitment of Sm proteins to sites of ectopic pole plasm. Overexpression of Oskar within the germline often leads to the formation of an ectopic focus of pole plasm (Zimyanin et al., 2007). Both VFP-SmB (Fig. 2E) and VFP-SmD3 (Fig. 2F) were convincingly recruited to such foci. We therefore conclude that SmB and SmD3 are specific components of the pole plasm.
Fig. 2.
SmB and SmD3 colocalize with pole plasm markers. (A-D) nanos-Gal4-driven UAS-VFP-SmB (A-C) oocytes were stained with antibodies against Oskar (Osk), Staufen (Stau) and Tudor (Tud) (red). nanos-Gal4-driven UAS-VFP-SmD3 (D) oocytes were stained with antibodies against Oskar (red). Note the colocalization between posterior VFP-SmB, VFP-SmD3 and pole plasm markers. (E) Females co-expressing UAS-VFP-SmB and UAS-oskar driven by nanos-Gal4 were processed for immunofluorescence using Oskar antibodies. (F) Females co-expressing UAS-VFP-SmD3 and UAS-oskar driven by nanos-Gal4 were processed as in E. VFP-SmB and VFP-SmD3 were recruited to ectopic pole plasm formed as a consequence of Oskar overexpression (merge, arrow). (G,H) The localization of nanos-Gal4-driven UAS-VFP-SmB (left) or UAS-VFP-SmD3 (right) was examined in stau (G) or tud (H) mutant backgrounds. Posterior-enriched Sm localization was not detected in the mutants.
In order to determine the pathway by which Sm proteins are sorted to the pole plasm, we examined the localization of VFP-SmB and VFP-SmD3 in several mutant backgrounds. In stau mutants, oskar mRNA is not maintained at the oocyte posterior (St Johnston et al., 1991) and, consequently, these egg chambers do not express Oskar protein. VFP-SmB and VFP-SmD3 were not observed at the oocyte posterior in stau mutants, indicating a requirement for Oskar expression and pole plasm formation in their posterior enrichment (Fig. 2G). In tud mutants, oskar mRNA and protein are expressed and localize to the oocyte posterior (Thomson and Lasko, 2004), resulting in the formation of a partial pole plasm. Interestingly, neither VFP-SmB nor VFP-SmD3 was convincingly enriched at the oocyte posterior in tud mutants (Fig. 2H). Lastly, we examined the localization of these proteins in dart5 mutants. As expected, given the Tud-delocalization phenotype in dart5 egg chambers (Anne et al., 2007; Gonsalvez et al., 2006), neither Sm protein was enriched at the oocyte posterior in dart5 mutants (see Fig. S2 in the supplementary material). Collectively, the results suggest a specific requirement for Tud or a fully functional pole plasm in the efficient sorting of VFP-SmB and VFP-SmD3 to the oocyte posterior.
Sm proteins are components of the oskar mRNP
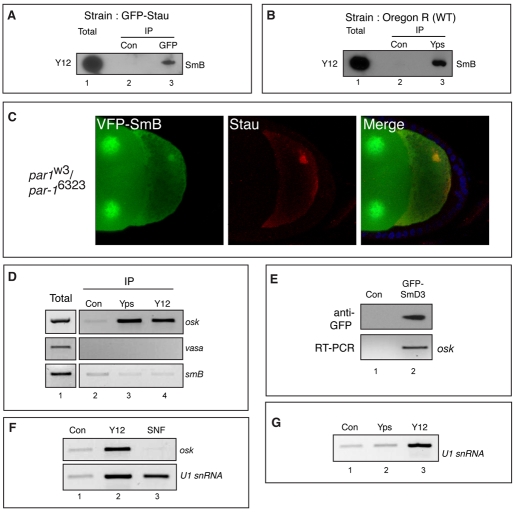
Although SmB and Tud colocalize at the posterior pole, we could not detect an association between these factors by co-immunoprecipitation (data not shown). This suggests that either Tud and Sm proteins do not associate in vivo or, if they do, the complex might be insoluble under our experimental conditions. Surprisingly, however, we detected an in vivo association between SmB and two known components of the oskar mRNP: Stau (Fig. 3A) and Yps (Fig. 3B) (St Johnston et al., 1991; Wilhelm et al., 2000). These findings suggest that Sm proteins associate with oskar mRNA in vivo. To test this hypothesis, we examined the localization of VFP-SmB in par-1 mutant egg chambers. In par-1 mutants, the polarity of the oocyte is disrupted, resulting in localization of oskar mRNPs at the oocyte center (Shulman et al., 2000; Tomancak et al., 2000). If SmB is a component of the oskar mRNP, it should track to the center of the oocyte in par-1 mutants. Consistent with this interpretation, VFP-SmB colocalized with Stau (used here as an oskar mRNP marker) in the center of par-1 oocytes (Fig. 3C).
Fig. 3.
SmB and SmD3 are components of the oskar mRNP. (A) Lysates from ovaries of GFP-Stau-expressing flies were subjected to immunoprecipitation using a control antibody (lane 2) or an antibody against GFP (lane 3). The precipitates were run on a gel and analyzed by western blotting using Y12. In Drosophila, Y12 recognizes SmB. A fraction of the total lysate (5%, lane 1) was also analyzed. (B) Ovarian lysates from wild-type flies were subjected to immunoprecipitation using a control antibody (lane 2) or an antibody against Yps (lane 3). The layout is similar to A. SmB was specifically detected in the GFP-Stau and Yps pellets. (C) Egg chambers from strain par-1w3/par-1w6323; UAS-VFP-SmB, nos-Gal4 were processed for immunofluorescence using a Stau antibody (red). VFP-SmB (green) colocalized with the Stau focus in the center of the oocyte. (D) Ovarian lysates from wild-type flies were immunoprecipitated with a control antibody (lane 2) or antibodies against Yps (lane 3) or Y12 (lane 4). RNA was extracted from the precipitates and the presence of oskar, vasa and SmB mRNAs was analyzed by RT-PCR. RNA from the total fraction was similarly analyzed (lane 1). oskar mRNA was specifically enriched in the Yps and Y12 precipitate. (E) Ovarian lysates from flies expressing wild-type GFP-SmD3 were immunoprecipitated with a control antibody (lane 1) or antibodies against GFP (lane 2). oskar mRNA specifically precipitated with GFP-SmD3. (F) The experiment was set up as in D. The following were used in the immunoprecipitation: control antibody (lane 1), Y12 antibody (lane 2) or an antibody against Snf (lane 3). The precipitates were examined for the presence of oskar mRNA (top) or U1 snRNA (bottom). In contrast to Y12, the Snf antibody only precipitates U1 snRNA. (G) The experiment was set up as in D, using control (lane 1), Yps (lane 2) or Y12 (lane 3) antibody. The Yps precipitate does not contain U1 snRNA.
A previous report demonstrated that immunoprecipitation of Yps co-precipitated oskar mRNA (Wilhelm et al., 2000). Using a similar strategy, we found that immunoprecipitation of SmB also specifically co-precipitated oskar mRNA (Fig. 3D), whereas vasa and SmB mRNAs were only present at background levels in the same precipitates (Fig. 3D). Because antibodies that specifically recognize endogenous SmD3 are unavailable, we examined the ability of GFP-SmD3 to associate with oskar mRNA. Our results indicate that, like SmB, SmD3 is also complexed with oskar mRNA in vivo (Fig. 3E).
Several observations indicate that the association of SmB and SmD3 with oskar mRNA occurs outside the context of the spliceosome. First, antibodies directed against a shared component of U1 and U2 snRNPs (Snf) did not co-precipitate oskar mRNA (Fig. 3F). As expected, the Snf precipitates contained robust amounts of U1 snRNA (Fig. 3F). Second, whereas snRNAs could be detected in Sm precipitates, they were not enriched in the Yps precipitate (Fig. 3G). Third, the primers used in the RT-PCR reactions were designed to amplify spliced and unspliced isoforms of oskar; however, only spliced oskar mRNA could be detected in the Sm precipitates. These results suggest that SmB and SmD3, but not core splicing factors, are associated with cytoplasmic spliced oskar mRNA.
A mutant form of SmD3 fails to localize to the pole plasm
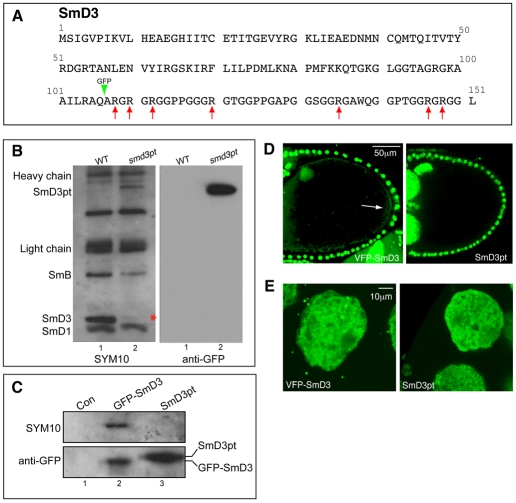
Several large-scale screens have resulted in the production of GFP-tagged ‘protein-trap’ alleles of numerous genes (Buszczak et al., 2007; Kelso et al., 2004; Morin et al., 2001; Quinones-Coello et al., 2007). The general approach utilizes a transposon bearing a GFP coding sequence flanked by splice donor and acceptor sites. If the construct integrates into an intron, it can produce an in-frame GFP-tagged version of an endogenous protein. One such allele (Flytrap database ID: P01793) containing an insertion in the SmD3 gene was obtained for these studies (Quinones-Coello et al., 2007). The position of the tag within the SmD3 coding region was verified by sequencing and is indicated in Fig. 4A (arrowhead). Henceforth, we refer to this allele as SmD3pt.
Fig. 4.
SmD3pt is hypomethylated and does not localize to the pole plasm. (A) The amino acid sequence of Drosophila SmD3. The green arrow indicates the position of the GFP tag within SmD3pt. The red arrows indicate arginine residues that were mutated to lysine in the GFP-SmD3(R-K) transgene. (B) Total lysates were prepared from wild type (lane 1) or SmD3pt homozygotes (lane 2) and snRNPs were immunoprecipitated using Y12. The methylation status of the Sm proteins was determined by blotting with SYM10 (left blot). The blot was subsequently stripped and probed with a GFP antibody (right-hand blot). SmD3pt lysates contain negligible amounts of wild-type SmD3 protein (asterisk). Methylation of SmD3pt is reduced in comparison to wild-type SmD3 (lane 1 versus lane 2). (C) S2 cells were transfected with the following: empty vector (lane 1), a vector expressing GFP-SmD3 (lane 2), or a vector expressing Smd3pt (lane 3). Lysates were prepared and the tagged proteins were immunoprecipitated using a monoclonal GFP antibody. The precipitates were first probed with SYM10 (top blot). The blot was then stripped and probed with a polyclonal GFP antibody (bottom blot). The small size difference between GFP-SmD3 and Smd3pt is due to the P-element-based linker present in Smd3pt. In contrast to GFP-SmD3, Smd3pt displays very little SYM10 reactivity. (D) Egg chambers expressing UAS-VFP-SmD3 driven using da-Gal4 were compared with egg chambers from SmD3pt homozygotes. Unlike VFP-SmD3, SmD3pt is not enriched at the oocyte posterior (arrow). (E) The same egg chamber that was processed in C is pictured. Nurse cell nuclei and cytoplasm are shown. VFP-SmD3 localizes to cytoplasmic foci known as U-bodies, whereas SmD3pt does not. Scale bars: 50 μm in D; 10 μm in E.
Despite the presence of the insertion within SmD3, SmD3pt is homozygous viable. RT-PCR analysis from homozygotes revealed that the vast majority of SmD3pt mRNA includes the GFP-trap exon, whereas exon-skipping accounts for a minute amount of the wild-type isoform (data not shown). In order to determine the methylation status of the mutant protein, snRNPs were immunoprecipitated from lysates of wild-type and SmD3pt flies and analyzed by western blotting with the anti-sDMA-specific SYM10 antibody (Fig. 4B). Consistent with the RT-PCR result, mutant lysates contained very little wild-type SmD3 (Fig. 4B, asterisk). Furthermore, in comparison to the SmD3 present in wild-type lysates, SmD3pt was appreciably under-methylated. By contrast, methylation of SmD1 was unaffected (Fig. 4B). Interestingly, the methylation of SmB was consistently reduced in the mutant lysates (Fig. 4B). SmB and SmD3 are known to heterodimerize in vivo (Raker et al., 1996). It is therefore plausible that their methylation is interdependent. In a parallel experiment, constructs expressing SmD3 with an N-terminal GFP tag (GFP-SmD3), or one that is identical to the protein-trap allele (Smd3pt), were transfected into S2 cells. GFP-tagged proteins were immunoprecipitated from lysates of these samples and their methylation analyzed. In contrast to GFP-SmD3, Smd3pt displayed very little SYM10 reactivity (Fig. 4C), confirming the hypomethylated status of the protein-trap isoform.
Next, we examined the localization of SmD3pt in egg chambers. In contrast to VFP-SmD3, SmD3pt was not detected at the posterior of stage 10 oocytes (Fig. 4D). Sm proteins have also been shown to localize to cytoplasmic foci known as U-bodies (Liu and Gall, 2007). Unlike VFP-SmD3, SmD3pt failed to accumulate in these cytoplasmic foci (Fig. 4E).
oskar mRNA is delocalized in SmD3pt oocytes
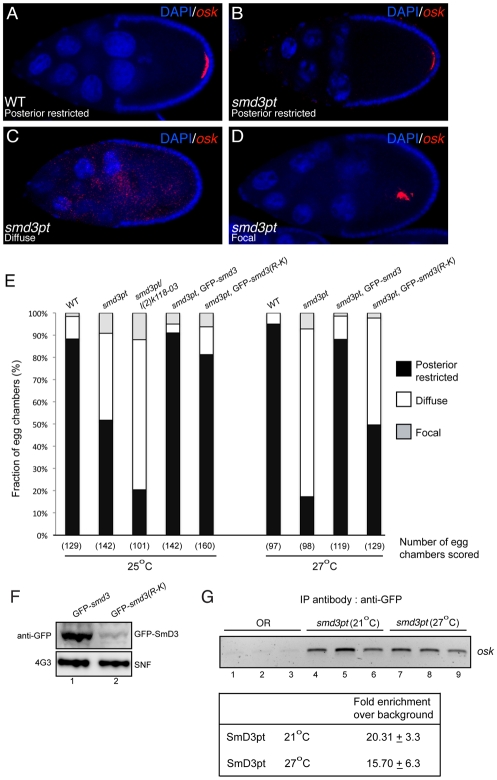
The finding that SmD3 is associated with oskar mRNA in vivo prompted us to examine the localization of this RNA in SmD3pt mutants. Unlike stage 9 and 10 wild-type egg chambers, in which oskar mRNA localized in a crescent at the posterior of the oocyte, the mRNA was delocalized to varying degrees in the mutants (Fig. 5A-D). In most egg chambers that contained delocalized oskar mRNA, the message was diffusely distributed throughout the oocyte (Fig. 5C). In a few instances, oskar mRNA was found in a tight focus or aggregate in the center of the oocyte (Fig. 5D). The penetrance of the oskar mRNA-delocalization phenotype in SmD3pt mutants is temperature sensitive; the defect was alleviated at temperatures below 25°C and greatly exacerbated at 27°C (Fig. 5E). A similar increase in the penetrance of the mutant phenotype was also observed at 25°C when the SmD3pt allele was brought in trans over a strong loss-of-function mutant, SmD3l(2)k118-03 (Fig. 5E) (Schenkel et al., 2002). Importantly, the posterior localization of oskar mRNA in the mutant background was rescued by expressing wild-type GFP-SmD3 (Fig. 5E).
Fig. 5.
oskar mRNA is delocalized in SmD3pt oocytes. (A-D) Stage 10 egg chambers from wild type (A) or SmD3pt homozygotes (B-D) raised at 25°C were processed for in situ hybridization using a probe against oskar mRNA (red). The egg chambers were counterstained with DAPI (blue). (E) The localization of oskar mRNA was scored under the indicated conditions. l(2)k118-03 is a strong loss-of-function SmD3 allele. oskar mRNA is delocalization to a greater extent when SmD3pt homozygotes are raised at 27°C. Expression of wild-type GFP-SmD3 completely rescues oskar mRNA localization, whereas rescue with GFP-SmD3(R-K) is incomplete. (F) Lysates were prepared from ovaries of flies expressing GFP-SmD3 (lane 1) or GFP-SmD3(R-K) (lane 2). The expression level of the transgenes was analyzed by western blotting using a GFP antibody (top blot). The blot was subsequently stripped and the load was verified by blotting with an antibody against Snf (bottom blot). (G) Lysates prepared from wild-type females (lanes 1, 2 and 3) or SmD3pt females raised at 21°C (lanes 4, 5 and 6) or 27°C (lanes 7, 8 and 9) were subjected to immunoprecipitation in triplicate using a polyclonal GFP antibody. The precipitating RNAs were extracted and oskar mRNA levels were analyzed using reverse transcriptase followed by qualitative (top) or quantitative (bottom) PCR. The difference in the amount of oskar mRNA associated with SmD3pt at 21°C versus 27°C was not statistically significant.
Hachet and Ephrussi demonstrated that splicing of oskar mRNA is crucial for its posterior localization (Hachet and Ephrussi, 2004). We examined whether oskar mRNA was properly spliced in SmD3pt mutants by RT-PCR (see Fig. S3 in the supplementary material). Despite the high sensitivity of RT-PCR, unspliced oskar pre-mRNA could not be detected in wild-type or SmD3pt oocytes. We therefore conclude that splicing of oskar pre-mRNA is efficient, and that the delocalized cytoplasmic message in the mutants corresponds to spliced mRNA.
In contrast to oskar mRNA localization, the translational regulation of the message was relatively unaffected in SmD3pt mutants. As expected, mutants that contained diffusely localized oskar mRNA did not express Oskar protein (data not shown). However, egg chambers that contained a central focus or aggregate of oskar mRNA contained Oskar protein within the same focus (data not shown). Similar results have been observed in other mutants that also produce a focus or aggregation phenotype (Krauss et al., 2009; Yano et al., 2004).
In order to test whether the oskar mRNA-delocalization phenotype resulted from reduced SmD3 arginine methylation, we constructed a methylation-null GFP-SmD3 transgene [GFP-SmD3(R-K)] (Fig. 4A, red arrows). Because wild-type GFP-SmD3 completely rescued oskar mRNA delocalization, we constructed the methylation-null transgene using the same transcriptional elements. Additionally, as both transgenes contained a ΦC31 recombination site, they were integrated into the same genomic locus (Bischof et al., 2007), eliminating the possible influence of position effect on transgene expression. Interestingly, although GFP-SmD3(R-K) fully rescued oskar mRNA localization at 25°C, the rescue at 27°C was incomplete (Fig. 5E). A closer examination revealed that, despite the aforementioned controls, GFP-SmD3(R-K) was several-fold reduced in expression level, compared with GFP-SmD3 (Fig. 5F). It is therefore possible that mutating the C-terminal arginine residues of SmD3 to lysine affects the stability of the protein. Despite the lower expression level of GFP-SmD3(R-K), however, the transgene still retains the ability to partially rescue oskar mRNA localization. Thus, the safest conclusion is that methylation of SmD3 plays a minimal role, if any, in the localization of oskar mRNA. Consistent with this interpretation, oskar mRNA is properly localized in dart5 mutants (data not shown), a background in which SmD3 is unmethylated (Gonsalvez et al., 2006).
The observed delocalization of oskar mRNA might be due to an inability of SmD3pt to associate with the message. Unfortunately, an antibody that specifically recognizes endogenous SmD3 is unavailable, such that we cannot compare the RNA association levels of wild-type SmD3 versus SmD3pt. However, because the oskar mRNA-delocalization phenotype is temperature sensitive, we tested whether loss of SmD3pt binding correlated with message delocalization. In contrast to females raised at 27°C, those raised at 21°C do not display a significant oskar mRNA-delocalization phenotype (data not shown). Ovarian lysates were prepared from wild-type (Oregon-R) or SmD3pt females raised at either 21°C or 27°C. The lysates were subjected to immunoprecipitation using anti-GFP antibodies. The co-precipitating RNAs were extracted and the presence of oskar mRNA was analyzed by reverse transcription followed by qualitative and quantitative PCR (Fig. 5G). Although we observed a slight decrease in the amount of oskar mRNA associated with SmD3pt at 27°C versus 21°C, the difference was not statistically significant.
Polarity of SmD3pt egg chambers
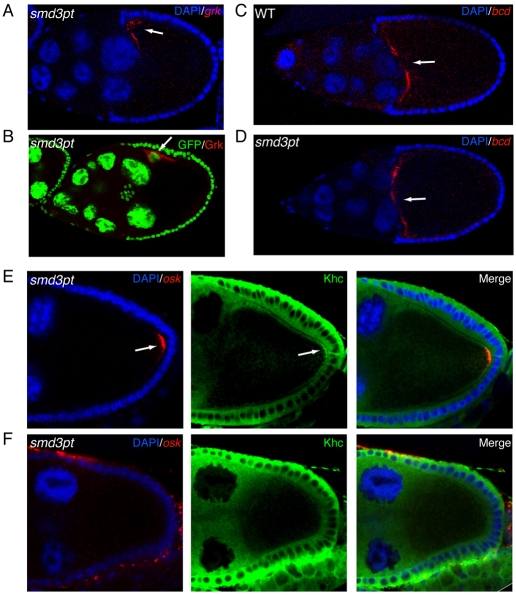
We next determined the specificity of the oskar mRNA-delocalization phenotype by examining the localization of gurken and bicoid mRNAs in mutant oocytes. In wild-type egg chambers, gurken mRNA localizes to the dorsal anterior corner of the oocyte (Gonzalez-Reyes et al., 1995; Neuman-Silberberg and Schupbach, 1993). No defect was observed in the localization of gurken mRNA or protein in the mutants (Fig. 6A,B). Similarly, in wild-type, as in mutant egg chambers bicoid mRNA localized to the anterior margin of the oocyte (Fig. 6C,D). Furthermore, even in egg chambers in which oskar mRNA was delocalized, the localization of gurken and bicoid mRNAs was unaffected (see Fig. S4 in the supplementary material). Because the localization of gurken and bicoid mRNAs relies on a polarized microtubule network (Duncan and Warrior, 2002; Januschke et al., 2002; Pokrywka and Stephenson, 1991), this finding suggests that the overall polarity of the egg chamber is unaffected in SmD3pt mutants. Furthermore, staining of SmD3pt oocytes with phalloidin did not reveal defects in the architecture of the actin cytoskeleton (see Fig. S5 in the supplementary material).
Fig. 6.
Polarity is unaffected in SmD3pt egg chambers. (A) Egg chambers from SmD3pt homozygotes were processed for in situ hybridization using a probe against gurken (grk) mRNA (red) and were counterstained with DAPI (blue). (B) Egg chambers from SmD3pt homozygotes were processed for immunofluorescence using an antibody against Gurken protein (red). The green fluorescence of SmD3pt is also shown. gurken mRNA and protein were localized to the dorsal-anterior corner of the oocyte (arrow). (C,D) Egg chambers from wild type (C) or SmD3pt homozygotes (D) were processed for in situ hybridization using a probe against bicoid (bcd) mRNA (red) and were counterstained with DAPI (blue). bicoid mRNA localized to the anterior margin of the oocyte in both instances (arrow). (E,F) SmD3pt mutants were processed for in situ hybridization using a probe against oskar mRNA (red) and for immunofluorescence using an antibody against Khc (green). In contrast to egg chambers containing localized oskar mRNA (E), egg chambers in which oskar mRNA was delocalized failed to accumulate posterior Khc (F).
The primary motor responsible for transporting oskar mRNA within the oocyte is thought to be Kinesin heavy chain (Khc). Khc localizes in a crescent at the posterior of wild-type stage 9 and 10a oocytes, and in Khc mutants oskar mRNA is mislocalized around the entire cortex of the oocyte (Brendza et al., 2002; Cha et al., 2002; Palacios and St Johnston, 2002). Localization of oskar mRNA and of Khc were simultaneously visualized in wild type and SmD3pt mutants. As expected, oskar mRNA and Khc colocalized at the posterior of stage 9 and 10a wild-type oocytes (data not shown). Similar results were obtained in SmD3pt mutants that contained properly localized oskar mRNA (Fig. 6E, arrow). However, Khc was never enriched at the oocyte posterior in egg chambers with delocalized oskar mRNA (Fig. 6F). As previously noted, such egg chambers do not express Oskar protein. Importantly, Oskar protein participates in a feedback loop that reinforces the posterior recruitment of microtubule plus ends (Zimyanin et al., 2007). Consequently, Khc, a plus-end-directed motor, is not enriched at the posterior of egg chambers that do not express Oskar protein (Zimyanin et al., 2007). Consistent with this interpretation, SmD3pt oocytes that contain a central focus of oskar mRNA and protein recruit Khc to the same focus (see Fig. S6 in the supplementary material).
SmD3 is required for germ cell specification and embryonic patterning
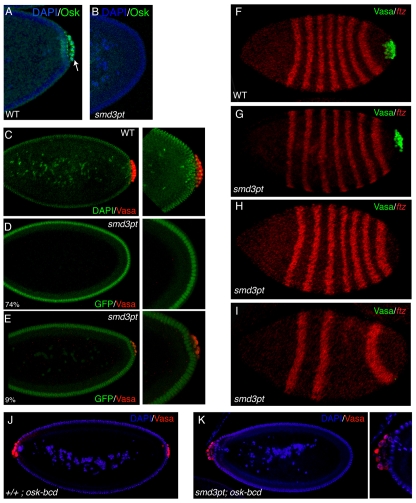
Proper localization of oskar mRNA during oogenesis is required for germ cell formation and patterning of the embryo. We extended our analysis to embryos from SmD3pt mothers raised at 27°C (referred to as SmD3pt embryos). In contrast to embryos derived from wild-type females, 74% of SmD3pt embryos contained neither germ cells nor posterior Oskar protein (Fig. 7A,B). Similar results were obtained when SmD3pt embryos were stained for Vasa, an essential pole plasm component and germ cell marker (Fig. 7C,D) (Hay et al., 1988; Lasko and Ashburner, 1988). Of note, a small percentage of mutant embryos (9%) contained fewer germ cells than their wild-type counterparts (Fig. 7E).
Fig. 7.
SmD3 is required for germ cell specification and embryonic patterning. (A,B) Embryos from wild-type (A) or SmD3pt (B) females were processed for immunofluorescence using an antibody against Oskar (green) and were counterstained with DAPI (blue). In contrast to wild type, most SmD3pt embryos contained neither germ cells nor posterir Oskar. (C-E) Embryos from wild-type (C) or SmD3pt (D-E) females raised at 27°C were processed for immunofluorescence using an antibody against Vasa (red). Wild-type embryos were counterstained with DAPI (green) and the green fluorescence of SmD3pt embryos is indicated. Most SmD3pt embryos (74%) did not contain germ cells (D). A small percentage (9%) of embryos contained many fewer germ cells than the wild type (E). (F-I) Embryos from wild-type (F) or SmD3pt (G-I) females were processed for in situ hybridization using a probe against fushi tarazu (ftz, red) and immunofluorescence using an antibody against Vasa (green). (G) An SmD3pt embryo that contains germ cells and proper patterning. (H) An SmD3pt embryo that lacks germ cells yet retains all posterior segments. (I) An SmD3pt embryo lacking both germ cells and posterior segments. (J,K) Embryos from wild-type (J) or SmD3pt (K) females expressing UAS-osk-bcd driven by nanos-Gal4 were processed for immunofluorescence using an antibody against Vasa (red) and counterstained with DAPI (blue). Although SmD3pt embryos were able to form anterior germ cells (34.3±1.6%), anterior germ cell formation was less efficient than in wild type (52.5±2.5%). These results are significant at P<0.0005.
We next examined the patterning of SmD3pt embryos by simultaneously visualizing fushi tarazu (ftz) mRNA (a marker for patterning) and Vasa protein (Fig. 7F-I). As with wild-type embryos, SmD3pt embryos that contained germ cells were properly patterned (Fig. 7F,G). These represent embryos that contain wild-type levels of Oskar activity. Interestingly, of those SmD3pt embryos that were devoid of germ cells, 26% retained proper patterning (Fig. 7H), whereas the rest were missing posterior segments (Fig. 7I). The embryos that retained proper patterning, yet were devoid of germ cells, are likely to contain a small amount of Oskar activity. This is consistent with previous findings suggesting that germline specification is more sensitive than posterior patterning to a reduction in Oskar activity (Lehmann and Nusslein-Volhard, 1986; Rongo et al., 1995). Embryos that lack both germ cells and posterior segments are likely to contain no Oskar activity.
We also analyzed whether SmD3 is required for germ cell formation apart from its role in oskar mRNA localization. We tested this by artificially targeting Oskar protein to the anterior of SmD3pt embryos. Oskar has been successfully targeted to the anterior pole of embryos by tethering the localization sequences from bicoid mRNA to the oskar coding sequence (Ephrussi and Lehmann, 1992). Anterior Oskar protein induces the formation of ectopic germ cells (Ephrussi and Lehmann, 1992; Tanaka and Nakamura, 2008). Mutants that function solely in oskar mRNA localization are capable of forming anterior germ cells. However, those mutants that also function downstream of oskar localization in germ cell specification are compromised for anterior germ cell formation. Interestingly, SmD3pt mutants appear to fall into the latter category. Out of 112 wild-type embryos expressing the oskar-bicoid (osk-bcd) transgene, 53% formed anterior germ cells (Fig. 7J). SmD3pt mutants were also capable of forming anterior germ cells (Fig. 7K). However, out of 138 embryos lacking posterior germ cells, only 34% contained anterior germ cells. This finding implies that, in addition to participating in oskar mRNA localization, SmD3 also functions separately in germline specification. However, firmer conclusions will require an SmD3 allele that is not compromised for oskar mRNA localization, yet is defective in germline specification.
DISCUSSION
Sm proteins and germ granule localization
Previous studies in C. elegans, Xenopus and mice showed that Sm proteins localize to cytoplasmic granules that are functionally similar to the Drosophila pole plasm (Barbee et al., 2002; Bilinski et al., 2004; Chuma et al., 2003). Using VFP-tagged constructs, we demonstrate that SmB and SmD3 localize to the Drosophila pole plasm. Does the localization of the tagged constructs reflect the true localization of the endogenous proteins? Several lines of evidence suggest that it does. VFP-SmB rescues the lethality of a transposon insertion in SmB and produces viable adults in which the tagged protein is properly localized (see Fig. S1C in the supplementary material). Expression of VFP-SmD3 or GFP-SmD3 rescues the oskar mRNA-delocalization phenotype of SmD3pt mutants and both constructs localize to the pole plasm (Fig. 4D, Fig. 5E; data not shown). Additionally, the transgenes are not overexpressed in comparison to their endogenous counterparts (see Fig. S1D in the supplementary material). Thus, the posterior enrichment of VFP-SmB and VFP-SmD3 does not appear to be the result of a tagging artifact.
Our results suggest that germ granules in Drosophila contain a novel type of Sm complex. SmB and SmD3, but not the other Sm proteins, have been shown to co-purify with human telomerase RNPs (Fu and Collins, 2006). Furthermore, the U7 snRNP, which is required for nuclear processing of histone mRNA, specifically lacks SmD1 and SmD2 (Pillai et al., 2001). Thus, there is precedence for the formation of non-canonical Sm complexes that contain subsets of the seven core Sm proteins.
oskar mRNA localization
The splicing function of Sm proteins is required for cell viability. Therefore, examination of Sm protein function in germline specification requires a viable mutant allele that is not compromised for splicing. Such an allele is not available for SmB. However, we demonstrate that SmD3pt is homozygous viable and defective in oskar mRNA localization. In early egg chambers, oskar mRNA occupies the entire volume of the ooplasm (Ephrussi et al., 1991; Kim-Ha et al., 1991). By stage 8, the message can be detected at the anterior margins of the oocyte and also within a transient central focus (Cha et al., 2002; Ephrussi et al., 1991; Kim-Ha et al., 1991). By stage 9, oskar mRNA localizes to the posterior pole of the oocyte where it remains anchored for the remainder of oogenesis (Ephrussi et al., 1991; Kim-Ha et al., 1991). In SmD3pt mutants, the localization of oskar mRNA is identical to that in wild type until stage 8 (data not shown). A significant fraction of stage 9 and 10 egg chambers, however, show varying patterns of oskar mRNA delocalization (Fig. 5). The penetrance of the mutant phenotype is temperature sensitive but is not significantly different between stage 9 and 10 egg chambers. Consistent with oskar mRNA delocalization, Stau, a core component of the oskar mRNP, was also delocalized in SmD3pt egg chambers (see Fig. S7 in the supplementary material).
Why is oskar mRNA delocalized in mutant oocytes? Smd3pt is hypomethylated in comparison to the wild-type protein. Our results indicate, however, that loss of methylation is unlikely to be the cause of oskar mRNA delocalization (Fig. 5E). It is also possible that the GFP insertion within Smd3pt somehow compromises the activity of the protein. Using anti-GFP antibodies, we found that Smd3pt is able to associate with oskar mRNA in vivo (Fig. 5G). We exploited the temperature-sensitive nature of the SmD3pt allele to ask whether oskar mRNA delocalization correlates with loss of Smd3pt binding. We found no significant difference in the association of SmD3pt with oskar mRNA at permissive (21°C) and non-permissive (27°C) temperatures (Fig. 5G). We conclude that when oskar mRNA is delocalized, SmD3pt remains associated with the message.
How do SmD3 and SmB function in oskar mRNA localization? In considering this question, it is worth examining the function of Sm proteins in snRNP assembly. An early event in assembly of spliceosomal snRNPs involves the association of an Sm core with the snRNA (Matera et al., 2007). By binding the snRNA, the Sm proteins are thought to aid in the three-dimensional folding of the RNA such that additional components can be specifically added to the maturing snRNP (Beggs, 2005; Kambach et al., 1999). By analogy, SmD3 and SmB might bind oskar mRNA early in the life cycle of the message, thereby enabling proper folding of the RNA and subsequent recruitment of additional essential components. When SmD3pt is brought in trans over a strong loss-of-function SmD3 mutant, the penetrance of oskar mRNA delocalization is greatly increased (Fig. 5). By contrast, removing one copy of SmB does not exacerbate the SmD3pt mutant phenotype (data not shown). Thus, SmB and SmD3 do not appear to function redundantly in oskar mRNA localization.
The role of microtubules in oskar mRNA localization
Unlike oskar mRNA, bicoid and gurken mRNAs were properly localized in SmD3pt egg chambers (Fig. 6). Additionally, no defect was observed in the repositioning of the oocyte nucleus to the dorsal-anterior corner of SmD3pt egg chambers (data not shown). These processes are microtubule dependent and require a properly polarized oocyte (Duncan and Warrior, 2002; Januschke et al., 2002; Pokrywka and Stephenson, 1991), suggesting that overall polarity is maintained in SmD3pt egg chambers.
In contrast to bicoid and gurken mRNAs, the plus-end-directed microtubule motor, Khc, was not enriched at the posterior pole in SmD3pt mutants that contained delocalized oskar mRNA. One interpretation suggests that microtubule plus ends are not anchored at the posterior in SmD3pt mutants. According to this view, the defect in oskar mRNA localization would be a secondary consequence of prior defects in the localization of microtubule plus ends. This hypothesis posits that the primary function of SmD3 is in the regulation of microtubule polarity. We cannot entirely rule out this possibility. However, the intertwined nature of oocyte plus end polarity and the Oskar pathway causes us to favor a slightly different hypothesis. Oskar protein participates in a positive-feedback mechanism that reinforces posterior recruitment of microtubule plus ends (Zimyanin et al., 2007). According to this view, an egg chamber that fails to express Oskar protein would fail to recruit sufficient microtubule plus ends to the posterior pole. Thus, Khc would appear delocalized in these egg chambers. We believe this to be the case in SmD3pt mutants. Consistent with the notion of an Oskar-Khc connection, SmD3pt egg chambers that contain a central focus of oskar mRNA and protein recruit Khc to the same focus (see Fig. S6 in the supplementary material).
Is the function of Khc compromised in SmD3pt egg chambers? The fact that Khc is not localized to the posterior pole of mutant egg chambers suggest that at least this aspect of its function is compromised. However, global functions of Khc in the female germline appear to be relatively unaffected in SmD3pt mutants. Loss of Khc results in delocalization of bicoid mRNA, gurken mRNA and protein, mispositioning of the oocyte nucleus, and mislocalization of oskar mRNA around the entire cortex of the oocytes (Cha et al., 2002; Januschke et al., 2002). None of these phenotypes was observed in SmD3pt mutants.
Conclusions
The Sm family of proteins is of ancient evolutionary origin. The Escherichia coli Sm ortholog, Hfq, functions to modulate the translation and stability of several RNAs, including mRNAs and tRNAs (Valentin-Hansen et al., 2004). Based on these ancestral functions, the involvement of eukaryotic Sm proteins in splicing is generally thought to be a derived function. Our finding that spliceosomal Sm proteins are also associated with oskar mRNA suggests that some of these ancestral functions in mRNA regulation have been retained. In the context of oskar, SmD3 is required for regulating the localization of the message. As a consequence of this function, hypomorphic mutants in SmD3 do not form germ cells and display defects in developmental patterning. This study represents the first demonstration of a eukaryotic Sm protein regulating the cytoplasmic fate of an mRNA.
Supplementary Material
Acknowledgements
We are deeply indebted to R. Padgett and J. McDonald for providing research space for G.B.G. during extended laboratory visits. We thank A. Ephrussi, P. Lasko, A. Nakamura, H. Salz, J. Steitz, D. St Johnston, M. Terns, J. Horabin and J. Wilhelm for providing antibodies and fly strains, without which this work would not have been possible; Karl Wenger and Sadanand Fulzele for allowing us to use their iCycler qPCR machine; J. Anne and B. Mechler for communicating results prior to publication; and S. Bortvedt, S. Lyons and C. Stanfield for technical assistance. This work was supported by NIH grants R01-GM053034 and R01-NS041617 (to A.G.M.) and F32HD055711 (to G.B.G.). Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.042721/-/DC1
References
- Anne J., Ollo R., Ephrussi A., Mechler B. M. (2007). Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development 134, 137-146 [DOI] [PubMed] [Google Scholar]
- Barbee S. A., Evans T. C. (2006). The Sm proteins regulate germ cell specification during early C. elegans embryogenesis. Dev. Biol. 291, 132-143 [DOI] [PubMed] [Google Scholar]
- Barbee S. A., Lublin A. L., Evans T. C. (2002). A novel function for the Sm proteins in germ granule localization during C. elegans embryogenesis. Curr. Biol. 12, 1502-1506 [DOI] [PubMed] [Google Scholar]
- Beggs J. D. (2005). Lsm proteins and RNA processing. Biochem. Soc. Trans. 33, 433-438 [DOI] [PubMed] [Google Scholar]
- Bilinski S. M., Jaglarz M. K., Szymanska B., Etkin L. D., Kloc M. (2004). Sm proteins, the constituents of the spliceosome, are components of nuage and mitochondrial cement in Xenopus oocytes. Exp. Cell Res. 299, 171-178 [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312-3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell R. E., Mahowald A. P. (1985). tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell 43, 97-104 [DOI] [PubMed] [Google Scholar]
- Breitwieser W., Markussen F. H., Horstmann H., Ephrussi A. (1996). Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 10, 2179-2188 [DOI] [PubMed] [Google Scholar]
- Brendza R. P., Serbus L. R., Saxton W. M., Duffy J. B. (2002). Posterior localization of dynein and dorsal-ventral axis formation depend on kinesin in Drosophila oocytes. Curr. Biol. 12, 1541-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M., Paterno S., Lighthouse D., Bachman J., Planck J., Owen S., Skora A. D., Nystul T. G., Ohlstein B., Allen A., et al. (2007). The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175, 1505-1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B. J., Serbus L. R., Koppetsch B. S., Theurkauf W. E. (2002). Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat. Cell Biol. 4, 592-598 [DOI] [PubMed] [Google Scholar]
- Chuma S., Hiyoshi M., Yamamoto A., Hosokawa M., Takamune K., Nakatsuji N. (2003). Mouse Tudor Repeat-1 (MTR-1) is a novel component of chromatoid bodies/nuages in male germ cells and forms a complex with snRNPs. Mech. Dev. 120, 979-990 [DOI] [PubMed] [Google Scholar]
- Cote J., Richard S. (2005). Tudor domains bind symmetrical dimethylated arginines. J. Biol. Chem. 280, 28476-28483 [DOI] [PubMed] [Google Scholar]
- Duncan J. E., Warrior R. (2002). The cytoplasmic dynein and kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr. Biol. 12, 1982-1991 [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Lehmann R. (1992). Induction of germ cell formation by oskar. Nature 358, 387-392 [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Dickinson L. K., Lehmann R. (1991). Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66, 37-50 [DOI] [PubMed] [Google Scholar]
- Extavour C. G., Akam M. (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869-5884 [DOI] [PubMed] [Google Scholar]
- Findley S. D., Tamanaha M., Clegg N. J., Ruohola-Baker H. (2003). Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development 130, 859-871 [DOI] [PubMed] [Google Scholar]
- Fu D., Collins K. (2006). Human telomerase and Cajal body ribonucleoproteins share a unique specificity of Sm protein association. Genes Dev. 20, 531-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez G. B., Rajendra T. K., Tian L., Matera A. G. (2006). The Sm-protein methyltransferase, dart5, is essential for germ-cell specification and maintenance. Curr. Biol. 16, 1077-1089 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A., Elliott H., St Johnston D. (1995). Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375, 654-658 [DOI] [PubMed] [Google Scholar]
- Gunkel N., Yano T., Markussen F. H., Olsen L. C., Ephrussi A. (1998). Localization-dependent translation requires a functional interaction between the 5′ and 3′ ends of oskar mRNA. Genes Dev. 12, 1652-1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet O., Ephrussi A. (2001). Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr. Biol. 11, 1666-1674 [DOI] [PubMed] [Google Scholar]
- Hachet O., Ephrussi A. (2004). Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature 428, 959-963 [DOI] [PubMed] [Google Scholar]
- Hay B., Jan L. Y., Jan Y. N. (1988). A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 55, 577-587 [DOI] [PubMed] [Google Scholar]
- Hayashi K., de Sousa Lopes S. M., Surani M. A. (2007). Germ cell specification in mice. Science 316, 394-396 [DOI] [PubMed] [Google Scholar]
- Januschke J., Gervais L., Dass S., Kaltschmidt J. A., Lopez-Schier H., St Johnston D., Brand A. H., Roth S., Guichet A. (2002). Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr. Biol. 12, 1971-1981 [DOI] [PubMed] [Google Scholar]
- Kambach C., Walke S., Young R., Avis J. M., de la Fortelle E., Raker V. A., Luhrmann R., Li J., Nagai K. (1999). Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell 96, 375-387 [DOI] [PubMed] [Google Scholar]
- Kelso R. J., Buszczak M., Quinones A. T., Castiblanco C., Mazzalupo S., Cooley L. (2004). Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 32, D418-D420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J., Smith J. L., Macdonald P. M. (1991). oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell 66, 23-35 [DOI] [PubMed] [Google Scholar]
- Kim-Ha J., Kerr K., Macdonald P. M. (1995). Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell 81, 403-412 [DOI] [PubMed] [Google Scholar]
- Krauss J., Lopez de Quinto S., Nusslein-Volhard C., Ephrussi A. (2009). Myosin-V regulates oskar mRNA localization in the Drosophila oocyte. Curr. Biol. 19, 1058-1063 [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. (1988). The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335, 611-617 [DOI] [PubMed] [Google Scholar]
- Lehmann R., Nusslein-Volhard C. (1986). Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141-152 [DOI] [PubMed] [Google Scholar]
- Liu J. L., Gall J. G. (2007). U bodies are cytoplasmic structures that contain uridine-rich small nuclear ribonucleoproteins and associate with P bodies. Proc. Natl. Acad. Sci. USA 104, 11655-11659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald A. P. (2001). Assembly of the Drosophila germ plasm. Int. Rev. Cytol. 203, 187-213 [DOI] [PubMed] [Google Scholar]
- Martin K. C., Ephrussi A. (2009). mRNA localization: gene expression in the spatial dimension. Cell 136, 719-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera A. G., Terns R. M., Terns M. P. (2007). Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 8, 209-220 [DOI] [PubMed] [Google Scholar]
- Micklem D. R., Dasgupta R., Elliott H., Gergely F., Davidson C., Brand A., Gonzalez-Reyes A., St Johnston D. (1997). The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol. 7, 468-478 [DOI] [PubMed] [Google Scholar]
- Micklem D. R., Adams J., Grunert S., St Johnston D. (2000). Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J. 19, 1366-1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. E., Dillon S. T., Boswell R. E. (2001). The RNA-binding protein Tsunagi interacts with Mago Nashi to establish polarity and localize oskar mRNA during Drosophila oogenesis. Genes Dev. 15, 2886-2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. (2001). A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050-15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman-Silberberg F. S., Schupbach T. (1993). The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell 75, 165-174 [PubMed] [Google Scholar]
- Newmark P. A., Mohr S. E., Gong L., Boswell R. E. (1997). mago nashi mediates the posterior follicle cell-to-oocyte signal to organize axis formation in Drosophila. Development 124, 3197-3207 [DOI] [PubMed] [Google Scholar]
- Palacios I. M., St Johnston D. (2002). Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development 129, 5473-5485 [DOI] [PubMed] [Google Scholar]
- Palacios I. M., Gatfield D., St Johnston D., Izaurralde E. (2004). An eIF4AIII-containing complex required for mRNA localization and nonsense-mediated mRNA decay. Nature 427, 753-757 [DOI] [PubMed] [Google Scholar]
- Pillai R. S., Will C. L., Luhrmann R., Schumperli D., Muller B. (2001). Purified U7 snRNPs lack the Sm proteins D1 and D2 but contain Lsm10, a new 14 kDa Sm D1-like protein. EMBO J. 20, 5470-5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrywka N. J., Stephenson E. C. (1991). Microtubules mediate the localization of bicoid RNA during Drosophila oogenesis. Development 113, 55-66 [DOI] [PubMed] [Google Scholar]
- Quinones-Coello A. T., Petrella L. N., Ayers K., Melillo A., Mazzalupo S., Hudson A. M., Wang S., Castiblanco C., Buszczak M., Hoskins R. A., et al. (2007). Exploring strategies for protein trapping in Drosophila. Genetics 175, 1089-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raker V. A., Plessel G., Luhrmann R. (1996). The snRNP core assembly pathway: identification of stable core protein heteromeric complexes and an snRNP subcore particle in vitro. EMBO J. 15, 2256-2269 [PMC free article] [PubMed] [Google Scholar]
- Rongo C., Gavis E. R., Lehmann R. (1995). Localization of oskar RNA regulates oskar translation and requires Oskar protein. Development 121, 2737-2746 [DOI] [PubMed] [Google Scholar]
- Santos A. C., Lehmann R. (2004). Germ cell specification and migration in Drosophila and beyond. Curr. Biol. 14, R578-R589 [DOI] [PubMed] [Google Scholar]
- Schenkel H., Hanke S., De Lorenzo C., Schmitt R., Mechler B. M. (2002). P elements inserted in the vicinity of or within the Drosophila snRNP SmD3 gene nested in the first intron of the Ornithine Decarboxylase Antizyme gene affect only the expression of SmD3. Genetics 161, 763-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A. J., Adams J. H., Davidson C. M., Micklem D. R., Haseloff J., St Johnston D., Brand A. H. (1998). Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev. 12, 1847-1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J. M., Benton R., St Johnston D. (2000). The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell 101, 377-388 [DOI] [PubMed] [Google Scholar]
- Smith J. L., Wilson J. E., Macdonald P. M. (1992). Overexpression of oskar directs ectopic activation of nanos and presumptive pole cell formation in Drosophila embryos. Cell 70, 849-859 [DOI] [PubMed] [Google Scholar]
- St Johnston D. (2005). Moving messages: the intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 6, 363-375 [DOI] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nusslein-Volhard C. (1991). Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell 66, 51-63 [DOI] [PubMed] [Google Scholar]
- Tanaka T., Nakamura A. (2008). The endocytic pathway acts downstream of Oskar in Drosophila germ plasm assembly. Development 135, 1107-1117 [DOI] [PubMed] [Google Scholar]
- Thomson T., Lasko P. (2004). Drosophila tudor is essential for polar granule assembly and pole cell specification, but not for posterior patterning. Genesis 40, 164-170 [DOI] [PubMed] [Google Scholar]
- Tomancak P., Piano F., Riechmann V., Gunsalus K. C., Kemphues K. J., Ephrussi A. (2000). A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat. Cell Biol. 2, 458-460 [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Eriksen M., Udesen C. (2004). The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol. Microbiol. 51, 1525-1533 [DOI] [PubMed] [Google Scholar]
- van Eeden F. J., Palacios I. M., Petronczki M., Weston M. J., St Johnston D. (2001). Barentsz is essential for the posterior localization of oskar mRNA and colocalizes with it to the posterior pole. J. Cell Biol. 154, 511-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzo N. F., Ephrussi A. (2002). Oskar anchoring restricts pole plasm formation to the posterior of the Drosophila oocyte. Development 129, 3705-3714 [DOI] [PubMed] [Google Scholar]
- Vanzo N., Oprins A., Xanthakis D., Ephrussi A., Rabouille C. (2007). Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev. Cell 12, 543-555 [DOI] [PubMed] [Google Scholar]
- Wang C., Lehmann R. (1991). Nanos is the localized posterior determinant in Drosophila. Cell 66, 637-647 [DOI] [PubMed] [Google Scholar]
- Webster P. J., Liang L., Berg C. A., Lasko P., Macdonald P. M. (1997). Translational repressor bruno plays multiple roles in development and is widely conserved. Genes Dev. 11, 2510-2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. E., Smibert C. A. (2005). Mechanisms of translational regulation in Drosophila. Biol. Cell 97, 235-252 [DOI] [PubMed] [Google Scholar]
- Wilhelm J. E., Mansfield J., Hom-Booher N., Wang S., Turck C. W., Hazelrigg T., Vale R. D. (2000). Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes. J. Cell Biol. 148, 427-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T., Lopez de Quinto S., Matsui Y., Shevchenko A., Ephrussi A. (2004). Hrp48, a Drosophila hnRNPA/B homolog, binds and regulates translation of oskar mRNA. Dev. Cell 6, 637-648 [DOI] [PubMed] [Google Scholar]
- Zimyanin V., Lowe N., St Johnston D. (2007). An oskar-dependent positive feedback loop maintains the polarity of the Drosophila oocyte. Curr. Biol. 17, 353-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.