Abstract
Lung disease causes most of the morbidity and mortality in cystic fibrosis (CF). However, understanding its pathogenesis has been hindered by lack of an animal model with characteristic features of CF. To overcome this problem, we recently generated pigs with targeted CFTR genes. We now report that, within months of birth, CF pigs spontaneously develop hallmark features of CF lung disease including airway inflammation, remodeling, mucus accumulation, and infection. Their lungs contained multiple bacterial species, suggesting an equal opportunity host defense defect. In humans, the temporal and causal relationships between inflammation and infection have remained uncertain. To investigate these processes, we studied newborn pigs. Their lungs showed no inflammation, but were less often sterile than controls. Moreover, after intrapulmonary bacterial challenge, CF pigs failed to eradicate bacteria as effectively as wild-type pigs. These results suggest that impaired bacterial elimination is the pathogenic event that initiates a cascade of inflammation and pathology in CF lungs. Finding that CF pigs have a bacterial host defense defect within hours of birth provides an opportunity to further investigate pathogenesis and to test therapeutic and preventive strategies before secondary consequences develop.
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel (1-4). People with CF develop pancreatic, intestinal, hepatic, vas deferens, and lung disease. Yet, despite much investigation, the understanding of how loss of CFTR causes disease in these organs remains uncertain and controversial. This is especially true for pulmonary disease, which is characterized by airway inflammation and infection (2-7).
Two obstacles have hindered insight into the pathogenesis of CF lung disease. First, it has not been possible to investigate respiratory tract abnormalities at their onset in humans; that is, beginning at birth, before treatments and chronic abnormalities confound investigation. Second, a suitable animal model has not been available; mice with targeted CFTR gene alterations do not develop the clinical manifestations typical of human CF (8). To circumvent these limitations, we recently targeted the porcine CFTR gene to produce null and ΔF508 alleles (9,10). At birth, CFTR−/− pigs displayed intestinal lesions (meconium ileus and microcolon), exocrine pancreatic destruction, gallbladder abnormalities, and early focal biliary cirrhosis similar to that seen in human CF. Like the lungs of human infants (11,12), porcine CF lungs lacked cellular inflammation and submucosal gland changes.
We hypothesized that CF lung disease would not be unique to humans and that CF pigs would develop abnormalities with time. If they did, we wished to know which of the two characteristic features of CF lung disease, inflammation and infection, would appear first. This has been a persistent question in CF (13-20); the answer has important implications for the pathogenesis of lung disease and for preventive and therapeutic strategies.
RESULTS
CF pigs can survive after surgery for meconium ileus
For CF pigs to survive, it was necessary to circumvent the meconium ileus that occurred with 100% penetrance (10). In humans, surgery is often required to relieve CF intestinal obstruction (21), and this also was true for pigs. We performed an ileostomy or cecostomy on newborn CF and non-CF pigs. Following surgery, animals were raised in a clean, but not germ-free environment and were segregated at birth from other pigs to avoid exposure to porcine viral and bacterial pathogens.
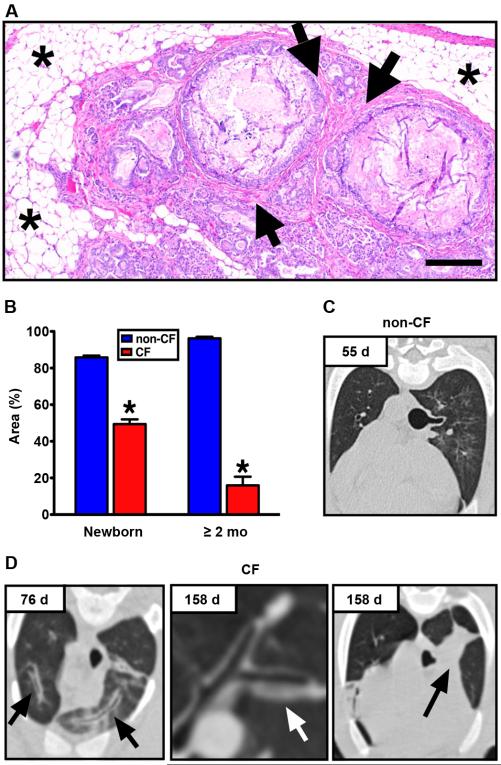
Here we report data from five CF pigs (3 CFTR−/− and 2 CFTR−/ΔF508) that survived ~2 months or more. CFTR−/ΔF508 pigs had similar gastrointestinal and pulmonary histopathological lesions and nasal voltage measurements as CFTR−/− pigs (fig. S1, S2), and hereafter CFTR−/− and CFTR−/ΔF508 pigs are collectively referred to as CF pigs. The CF animals were euthanized because of respiratory disease or due to complications of gastric ulcers, which are common in swine and are exacerbated by pulmonary disease and stress (22,23) (table S1). Necropsy revealed a fatty pancreas, and microscopic examination revealed distended pancreatic ducts with intervening fibrosis closely matching Dorothy Andersen’s descriptions of “cystic fibrosis of the pancreas” (Fig. 1A) (24). The pancreatic parenchymal loss had progressed since birth (Fig. 1B)(25). The microcolon, seen at birth, had reverted to a normal size and structure. The microgallbladder with mucinous changes and the heterogeneous focal biliary cirrhosis had not substantially changed from that of newborn CFTR−/− pigs (25).
Fig. 1. CF pigs develop pancreatic and pulmonary disease.
(A) Pancreas from Case #1. Adipose tissue (asterisks) surrounded degenerative exocrine lobules, large obstructed ducts and fibrosis (arrows), HE stain, scale bar, 240 μm. (B) Lobular exocrine tissue in newborn pigs and pigs greater than 2 months age (data from newborn pigs was previously published in (25)). Low magnification images of pancreatic parenchyma and interlobular connective tissue were quantified, and lobular tissue area was recorded as a percent of the total area for each image (*, P < 0.01, Mann-Whitney test). Chest X-ray computed tomography from (C) non-CF and (D) CF pigs (Case #5, left; Case #4, middle and right). Airway wall thickening (arrows) appeared with time in CF pigs. At 158 d, obstruction of tracheal bronchus was observed in Case #4 with collapse of the associated lung segment (black arrow).
CF pigs develop lung abnormalities with time
We performed sequential bronchoalveolar lavage (BAL) on several animals and found that cell counts and cytokines from CF pigs did not differ from control samples (fig. S3). In contrast, chest X-ray computed tomography showed airway walls with progressive thickening and frequent scattered infiltrates, with the tracheal lobe often showing the most involvement (Fig. 1C, 1D). These changes are also observed in patients with CF (2).
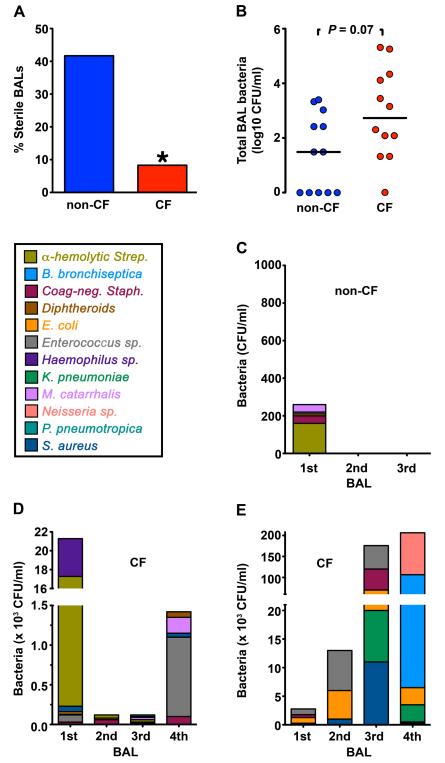
To test for the presence of bacteria, we cultured BAL liquid. BAL liquid from CF pigs was less frequently sterile compared to wild-type littermates (Fig. 2A). It also tended to contain more bacteria (Fig. 2B). Cultures from both CF and non-CF BAL yielded a variety of bacterial species, which differed from animal to animal (Fig. 2C-2E) and included Staphylococcus aureus, which frequently causes CF lung infections (26) (Fig. 2C-2E, S4). The total numbers and species of bacteria recovered from individual CF pigs varied from one BAL to another. Collectively, these data suggest that the CF porcine lung has an impaired ability to eradicate bacteria, consistent with data from humans showing that many different bacteria can infect CF lungs, especially early in the course of disease (26,27).
Fig. 2. CF pig lungs contain bacteria.
Data are from BAL, with several animals having multiple lavages at 4-6 week intervals. (A) Percentage of sterile BAL liquid samples obtained over time from non-CF and CF animals. * P < 0.05. (B) Number of bacteria recovered in tracheal lobe BAL samples obtained over time from non-CF and CF animals. Horizontal line denotes median. n=7 non-CF and n=5 CF animals. Each data point represents a BAL sample from an animal. Several animals had sequential BAL over time and are represented more than once. (C-E) Serial BAL cultures from a non-CF pig (C) and two CF pigs (Case #5, D; Case #4, E). Data are color-coded to indicate individual species of bacteria.
The lungs of CF pigs develop histopathological changes like those in human CF
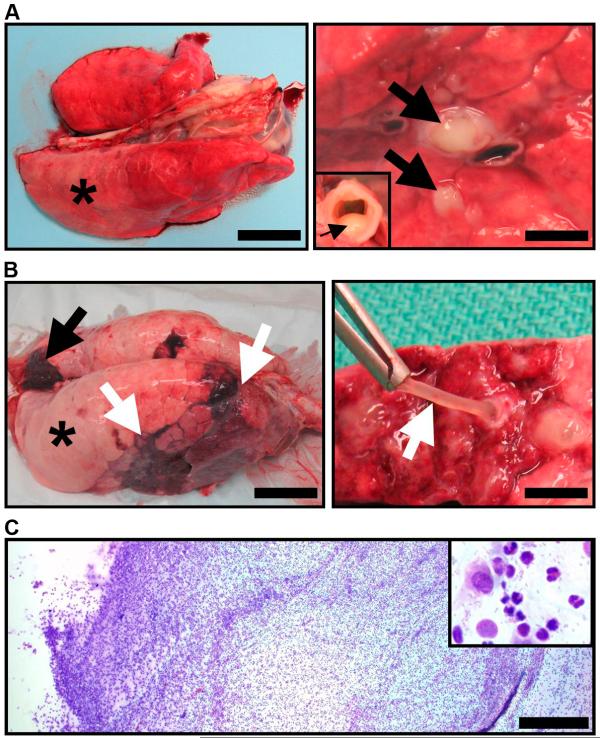
After removal at necropsy, some regions of CF lungs did not deflate, and lungs from one pig showed areas of atelectasis and pneumonia (Fig. 3A, 3B). These features are characteristic of airway obstruction. Some airways appeared normal, whereas others had striking features. Purulent exudate occluded some bronchi and partially filled the trachea (Fig. 3A). Other airways were filled with tenacious mucus that retained its shape when retracted, and contained neutrophils, some macrophages, and bacteria (Fig. 3B, 3C).
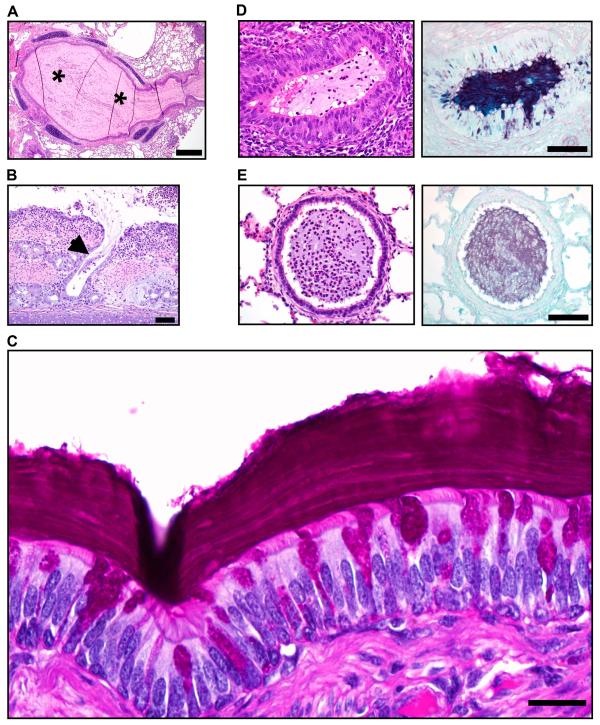
Fig. 3. CF pigs spontaneously develop lung disease.
(A) Lung, Case #1. Right caudal lung did not collapse (*)(scale bar, 3.4 cm) and sectioning revealed purulent material in obstructed airways (arrows)(scale bar, 2.9 mm) and tracheal lumen (arrow, inset). (B) Lung, Case #4. Lungs had atelectasis (black arrow), hyperinflation (asterisk) and pneumonia (white arrows, left panel, scale bar, 5 cm). Airways were obstructed by tenacious and resilient mucus (white arrow, right panel, scale bar, 6.6 mm). (C) Mucus cytology, Case #4. Mucus had a high cellularity composed primarily of degenerative neutrophils (inset) with macrophages and bacteria (scale bar, 0.4 mm).
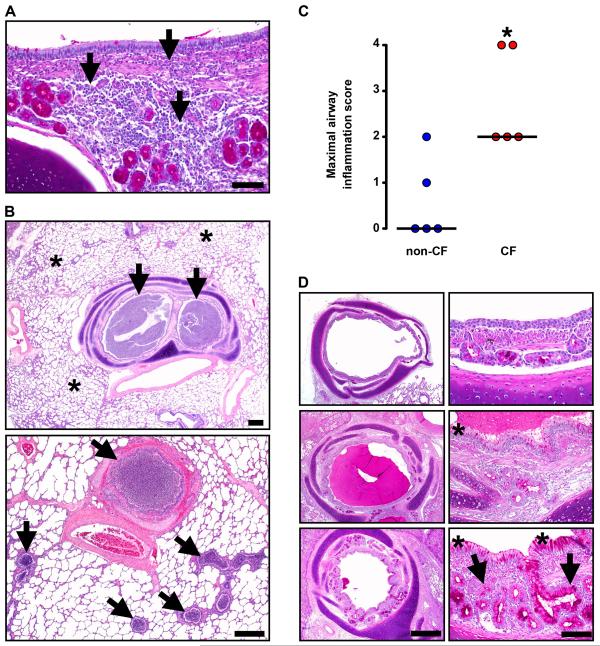
Inflammation, remodeling, and mucus accumulation are three features that characterize human CF airways (24,28,29). We examined the CF lungs for each of these. Microscopic examination revealed a range of inflammation severity within CF pig airways. Inflammation varied from none, to mild mononuclear (Fig. 4A), to severe neutrophilic infiltration that occasionally ulcerated the airway wall with abscess formation or destroyed submucosal glands (fig. S5A, S5B). In addition, some bronchi and small bronchioles contained purulent exudate (Fig. 4B). Inflammation centered on the airways and generally spared alveoli, although at times airway obstruction produced foci of hyperinflation and atelectasis (fig. S5C). Pneumonia was occasionally observed (fig. S5D). In contrast, non-CF lungs showed minimal cellular inflammation (Fig. 4C).
Fig. 4. Lung disease in CF pigs replicates that in human CF.
(A) Case #3. Infiltration of the airway wall by lymphocytes and plasma cells (arrows), PAS stain, scale bar, 80 μm. (B) Case #1. Obstruction (arrows) of bronchi and small bronchioles was a striking feature in otherwise unaffected lung (asterisks). HE stain, scale bars, 0.75 mm. (C) Airways from non-CF and CF pigs were scored for the most severe degree of leukocytic infiltration detected with the following scale: 0 – no leukocytes detected; 1 – rare individual to scattered leukocytes occasionally seen in airway wall with lack of cellular aggregates; 2 – grade 1 plus minor to moderate leukocyte aggregates detected in wall ± rare in lumen; 3 – grade 2 plus in multiple airways, with early wall injury, and luminal leukocytic aggregates; and 4 – grade 3, plus airway wall destruction, leukocytes filling and obstructing airways. For grades 1 and 2, leukocytes were primarily lymphocytes and plasma cells, and for grades 3 and 4, leukocytes were primarily neutrophils. Each point represents a sample from a different animal. CF pigs airways had more severe scores of airway infiltration (*P <0.05, Mann-Whitney, horizontal line indicates the median). (D) Case #4. Airways (left panels, scale bar, 0.7 mm) ranged from relatively unaffected (top) to severe disease (bottom, note that luminal mucocellular plug was removed at necropsy) with airway wall thickening. Surface epithelium (right panels, scale bar, 70 μm) ranged from near normal (top) to mucinous and hyperplastic change (asterisks) in moderate to severe disease (middle and bottom panels). Note that hypertrophy/hyperplasia of submucosal glands (arrows, bottom right) was uncommon and generally restricted to the most severe and chronically affected airways.
Airway remodeling was heterogeneous. Within individual animals it ranged from none (Fig. 4D, top), to mild (middle), to severe (bottom), with thickened airway walls, goblet cell hyperplasia of surface epithelium, and, uncommonly, expansion of submucosal glands.
Mucus distended airway lumens (Fig. 4D, 5A); however, we rarely found mucus filling, obstructing, or dilating submucosal gland ducts; Fig. 5B shows one example. More commonly, the mucus appeared to arise from goblet cells in the surface epithelium (Fig. 5C). Peripheral bronchioles, which lack submucosal glands, also sometimes showed luminal mucus adjacent to proliferative and mucinous epithelia (Fig. 5D). However, we occasionally found mucus in bronchioles lacking mucinous changes, suggesting that the mucus might have migrated from larger airways (Fig. 5E). Consistent with a surface epithelial origin, the mucus sometimes had a distinctive lamellar appearance, reminiscent of growth rings in trees, suggesting progressive deposition over time (Fig. 5C). This striated appearance resembled the gallbladder and intestinal mucus of newborn CF pigs (25). These changes differed strikingly from those in non-CF pigs (fig. S6). Thus, CF pigs spontaneously develop lung disease within the first few months of life, and the abnormalities replicate those in humans with CF (table S2).
Fig. 5. Mucus accumulates in CF airways.
(A) Case #4. Some airways were completely obstructed by mucocellular material (asterisks, HE stain, scale bar, 0.44 mm). (B) Case #1. Submucosal gland ducts were rarely dilated with stringy mucocellular debris (arrow, HE stain, scale bar, 75 μm). (C) Case #1. Some bronchial walls were lined by mucus with a distinct lamellar appearance (PAS stain, scale bar, 22 μm). (D) Bronchioles, Case #1. An obstructed bronchiole (which lack submucosal glands) with proliferative and mucinous changes in surface epithelium, HE (left panel) and ABPY (right) stains, scale bar, 71 μm. (E) Bronchioles, Case #1. Some obstructed bronchioles lacked proliferative and mucinous changes to epithelium, HE (left) and ABPY (right) stains, scale bar, 71 μm.
The lungs of newborn CF pigs lack inflammation
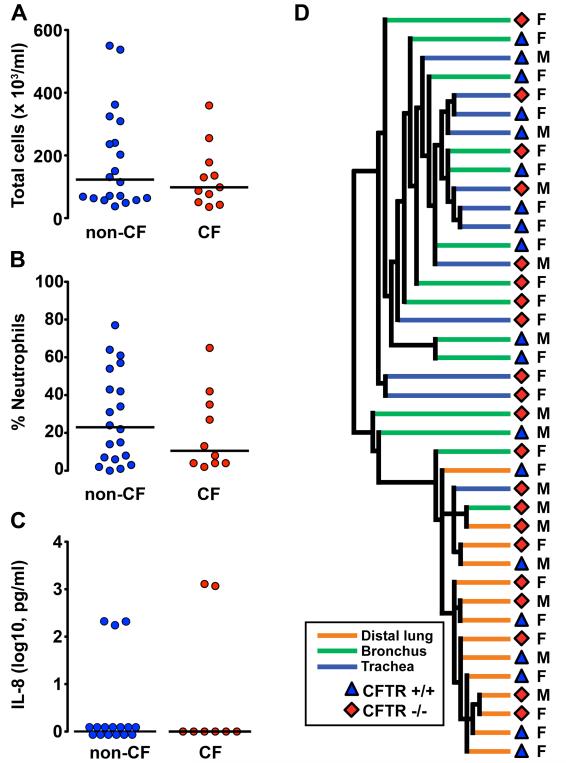
The presence of pulmonary disease in CF pigs a few months old raised questions about how the disease begins. We were interested in inflammation because it is a prominent manifestation in the CF pigs as well as in the lungs of children and adults with CF (7,24,28,29). Moreover, a persistent question in CF research has been whether inflammation precedes infection or vice versa (13,14,16-20,30). To answer this question, we studied newborn pigs (6-12h old). Our earlier histopathologic examination of lungs revealed no evidence of inflammation at birth (10). BAL liquid showed no difference in leukocyte cell counts or IL-8 levels between non-CF and CF pigs (Fig. 6A-6C) (10).
Fig. 6. At birth, CF pig lungs lack inflammation.
(A) Total cell counts, (B) Neutrophil percentages, and (C) IL-8 levels in BAL liquid obtained from non-CF and CF piglets within 6-12 h following birth. Each point represents a sample from a different animal. Horizontal line denotes median. No statistically significant differences were observed between non-CF and CF BAL liquid total cell counts, neutrophil percentages, or IL-8 levels. (D) Unsupervised hierarchical clustering of mRNA samples from trachea, bronchus, and distal lung samples obtained from newborn piglets (6-12 h old). Samples segregated into two clusters: airways (trachea and bronchus) and distal lung, but there was no clustering based on CFTR genotype. M and F refer to male and female pigs.
We also evaluated mRNA expression profiles in trachea, bronchi, and distal lung. Unsupervised hierarchical clustering segregated samples into two clusters: airways (trachea and bronchus) and distal lung (Fig. 6D), but there was no clustering based on CFTR genotype. Only a small number of differentially expressed genes were identified between non-CF and CF samples (table S3, S4, S5, and Supplementary Material). However, the number of differentially expressed genes decreased as the P-value and fold change stringency were increased, and expression was not consistently altered across the different tissues. We used Gene Set Enrichment Analysis (GSEA) to further query changes in gene expression using 2,775 publicly available, curated gene sets (table S4). This analysis failed to identify any gene sets that significantly correlated with the CF genotype. These results offer no evidence of a pathway, gene network, or signaling cascade consistent with inflammation in the lungs of newborn CF pigs. Moreover, comparison of these expression profiles with previously published data from CF cells did not reveal genes common to the studies that were consistently differentially expressed across the CF pig samples (table S6). Together, the results indicate a lack of inflammation in the lungs of newborn CF pigs.
Lungs of newborn CF pigs have an impaired ability to eradicate bacteria
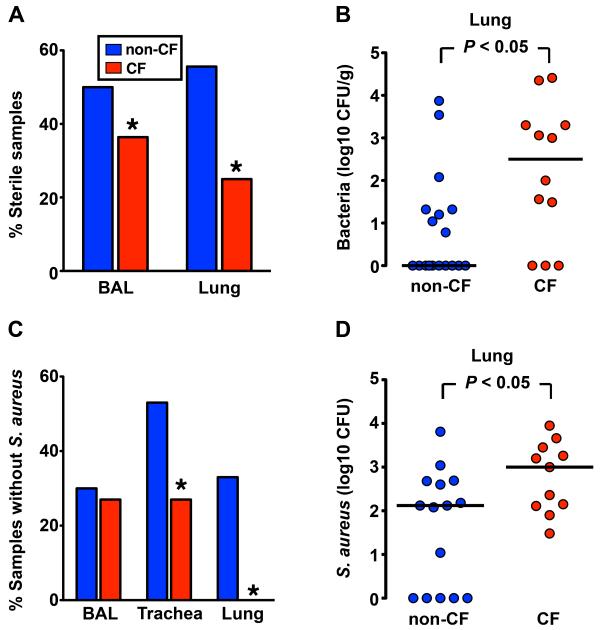
To learn whether these normal-appearing newborn lungs exhibit a host defense defect, we cultured BAL obtained shortly after birth and found that BAL from CF pigs was less frequently sterile and tended (not statistically significant) to have more bacteria than BAL from wild-type pigs (Fig. 7A, S7A). Because the BAL procedure might introduce contaminating bacteria or inadequately recover intrapulmonary bacteria, we removed sections of lung under sterile conditions and cultured them. Whereas more than 50% of wild-type lungs were sterile, only 25% of CF lungs had sterile cultures, and more bacteria were present in CF lungs (Fig. 7A, 7B). The species of bacteria were similar in the two groups and may have entered the lung during passage through the birth canal or via aspiration (fig. S7B). These data suggest that CF pigs have a defect in sterilizing their lungs.
Fig. 7. Newborn CF piglets fail to eradicate bacteria.
(A) Percentage of BAL liquid and lung homogenate samples that were sterile (no culturable bacteria) from newborn non-CF and CF pigs. BAL liquid n=20 non-CF and n=11 CF; lung homogenate n=18 non-CF and n=12 CF. Data are from 6 litters. * P < 0.05. (B) Quantitative microbiology on lung homogenate tissue samples. 6-12 h-old piglets were euthanized and lungs were sterilely removed. Each point represents an individual animal. Horizontal line denotes median. (C) Percentage of BAL liquid, trachea, and lung homogenate samples that contained no S. aureus. Samples were obtained from newborn non-CF and CF piglets after intrapulmonary challenge with S. aureus. (* P < 0.05 Chi-square test). (D) Bacteria recovered from tracheal lobe after S. aureus intrapulmonary challenge. Each point represents a different animal. Horizontal line denotes median. Data are from 5 litters. n=15 non-CF and n=11 CF.
To test this hypothesis directly, we challenged newborn pigs by aerosolizing a defined inoculum of S. aureus into the airways. We chose S. aureus because it commonly infects human infants and children (26), and we found it in the lungs of older pigs (fig. S4). We used an organism that we recovered from one of the older CF pigs. Four hours after delivering the S. aureus, non-CF pigs had tracheal washes that were sterile more often than CF pigs, although BAL cultures did not readily segregate by genotype (Fig. 7C, S7C). Importantly, direct culture of lung showed that CF pigs failed to completely eliminate the S. aureus as frequently as non-CF littermates, and CF pigs had more bacteria in their lungs (Fig. 7C, 7D). Thus within hours of birth, the CF lung manifests a host defense defect in eradicating bacteria.
DISCUSSION
Our data indicate that, with time, CF pigs spontaneously develop lung disease that largely replicates that in humans, including the hallmark features of inflammation, remodeling, mucus accumulation and infection. Moreover, within hours of birth, the CF pig lung already has a defect in eradicating bacteria. Although it is not possible to determine whether CF pigs or humans have more severe lung disease within the first months of life, the pulmonary changes in these two species differ from findings in mice which lack typical features of human CF lung disease. These observations indicate that the pulmonary consequences of CFTR loss are not unique to primates. Our results also suggest that comparative studies of humans, pigs, mice, and ferrets (31) may aid understanding of pathogenesis.
Inflammation is a key participant in CF lung destruction (7,24,28,29,32). Yet, the chicken and egg conundrum about which comes first, inflammation or bacteria, has remained a persistent question. Our data indicate that shortly after birth the CF lung has an impaired ability to eradicate bacteria in the absence of inflammation. This defect suggests that chronic, perhaps low-level bacterial stimulation in the CF lung generates inflammation, leading to the hallmark features of CF lung disease. How does our study relate to earlier work on the relationship between inflammation and infection? Examinations of BAL liquid from infants and children with CF have yielded conflicting data regarding the presence of inflammation and infection early in CF lung disease (13,14,30). In vitro models of airway epithelial cell lines and primary cultures produced data both for and against inherent CF inflammation (16-19). Studies of human fetal trachea transplanted into mice suggested that inflammation might occur spontaneously in developing CF airways (20). Compared to earlier work, our in vivo studies have the advantages of avoiding culture conditions, serial passage of cells, and expression of recombinant CFTR that might unexpectedly influence results. In addition, our data were collected within a few hours of birth; because newborns display impaired bacterial elimination, studies done even a few days after birth may not allow unambiguous interpretations. Our present work cannot however address the possibility that the CF lung might have an exaggerated inflammatory response to infection (6).
There were few abnormalities in CF BAL liquid despite X-ray and histopathologic evidence of disease. This difference is interesting because BAL liquid from humans with CF can show evidence of inflammation at what appear to be early stages of the disease (13,14,30). The lack of BAL abnormalities in pigs might relate to heterogeneity of the lung disease and/or to inadequate sampling by BAL. However, the relationship between BAL results and histopathology remains unknown in humans with CF. It is also possible that viral infections or environmental factors to which the CF pigs were not exposed might increase evidence of inflammation in BAL liquid of humans. These considerations and the impaired elimination of bacteria at birth suggest a possible benefit of preventive antibacterial strategies initiated very early in babies with CF, perhaps even before evidence of inflammation in BAL liquid.
Our data suggest that CF produces an equal opportunity host defense defect because porcine CF lungs contained a variety of bacterial species. In addition, the numbers and species of bacteria varied over time, suggesting a dynamic interaction between host and bacteria. This may result because the lung has multiple defenses against bacteria; some may be unaffected by CF, and others may be partially disrupted. Thus, residual defenses may provide variable protection. In this regard, it is interesting that we did not culture Pseudomonas aeruginosa, an important pathogen in humans with CF and an ubiquitous environmental organism (2,26). In human CF, many bacteria other than P. aeruginosa infect patients (26,27), and during the first years of life, S. aeureus is at least twice as common as P. aeruginosa (33,34). Thus, the CF host defense defect may allow infection with a diversity of bacterial species, and then time, selective pressures of pharmaceutical antibiotics, and residual host defense mechanisms may constrain the bacterial community to one or a few predominant species. Whether CF pigs will be less or more susceptible to P. aeruginosa lung infection remains unknown.
The observation that the lungs and gastrointestinal organs of CF pigs share many features with those of human CF suggests that these animals might be of value in better understanding the disease and in testing therapeutic and preventive strategies for multiple organs. Finding that CF pigs have a bacterial host defense defect within hours of birth could also provide an opportunity to explore the pathogenesis of the airway disease before secondary consequences develop.
MATERIALS AND METHODS
Animals
We previously reported production of CFTR+/−, CFTR+/δF508, and CFTR−/− pigs (9,10). For this study, animals were crossed and the progeny studied. Standard procedures for animal husbandry were used throughout. All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Iowa and the University of Missouri. Within 6-12 h of birth, control and CF piglets underwent surgical placement of an ileostomy or cecostomy to prevent complications from meconium ileus. In the immediate perioperative period, animals were maintained on intravenous fluids, received carprofen (4 mg/kg every 12 h, IV/SC, Vericore Ltd.) for analgesia, and were treated with prophylactic antibiotics: metronidazole (22 mg/kg every 8 h, IV, SCS Pharmaceuticals), gentamicin (3 mg/kg every 24 h, IV, Hospira, Inc.), and ampicillin (10 mg/kg every 8 h, IV, Abraxis Pharmaceuticals). Thereafter, piglets were fed milk replacer and weaned to a solid diet. Animals received: a) Oral pancreatic enzyme replacement therapy (PancreVed, Vedco) with meals (4000 IU lipase/120 mL milk replacer or ~10,000 IU lipase/kg/day divided between meals). b) Oral fat-soluble vitamins (PancreVed, Vedco). c) Either an oral H2 blocker (1 mg/kg) (famotidine) or oral proton pump inhibitor (1 mg/kg) (omeprazole, Sandoz) once a day. d) Oral polyethylene glycol 3350 (Paddock Laboratories, MinneapPharmaceuticals) with each meal titrated to maintain soft stools.
Seven littermates served as controls. There were 6 CFTR+/+ and 1 CFTR+/− animals and 4 males and 3 females. These pigs underwent surgical placement of an ostomy and received similar treatments to those of the CF animals including administration of an H2 blocker or proton pump inhibitor. Their chest computed tomography scans revealed no abnormalities. They were euthanized at 55, 67, 67, 83, 83, 84, and 100 days old. Necropsy revealed normal lungs. We also observed changes in the stomach ranging from gastric erosions to moderate ulcers.
Tissue histopathology
At necropsy, all pigs were examined for gross lesions and findings documented. Cytology specimens were stained with Wright-Giemsa stain. Tissues from pigs were fixed in 10% neutral buffered formalin (48-96 h). Tissues were then routinely processed, embedded, sectioned (4 μm), and stained with hematoxylin and eosin (HE) for general examination. Additional sections were selectively stained with Periodic acid-schiff (PAS) or modified Gram’s stain (GS). All tissues were examined by a pathologist familiar with the model.
Bronchoalveolar lavage liquid collection and analysis
For bronchoalveolar lavage (BAL) in the older pigs, animals were anesthetized with ketamine (15-20 mg/kg, IM), xylazine (1-1.5 mg/kg, IM), and intravenous propofol. A flexible fiberoptic bronchoscope (Pentax FB-10X) was inserted orally and passed through the vocal cords. The suction channel was not used until the tip of the bronchoscope was past the vocal cords. The bronchoscope tip was gently inserted and wedged into the tracheal bronchus airway. Three aliquots (10 ml each) of sterile saline were instilled into the airway and lavage liquid was recovered with intermittent suction. Similar volumes of fluid were recovered from non-CF and CF animals. The BAL liquid was pooled and immediately placed on ice, transported to the laboratory for processing (cell counts and microbiology studies) and stored at -80°C for subsequent analysis.
BAL was performed in newborn pigs immediately after euthanasia and aseptic removal of the lung. A 5-ml aliquot of sterile saline was instilled into the mainstem bronchus. This was repeated 3 times. A similar amount of liquid was recovered from both non-CF and CF newborn piglets.
The total number of recovered cells in BAL liquid was quantified with a hemacytometer and morphologic differentiation of cells was performed on cytospin preparations that were stained with Diff-Quick Stain kit (Baxter). Microbiologic studies were performed on collected BAL liquid, and cytokine ELISAs were performed on BAL liquid that was centrifuged for 10 m at 1600 × g.
Microbiologic studies
Standard microbiologic techniques were utilized to identify and quantify bacteria present in BAL liquid and lung homogenate samples. Samples were serially diluted and plated onto blood agar (tryptic soy agar with sheep blood; Remel), Colombia colistin-nalidixic acid agar (Remel), Chocolate agar (Remel), Mannitol Salt Agar (Remel), MacConkey agar (Remel), and Burkholderia cepacia selective agar (Remel). Organisms were identified with standard microbiological procedures. Some identifications were confirmed by API 20E or API 20NE (bioMérieux), Vitek (bioMérieux) or 16S sequencing (University of Iowa Clinical Microbiology Laboratory and Iowa State University Diagnostic Laboratory). Quantification of colony-forming units (CFU) was performed.
Cytokine analysis
IL-8 and TNF-α were assayed with standard sandwich ELISAs (R&D Systems).
Newborn piglet bacterial challenge experiments
Intrapulmonary bacterial challenge experiments were performed on newborn piglets shortly after birth (8-10 h). Staphylococcus aureus 43 (an isolate obtained from the lungs of Case #1) was made chloramphenicol resistant to aid in bacterial recovery from the airways by introduction of plasmid pCM1. The plasmid was moved into isolate 43 by transduction with bacteriophage a80 as described previously (35). After administration of ketamine (15 mg/kg, IM) and xylazine (1.0 mg/kg, IM), S. aureus (1.9 × 105 ± 3.3 × 104 cfu in a total volume of 0.1 ml 0.45% sodium chloride) was delivered into the airways via an atomizer (MADgic laryngo-tracheal atomizer; Wolfe Tory Medical, Inc.) following passage of the atomizer tip just distal to the vocal cords. Four hours later, the animals were euthanized and the trachea and lungs were sterilely removed from the chest. The distal trachea was placed into 1 ml of PBS and vortexed for 20 s to recover bacteria. The tracheal lobe was homogenized in 5 ml of PBS. BAL was performed on the right and left lungs (5 ml × 3 times on each side). BAL liquid recovery was similar between the groups. The tracheal wash, tracheal lobe homogenate, and BAL liquid samples were serially diluted in sterile saline. Each dilution was plated onto tryptic soy agar (TSA, Remel) plates containing 20 μg/ml chloramphenicol (Sigma). Plates were incubated in the dark under aerobic conditions at 37° C for 48 h. Quantification was done by counting the number of CFU on each plate.
Measurement of nasal Vt
The transepithelial voltage (Vt) across the nasal epithelium was measured as described (36,37) under propofol anesthesia with the animal spontaneously breathing. The reference electrode was a small Ringer’s solution-filled catheter inserted into the leg muscle (25 g needle). The exploring electrode was a 6 french Foley catheter filled with Ringer’s solution (148 mM NaCl, 2.4 mM KH2PO4, 0.4 mM K2PO4, 2.25 mM CaCl2, 1.2 mM MgCl2) inserted ~ 4 cm into the nasal cavity, and the nasal mucosa was perfused at a rate of 5 ml/m using Ringer’s solution to obtain baseline Vt. Subsequent perfusion solutions included: Ringer’s solution containing 100 μM amiloride; Cl−-free Ringer’s solution containing gluconate substituted for Cl− plus amiloride; Cl−-free Ringer’s solution containing amiloride plus 10 μM isoproterenol; Cl−-free Ringer’s solution containing amiloride, isoproterenol, plus 100 μM ATP; Cl−-free Ringer’s solution containing amiloride, isoproterenol, ATP, plus 100 μM GlyH-101. Voltage was measured with a voltmeter connected to a strip chart recorder. Each solution was perfused for 3-5 m. Following completion of the measurements, the epithelium was disrupted by brushing, and Vt was measured again to determine the zero value of Vt.
CT scanning
All chest X-ray computed tomographic (CT) imaging was performed on sedated, spontaneously breathing animals with a 64-slice high-resolution CT (HRCT) scanner (SOMATOM 64, Siemens).
Microarray analysis
Trachea, bronchus, and distal lung tissue samples were dissected from newborn piglets within 12 h of birth. Samples were cut into ~5 mm3 pieces and stored in RNAlater RNA Stabilization Reagent (Ambion) using the manufacturer’s recommended protocols. Total RNA was isolated with TRIzol Reagent (Invitrogen). Only RNA samples attaining a minimum of 7.0 RNA Integrity Number on the Agilent 2100 Bioanalyzer (Agilent Technologies) were processed. 5 μg of total RNA was used to generate biotinylated cRNA using the Affymetrix Gene Chip one-cycle target labeling kit (Affymetrix, Inc.) according to the manufacturer’s recommended protocols, and then hybridized to the Affymetrix Porcine GeneChip (23,937 probe sets that interrogate approximately 23,256 transcripts from 20,201 S. scrofa genes). Each tissue hybridization (trachea, bronchus, and distal lung) was performed on a separate day, with all genotypes represented in each run. The arrays were washed, stained, and scanned with Affymetrix Model 450 Fluidics Station and Affymetrix Model 3000 scanner, and data collected using the GeneChip Operating Software (GCOS) v.1.4, using the manufacturer’s recommended protocols. The following analysis tools were used to analyze the experimental data: Gene Pattern (38) (unsupervised hierarchical clustering), Partek Genomics Suite (39) (oneway ANOVA analysis), and Gene Set Enrichment Analysis (40) (GSEA), Ingenuity Pathway Analysis (41) (IPA) and Database for Annotation, Visualization and Integrated Discovery (42) (DAVID) to aid in identifying differentially expressed genes by pathway analysis.
Statistical analysis
Data are presented as either mean ± standard error of the mean (SEM) or individual data points and median. Statistical analyses used Mann-Whitney or Chi-square tests. Statistical significance was considered P < 0.05. Prior to log transformation, 1 was added to all data values.
Supplementary Material
Fig. S1. CFTR−/ΔF508 newborn piglets have similar histopathology as CFTR−/− piglets.
Fig. S2. CFTR−/ΔF508 newborn piglets have similar nasal electrophysiology as CFTR−/− piglets.
Fig. S3. Bronchoalveolar lavage (BAL) neutrophil and IL-8 levels in non-CF and CF animals.
Fig. S4. Microbiology profile of BAL liquid obtained from non-CF and CF animals.
Fig. S5. Airway wall ulceration, obstruction, atelectasis, and pneumonia.
Fig. S6. Lungs from non-CF pigs ≥ 2 months of age.
Fig. S7. Lung microbiology in newborn CF pigs.
Table S1. Summary of CF pigs.
Table S2. Review of pulmonary lesions in human CF infants (less than 6 months) and CF pigs.
Table S3. Number of differentially expressed genes in each tissue, after the implementation of filters.
Table S4. Gene set enrichment analysis of data from CF and non-CF tissues.
Table S5. Differentially expressed genes based on a P-value cut-off <0.05 and a >2-fold change.
Table S6. Comparison of microarray data for porcine tissue with array profiles from other studies.
Aknowledgements
We thank E. Bagnall, M. Bland, P. Cooper, B. Diestelmeier, K. Dobbs, T. Fagan, N. Gansemer, M. Griffin, K. Heilmann, C. Hochstedler, M. Hudson, J. Kinyon, P. Korsakov, D. Krotz, X. Li, S. Malerich, T. Mayhew, K. Munson, C. Murphy, P. Naumann, M. Parker, M. Parsons, M. Rector, L. Reznikov, A. Rieke, J. Rodgers, P. Ruff, L. Schneider, J. Sieren, K. Singletary, T. Smith, E. Solin, M. Sturm, T. Wallen, and D. Wax for excellent assistance; K. Chaloner, D. Hornick, G. McLennan, and T. Yahr for insightful discussions; CFF Therapeutics and R. Bridges for the gift of GlyH-101. Funding: This work was supported by the NHLBI (HL51670 and HL91842) and the CFF. DAS is a Parker B. Francis Fellow and was supported by NIAID (AI076671). MJW is an HHMI Investigator.
Footnotes
Accession numbers: GSE21071
REFERENCES AND NOTES
- 1.Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic Fibrosis. In: Scriver CR, et al., editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2001. pp. 5121–5189. [Google Scholar]
- 3.Quinton P. Physiological basis of cystic fibrosis: a historical perspective. Physiol. Rev. 1999;79(Suppl. 1):S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N. Engl. J. Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 5.Guggino WB. Cystic fibrosis and the salt controversy. Cell. 1999;96(5):607–610. doi: 10.1016/s0092-8674(00)80570-x. [DOI] [PubMed] [Google Scholar]
- 6.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can’t they clear the infection? Respir. Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elston C, Geddes D. Inflammation in cystic fibrosis--when and why? Friend or foe? Semin. Respir. Crit. Care Med. 2007;28(3):286–294. doi: 10.1055/s-2007-981649. [DOI] [PubMed] [Google Scholar]
- 8.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 1999;79(Suppl. 1):S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 9.Rogers CS, et al. Production of CFTR null and ΔF508 heterozygous pigs by AAV-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Invest. 2008;118(4):1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturgess J, Imrie J. Quantitative evaluation of the development of tracheal submucosal glands in infants with cystic fibrosis and control infants. Am. J. Pathol. 1982;106:303–311. [PMC free article] [PubMed] [Google Scholar]
- 12.Oppenheimer EH. Similarity of the tracheobronchial mucous glands and epithelium in infants with and without cystic fibrosis. Hum. Pathol. 1981;12(1):36–48. doi: 10.1016/s0046-8177(81)80240-7. [DOI] [PubMed] [Google Scholar]
- 13.Khan TZ, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 14.Balough K, et al. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Ped. Pulmonol. 1995;20:63–70. doi: 10.1002/ppul.1950200203. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong D, et al. Severe viral respiratory infections in infants with cystic fibrosis. Ped. Pulmonol. 1998;26(6):371–379. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Aldallal N, et al. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;166(9):1248–1256. doi: 10.1164/rccm.200206-627OC. [DOI] [PubMed] [Google Scholar]
- 17.Perez A, et al. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292(2):L383–395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 18.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281(1):L71–78. doi: 10.1152/ajplung.2001.281.1.L71. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava M, et al. Digitoxin mimics gene therapy with CFTR and suppresses hypersecretion of IL-8 from cystic fibrosis lung epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101(20):7693–7698. doi: 10.1073/pnas.0402030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirouvanziam R, et al. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 2000;23(2):121–127. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 21.Del Pin CA, Czyrko C, Ziegler MM, Scanlin TF, Bishop HC. Management and survival of meconium ileus. A 30-year review. Ann. Surg. 1992;215(2):179–185. doi: 10.1097/00000658-199202000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trobo JI, et al. Hemorrhagic gastroesophageal ulceration by pulmonary infection in extrahepatic cholestatic pigs. J. Med. 1994;25(3-4):251–254. [PubMed] [Google Scholar]
- 23.Straw BE. Diseases of Swine. 9th ed C.H.I.P.S.; Weimar, Texas: 2006. [Google Scholar]
- 24.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease. A clinical and pathologic study. Am. J. Dis. Child. 1938;56:344–399. [Google Scholar]
- 25.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am. J. Pathol. 2010;176(3) doi: 10.2353/ajpath.2010.090849. DOI # 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razvi S, et al. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest. 2009;136(6):1554–1560. doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- 27.Harris JK, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2007;104(51):20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedrossian CWM, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum. Pathol. 1976;7:195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 29.Esterly JR, Oppenheimer EH. Observations in cystic fibrosis of the pancreas. 3. Pulmonary lesions. Johns Hopkins Med. J. 1968;122(2):94–101. [PubMed] [Google Scholar]
- 30.Armstrong DS, et al. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr. Pulmonol. 2005;40(6):500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, et al. AAV targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Invest. 2008;118(4):1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenfeld M, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Ped. Pulmonol. 2001;32(5):356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 33.Harrison F. Microbial ecology of the cystic fibrosis lung. Microbiology. 2007;153(Pt 4):917–923. doi: 10.1099/mic.0.2006/004077-0. [DOI] [PubMed] [Google Scholar]
- 34.Valenza G, et al. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 2008;7(2):123–127. doi: 10.1016/j.jcf.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 36.Zabner J, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Invest. 1996;97(6):1504–1511. doi: 10.1172/JCI118573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Standaert TA, et al. Standardized procedure for measurement of nasal potential difference: an outcome measure in multicenter cystic fibrosis clinical trials. Pediatr. Pulmonol. 2004;37(5):385–392. doi: 10.1002/ppul.10448. [DOI] [PubMed] [Google Scholar]
- 38.Gene Pattern Broad Institute Home page. http://www.broadinstitute.org/cancer/software/genepattern/.
- 39.Partek: turning data into discovery Home page. http://www.partek.com/.
- 40.Gene Set Enrichment Analsysis Home page. http://www.broadinstitute.org/gsea/.
- 41.Ingenuity Systems Home page. http://www.ingenuity.com/.
- 42.DAVID Bioinformatics Resources 6.7 Home page. http://david.abcc.ncifcrf.gov/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CFTR−/ΔF508 newborn piglets have similar histopathology as CFTR−/− piglets.
Fig. S2. CFTR−/ΔF508 newborn piglets have similar nasal electrophysiology as CFTR−/− piglets.
Fig. S3. Bronchoalveolar lavage (BAL) neutrophil and IL-8 levels in non-CF and CF animals.
Fig. S4. Microbiology profile of BAL liquid obtained from non-CF and CF animals.
Fig. S5. Airway wall ulceration, obstruction, atelectasis, and pneumonia.
Fig. S6. Lungs from non-CF pigs ≥ 2 months of age.
Fig. S7. Lung microbiology in newborn CF pigs.
Table S1. Summary of CF pigs.
Table S2. Review of pulmonary lesions in human CF infants (less than 6 months) and CF pigs.
Table S3. Number of differentially expressed genes in each tissue, after the implementation of filters.
Table S4. Gene set enrichment analysis of data from CF and non-CF tissues.
Table S5. Differentially expressed genes based on a P-value cut-off <0.05 and a >2-fold change.
Table S6. Comparison of microarray data for porcine tissue with array profiles from other studies.