Abstract
HDL and its major protein component apolipoprotein A-I (apoA-I) exert anti-inflammatory effects, inhibit monocyte chemotaxis/adhesion, and reduce vascular macrophage content in inflammatory conditions. In this study, we tested the hypothesis that the apoA-I mimetic 4F modulates the function of monocyte-derived macrophages (MDMs) by regulating the expression of key cell surface receptors on MDMs. Primary human monocytes and THP-1 cells were treated with 4F, apoA-I, or vehicle for 7 days and analyzed for expression of cell surface markers, adhesion to human endothelial cells, phagocytic function, cholesterol efflux capacity, and lipid raft organization. 4F and apoA-I treatment decreased the expression of HLA-DR, CD86, CD11b, CD11c, CD14, and Toll-like receptor-4 (TLR-4) compared with control cells, suggesting the induction of monocyte differentiation. Both treatments abolished LPS-induced mRNA for monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 (MIP-1), regulated on activation, normal T-expressed and presumably secreted (RANTES), IL-6, and TNF-α but significantly upregulated LPS-induced IL-10 expression. Moreover, 4F and apoA-I induced a 90% reduction in the expression of CD49d, a ligand for the VCAM-1 receptor, with a concurrent decrease in monocyte adhesion (55% reduction) to human endothelial cells and transendothelial migration (34 and 27% for 4F and apoA-I treatments) compared with vehicle treatment. In addition, phagocytosis of dextran-FITC beads was inhibited by 4F and apoA-I, a response associated with reduced expression of CD32. Finally, 4F and apoA-I stimulated cholesterol efflux from MDMs, leading to cholesterol depletion and disruption of lipid rafts. These data provide evidence that 4F, similar to apoA-I, induces profound functional changes in MDMs, possibly due to differentiation to an anti-inflammatory phenotype.
Keywords: high-density lipoprotein, phagocytosis, adhesion, cytokines, anti-inflammatory
monocytes, macrophages, and dendritic cells (DCs) play critical roles in regulating the response to infection by populating peripheral tissues and by acting as sensors and effectors of local tissue injury. Monocytes normally migrate into tissues at a rate that is higher than that required to replenish resident tissue macrophages (22, 43). In response to inflammatory stimuli, monocytes have two possible fates. They may either undergo differentiation to form DCs or they may become macrophages (43). DCs respond to bacterial infection by coupling innate and adaptive immune responses. Monocytes that do not commit to DC formation become macrophages (5).
Macrophages are versatile cells that play a key role in the development of inflammation and the regulation of immune responses. They are a heterogenous group of cells whose activation state is affected by their environment. Two principal phenotypes have been described, including the classically activated state M1 induced by type I cytokines (e.g., TNF) or bacterial products (e.g., LPS) and an alternative anti-inflammatory phenotype M2 induced by IL-4, IL-10, or glucocorticoid hormones (28, 31). Classically activated macrophages respond to type 1 inflammatory cytokines and bacterial products such as LPS. These molecules interact with receptors on macrophage cell surfaces and initiate a signaling cascade that leads to the transcription of inflammatory genes. Sustained activation of these responses may lead to different disease states depending on the site of action. In contrast, M2 macrophages are immunomodulatory and anti-inflammatory. They respond to a different panel of cytokines and are characterized by a phenotype that is distinct from the M1 type (28). Macrophage classes can switch from one state to another, thus ensuring that inflammatory responses, tissue injury, and wound healing are tightly regulated (42).
Macrophages can phagocytose atherogenic apolipoprotein (apo)B-containing lipoproteins to form foam cells, stimulate the production of inflammatory cytokines, and recruit other cell types in the development of complex atherosclerotic lesions (26). Their involvement in innate immune responses is crucial to host defense. These phenomena are initiated and sustained by different cell surface receptors and ligands.
HDL and its major protein component, apoA-I, exert prominent anti-inflammatory and antiatherogenic effects that contribute to cardiovascular risk reduction in humans (11). HDL has been shown to inhibit foam cell formation and prevent aortic lesions (26). A recent study (19) has shown that apoA-I plays an important role in innate immunity by modulating the properties of monocyte-derived macrophages (MDMs). Thus raising serum HDL represents an important therapeutic goal. However, obtaining sufficient quantities of the lipoprotein is impractical. Other approaches to raise HDL, including apolipoprotein-based therapies, are currently under development. ApoA-I mimetic peptides, previously designed in our laboratories, represent an emerging area of HDL therapy (2, 9). The ApoA-I mimetic 4F, whose structure is based on the helical repeating domains of apoA-I (9), dramatically inhibits atherosclerotic lesion formation in dyslipidemic mice and rabbits (33, 49). 4F mediates this response, in part, by enhancing the turnover of macrophages in the blood vessel wall (34). A similar effect of 4F on macrophage trafficking has been demonstrated in the vessel wall of influenza A-infected mice (50).
Sepsis-induced inflammatory injury is also associated with a reduction in plasma apoA-I/HDL levels (13). Clinical and basic research studies (21, 39, 53) show that increasing circulating HDL reduces complications associated with endotoxemia. Analogous to apoA-I, 4F inhibits inflammatory responses to LPS both in vitro and in vivo (17, 39). It has been proposed that 4F reduces the cytotoxic effects of endotoxin by directly binding to the lipid A component of LPS, thus preventing its interaction with Toll-like receptor-4 (TLR-4) on target cell surfaces (17). In the current study, we demonstrate that 4F, like apoA-I, alters macrophage function, possibly due to the induction of an anti-inflammatory type of macrophage.
MATERIALS AND METHODS
Cell culture reagents and antibodies.
Cell culture medium RPMI 1640 was obtained from American Tissue Culture Collection (ATCC). All other cell culture materials were obtained from Cellgro. LPS and FCS were purchased from Sigma Chemical. Ficoll (Fico/Lite LymphoH) was obtained from Atlanta Biologicals. Conjugated antibodies were obtained from BD Biochemicals. THP-1 monocytes were obtained from ATCC and maintained in RPMI medium supplemented with 10% FCS.
Peptide synthesis.
The apoA-I mimetic 4F, an 18-residue class A amphipathic helical peptide with the sequence Ac-DWFKAFYDKVAEKFKEAF-NH2, and a control peptide, Sc-4F with the same amino acids but different sequence Ac-DWFAKDYFKKAFVEEFAK-NH2, were synthesized by the solid phase peptide synthesis method (9). Peptide purity was assessed by mass spectral analysis and analytical HPLC. Peptide concentration was determined using ε280 = 7,300 M−1/cm−1. The peptide was endotoxin free as determined by the Limulus assay.
Purification of apoA-I.
As a control for 4F, human apoA-I was purified from plasma obtained from American Red Cross donors as previously described (1). Briefly, HDL was isolated from plasma by density gradient centrifugation (7). After delipidation and lyophilization of HDL, apoA-I was purified by preparative HPLC on a reverse-phase column. The purity was checked by mass spectral analysis and SDS electrophoresis. ApoA-I concentration was determined using ε280 = 31,500 M−1/cm−1. ApoA-I was endotoxin free as determined by the Limulus assay.
Monocyte cell culture.
Human peripheral blood mononuclear cells were isolated from the blood of normal volunteers (recruited with the approval of the Institutional Review Board of the University of Alabama at Birmingham) by Ficoll gradient according to standard procedures (46). Adherent MDMs were washed with fresh medium and incubated with medium alone or medium containing apoA-I or 4F. Medium (with and without 4F/apoA-I) was replenished on day 3. Different concentrations of apoA-I or 4F (up to 50 μg/106 cells) and exposure periods (up to 7 days) were included in the experimental protocol. The maximum effects on monocyte differentiation were observed at 50 μg/106 cells after 7 days. Therefore, further studies were carried out under these conditions. THP-1 monocytes were obtained from ATCC and were grown in RPMI media (ATCC) containing 10% FBS and antibiotics. They were differentiated into macrophages by the addition of PMA.
Flow cytometric analysis.
MDMs (2 × 105 cells) were incubated with optimal concentrations of antibodies to macrophage surface markers (HLA-DR, CD86, CD11b, CD11c, CD14, TLR-4, and CD49d) or control PE-, FITC-, or APC-labeled irrelevant antibodies of the same isotype for 20 min at 4°C using our standard protocols (46). After being stained, the cells were washed with 2 ml PBS, fixed with 1% paraformaldehyde, and analyzed by flow cytometry. FACS data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Gene expression measurements.
Monocytes were isolated from three independent normal blood donor buffy coats (Research Blood Components) by Ficoll gradient and adherence. They were treated with 1) vehicle; 2) 4F; 3) apoA-I; and 4) MCSF + LPS + IFNγ, which induces an M1 phenotype; or 5) MCSF + IL-4, which induces an M2 phenotype, and they were maintained in RPMI supplemented with 10% FBS for 7 days. RNA isolation and real-time PCR for CCR7, IL2RA, CXCL11, and CCL19 (upregulated in M1 type) and for CTSC, TLR-5, MRC1, and FABP4 (downregulated in M1 type; Ref. 29) were carried out to characterize the MDM phenotype obtained by treatment with 4F or apoA-I. The expression levels of three genes important for cholesterol efflux, ABCAI, ABCG1, and PPAR, were also monitored by real-time PCR using an established protocol (45).
LPS (1 μg/ml) was added to MDMs that were pretreated (7 days) with vehicle, 4F (50 μg/106 cells), apoA-I (50 μg/106 cells), or 4F plus apoA-I (50 μg/106 cells each), and mRNA levels for MCP-1 (CCL2), MIP-1 (CCL3), regulated on activation normal T-expressed and presumably secreted (RANTES; CCL5), IL-6, IL-10, and TNF-α were measured by real-time PCR (45).
Phagocytosis assay.
Phagocytic activity of day 7 MDMs was determined as described previously (47). Briefly, FITC-labeled latex microspheres (Polysciences, Warrington, PA) were added to cells (2 × 106/ml) in DMEM without serum at a ratio of 10 microspheres/cell and incubated for 30 min at 37°C in a 5% CO2 incubator. Control phagocytic activity was determined by treating cells with FITC-latex beads at 4°C. After being washed to remove free microspheres, the cells were fixed with 1% paraformaldehyde and the uptake of FITC-labeled microspheres was analyzed by FACS. Previous studies (47) showed that a shift in fluorescence intensity reflects bead uptake and not cell surface adherence.
Monocyte adhesion assay.
The ability of MDMs treated with vehicle, 4F, or apoA-I to adhere to LPS-activated human umbilical vein endothelial cell (HUVEC) monolayers was determined using the Vybrant cell adhesion assay kit (Molecular Probes; Ref. 17). Briefly, HUVECs were grown in 24-well plates in M199 medium supplemented with 10% FBS, glutamine, nonessential amino acids, and antibiotics. When cells reached 90% confluency, they were stimulated with LPS (1 μg/ml) for 6 h and washed, and the media were replaced. Concurrently, MDMs that were pretreated (7 days) with vehicle, 4F, or apoA-I were loaded with the fluorescent dye calcein acetoxymethyl ester for 30 min at 37°C and adhesion was measured as detailed by Gupta et al. (17). MDMs were washed to remove unincorporated dye and incubated with HUVECs for 60 min at 37°C. Nonadherent MDMs were removed by gentle washing, and fluorescence was measured at λ520. The fluorescence of control (vehicle treated) MDMs was designated 100%.
Transendothelial migration.
HUVECs were seeded on gelatin-coated 6.5-mm Transwell filters with a 5-μm pore size and grown to confluency in M199 medium supplemented with 10% FBS, glutamine, nonessential amino acids, and antibiotics. After 48 h, the medium and nonadherent cells were removed. Concurrently, monocytes treated with 4F, apoA-I, or vehicle for 7 days were labeled with calcein for 30 min at 37°C. Fresh RPMI medium (500 μl) supplemented with 10% FBS and antibiotics was added to the lower compartment of a fresh 24-well plate. The Transwell filters with HUVECs were transferred to the fresh plates, 105-labeled monocytes in 100 μl of RPMI buffer were added to the filters, and the plates were incubated for 2 h at 37°C. Fluorescence in the lower chamber was measured at λ520 and expressed as percentage of cells migrated.
Peptide-mediated cholesterol efflux from MDMs.
Peptide-mediated cholesterol efflux was measured in MDMs as described by Kritharides et al. (20). Briefly, MDMs grown in RPMI medium were plated at a density of 1 × 106 cells per well in a 6-well plate in RPMI containing 10% FBS, 50 μmol/l β-mercaptoethanol, and 50 ng/ml of PMA. Cells were incubated with [14C]cholesterol containing acetylated-LDL (Ac-LDL) for 24 h to load the macrophages with cholesterol. Equilibrated cholesterol enriched cells were washed with PBS and incubated with 1 ml of medium containing 4F or apoA-I (50 μg/ml). After 12 h, the medium was removed and counted. Cells were lysed and counted, and protein content was determined by the method of Lowry et al. (25). Data are expressed as percent cholesterol effluxed.
Immunocytochemical staining of rafts.
Rafts on differentiated monocytes were visualized using the Vybrant lipid raft labeling kit from Molecular Probes. Briefly, lipid rafts were labeled with Alexafluor 488-CT-B conjugate (1 μg/ml; 15 min 4°C), washed, cross-linked with anti-CT-B antibody (1:200; 15 min 4°C), and counterstained with DAPI (1:500 5 min at room temperature) to identify the nuclei. Controls included cells stained with no primary antibody or with an irrelevant antibody. Cells were analyzed by Nikon Eclipse TE-2000 U microscopy using NIS Elements Imaging Software (Nikon), and differences in raft expression between treatment groups were determined using ImageJ (Image Processing and Analysis in Java; NIH).
Statistical methods.
All results are reported as means ± SE. Statistical analysis was performed using SigmaStat 3.5 software (Systat Software). Differences between the groups were assessed by one-way ANOVA with post hoc testing (Student-Neuman-Keuls test). A P value <0.05 was considered statistically significant.
RESULTS
We previously reported that 4F inhibits both LPS-induced VCAM-1 expression in HUVECs and the binding of THP-1 monocytes to LPS-stimulated HUVECs (17). These effects were attributed to a binding interaction between 4F and LPS that resulted in the neutralization of endotoxin. In this study, we examined the direct effects of 4F on the phenotype and function of MDMs.
4F alters expression of cell surface markers.
To determine whether macrophage activation is influenced by 4F or apoA-I, we monitored the effects of these treatments on phenotypic markers on human primary monocytes and THP-1-derived monocytes by flow cytometry. In initial studies, we assessed the concentration-dependent effects of 4F and apoA-I (10–50 μg/106 cells) on MDM cell surface markers after 7 days in culture. Sc-4F (control peptide) was also included in these initial studies as a negative control. Supplemental Fig. S1 shows that 4F maximally reduced expression of HLA-DR, CD86, CD11b, and CD11c at a dose of 50 μg/106 cells (supplemental data for this article are available online at the Am J Physiol Cell Physiol website). apoA-I-treated cells showed a similar dose response (data not shown). In subsequent studies, this concentration of 4F or apoA-I was used to monitor the time dependence for down-regulation of cell surface markers. Supplemental Fig. S2 shows the effects of 4F on the expression of HLA-DR, CD86, CD11b, and CD11c by FACS analysis after 3 and 7 days. A significant reduction in the expression of cell surface markers was observed after 7 days but not at earlier time points. ApoA-I-treated cells showed a similar time dependence for downregulation of surface markers (data not shown). Therefore, further studies were carried out with MDMs treated with 50 μg/106 cells of 4F or apoA-I for 7 days. The MDM phenotype was not altered by Sc-4F treatment compared with vehicle treatment (data not shown).
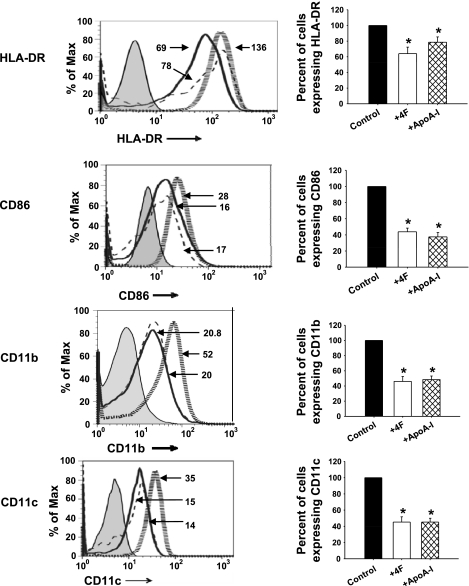
4F and apoA-I significantly reduced both the number of cells expressing HLA-DR, CD86, CD11b, and CD11c and their mean fluorescent intensity compared with vehicle-treated cells (Fig. 1). Quantitative analyses of histograms indicated that 4F and apoA-I each reduced the expression of HLA-DR by 40% and CD86, CD11b, and CD11c by 50%. These results suggest that 4F and apoA-I attenuate the expression of surface markers characteristic of activated macrophages. Similar results were also obtained with the human macrophage cell line THP-1-derived macrophages (data not shown).
Fig. 1.
4F and apoliprotein (apo)A-I alter macrophage phenotype. Primary human monocytes were treated with 4F (50 μg/106 cells), apoA-I (50 μg/106 cells), or vehicle control for 7 days. Cells were stained with antibodies to HLA-DR, CD86, CD11b, and CD11c, and expression of these surface markers was monitored by flow cytometry. Left: monocyte-derived macrophage (MDM) surface marker expression, depicted as a representative histogram for each marker under different treatment conditions. Hatched gray lines, control cells; solid black lines, cells + 4F; dashed lines, cells + apoA-I. Numbers depict the mean fluorescence intensity of the corresponding histogram. Right: changes in percentage of cells expressing each marker. Percentage of cells expressing a particular marker is calculated with respect to control cells, designated 100%. Data are means ± SE; n = 5. *P < 0.05, significant difference between control samples and treatment groups.
4F attenuates inflammatory responses of LPS.
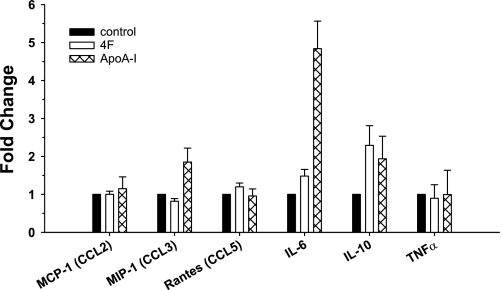
The 4F-induced changes in MDM cell surface markers suggested that the peptide may also modulate macrophage function. This is supported by measurements of mRNA levels for representative chemokines and cytokines involved in the inflammatory response in MDMs pretreated with vehicle, 4F alone (50 μg/106 cells), apoA-I alone (50 μg/106 cells), or 4F (50 μg/106 cells) plus apoA-I (50 μg/106 cells) (for 7 days). These MDMs were subsequently treated with media alone or stimulated with LPS for an additional 18 h. Treatment of monocytes with 4F for 7 days induced a twofold upregulation of IL-10 without significantly altering mRNA levels of MCP-1 (CCL2), MIP-1 (CCL3), RANTES (CCL5), and cytokines IL-6 and TNF-α. Similar to 4F, apoA-I induced increased expression of mRNA for the anti-inflammatory IL-10 (2-fold), but, unlike 4F, apoA-I also induced increased mRNA expression of IL-6 (4.5-fold; Fig. 2).
Fig. 2.
4F enhances IL-10 mRNA expression in MDMs. Primary human monocytes were pretreated with 4F (50 μg/106 cells), apoA-I (50 μg/106 cells), or vehicle for 7 days. 4F induces a 2-fold upregulation of IL-10 with no significant effect on the other genes, whereas apoA-I induces an upregulation of IL-6 (4.5-fold) and IL-10 (2-fold). Data are means ± SE; n = 5.
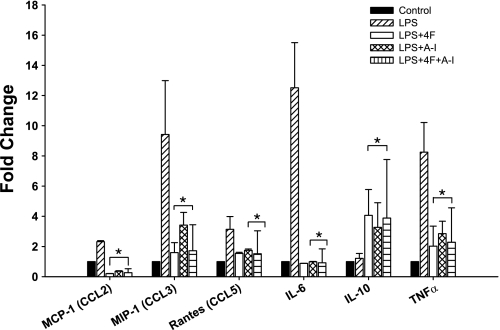
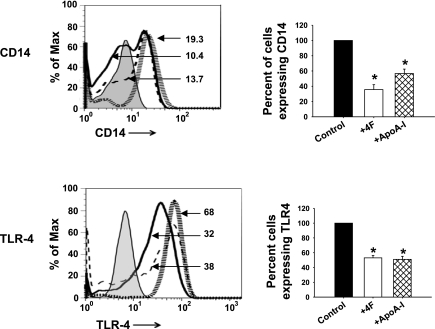
Compared with cells treated with vehicle alone, LPS stimulation of vehicle-treated MDMs induced a significant increase in mRNA levels of MCP-1 (2-fold), MIP-1 (9-fold), RANTES (3-fold), IL-6 (12-fold), and TNF-α (8-fold) but did not upregulate the anti-inflammatory cytokine IL-10 (Fig. 3). In striking contrast, compared with cells treated with vehicle plus LPS, stimulation of MDMs treated with 4F or apoA-I with LPS downregulated mRNA for the proinflammatory chemokines and cytokines MCP-1, MIP-1, RANTES, IL-6, and TNF-α and sharply upregulated (4-fold increase) IL-10 mRNA (Fig. 3). Moreover, simultaneous treatment of MDMs with 4F plus apoA-I did not further enhance these effects of 4F or apoA-I, suggesting that 4F and apoA-I likely mediate their effects via a similar mechanism of action (Fig. 3). To elucidate the mechanism of 4F and apoA-I downregulation of LPS responsiveness, we determined the effects of these treatments on CD14 and TLR-4 expression, two cell surface receptors required for LPS signaling. Figure 4 shows that both 4F and apoA-I reduced the percentage of cells expressing CD14 and TLR-4 by 50%. Similar results were obtained with THP-1-derived macrophages (data not shown).
Fig. 3.
4F abolishes LPS-induced inflammatory chemokine and cytokine mRNA upregulation and enhances IL-10 mRNA expression. Primary human monocytes were pretreated with 4F (50 μg/106 cells), apoA-I (50 μg/106 cells), or vehicle for 7 days stimulated with LPS (1.0 μg/ml) for 18 h. Treatment with 4F, apoA-I, or 4F + apoA-I significantly decreased LPS-induced monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 (MIP-1), regulated on activation normal T-expressed and presumably secreted (RANTES), IL-6, and TNF-α and enhanced IL-10 mRNA. Data are means ± SE; n = 5. *P < 0.05, significant difference between LPS-treated and 4F-, apoA-I-, or 4F + apoA-I-treated cells.
Fig. 4.
4F attenuates Toll-like receptor-4 (TLR-4) and CD14 expression. Pretreatment of MDMs with 4F (50 μg/106 cells) or apoA-I (50 μg/106 cells) for 7 days downregulates surface CD14 and TLR-4 compared with vehicle control as measured by flow cytometry. Left: MDM surface marker expression, depicted as a representative histogram for each marker under different treatment conditions. Hatched gray lines, control cells; solid black lines, cells + 4F; dashed lines, cells + apoA-I. Numbers depict the mean fluorescence intensity of the corresponding histogram. Right: changes in percentage of cells expressing the markers. Percentage of cells expressing a particular marker is calculated with respect to control cells, designated 100%. Data are means ± SE; n = 5. *P < 0.05, significant difference between control samples and treated cells.
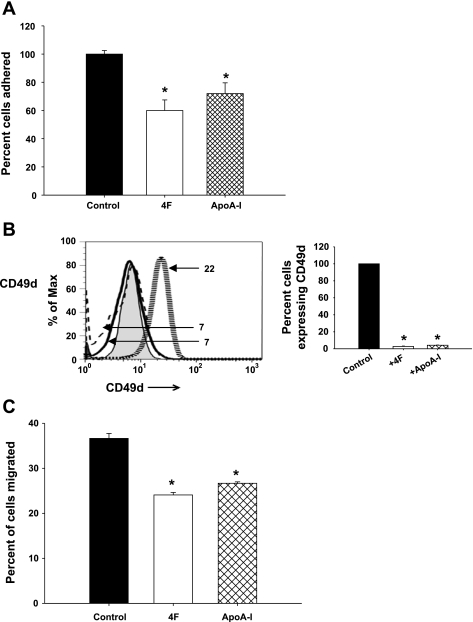
4F inhibits the adhesion of monocytes to endothelial cells.
Results of the current study suggest that 4F exerts additional anti-inflammatory effects that are independent of LPS binding and neutralization. Since 4F and apoA-I treatment altered cytokine/chemokine secretion by MDMs (Fig. 2) and the expression of cell surface markers (Fig. 1), we assessed the effects of these treatments on functional responses (adhesion and phagocytic activity) of MDMs. As seen in Fig. 5A, there was a 40% decrease in the binding of 4F-treated MDMs to HUVEC monolayers and a 35% decrease for apoA-I-treated MDMs.
Fig. 5.
4F inhibits monocyte adhesion to and transmigration through human umbilical vein endothelial cells (HUVECs). A: MDMs were treated with 4F (50 μg/106 cells), apoA-I (50 ug/106 cells), or vehicle control for 7 days and loaded with calcein and their adhesion to LPS-stimulated HUVECs was measured. Data are means ± SE. *P < 0.001, 4F and apoA-I vs. control. B: MDMs pretreated with 4F and apoA-I express decreased levels of CD49d compared with control-treated cells. Numbers depict the mean fluorescence intensity of the corresponding histogram. Percentage of cells expressing a particular marker is calculated with respect to control cells, designated 100%. Hatched gray lines, control cells; solid black lines, cells + 4F; dashed lines, cells + apoA-I. C: pretreatment of MDMs with 4F (50 μg/106 cells), apoA-I (50 ug/106 cells), or vehicle control for 7 days inhibits transendothelial migration. HUVECs were plated on Transwell filters, and calcein-loaded MDMs pretreated with vehicle, 4F, or apoA-I were added to the filters. Fluorescence of the migrated cells (measured in the lower chamber) is expressed as a percentage of cells loaded on the filter. Transendothelial migration of 4F-treated cells is 34% less and apoA-I treated cells is 27% less than controls. Data are means ± SE; n = 6. *P < 0.01, compared with controls.
Leukocyte-endothelial interactions are highly dependent on the binding of integrins to adhesion molecules. CD49d (α4-integrin) forms a heterodimer with CD29 (β1-integrin) or CD18 (β2-integrin) to form very late antigen-4, which binds to VCAM-1 and ICAM-1 on target cells (30). Since our data showed that pretreatment of MDMs with 4F reduced their adhesion to HUVECs, we tested the effects of 4F treatment on the expression of the VCAM-1 ligand CD49d. Figure 5B shows that treatment of MDMs with 4F or apoA-I for 7 days decreased CD49d expression by 95% compared with vehicle control.
4F inhibits transendothelial migration.
The impaired adhesion of 4F- and apoA-I-treated MDMs to endothelial cells, possibly due to a downregulation of CD49d, suggested that their transendothelial migration could also be reduced compared with vehicle-treated MDMs. As seen in Fig. 5C, the migration of these MDMs through HUVECs was reduced by ∼34% for 4F-treated monocytes and by 27% for apoA-I-treated monocytes. These results further support our hypothesis that 4F and apoA-I act directly on monocytes and modulate their function.
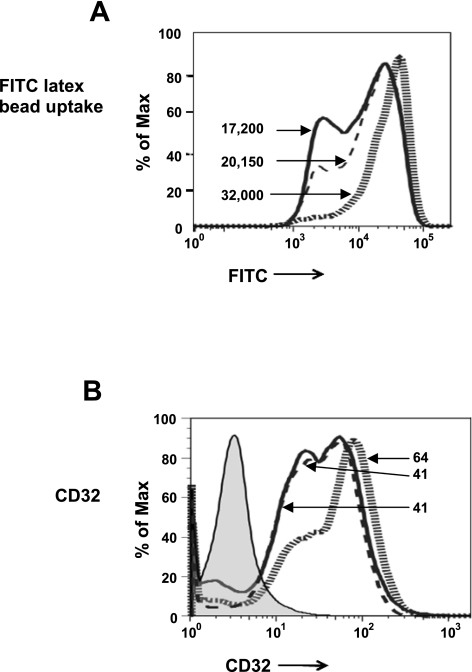
4F treatment impairs phagocytosis.
Phagocytosis of FITC-labeled latex beads by MDMs was measured by flow cytometry. MDMs treated with 4F or apoA-I exhibited reduced phagocytosis of FITC beads compared with cells treated with vehicle (Fig. 6A). While phagocytic activity was observed in MDMs from all treatment groups, the number of beads phagocytosed per cell was significantly reduced in MDMs pretreated with either 4F or apoA-I compared with vehicle treatment (Fig. 6A). The decrease in phagocytosis in 4F- or apoA-I-treated cells was associated with a reduction in the expression of CD32, an Fcγ receptor known to play a role in phagocytosis Fig. 6B; Ref. 18). Similar results were obtained with THP-1-derived macrophages (data not shown).
Fig. 6.
4F and apoA-I decrease the phagocytic activity of macrophages. Pretreatment of MDMs with 4F (50 μg/106 cells), apoA-I (50 ug/106 cells), or vehicle control for 7 days inhibits phagocytosis of FITC-labeled latex beads as indicated by reduced mean fluorescence intensity (A) and decreases the expression of CD32 (B). Numbers depict the mean fluorescence intensity of the corresponding histogram. Hatched gray lines, control cells; solid black lines, cells + 4F; dashed lines, cells + apoA-I.
4F induces the formation of an anti-inflammatory macrophage with predominantly M2 characteristics.
The above studies suggest that 4F and apoA-I induce anti-inflammatory properties in macrophage. Previous studies by Martinez et al. (29) have shown that patterns of gene expression differ between M1 and M2 macrophages. To determine if 4F or apoA-I treatment induces a particular type of macrophage phenotype, we compared the mRNA expression levels of eight genes (known to be differentially expressed in M1 and M2 macrophages) in MDMs treated with 4F or apoA-I to polarized macrophages obtained by treatment with macrophage colony-stimulating factor (MCSF) + IL-4 (control M2 type) or MCSF + LPS + IFNγ (control M1 type). As shown in Table 1, the profile of mRNA expression of MDMs treated with 4F or apoA-I more closely resembled that of control M2 macrophages than M1 macrophages in the following respects: 1) mRNA expression of CCR7, IL2RA, CXCL11, and CCL19 was significantly increased in control M1 macrophages (treated with MCSF + LPS + IFNγ) compared with untreated cells. However, these genes were not upregulated in either control M2 macrophages (treated with MCSF + IL-4) or MDMs treated with 4F or apoA-I. 2) mRNA levels of FABP4 was significantly increased in control M2 macrophages and in 4F-treated MDMs compared with control M1 macrophages. 3) mRNA levels of TLR-5 were similar in 4F- or apoA-I-treated and control M2 type macrophages, whereas this gene was significantly downregulated in control M1 macrophages. 4) However, the expression of CTSC and MRC1 in apoA-I-treated MDMs were significantly downregulated, similar to control M1 type, unlike 4F-treated and control M2 type macrophages. Noteably, 4F-treated MDMs exhibited characteristics of the M2 type macrophages for all eight genes studied here, while apo A-I-treated MDMs showed predominantly M2 phenotype but they shared some characteristics of the M1 phenotype (CTSC, MRC1, and FABP4 were downregulated as in the M1 type). To our knowledge, this is the first study to demonstrate an effect of 4F on the differentiation of MDMs.
Table 1.
Analysis of gene expression in 4F- and apoA-I-treated MDMs
| Gene | 4F | apoA-I | MCSF + IL-4 (M2 type) | MCSF + LPS (M1 type) |
|---|---|---|---|---|
| CCR7 | 0.88 ± 0.21 | 0.92 ± 0.14 | 0.71 ± 0.22 | 3.52 ± 1.6 |
| IL2RA | 0.72 ± 0.12 | 0.79 ± 0.05 | 0.69 ± 0.34 | 4.29 ± 1.5 |
| CXCL11 | 0.76 ± 0.07 | 0.72 ± 0.23 | 1.19 ± 0.31 | 62.65 ± 12.3 |
| CCL19 | 0.84 ± 0.14 | 0.74 ± 0.24 | 1.00 ± 0.17 | 33.98 ± 10.2 |
| CTSC | 0.73 ± 0.14 | 0.58 ± 0.21 | 1.23 ± 0.24 | 0.58 ± 0.24 |
| MRC1 | 0.71 ± 0.26 | 0.30 ± 0.05 | 1.09 ± 0.34 | 0.11 ± 0.06 |
| TLR-5 | 0.94 ± 0.07 | 0.69 ± 0.14 | 0.92 ± 0.16 | 0.13 ± 0.04 |
| FABP4 | 9.39 ± 1.30 | 0.26 ± 0.07 | 3.36 ± 0.74 | 0.64 ± 0.16 |
Values are means ± SE of 3 independent measurements. Values shown are a fold change of the individual genes in treated monocyte-derived macrophages (MDMs) compared with their respective control MDMs. Eight genes associated with M1 or M2 type of macrophage phenotype are listed. apoA-I, apolipoprotein A-I; MCSF, macrophage colony-stimulating factor.
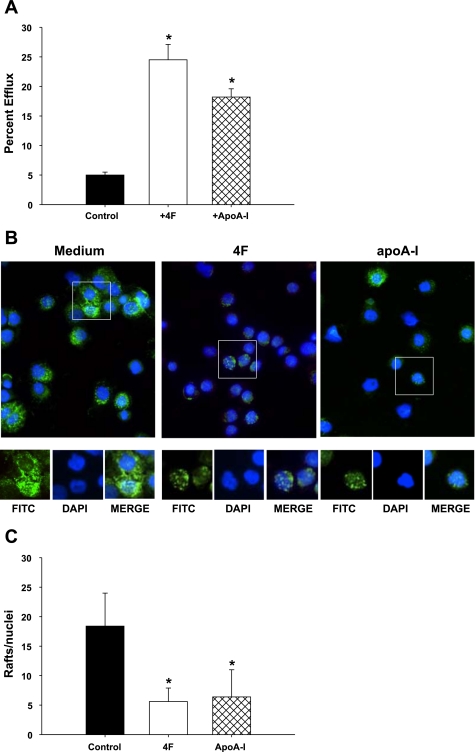
4F stimulates cholesterol efflux and reduces lipid rafts on monocytes.
The effects of 4F or apoA-I on MDM gene transcription include prominent changes in the expression of membrane-associated proteins. Rafts are cholesterol and sphingomyelin-rich microdomains of plasma membranes. Apo-AI and 4F are amphipathic molecules that mediate macrophage cholesterol efflux by a mechanism that is lipid raft dependent (14, 15). Since rafts are enriched in cholesterol, an increase in efflux activity may destabilize these structures and associated proteins including CD32 and CD14. In the current study, we found that 4F and apoA-I treatment of MDMs was associated with a reduction in the expression of CD14 and CD32 (Figs. 4 and 6B). To examine the possible mechanisms underlying the effects of 4F and apoA-I, we first monitored the effects of these treatments on cholesterol efflux from [14C]cholesterol-loaded primary human monocytes and THP-1-derived macrophages. MDMs treated with 4F and apoA-I exhibited significantly higher levels of cholesterol efflux (25 and 18%, respectively; Fig. 7A) than control cells treated with media alone (5%). Similar results were obtained with THP-1-derived macrophages. 4F and apoA-I are known to efflux cholesterol via both ABCAI and ABCG1 transporters (52). Both 4F and apoA-I upregulated genes for ABCA1 ( 2-fold), ABCG1 ( 2-fold), and PPARγ (2-fold), molecules that play an important role in cholesterol efflux.
Fig. 7.
4F stimulates cholesterol efflux and decreases membrane-associated rafts. A: primary human monocytes were loaded with [14C]cholesterol and treated with 4F + apoA-I for 18 h. Cholesterol effluxed from the cell was measured and expressed as a percentage of total cholesterol (cell + medium). Black bars, vehicle; open bars, cells + 4F; hatched bars, cells + apoA-I . Data are means ± SE; n = 6. *P < 0.001, significant difference between control samples and treated cells. B: cells were treated with medium alone, 4F (50 μg/106 cells), or apoA-I (50 ug/106 cells) for 4 days and stained for lipid rafts and nuclei using Alexa fluor 488-CT-B conjugate (FITC) and DAPI, respectively. C: number of cells expressing lipid rafts, determined by ImageJ analysis, and expressed as rafts/nuclei, was significantly reduced in cells treated with 4F and apoA-I compared with medium alone (5.6 ± 2.3, 6.4 ± 4.6, and 18.4 ± 5.6, respectively). *P < 0.008. Data represent 4 experiments.
We next determined whether 4F and apoA-I induce the loss of lipid raft expression in MDM and THP-1 cells. Cells were incubated with media alone, 4F, or apoA-I for 4 days and stained for the presence of lipid rafts. Immunocytochemical analysis showed that cells treated with 4F or apoA-I expressed equivalent levels of lipid rafts/cell (5.6 ± 2.3 and 6.4 ± 4.6, respectively) but that the markers of rafts/cell in both treated groups were significantly lower than in control cells treated with medium alone (18.4 ± 5.6; P < 0.003 and P < 0.008, respectively; Fig. 7, B and C).
DISCUSSION
Both apoA-I and the apoA-I mimetic peptide 4F possess anti-inflammatory and atheroprotective properties (17, 33, 37, 50). We (17) previously reported that 4F attenuates LPS-induced monocyte adhesion to cultured HUVECs. 4F also reduces macrophage content in fatty lesions of dyslipidemic mice (34) and inhibits macrophage trafficking in the vessel wall of mice infected with influenza A virus (50). These protective effects of 4F have been ascribed to the ability of the peptide to improve HDL function (3, 36, 38). Since monocytes/macrophages contribute to vascular injury under hyperlipidemic and septic conditions, a goal of these studies was to test whether 4F and/or apoA-I directly modulate phenotypic and functional changes in MDMs.
In the context of inflammatory vascular disease, monocytes undergo differentiation to form DCs or macrophages. In vitro treatment of monocytes with GM-CSF + IL-4 induces a DC phenotype that is characterized by upregulation of HLA-DR and CD86. This response maybe subject to regulation by HDL and/or apoA-I, since a previous report (19) shows that apoA-I inhibits the differentiation of monocytes to a DC phenotype. We have observed that 4F inhibits the GM-CSF + IL-4-induced differentiation of primary human monocytes (10). In the current study, 4F treatment, in the absence of GM-CSF + IL-4, also reduced expression of HLA-DR, CD86, CD11b, and CD11c in monocytes. A similar result was obtained with apoA-I treatment. Such changes in cell surface markers are associated with the adoption of an anti-inflammatory macrophage phenotype (16). Induction of an anti-inflammatory phenotype in 4F-treated MDMs is further supported by the prominent induction of the anti-inflammatory IL-10 and a significant decrease in inducible proinflammatory chemokines and cytokines, MCP-1, MIP-1, RANTES, IL-6, and TNF-α (29). The anti-inflammatory and atheroprotective effects of IL-10 have been demonstrated in IL-10 null mice as well as in mice that are induced to overexpress IL-10 by adenoviral transfection (41, 51). Our results show that 4F/apoA-I upregulates the anti-inflammatory cytokine IL-10 in MDMs. By this mechanism, apoA-I mimetic peptide treatment may reduce tissue injury by inhibiting the recruitment of additional immune cells to sites of inflammation.
To test the hypothesis that these changes are related to changes in the activation properties of MDMs, we measured the cell surface expression of CD14 and TLR-4, cell surface receptors for LPS. Flow cytometry studies showed that both 4F and apoA-I inhibited expression of these receptors. Our results confirm those of a previous study (39) showing that treatment of human monocytes with apoA-I, in the form of reconstituted HDL, reduced CD14 expression. However, an effect of apoA-I on TLR-4 expression was not reported in this study. IL-10 was previously shown to inhibit TLR-4 mRNA expression in human monocytes (4). Thus, it is possible that downregulation of these receptors by IL-10 may be an additional mechanism to explain the anti-inflammatory effects of 4F and apoA-I.
Increased adhesion of monocytes to the blood vessel wall plays an important role in LPS-mediated inflammation as well as in the initiation and progression of atherosclerotic lesions. CD49d (α4-integrin), the ligand for the adhesion molecule VCAM-1 (40), and CD11b and CD11c, expressed on the cell surface of monocytes and neutrophils, induce the adhesion of monocytes to endothelial cells. Downregulation of these molecules on monocytes would inhibit the adhesion of monocytes to endothelial cells, which, in turn, would reduce transendothelial migration and lesion formation. The decrease in CD49d, CD11b, and CD11c expression observed in MDMs treated with 4F or apoA-I would therefore be expected to reduce monocyte adhesion and transendothelial migration. CD49d expression in 4F-treated MDMs was reduced by almost 95%, while MDM adhesion to HUVEC monolayers was only reduced by 45–50%. This differential response is likely explained by the presence of other ligands on the MDM cell surface that participate in binding to HUVECs but may not be subject to regulation by 4F or apoA-I. In addition to this reduced adhesion of MDMs to endothelial cells, we report here that 4F also inhibited MDM transendothelial migration by 34% compared with untreated cells. Taken together, our data strongly indicate an additional role for 4F in the inhibition of MDM recruitment to sites of inflammation.
Phagocytosis plays a key role in inflammation and immunity. Phagosome formation includes, as a first step, the engulfment of foreign particles in a membrane-bound vacuole (54). Lipids (particularly sphingolipids, cholesterol, and phospholipids) play a key role in the segregation of microdomains or rafts that are involved in the recruitment of Fcγ receptors that are required for phagocytosis (54). Earlier studies from our laboratory (12) showed that 4F preferentially binds to sphingolipid-rich, cholesterol-rich domains (rafts). 4F and apoA-I are amphipathic helical molecules that bind to lipids, especially phospholipids, with high affinity to form disk-like particles. Phospholipids and cell membrane-associated cholesterol are thus extracted to form “nascent HDL-like” particles. Such depletion of cholesterol content from rafts by apoA-I has been recently demonstrated in monocytes (14, 32). In the process, depletion of cholesterol from lipid rafts may interfere with the recruitment and sequestration of Fcγ receptors to the cell membrane. The important role of cholesterol in Fcγ receptor-mediated phagocytosis has been demonstrated by Loike et al. (23), who showed that statins inhibit phagocytosis by virtue of their ability to inhibit cholesterol biosynthesis. The results of this study underscore the importance of cellular cholesterol in the regulation of Fcγ receptor-mediated phagocytosis. Our studies show an association among 4F- or apoA-I-mediated cholesterol depletion, decreased raft formation, and subsequent loss of the Fcγ receptor CD32. This may, in part, explain the observed inhibition of phagocytosis reported here. Furthermore, this also correlates well with the observed 4F- or apoA-I-induced decrease in cell surface CD14 and TLR-4. Both CD14 and TLR-4 are associated with rafts either constitutively or upon activation (15). A depletion of rafts by 4F or apoA-I could result in a loss of CD14 and TLR-4, leading to the inhibition of LPS-induced inflammatory responses.
The results of the current study provide additional insight into the mechanisms of 4F and apoA-I action by demonstrating that these treatments influence functional properties of macrophages. We show, for the first time, that 4F induces the differentiation of MDMs to an anti-inflammatory phenotype. Distinct populations of activated macrophages (31, 48) have been identified, with inflammatory mediators playing an important role in directing phenotypic differentiation. These macrophage activation states are characterized by differences in cell morphology and function. LPS and other inflammatory mediators induce classically activated M1 type macrophages to release cytokines and chemokines that induce tissue injury at sites of inflammation. M1 macrophages can be converted to alternatively activated M2 macrophages by anti-inflammatory cytokines such as IL-4 or IL-10 (31, 48). M2 macrophages play a critical role in dampening local inflammatory responses by promoting collagen synthesis and tissue repair (31). Functional responses of M1 or M2 macrophages are significantly different. These cellular subtypes may be induced by exposure to different agents and exhibit differences in their phenotype and function. Macrophage subtypes further exhibit plasticity in that they can switch from one state to another, thus ensuring that inflammatory responses, tissue injury, and wound healing are tightly regulated (42). The data presented here further suggest that 4F/apoA-I induce monocytes to differentiate to an alternatively activated macrophage with predominantly M2-characteristics (29).
Our results suggest that these responses are mediated, in part, via transcriptional upregulation of the anti-inflammatory cytokine IL-10, which is known to induce an M2 phenotype in macrophages (27). It is further proposed that anti-inflammatory effects of 4F and apoA-I action are related to their ability to mediate cholesterol removal from cells. Lipid rafts are cholesterol-enriched microdomains that play a critical role in mediating a variety of cellular responses (15). The coordinated response of monocytes/macrophages to inflammatory stimuli is thought to be due to the localization of specific proteins in these structures (24). Fcγ receptors (CD32), CD14, TLR-4, HLA-DR, CD49d, CD11b, and CD11c have been identified as components of lipid rafts either constitutively or upon stimulation (6, 15, 44). Disruption of lipid rafts in THP-1 cells has also been shown to result in an anti-inflammatory phenotype accompanied by an increase in IL-10 (8). Our data show that 4F, similar to apoA-I, depletes cell cholesterol and disrupts lipid rafts. Signaling mechanisms linking raft depletion with the induction of IL-10 are unclear at this time and are the focus of ongoing studies in the laboratory. It follows that 4F treatment was associated with a reduction in these raft-associated proteins, a 2-fold increase in IL-10, an attenuated inflammatory response to LPS, and a reduction in phagocytic activity. By this mechanism, 4F-mediated cholesterol efflux may impair the assembly of lipid raft complexes, thus attenuating proinflammatory responses of monocytes/macrophages to cell stimulation. This would also, partly, explain the lack of an additive effect of 4F and apoA-I under the experimental conditions. Disruption of the rafts by either one of these treatments would modulate the activity of the raft-associated proteins mentioned above. It is proposed that these 4F-mediated changes underlie the observed decrease in vascular macrophage content in dyslipidemic and influenza-infected mice (34, 50).
In the context of inflammation, HDL levels and function are compromised, thus limiting the protective effects of endogenous apoA-I. Previous studies (35) show that 4F increases circulating levels of HDL and improves its anti-inflammatory properties. Our data show, for the first time, that 4F and apoA-I exert vasculoprotective effects by altering the activation state of MDMs as well as reducing their ability to migrate to other sites of inflammation. The use of apoA-I as a therapeutic agent, however, is impractical, and new strategies to raise apoA-I and HDL levels are required. 4F, an apoA-I mimetic peptide, is one such candidate therapy and is currently undergoing clinical evaluation for coronary risk reduction. This study suggests that 4F also holds promise for the management of other inflammatory diseases including sepsis.
GRANTS
This work was supported by National Institutes of Health Grants GM-082952 (to G. Datta and C. R. White), DK-070040 (to C. R. White), DK-074033 and AJ-1083539 (to L. E. Smythies), HD-059142 (to A. Maheshwari) and HL-34343 (to G. M. Anantharamaiah); by American Health Association Grant AHA-0565205 (to G. Datta); by the Crohn's and Colitis Foundation of America (to L. E. Smythies); and by the Digestive Diseases Development Center Grants DK-064400 and RR-020136.
DISCLOSURES
G. M. Anantharamaiah is a principal in Bruin Pharma.
Supplementary Material
REFERENCES
- 1.Anantharamaiah GM, Hughes TA, Iqbal M, Gawish A, Neame PJ, Medley MF, Segrest JP. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res 29: 309–318, 1988 [PubMed] [Google Scholar]
- 2.Anantharamaiah GM, Jones JL, Brouillette CG, Schmidt CF, Chung BH, Hughes TA, Bhown AS, Segrest JP. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem 260: 10248–10255, 1985 [PubMed] [Google Scholar]
- 3.Anantharamaiah GM, Mishra VK, Garber DW, Datta G, Handattu SP, Palgunachari MN, Chaddha M, Navab M, Reddy ST, Segrest JP, Fogelman AM. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J Lipid Res 48: 1915–1923, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Armstrong L, Medford AR, Hunter KJ, Uppington KM, Millar AB. Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol 136: 312–319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 392: 245–252, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bouillon M, El Fakhry Y, Girouard J, Khalil H, Thibodeau J, Mourad W. Lipid raft-dependent and -independent signaling through HLA-DR molecules. J Biol Chem 278: 7099–7107, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chung BH, Segrest JP, Ray MJ, Brunzell JD, Hokanson JE, Krauss RM, Beaudrie K, Cone JT. Single vertical spin density gradient ultracentrifugation. Methods Enzymol 128: 181–209, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri J. Implications of lipid raft disintegration: enhanced anti-inflammatory macrophage phenotype. Surgery 136: 169–175, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res 42: 1096–1104, 2001 [PubMed] [Google Scholar]
- 10.Datta G, White CR, Chaddha M, Palgunachari MN, Anantharamaiah GM, Smythies LE. The apoA-I mimetic peptide, 4F, inhibits LPS-induced inflammatory reactions through dysregulation of the NFkB activation pathway (Abstract). Circulation 116: 209, 2007. [Google Scholar]
- 11.Dimayuga P, Zhu J, Oguchi S, Chyu KY, Xu XO, Yano J, Shah PK, Nilsson J, Cercek B. Reconstituted HDL containing human apolipoprotein A-1 reduces VCAM-1 expression and neointima formation following periadventitial cuff-induced carotid injury in apoE null mice. Biochem Biophys Res Commun 264: 465–468, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Epand RM, Epand RF, Sayer BG, Melacini G, Palgulachari MN, Segrest JP, Anantharamaiah GM. An apolipoprotein AI mimetic peptide: membrane interactions and the role of cholesterol. Biochemistry 43: 5073–5083, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Feingold KR, Funk JL, Moser AH, Shigenaga JK, Rapp JH, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun 63: 2041–2046, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaus K, Kritharides L, Schmitz G, Boettcher A, Drobnik W, Langmann T, Quinn CM, Death A, Dean RT, Jessup W. Apolipoprotein A-1 interaction with plasma membrane lipid rafts controls cholesterol export from macrophages. FASEB J 18: 574–576, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gaus K, Rodriguez M, Ruberu KR, Gelissen I, Sloane TM, Kritharides L, Jessup W. Domain-specific lipid distribution in macrophage plasma membranes. J Lipid Res 46: 1526–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ Res 97: 236–243, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Vargas P, Ortiz-Munoz G, Lopez-Franco O, Suzuki Y, Gallego-Delgado J, Sanjuan G, Lazaro A, Lopez-Parra V, Ortega L, Egido J, Gomez-Guerrero C. Fcgamma receptor deficiency confers protection against atherosclerosis in apolipoprotein E knockout mice. Circ Res 99: 1188–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, Paik SG, Kim YS, Yang Y, Lim JS. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun 338: 1126–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kritharides L, Christian A, Stoudt G, Morel D, Rothblat GH. Cholesterol metabolism and efflux in human THP-1 macrophages. Arterioscler Thromb Vasc Biol 18: 1589–1599, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA 90: 12040–12044, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA 101: 11779–11784, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loike JD, Shabtai DY, Neuhut R, Malitzky S, Lu E, Husemann J, Goldberg IJ, Silverstein SC. Statin inhibition of Fc receptor-mediated phagocytosis by macrophages is modulated by cell activation and cholesterol. Arterioscler Thromb Vasc Biol 24: 2051–2056, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lopez JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. J Thromb Haemost 3: 1737–1744, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 26.Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A. Macrophage diversity and polarization: in vivo veritas. Blood 108: 408–409, 2006 [Google Scholar]
- 28.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol 154: 4099–4112, 1995 [PubMed] [Google Scholar]
- 31.Mosser DM. The many faces of macrophage activation. J Leukoc Biol 73: 209–212, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 28: 2071–2077, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral administration of an Apo A-I mimetic peptide synthesized from d-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105: 290–292, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Navab M, Anantharamaiah GM, Hama S, Hough G, Reddy ST, Frank JS, Garber DW, Handattu S, Fogelman AM. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 25: 1426–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM. Oral D-4F causes formation of prebeta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 109: 3215–3220, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Buga GM, Fogelman AM. Peptide mimetics of apolipoproteins improve HDL function. J Clin Lipidol 1: 142–147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Hough G, Wagner A, Nakamura K, Garber DW, Datta G, Segrest JP, Hama S, Fogelman AM. Human apolipoprotein AI mimetic peptides for the treatment of atherosclerosis. Curr Opin Investig Drugs 4: 1100–1104, 2003 [PubMed] [Google Scholar]
- 38.Navab M, Yu R, Gharavi N, Huang W, Ezra N, Lotfizadeh A, Anantharamaiah GM, Alipour N, Van Lenten BJ, Reddy ST, Marelli D. High-density lipoprotein: antioxidant and anti-inflammatory properties. Curr Atheroscler Rep 9: 244–248, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, van der Poll T, ten Cate JW, van Deventer SJ. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med 184: 1601–1608, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira S, Zhou M, Mocsai A, Lowell C. Resting murine neutrophils express functional alpha 4 integrins that signal through Src family kinases. J Immunol 166: 4115–4123, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol 19: 2847–2853, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142: 481–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282: 480–483, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Sadra A, Cinek T, Imboden JB. Translocation of CD28 to lipid rafts and costimulation of IL-2. Proc Natl Acad Sci USA 101: 11422–11427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaik SS, Soltau TD, Chaturvedi G, Totapally B, Hagood JS, Andrews WW, Athar M, Voitenok NN, Killingsworth CR, Patel RP, Fallon MB, Maheshwari A. Low intensity shear stress increases endothelial ELR + CXC chemokine production via a focal adhesion kinase-p38β MAPK-NF-κB pathway. J Biol Chem 284: 5945–5955, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smythies LE, Maheshwari A, Clements R, Eckhoff D, Novak L, Vu HL, Mosteller-Barnum LM, Sellers M, Smith PD. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol 80: 492–499, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115: 66–75, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res 48: 2344–2353, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation 110: 3252–3258, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Von Der Thusen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/-− mice. FASEB J 15: 2730–2732, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Wool GD, Reardon CA, Getz GS. Apolipoprotein A-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J Lipid Res 49: 1268–1283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu A, Hinds CJ, Thiemermann C. High-density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock 21: 210–221, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Yeung T, Grinstein S. Lipid signaling and the modulation of surface charge during phagocytosis. Immunol Rev 219: 17–36, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.