Abstract
Of the three major protein variants produced by the UT-A gene (UT-A1, UT-A2, and UT-A3) UT-A1 is the largest. It contains UT-A3 as its NH2-terminal half and UT-A2 as its COOH-terminal half. When being part of UT-A1, UT-A3 and UT-A2 are joined by a segment, Lp, whose central part, Lc, is not part of UT-A3 or UT-A2 but is present only in UT-A1. Lc contains the phosphorylation sites S486 and S499 that are involved in protein kinase A-dependent activation, as well as the binding site for snapin, a protein involved in soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE)-mediated vesicle trafficking and fusion to the plasma membrane. We attached Lc to UT-A2 and UT-A3 to test how these phosphorylation sites influenced their urea transport activity. Adding Lc to UT-A2 conferred stimulation by cAMP to the cAMP-unresponsive UT-A2, and adding Lc to UT-A3 did not further enhance its already existing cAMP response. These findings suggest that the responsiveness to vasopressin that is observed with UT-A1 can be introduced into the unresponsive UT-A2 variant through the Lc segment that is unique to UT-A1. In UT-A3, however, the Lc segment plays no significant role in its activation by cAMP. In addition, the Lc segment also gave UT-A2 the ability to bind snapin and, in Xenopus oocytes, to be stimulated in its urea transport activity by snapin and syntaxins 3 and 4, in the same way as UT-A1.
Keywords: phosphorylation site, protein kinase A-dependent activation
the ut-a gene can give rise to several different protein variants that mediate the urea permeability in mammalian kidney cells, in particular of the descending limb of the loop of Henle and the inner medullary collecting duct (IMCD) (17). The different protein variants, each with a different physiological function, are obtained through alternative splicing, which is best understood by examining the gene's structure.
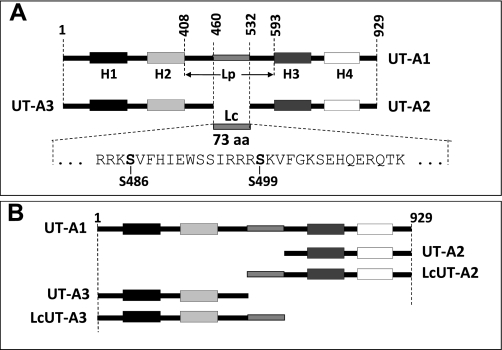
The UT-A gene is derived by the fusion of two ancestrally duplicated UT genes (1); thus it contains an internal tandem repeat of two homologous stretches, each encoding a urea-conducting channel unit, and each being preceded by an active promoter (15). This tandem arrangement gives rise to three major UT-A gene products. The first unit codes for UT-A3 and the second codes for UT-A2. UT-A1, the largest gene product with 929 amino acids (in the rat), contains UT-A3 (460aa) as its upstream half and UT-A2 (397aa) as its downstream half. When they are part of UT-A1, UT-A3 and UT-A2 are joined by a stretch that constitutes the cytoplasmic loop (Lp) of UT-A1. Lp consists of the cytoplasmic COOH-terminal domain of UT-A3 (52aa), the cytoplasmic NH2-terminal domain of UT-A2 (61aa) and a central hydrophilic linker region (Lc) (73aa). In addition to UT-A1 through UT-A3, the UT-A gene gives rise to a few additional minor splicing variants (20) whose physiological role is not known.
Arginine vasopressin (AVP, also known as antidiuretic hormone or ADH) is the key hormonal regulator of UT-A1 transport activity in vivo. Vasopressin increased transepithelial urea movements in isolated IMCD tubules (18, 24) by increasing UT-A1 phosphorylation and stimulating UT-A1 protein accumulation in the plasma membrane (9). When heterologously expressed in Madin-Darby canine kidney (MDCK) cells, UT-A1 mediated a urea permeability that was stimulated up to 20-fold over background by vasopressin and forskolin, an activator of adenylate cyclase (6, 7). In UT-A3-expressing MDCK cells, basolateral urea influx was enhanced as well (22, 23). In cRNA-injected oocytes, UT-A1 and UT-A3 also mediated cAMP-stimulated urea influx (8, 21). However, deletion of two conserved PKA consensus sites in the NH2-terminal domain of UT-A3 did not alter urea flux, suggesting that UT-A3 is phosphorylated by cAMP through a nonclassical pathway (21). UT-A2-mediated uptake was not stimulated by cAMP agonists when expressed in Xenopus oocytes (5, 19), but in UT-A2-expressing MDCK cells (16) urea flux was transiently increased by vasopressin and forskolin. However, this increase was not blocked by H89, an inhibitor of PKA (16). This contrasts with UT-A1, whose phosphorylation and flux stimulation in MDCK cells is at least partially blocked by H89 (6, 9).
The involvement of PKA in UT-A1-mediated transport activation and its sensitivity to H89 is likely due to the presence two PKA consensus sites, namely serines S486 and S499 (3). These two sites are absent in UT-A2 and UT-A3, as they are located in the Lc segment that is unique to UT-A1. The purpose of this study was to test whether adding the Lc segment to UT-A2 can confer cAMP- and PKA-dependent activation to UT-A2-mediated urea transport. In addition, we tested whether adding the Lc segment to UT-A2 would enable snapin to bind to UT-A2 in the same manner as it binds to UT-A1. The rationale for this test is our finding that snapin formed the link between intracellular vesicle-resident UT-A1 and the soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) machinery that mediates the fusion of transporter-containing vesicles with the plasma membrane (13).
Using cRNA-injected Xenopus oocytes, we found that the two PKA phosphorylation sites could indeed render UT-A2 sensitive to cAMP-mediated stimulation. We also found that fusing Lc to UT-A2 added the binding site for snapin through which the UT protein is coupled to the SNARE complex.
METHODS
Plasmid construction.
UT-A3 and UT-A2 comprise the NH2- and COOH-terminal portions, respectively, of UT-A1; they are linked by a 73 amino acid stretch (Lc, residues 460-532) that comprises the center of the cytosolic loop region. Rat UT-A1 cDNA was used as a template to amplify by PCR the desired extended UT-A2 and UT-A3 constructs from a UT-A1 cDNA template, by using primers corresponding to the appropriate boundaries of the Lc region. These constructs were (numbers according to the UT-A1 peptide sequence) the following: UT-A3 (wt, residues 1-459); LcUT-A3 (residues 1-532); rUT-A2 (wt; residues 533-929); and LcUT-A2 (residues 460-929). The UT-A3 versions also contained a COOH-terminal and the UT-A2 versions contained a NH2-terminal c-myc tag; these tags were not used for Western blot analysis in this study. Control experiments showed that they did not affect urea flux activities. The UT constructs were initially cloned into the pZero-2 cloning vector (Invitrogen), verified by sequencing, and then subcloned into the multiple cloning sites of the oocyte expression vector pGH19 and the mammalian expression vector pcDNA3. Figure 1 shows the different UT-A2 and UT-A3 constructs, the Lp and Lc regions, and their relation to UT-A1.
Fig. 1.
Schematic presentation of the UT-A protein variants UT-A2 and UT-A3 in relation to UT-A1. A: UT-A1 protein contains four hydrophobic domains H1 through H4 where the H1/H2 pair comprises UT-A3 and the H3/H4 pair comprises UT-A2. Portions of the cytoplasmic loop Lp, which connects H2 and H3 in UT-A1, are shared with either UT-A2 or UT-A3. However, its central portion, Lc, is unique to UT-A1. This 73-aa stretch contains the two PKA phosphorylation sites that are presumed to be involved in the cAMP-dependent activation of UT-A1. B: schematic presentation of the Lp- and Lc-fusion constructs of UT-A2 and UT-A3 used in this study.
Site-directed mutagenesis.
PKA consensus sites ([RK]-X-[ST]) were mutated from LcUT-A2 with the GeneTailor site-directed mutagenesis kit (Invitrogen), according to the manufacturer's instructions, by changing the serine (S) to alanine (A). To mutate these residues, the following oligonucleotides were used: S486A: 5′-ACCGTGTTCCCCAGGCGTAAGGCCGTGTTCCATA-3′; S499A: 5′-GGTCATCCATCCGGAGGAGGGCCAAAGTGTTTG-3′. A double mutant (S486A/S499A) construct was also generated. The constructs were verified by nucleotide sequence analysis.
cRNA injection and protein expression in Xenopus oocytes.
Female Xenopus laevis (Xenopus Express, Brooksville, FL) were acquired, retained, and used under protocols reviewed and approved by the Institutional Animal Care and Use Committees of Emory University. Ovary nodes were surgically removed from frogs anesthetized with MS-222 (Tricaine methane sulfonate). Oocytes (stages V-VI) were dissected by hand, and follicular layers were removed with a collagenase treatment for up to 1 h under mild agitation in a Ca-free Barth solution (in mM: 90 NaCl, 3 KCl, 0.82 MgSO4, and 5 HEPES, pH 7.6).
Linearized pGH19-based plasmids encoding UT-A1 and the different versions of UT-A2 and UT-A3 served as templates to synthesize cRNAs with T7 polymerase using the mMessage mMachine T7 Ultra kit (Ambion, Austin, TX). The cRNAs (2 ng/oocyte in 23 nl of water) were microinjected into the oocytes, obtained from two to three animals for each experimental series.Control oocytes were injected with the same volume (23 nl) of water. Injected oocytes were kept in OR3 medium at 18°C. Four days after injection, healthy oocytes were selected for urea influx measurements.
Isolation of total and plasma membranes from X. laevis oocytes.
Ten oocytes were rinsed in uptake solution (in mM: 200 mannitol, 2 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES buffer, 5 Tris·HCl, pH 7.4) and homogenized in 1 ml of the same solution supplemented with protease inhibitor cocktail (Sigma-Aldrich). Homogenates were centrifuged at 200 g for 10 min at 4°C to discard cell debris, and the supernatant was centrifuged at 15,000 g for 30 min at 4°C to pellet down total membrane. The pellets were collected and resuspended in ice-cold homogenization buffer (1% Triton X-100, 1 mM EDTA, and 150 mM NaCl, 20 mM HEPES, pH 7.4) containing protease inhibitor mixture at 4°C. SDS was added to a final concentration of 1%, and samples were sheared with a 25-gauge needle. Total protein concentration in each sample was measured by a modified Lowry assay (DC Protein Assay Kit, Bio-Rad) and adjusted to 1 μg/μl with the same homogenization buffer as for Western blot analysis.
To prepare pure plasma membranes from oocytes, we adapted a protocol from Leduc-Nadeau and coworkers (11). Accordingly, 20–40 oocytes were rinsed in MES-buffered saline solution (MBSS: 80 mM NaCl, and 20 mM MES, pH 6.0) and incubated for 15 min at room temperature (RT) in MBSS plus 0.005% subtilisin A (Sigma-Aldrich) under very mild agitation to partially digest the vitelline membrane. The oocytes were then washed three times in ice-cold MBSS. The oocyte plasma membranes were mechanically strengthened by 60-min (at 4°C) incubations with polymers, first with 1% colloidal silica (Ludox Cl; Sigma-Aldrich) and then with 0.1% polyacrylic acid (Sigma-Aldrich), with a thorough wash with MBSS between incubations. The oocytes were then transferred to an Eppendorff tube with 0.4 ml of ice-cold homogenization buffer (HB) (in mM: 5 MgCl2, 5 NaH2PO4, 1 EDTA, 80 sucrose, and 20 Tris pH 7.4). They were homogenized in this buffer with a 200-μl pipette until no particles (dark granules) were visible (15–20 pipettings). The homogenates were diluted to 1.5 ml with HB and centrifuged at 15 g for 30 s at 4°C. The supernatants were removed, leaving the bottom 75–100 μl that contain the plasma membranes, and diluted again with 1 ml of ice-cold Hb. This washing step was repeated, first at 15 g and then once each at 25 g and 35 g. Finally, the purified membranes were pelleted by centrifuging at 15,000 g for 30 min and then resuspended in 100 μl of HB. Total protein concentration in each sample was measured as above and adjusted to 1 μg/μl with the same homogenization buffer as for Western blot analysis.
Functional analysis of UT-A in X. laevis oocytes.
The methods of [14C]urea uptake into oocytes were described previously (13). Oocytes were removed from the OR3 medium and preincubated in 2 ml of uptake solution (in mM: 200 mannitol, 2 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES buffer, 5 Tris·HCl, pH 7.4) for 30 min at room temperature. The urea uptake experiment consisted of transferring the oocytes (8–10 per experimental condition) into uptake solution containing 2 μCi of [14C]urea/ml and 1 mM cold urea. They were removed at the appropriate time points and washed four times with ice-cold uptake solution containing 1 mM cold urea. Each individual cell was then dissolved in 10% SDS, followed by scintillation counting. For inhibition experiments, [14C]urea uptake was measured after a 30-min preincubation with 100 mM dimethylurea (DMU). Where not specifically indicated, urea uptake was measured over 3 min.
The effect of PKA agonists were examined by incubating the oocytes for 2 h in OR3 medium containing the membrane-permeant analog of cAMP 8-(4-chlorophenylthio)-3′,5′-cAMP (CPT-cAMP, 0.5 mM), the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (0.5 mM), and the activator of adenylate cyclase forskolin (0.05 mM). The oocytes were then transferred into uptake solution containing the same cocktail and kept there for the 30 min before the tracer uptake experiment; cocktail-containing solution was also used during the flux experiment. In control experiments (not shown) a 2-h exposure to the cocktail led to only a lightly stronger activation of urea fluxes than the 1-h exposure that had been reported elsewhere (5).
Cell culture and transfection.
Mouse IMCD3 (mIMCD3) cells were cultured in DMEMcontaining 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. They were grown to 70% confluence in six-well plates and transfected with 2 μg plasmid DNA/well. Transfection was carried out by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After a 48-h incubation, transfected cells were collected and used for immunoblotting, immunoprecipitation, or GST-pulldown assay studies.
Preparation of GST-snapin fusion protein and pulldown assays.
The full-length open reading frame encoding snapin was cloned into GST fusion vector pGEX-4T-2 and then expressed in Eschericia coli BL21-Codon plus-(DE3) (Stratagene) cells as described previously (13). GST-snapin fusion protein was immobilized on glutathione-Sepharose (Amersham Biosciences) and washed six times with binding buffer. Quantity and quality of GST-fusion protein were checked by Bradford protein assay or by SDS-PAGE analysis using Coomassie Brilliant Blue staining. UT-transfected mIMCD3 cells were washed with cold PBS containing 1 mM EDTA and then scraped into 0.5 ml of lysis/binding buffer containing 1.2% Triton X-100 and 1% protease inhibitor mixture. Cells were homogenized with a glass Teflon homogenizer (10 strokes at 900 rpm), sheared by passing the extract through a 25-gauge needle (5 times), and then centrifuged at 12,000 g for 45 min to remove insoluble material. The supernatant was resuspended in 0.5 ml of the same binding buffer (without Triton X-100) under gyration overnight and centrifuged at 14,000 g for 2 h at 4°C. Finally, the resultant supernatant containing 0.6% Triton X-100 was incubated with the immobilized GST-snapin bound resin under gyration overnight at 4°C. After extensive washing, the bound protein complexes were resolved on a 4–15% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The blots were probed with the indicated antibodies. Experiments were performed in duplicate or triplicate.
Immunodetection of UT-A proteins.
We used an antibody to an NH2-terminal peptide of UT-A1 to detect UT-A3 (2) and an antibody to a COOH-terminal peptide (10) to detect UT-A2 on Western blots.
Statistical methods.
The bar graphs of the urea fluxes show the means ± SD, obtained from at least 10 oocytes per experimental condition.
RESULTS
Expression of UTs induces urea flux in X. oocytes.
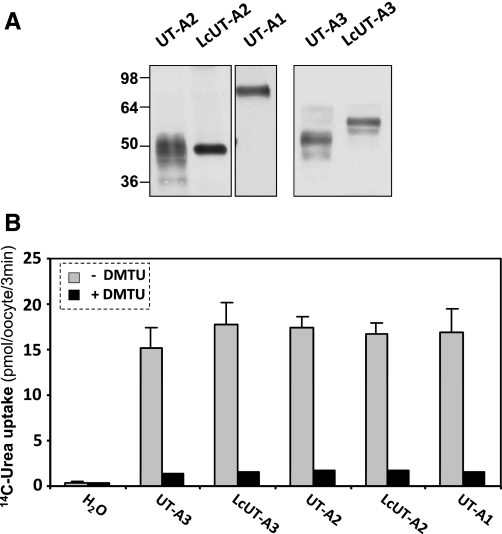
We tested all UT versions indicated in Fig. 1B for their protein expression (Fig. 2A) and their [14C]urea transport activity (Fig. 2B) in Xenopus oocytes. Basal urea flux rates and inhibition by DMU were essentially the same in UT-A1, wild-type UT-A2 and UT-A3, and the Lc constructs (LcUT-A2, and LcUT-A3).
Fig. 2.
Comparison of the urea transport activity of UT-A2 and UT-A3 with their Lc and Lp variants. A: Western blots of the proteins as expressed in oocytes. The expected molecular masses are 43.2 kDa for UT-A2, 51.6 kDa for LcUT-A2, 50.4 kDa for UT-A3, and 58.7 kDa for LcUT-A3 (see discussion for the apparent abnormal size of LcUT-A2). B: rates of [14C]urea influx into oocytes in the absence and presence of dimethylthiourea (DMTU). Values are averages ± SD obtained from 10 to 12 oocytes.
Effect of cAMP agonists on UT-A activity in X. oocytes.
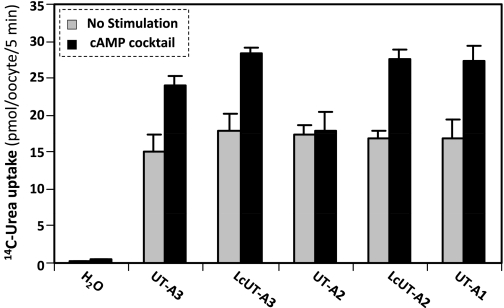
The rationale for this experimental series is to examine the role of the cytoplasmic loop in the cAMP-mediated regulation of urea transport. We have previously shown that mutating the PKA-phosphorylated S486 and S499 in the Lc segment (see Fig. 1A) of the cytoplasmic loop abolished the stimulation of UT-A1 activity by cAMP (3). As neither UT-A2 nor UT-A3 contains the Lc segment, Lc cannot contribute to any potentially observed cAMP sensitivity of these two UT-A variants. Accordingly, incubation with a cAMP-stimulating cocktail had no effect on urea uptake into oocytes expressing UT-A2 or into water-injected oocytes. In contrast, it significantly increased (by an average of 60%) urea uptake into oocytes expressing UT-A1, LcUT-A2, LcUT-A3, and UT-A3 (Fig. 3).
Fig. 3.
Stimulation of urea transport activity by cAMP. Fluxes were measured in untreated oocytes or oocytes preincubated with a cocktail consisting of 500 μM 8-(4-chlorophenylthio)-3′,5′-cAMP (CPT-cAMP), 500 μM IBMX, and 50 μM forskoliin (FSK). UT-A3, LcUT-A3, LcUT-A2, and UT-A1 were stimulated by an average of 60% (all at P < 0.01).
Lc segment confers cAMP activation to UT-A2.
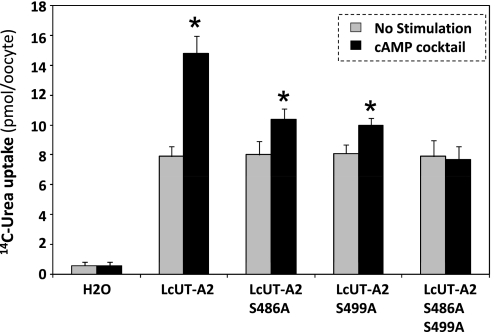
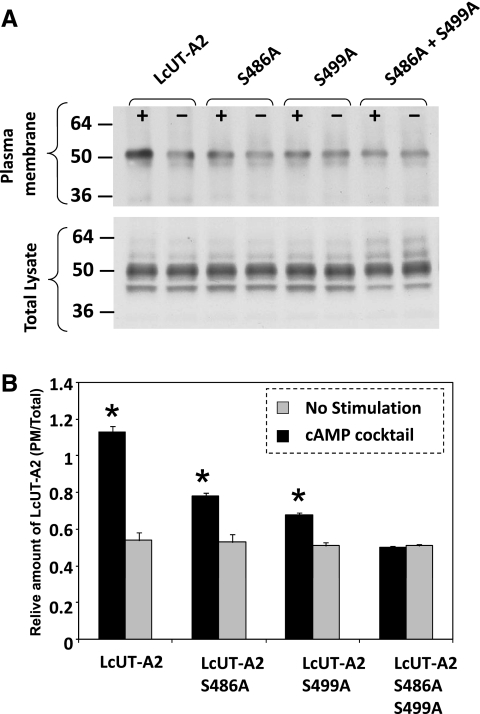
To test the function of the PKA phosphorylation sites in the Lc segment, we changed the serine residues S486 and S499 to alanine by site-directed mutagenesis. We also generated the double mutant S486A/S499A. As can be seen in Fig. 3, attaching the Lc segment to UT-A2 conferred cAMP activation to UT-A2. Furthermore, mutating the two PKA sites of LcUT-A2 in turn abolished the conferred cAMP sensitivity (Fig. 4). This Lc-mediated cAMP dependence of UT-A2 activity is due to increased levels of the protein in the plasma membrane. We demonstrated this by determining the amount of LcUT-A2 protein in highly purified plasma membranes of oocytes. As the two PKA sites on the Lc segment of LcUT-A2 are mutated away, there is a parallel decrease of Lc-UT-A2-mediated transport activity in oocytes (Fig. 4) and of the amount of LcUT-A2 in the plasma membrane (Fig. 5).
Fig. 4.
cAMP activation of urea transport activity mediated by LcUT-A2 and its mutants in which one or both PKA phosphorylation sites (S486 and S499) had been abolished by changing serine to alanine. *Statistically significant difference from no stimulation.
Fig. 5.
cAMP-dependent accumulation of Lc-UT-A2 in the oocyte plasma membrane. A: Western blots of purified plasma membrane preparations from sham-incubated (−) or cAMP-cocktail-incubated (+) oocytes. B: summary graph from three experiments. Values are averages of the ratios of the LcUT-A2 band densities from plasma membrane preparations to those from whole oocyte lysates. *Statistically significant difference from no stimulation.
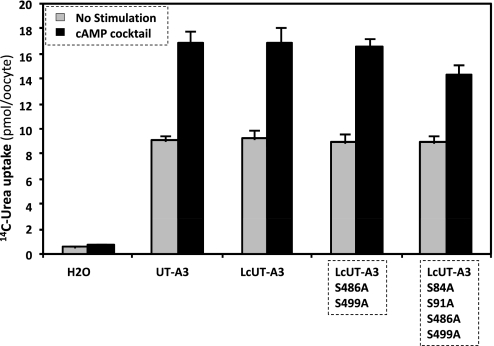
Lc segment has no effect on cAMP activation of UT-A3.
In contrast to UT-A2, UT-A3 activity is stimulated by cAMP (5, 8, 21). We confirmed this previously made observation and, when comparing LcUT-A3 with UT-A3, we found no difference between the two variants in their cAMP response (Fig. 6). Thus adding the PKA phosphorylation sites of Lc had no influence on UT-A3 activation, unlike with UT-A2. We tested this further by mutating S486 and S499 to alanine in LcUT-A3 (Fig. 6). Again, as expected from the notion that the PKA sites on Lc played no role in UT-A3 activation, this mutation did not change the cAMP response to LcUT-A3. We also mutated two additional consensus PKA phosphorylation sites on the NH2-terminal cytoplasmic domain of UT-A3 (S84A and S91A), which are analogous to the S85A and S92A mutations introduced by Smith and coworkers (21) on mouse UT-A3 and UT-A6 (a truncated variant of UT-A3) (21). In the same way as these mutations were without consequence for UT-A3 and UT-A6, they were of little consequence for LcUT-A3.
Fig. 6.
cAMP activation of urea transport activity mediated by UT-A3, LcUT-A3, and its PKA site mutants. The mutations consisted of abolishing S486 and S499 located on Lc (fourth column), plus putative PKA sites S84 and S91 on the cytoplasmic NH2 terminus of UT-A3 (fifth column).
Lc segment contains the binding site for snapin.
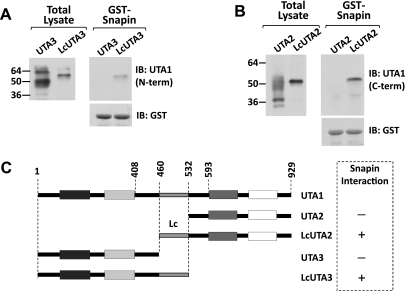
We have previously shown that the cytoplasmic loop of UT-A1 contains a binding site for snapin. Snapin controls UT-A activity by binding to UT-A1 and coupling it to the SNARE fusion machinery at the plasma membrane (13). We used UT-A2, UT-A3, and their Lc variants to determine on which segment of the cytoplasmic loop the snapin binding site is located, employing snapin-GST pulldown experiments. Wild-type UT-A2 and UT-A3 did not bind snapin. In contrast, the Lc variants were pulled down by the GST-snapin beads (Fig. 7). This demonstrates that the snapin binding site is located within this 73-residue stretch.
Fig. 7.
Snapin binding to the Lc segment. Mouse IMCD3 (mIMCD3) cells were transfected with UT-A2, UT-A3, or their Lc variants, and their cell lysates were subjected to glutathionine-S transferase (GST)-snapin pulldown experiments. A: immunoblots of total cell lysates and proteins pulled down by GST-snapin, developed for UT-A3 and its variants. GST immunoblots served as loading controls. B: immunoblots of total cell lysates and snapin-GST-pulled-down protein developed for UT-A2 and its variants. C: summary of experimental results (plus signs on right-side box) aligned with the schematic presentation of the different constructs.
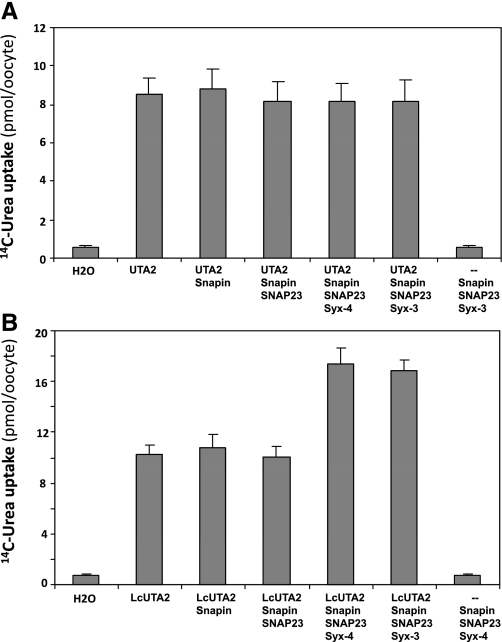
We also performed a functional test of snapin binding to the Lc segment. In our previous study we had shown that, in the presence of snapin, the t-SNARE components syntaxin-3 and syntaxin-4 enhanced UT-A1-mediated urea transport activity in oocytes (14). If the activation of UT-A1 was mediated by snapin binding to Lc, as suggested by the results of Fig. 7, snapin/syntaxin should enhance the activity of LcUT-A2 but not of UT-A2. We therefore compared the effect of snapin on urea fluxes in UT-A2- and LcUT-A2-injected oocytes, in the absence and presence of syntaxin-3 or -4. Figure 8A shows that UT-A2 activity was unaffected by co-injection with snapin and the two syntaxins. In contrast, LcUT-A2 activity was enhanced when co-expressed with snapin/syntaxin (Fig. 8B).
Fig. 8.
Effect of snapin coexpression on urea transport activity in oocytes mediated by UT-A2 and LcUT-A2. A: UT-A2 activity is not affected by co-injection with either snapin alone or any of the SNARE proteins. B: LcUT-A2 activity is enhanced by the presence of both syntaxin-3 and syntaxin-4.
DISCUSSION
The regulation of the renal UT-A1 urea transporters by the central antidiuretic hormone AVP is of key importance to the urinary concentrating mechanism and consequently overall water balance. The purpose of this study was to examine the role of UT-A1's large cytoplasmic loop in urea flux activation by the vasopressin pathway. The large cytoplasmic loop of UT-A1 (Lp) consists of three segments. The two outer (NH2- and COOH-terminal) segments are part of the UT variants UT-A3 and UT-A2, respectively, each of which are independently functioning urea-transport units. The central segment (Lc) is not shared with UT-A3 or UT-A2 and is unique to UT-A1 (see Fig. 1).
We used UT-A2 and UT-A3 as starting points for adding Lc. We were interested in the Lc segment for two reasons: 1) in a previous study we identified two cAMP-sensitive PKA phosphorylation sites (S486 and S499) in this segment of UT-A1 (3); and 2) the Lp loop is a prime candidate for binding snapin, a component of the SNARE fusion machinery through which UT-A1 is recruited from intracellular vesicles into the plasma membrane (13). By extending UT-A3 COOH terminally and UT-A2 NH2 terminally with Lc, we intended to test whether this UT-A1-specific segment conferred the same activation by cAMP and snapin as observed with UT-A1.
Adding the Lc segment to UT-A3 raised the molecular mass of LcUT-A3 by about 8.4 kDa, as expected, but not for UT-A2 (Fig. 2A). Instead, UT-A2 appeared on the Western blot as a broad band with the same molecular mass as LcUT-A2, for both rat and human UT-A2. This is unlikely due to degradation of UT-A2 protein because its molecular mass was larger than expected while LcUT-A2 exhibited the expected molecular mass. One possible explanation is that the increased size of UT-A2 is due to glycosylation.
We showed that exposure to cAMP agonists significantly increased urea flux in Xenopus oocytes expressing UT-A1 and UT-A3 but not UT-A2, which agrees with previously published reports (5, 19). We also found that adding the Lc segment conferred cAMP activation to UT-A2 and that mutating S486 and S499 in LcUT-A2 abolished this activation. This confirms our expectation that the Lc segment containing the two sites (S486 and S499) is essential for cAMP-mediated UT-A1 activation. It does not conflict with the observation that forskolin and vasopressin caused a transient increase of urea transport in UT-A2-expressing MDCK cells (16), as the authors concluded that this activation is independent of PKA activation but rather is caused by a rise in intracellular Ca levels.
Our experiments also showed that adding Lc to UT-A3 did not change the extent of activation by cAMP. Native UT-A3 does not possess the Lc segment but is still activated by cAMP in oocytes (21). We show here that UT-A3 was not further activated by adding Lc to the protein. The site on UT-A3 that is responsible for cAMP activation is not known. Smith and coworkers (21) mutated two potential PKA phosphorylation sites on UT-A3 and showed that they were not involved in PKA-mediated activation. Thus, while there appear to be two mechanisms by which LcUT-A3 could be activated, these two actions are not additive in their effects. The intrinsic activation of UT-A3 might be mechanistically related to the mechanism proposed for activation in UT-A3-expressing MDCK cells that involves casein kinase II, protein kinase C, and calmodulin (23). Nevertheless, the consideration that UT-A1 possesses all amino acid residues of UT-A3 (with the exception of its very COOH-terminal residue) and that UT-A1 activation by cAMP is completely abolished by mutating the Lc-residing S486 and S499 to alanines, it would appear that the mechanism(s) that are responsible for activating UT-A3 are of lesser importance for UT-A1 activation.
The Lc constructs that we used to test the effect of the cytoplasmic loop's PKA phosphorylation sites on UT-A2 activity also lend themselves to test the effect of the loop's snapin binding site. If, as we demonstrated with UT-A1 (13), snapin formed a link between UT-A1 and the SNARE machinery by binding to that loop, adding the entire loop to UT-A2 should enable UT-A2 to bind snapin, too. We showed here that, as expected, snapin did not recognize UT-A2 but it did bind to LcUT-A2. This probably means that in its native environment of the loop of Henle, UT-A2 does not utilize snapin but moves via a snapin-independent pathway to the plasma membrane. It also means that the snapin binding site of UT-A1 is located in the Lc segment. Because of its unique (among UT variants) snapin binding site, UT-A1 is the sole UT variant that gets connected via snapin to the AVP-regulated SNARE machinery. UT-A1 shares this feature with aquaporin AQP2, which can also bind snapin, and which, with the help of snapin, is connected to syntaxin-3 for delivery to the apical membrane (14).
We also demonstrated that the activation pattern in oocytes by coinjection with snapin/syntaxin was the same for Lc-UT-A2 (Fig. 8B) as for UT-A1 (14), whereas UT-A2 activity was unaffected by the presence of snapin/syntaxin (Fig. 8A). This provides further evidence in favor of Lc containing the functionally important snapin binding site.
The snapin binding site and the PKA phosphorylation sites are all located on the Lc segment with its 72 residues. As the precise location of the snapin binding site within Lc is not known, it is not possible to determine the distance between snapin binding and phosphorylation sites. This raises the possibility that these sites may be in close proximity to each other, and if so, that a possible relationship exists between UT-A1 phosphorylation status and snapin binding. However, this cannot be evaluated until the precise snapin binding site is determined.
It should be noted here that the nearly identical activation of UT-A2 activity in oocytes by syntaxin-3 and syntaxin-4 in the presence of snapin does not necessarily reflect the actual mechanism by which these two syntaxins influence UT-A1 trafficking in the IMCD. We have shown previously that the two syntaxins exhibited a strong differential selectivity for the aquaporins AQP2 and AQP3: the apical AQP2 was activated by syntaxin-3 and the basolateral AQP3 was activated by syntaxin-4; whereas we found no analogous syntaxin selectivity for the apical UT-A1 (14). Thus the pathway by which UT-A1 is moved into the apical membrane in response to vasopressin appears not to be identical to that for AQP2. Nevertheless, whereas the present data do not address the mechanistic question of exactly how UT-A1 is moved into the plasma membrane, they do show that one of the components, the snapin site on the Lc segment is involved and its function could be transferred to the UT-A2 protein.
If the snapin binding site of UT-A1 is an important component for directing UT-A1 to the apical SNARE machinery, its absence in UT-A3 could explain why UT-A3 is moved to the basolateral instead of the apical membrane. However, any signal that UT-A3 may possess, which guides it toward the basolateral membrane, is also shared with UT-A1. Thus one has to conclude that in their entirety, the apically directing signal elements on UT-A1 are stronger than any basolaterally directing signals.
Xenopus oocytes have been a very useful system to examine the function of transport proteins, including UT-A variants. Their plasma membrane permits proteins to be incorporated regardless of whether these are normally found in the apical or the basolateral membrane of polarized epithelial cells. In that respect, one might consider oocytes inadequate models of epithelial cells. However, whereas the unmodified oocyte membrane appears nonselective, we have been able to turn it into one selective for either apically or basolaterally sorted aquaporins (AQP2 and AQP3, respectively), by priming it with an apical or basolateral syntaxin (stx-3 or stx-4, respectively) (14). Still, the situation appears to be more complex in the case of the apically sorted UT-A1 and the basolateral UT-A3. Clearly, there are additional elements in the epithelial sorting machinery that are important for the trafficking of the UT-A variants and that are not present in the appropriate amounts in the oocyte membrane. Identifying these will be of great interest in future work.
Another potential difference between oocytes and epithelial cells could be elevated cAMP levels in oocytes compared to mammalian cells. Elevated cAMP levels in oocytes could intrinsically activate UT-A-mediated urea fluxes. However, judging from published cAMP values, there seem to be only minor differences: in oocytes, the volume-averaged cytoplasmic cAMP concentration is ∼1.0–1.5 μM (4), whereas a recent cAMP determination in neurons using a fluorescent dye yielded 1.5 μM (12).
In summary, our findings affirm the importance of the Lc segment of UT-A1 through which vasopressin regulates the UT-A1-mediated urea permeability of the IMCD. Our data demonstrate the functional importance of two classes of sites, namely two PKA phosphorylation sites and a snapin binding site, by showing that fusing the Lc segment to the Lc-lacking UT-A2 variant enabled it to exhibit the same regulatory behavior as UT-A1.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK-41707 and P01 DK-61521 and a grant from the Emory University Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Bagnasco SM. Gene structure of urea transporters. Am J Physiol Renal Physiol 284: F3–F10, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicirelli MF, Smith LD. Cyclic AMP levels during the maturation of Xenopus oocytes. Dev Biol 108: 254–258, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Fenton RA, Stewart GS, Carpenter B, Howorth A, Potter EA, Cooper GJ, Smith CP. Characterization of mouse urea transporters UT-A1 and UT-A2. Am J Physiol Renal Physiol 283: F817–F825, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol 291: C600–C606, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol 286: C1264–C1270, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol 10: 230–237, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Klein JD, Fröhlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680–2686, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Klein JD, Price SR, Bailey JL, Jacobs JD, Sands JM. Glucocorticoids mediate a decrease in AVP-regulated urea transporter in diabetic rat inner medulla. Am J Physiol Renal Physiol 273: F949–F953, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Leduc-Nadeau A, Lahjouji K, Bissonnette P, Lapointe JY, Bichet DG. Elaboration of a novel technique for purification of plasma membranes from Xenopus laevis oocytes. Am J Physiol Cell Physiol 292: C1132–C1136, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Mironov SL, Skorova E, Taschenberger G, Hartelt N, Nikolaev VO, Lohse MJ, Kugler S. Imaging cytoplasmic cAMP in mouse brainstem neurons. BMC Neurosci 10: 29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mistry AC, Mallick R, Fröhlich O, Klein JD, Rehm A, Chen G, Sands JM. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem 282: 30097–30106, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Mistry AC, Mallick R, Klein JD, Weimbs T, Sands JM, Fröhlich O. Syntaxin specificity of aquaporins in the inner medullary collecting duct. Am J Physiol Renal Physiol 297: F292–F300, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama Y, Naruse M, Karakashian A, Peng T, Sands JM, Bagnasco SM. Cloning of the rat Slc14a2 gene and genomic organization of the UT-A urea transporter. Biochim Biophys Acta 1518: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Potter EA, Stewart G, Smith CP. Urea flux across MDCK-mUT-A2 monolayers is acutely sensitive to AVP, cAMP, and [Ca2+]. Am J Physiol Renal Physiol 291: F122–F128, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sands JM. Renal urea transporters. Curr Opin Nephrol Hypertens 13: 525–532, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol Renal Fluid Electrolyte Physiol 253: F823–F832, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Shayakul C, Steel A, Hediger MA. Molecular cloning and characterization of the vasopressin-regulated urea transporter of rat kidney collecting ducts. J Clin Invest 98: 2580–2587, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CP. Mammalian urea transporters. Exp Physiol 94: 180–185, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Smith CP, Potter EA, Fenton RA, Stewart GS. Characterization of a human colonic cDNA encoding a structurally novel urea transporter, hUT-A6. Am J Physiol Cell Physiol 287: C1087–C1093, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Stewart GS, King SL, Potter EA, Smith CP. Acute regulation of mUT-A3 urea transporter expressed in a MDCK cell line. Am J Physiol Renal Physiol 292: F1157–F1163, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Stewart GS, Thistlethwaite A, Lees H, Cooper GJ, Smith C. Vasopressin regulation of the renal UT-A3 urea transporter. Am J Physiol Renal Physiol 296: F642–F648, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Wall SM, Han JS, Chou CL, Knepper MA. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 262: F989–F998, 1992. [DOI] [PubMed] [Google Scholar]