Abstract
The results presented here show that STC-1 cells, a model of intestinal endocrine cells, respond to a broad range of amino acids, including l-proline, l-serine, l-alanine, l-methionine, l-glycine, l-histidine, and α-methyl-amino-isobutyric acid (MeAIB) with a rapid increase in the intracellular Ca2+ concentration ([Ca2+]i). We sought to identify the mechanism by which amino acids induce Ca2+ signaling in these cells. Several lines of evidence suggest that amino acid transport through the Na+-coupled neutral amino acid transporter 2 (SNAT2) is a major mechanism by which amino acids induced Ca2+ signaling in STC-1 cells: 1) the amino acid efficacy profile for inducing Ca2+ signaling in STC-1 cells closely matches the amino acid specificity of SNAT2; 2) amino acid-induced Ca2+ signaling in STC-1 cells was suppressed by removing Na+ from the medium; 3) the nonmetabolized synthetic substrate of amino acid transport MeAIB produced a marked increase in [Ca2+]i; 4) transfection of small interfering RNA targeting SNAT2 produced a marked decrease in Ca2+ signaling in response to l-proline in STC-1 cells; 5) amino acid-induced increase in [Ca2+]i was associated with membrane depolarization and mediated by Ca2+ influx, since it depended on extracellular Ca2+; 6) the increase in [Ca2+]i in response to l-proline, l-alanine, or MeAIB was abrogated by either nifedipine (1–10 μM) or nitrendipine (1 μM), which block L-type voltage-sensitive Ca2+ channels. We hypothesize that the inward current of Na+ associated with the function of SNAT2 leads to membrane depolarization and activation of voltage-sensitive Ca2+ channels that mediate Ca2+ influx, thereby leading to an increase in the [Ca2+]i in enteroendocrine STC-1 cells.
Keywords: l-proline, intracellular calcium, transreceptor
the gastrointestinal (GI) tract is a sensory organ that responds to a variety of signals originating in the lumen, including nutrient and nonnutrient chemicals, microorganisms, drugs, and toxins. Detection of luminal contents by GI cells initiates a cascade of events involving hormonal and neural pathways and culminating in functional responses that ultimately regulate nutrient digestion and absorption, food intake, pancreatic insulin secretion, and metabolism. The endocrine cells of the GI tract, which produce and release more than 20 identified hormones (27), are thought to play a critical role in the integration and coordination of these physiological responses (11, 37) and in sensing nutrient and nonnutrient stimuli in the GI lumen (34, 35, 39). Despite its physiological importance, the molecular recognition events sensing the chemical composition of the luminal contents of the GI tract have remained incompletely understood.
Amino acids have been known for many years to regulate GI functions via the release of GI hormones from enteroendocrine cells (1, 43). It is increasingly recognized that amino acids are sensed in the GI tract by a variety of recently identified receptors and transporters (16, 17, 44), but the precise mechanisms involved remain incompletely understood. Previous studies indicated that enteroendocrine cells express transcripts encoding G protein-coupled receptors (GPCRs) of the type 2 taste receptor (T2R) family, α-subunits of gustducin and transducin, PLCβ2, and the cation channel transient receptor potential (TRP) M5 (TRPM5), which have been associated with chemosensory signaling (36, 46, 47). Much less is known about the expression and function in enteroendocrine cells of heterodimers of T1R1 and T1R3, thought to act as broadly tuned amino acid sensors in the mouse (24, 44). Furthermore, the extracellular Ca2+-sensing receptor [CaR; first cloned and characterized by Brown et al. (3)], an important regulator of Ca2+ homeostasis expressed in a variety of tissues, including the GI tract (13), has been implicated in sensing aromatic amino acids (9, 13, 31, 49). Indeed, previous studies demonstrated that l-phenylalanine and l-tryptophan induce a leftward shift of the concentration-response curves for agonist (extracellular Ca2+)-induced intracellular Ca2+ mobilization through the CaR (9). In addition to these sensing mechanisms through GPCR signaling (33), it is increasingly recognized that Na+-coupled amino acid transporters can also operate as amino acid sensors, e.g., by regulating the membrane potential of excitable cells, including enteroendocrine cells. Indeed, the term transreceptors has been proposed to emphasize the dual function (transporters/receptors) of these membrane proteins (16, 17).
Cultured cell lines have been used extensively to investigate basic questions related to the organization, signaling pathways, and function of intestinal cells. In this context, mouse enteroendocrine STC-1 cells have provided a model system for studying Ca2+ signaling in response to GI peptides (6, 7, 14, 25, 38), bitter agonists (8, 47), and nutrients, including amino acids (7, 18, 19, 21, 22). For example, Liddle (18) reported that l-phenylalanine stimulates a rapid increase in intracellular Ca2+ concentration ([Ca2+]i) in STC-1 cells, but the precise mechanism(s) remained undefined. Recently, another study concluded that the stimulatory effect of l-phenylalanine on [Ca2+]i in STC-1 cells is mediated by the CaR (15), but the effect of this amino acid was elicited at high concentrations, and other l-amino acids were apparently not tested.
The experiments presented here were designed to define the molecular pathway(s) by which externally applied amino acids can initiate Ca2+ signaling in STC-1 cells. We found striking stimulation of Ca2+ signaling in response to l-proline, l-serine, l-alanine, l-methionine, l-glycine, l-histidine, and α-methyl-amino-isobutyric acid (MeAIB) in STC-1 cells. Replacement of external Na+ by N-methyl-d-glucamine (NMDG) abolished amino acid-induced increase in [Ca2+]i. These characteristics closely match properties of the Na+-dependent neutral amino acid transporter 2 (SNAT2) and imply a major role for SNAT2 as an amino acid sensor in these cells. In line with this conclusion, small interfering RNA (siRNA)-mediated knock down of SNAT2 greatly decreased amino acid-induced Ca2+ signaling in STC-1 cells. Interestingly, removal of external Ca2+, or addition of an L-type voltage-sensitive Ca2+ channel (VSCC) blocker, abolished the amino acid-induced increase in [Ca2+]i. Consequently, we propose that the inward current of Na+ associated with the function of SNAT2 leads to membrane depolarization and activation of VSCC that mediate Ca2+ influx, thereby leading to an increase in the [Ca2+]i in enteroendocrine STC-1 cells.
MATERIALS AND METHODS
Cell Culture
The STC-1 cell line (a gift from D. Hanahan, University of California at San Franscisco) is derived from an intestinal endocrine tumor that developed in a double-transgenic mouse expressing the rat insulin promoter linked to the simian virus 40 large T antigen and the polyoma small T antigen (32). STC-1 and human embryonic kidney (HEK)-293 cells were grown in Dulbecco's modified Eagle's medium (Sigma, D5796), supplemented with 10% fetal bovine serum with penicillin (10 U/ml), streptomycin (10 μg/ml), and amphotericin B (25 ng/ml) and maintained in humidified incubator under 10% CO2 and 90% air at 37°C. Upon reaching 75–100% confluency, cells were subcultured by trypsinization. For experimentation, cells were subcultured onto glass slides and studied at 75–100% confluency. HEK-293 cells stably expressing human CaR (hCaR) were constructed by transfection with a plasmid coding for hCaR (50), followed by selection under continuous exposure to G 418 (0.5 mg/ml; Calbiochem). Single clones were isolated and maintained under continuous selection.
Solutions
Standard saline consisted of Hanks' buffered salt solution (HBSS; Invitrogen) without phenol red, supplemented with 0.5 mM CaCl2 and 20 mM HEPES buffer. Final concentrations (in mM) were as follows: 138 NaCl, 4 NaHCO3, 0.3 Na2HPO4, 5 KCl, 0.3 KH2PO4, 1.8 mM CaCl2, 0.5 MgCl2, 0.4 MgSO4, 5.6 d-glucose, 20 HEPES, pH 7.4. Solutions of varying Ca2+ concentrations were made starting with Ca2+-free HBSS to which the appropriate Ca2+ was added. In experiments with gadolinium, solutions were phosphate, Ca2+, and glucose free. Amino acids were made in HBSS in stock solutions of 50 or 100 mM, pH adjusted to 7.4, and used within 3 days of preparation, with the exception of l-cysteine, which was made immediately before use. In experiments using 10 mM l-proline, a stock solution of 1 M was made.
Measurement of [Ca2+]i and Membrane Potential
Cell plating.
STC-1 cells were plated onto 10-mm by 22-mm glass coverslips (fluorimetry), or 18-mm-diameter circular coverslips (single-cell imaging), which were placed inside 35-mm plastic Petri dishes filled with growth media and placed in the incubator (as described in Cell Culture).
Fura-2 loading.
Cells were removed from the incubator, washed once with HBSS, then incubated in HBSS containing 5 μM fura-2 AM for 30 min (fluorimetry) or 45 min (single-cell imaging) at 37°C. Cells were than washed with HBSS and kept at 37°C for at least 5 min before the start of the experiment.
Fluorimetry.
Coverslips containing cells were mounted in a standard 1-cm path-length cuvette filled with HBSS (37°C) using a special holder (ANO-2100, Hitachi Instruments). The cuvette was placed in a fluorimeter (F-2000, Hitachi Instruments) with a heated water jacket (37°C), and solution was continuously stirred with the aid of a small magnetic stir bar. Small volumes of concentrated test solutions were introduced into the bottom one-third of the cuvette with a Hamilton syringe. All concentrations reported are the final steady-state mixed value. Injection was completed within 1 s. Measurements of mixing kinetics showed that introduced test solutions were completely mixed (at the level of the detection window, about the middle one-third of the cuvette) within 2 s, and with no sizable overshoot. The size of the detection window allowed measurement on the order of 105 cells.
Measurement of [Ca2+]i.
Excitation was set to 340 nm and 380 nm, and emission signal collected at 380 nm, all with a 10-nm bandwidth. Samples were taken every 0.5 s using associated software (“F-2000 Intracellular Cation Measurement System”, Hitachi Instruments). At the end of each assay, digitonin was added to permeabilize the cells and cause a maximum intracellular Ca2+ signal, followed immediately by addition of EGTA to obtain a minimum signal. Using these values, and a Kd for fura-2 of 224 nM, the software converted the 340-nm and 380-nm intensities into [Ca2+]i values. In solutions with <0.5 mM Ca2+, or >5 mM Ca2+, calibrations were performed using values from sister cultures at 1.8 mM external Ca2+. Cells were washed, loaded with fura-2, and rinsed in HBSS containing the same Ca2+ concentration as the intended experimental starting value. In experiments requiring <0.5 mM Ca2+, to aid in the prevention of depletion of intracellular Ca2+ stores, STC-1 cells were washed, loaded with fura-2, and rinsed with solutions containing 0.5 mM Ca2+, and then incubated in the lower Ca2+ solution for 2 min before the start of the assay. In experiments requiring conditioning of cells with amino acid, the amino acid was added 2 min before the start of the assay.
Measurement of membrane potential.
Cells were loaded with the voltage-sensitive indicator di-8-aminonaphthylethenylpyridinium (di-8-ANEPPS; Invitrogen) at 5 μM for 20 min and washed with HBSS before being inserted into the fluorimeter. Excitation was set at 480 nm, with a bandwidth of 20 nm, and emission signals were collected at 560 and 620 nm, with a 20-nm bandwidth. Samples were taken every 0.5 s, and the ratio of the 560-nm signal to 620-nm signal was calculated at each time point. Voltage changes were monitored as percent change in ratio relative to baseline values. These changes in ratio are linear with respect to changes in voltage (2).
mRNA Amplification
To detect the expression of sequences encoding CaR (accession U20759), transient receptor potential canonical 1 (TRPC1; accession U31110), T1R3 (accession AF456324), SNAT2 (accession NM_181090), and actin (accession V01217J00691), we employed reverse transcriptase-PCR (RT-PCR) with specific oligonucleotides primers designed using MacVector v11.02 (MacVector). The cDNA sequences used to design the primers were for CaR forward primer (843–864) 5′-GAGCCCCTCACAAGGAGATTG-3′, CaR reverse primer (1448–1470) 5′-CCAGGTCACCACACTCATCAAAG-3′; TRPC1 forward primer (2235–2259) 5′-ACTCTGGTATGAAGGGTTGGAAGAC-3′, TRPC1 reverse primer (2677–2697) 5′-GAACAAAGCAAAGCAGGTGCC-3′; T1R3 forward primer (1003–1024) 5′-TCCCATTATGTGGAGACTCGCC-3′, T1R3 reverse primer (1448–1426) 5′-CCGTTGAAGGTGCCTACAGTATG-3′; SNAT2 forward primer (803–824) 5′-TTGGGACATAAGGCATACGGTC-3′, SNAT2 reverse primer (1288–1309) 5′-GGGATGGCAGACAAAGGAAAAC-3′; and actin forward primer (134–153) 5′-TGGGTATGGGTCAGAAGGAC-3′, actin reverse primer (618–636) 5′-AATGTCACGCACGATTTCC-3′. RT-PCR was performed on 5 μg of total RNA extracted from STC-1 cells using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized at 45°C by using the designed CaR, TRPC1, T1R3, SNAT2, and actin antisense oligonucleotides described above and ThermoScript Reverse Transcriptase (Invitrogen) under the conditions suggested by the manufacturer. A fraction of the obtained cDNAs was amplified by PCR using Platinum Taq DNA Polymerase High Fidelity (Invitrogen), as suggested by the manufacturer, and the sense and antisense primers specific for CaR, TRPC1, T1R3, SNAT2, and actin indicated above. As controls, in a set of reactions, the sense primers for CaR, TRPC1, T1R3, SNAT2, and actin were not added to the obtained cDNAs during the PCR amplification step. The products of PCR were resolved in 2.0% NuSieveGTG Agarose (FMC BioProducts)-1× TBE buffer (100 mM Tris, 90 mM boric acid, 1 mM EDTA, pH 8.4). The gel was stained for 60 min with ethidium bromide (0.5 μg/ml) in 1× TBE, follow with two 15-min washes with distilled water. The gel was viewed and images captured using a luminescent image analyzer LAS-4000 mini (Fujifilm Life Sciences). The predicted sizes of the RT-PCR products for CaR, TRPC1, T1R3, SNAT2, and actin are 627, 462, 423, 506, and 502 bp, respectively.
Transfection of siRNA Into STC-1 Cells
siRNA directed against taste receptor subunit T1R3, was purchased from Dharmacon (TAS1R3), and transfected at 100 nM into STC-1 cells using Lipofectamine 2000 (Invitrogen) with the reverse transfection protocol (as described by Invitrogen). Cells were used 2 and 3 days after transfection. siRNA against SNAT2 was purchased from Applied Biosystems (Ambion, Slc38a2, rat) and transfected at 100 nM into STC-1 cells using the reverse transfection protocol. Cells were used 3 and 4 days after transfection.
HEK-293 Cell Culture, Transfections, and Indirect Immunofluorescence
Semiconfluent HEK-293 cells grown (see Cell Culture above) in 35-mm plastic dishes were transiently transfected with 1 μg of a cDNA encoding the hCaR using Lipofectamine Plus (Invitrogen), as previously described (50). Immunofluorescence analysis of the cells transiently transfected was performed 18 h posttransfection.
Indirect immunofluorescence was performed in 10% buffered formalin phosphate fixed cells that were permeabilized with 0.3% Triton X-100-PBS during 7 min at 25°C to detect surface and intracellular CaR expression (total). Parallel cultures fixed under the same conditions were not permeabilized to detect the presence of the CaR at the cell surface. After extensive PBS washing, the fixed cells were incubated for 2 h at 25°C in blocking buffer (PBS-3% BSA) with (total) or without (surface) 0.05% Tween 20. Subsequently, the cells were stained at 25°C for 4 h with a rabbit antibody raised against a peptide corresponding to amino acid residues 12–27 in the extracellular domain of the CaR (Affinity Bioreagents) and diluted in PBS-3% BSA. The cells were then extensively washed with PBS-0.05% with (total) or without (surface) 0.05% Tween 20 and stained at 25°C for 60 min with Alexa Fluor 488-conjugated chicken-anti-rabbit (Invitrogen) diluted in PBS-3% BSA and washed again with PBS-with (total) or without (surface) 0.05% Tween 20. Finally the samples were mounted with a gelvatol-glycerol solution containing 2.5% 1,4-diazobicyclo-[2.2.2]octane (29). The samples were examined and images captured using a LSM 510 Meta confocal microscope (Carl Zeiss, Germany). The selected cells displayed in the appropriate figures were representative of 80% of the population of positive cells.
Data Expression
Data are expressed by means ± SE. Statistical significance was examined by Student's t-test. A P value of <0.05 was considered statistically significant.
RESULTS
Role of the CaR in the Stimulation of Ca2+ Signaling Induced by l-Phenylalanine in STC-1 Cells
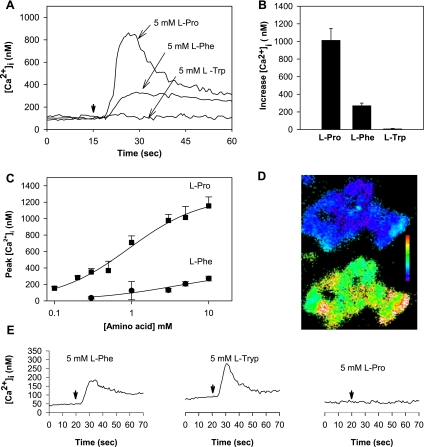
STC-1 cells loaded with the fluorescent Ca2+ indicator fura-2 AM were stimulated with 5 mM l-phenylalanine, and the changes in [Ca2+]i were continuously recorded. The baseline level of [Ca2+]i in these cells was 131.7 ± 4.3 nM (n = 110). As shown in Fig. 1A, addition of l-phenylalanine induced an approximately twofold increase in [Ca2+]i after a short (3-s) delay, in agreement with previous results. Since l-tryptophan has been shown to be as potent as l-phenylalanine in inducing Ca2+ signaling through the hCaR (9, 49), we also tested the effect of this amino acid on [Ca2+]i in STC-1 cells. Surprisingly, we found that addition of 5 mM l-tryptophan failed to produce any detectable increase in [Ca2+]i in STC-1 cells (Fig. 1A). Similarly, addition of the potent CaR agonist Gd3+ to STC-1 cells, at concentrations as large as 100 μM, did not induce any change in [Ca2+]i in these cells (results not shown). In striking contrast, l-proline, an amino acid that has been reported to be much less potent than aromatic amino acids in stimulating Ca2+ signaling via the hCaR, induced a striking increase in [Ca2+]i in STC-1 cells, followed by a slower recovery toward baseline (Fig. 1A). The superiority of l-proline over l-phenylalanine and l-tryptophan (all at 5 mM) in increasing [Ca2+]i was confirmed in multiple, independent experiments (Fig. 1B). The mean values obtained were as follows: l-proline 1,014.2 ± 134.0 nM (n = 13); l-phenylalanine 207.2 ± 26.2 nM (n = 4), and l-tryptophan 8.0 ± 4.9 nM (n = 5). We substantiated that l-proline is strikingly more effective than l-phenylalanine in increasing peak [Ca2+]i over a wide concentration range (Fig. 1C). Digital fluorescence image analysis to measure [Ca2+]i in single cells revealed that most (96.4%, n = 110) STC-1 cells in the population exhibited a rapid and transient increase in [Ca2+]i in response to 5 mM l-proline (Fig. 1D). A smaller proportion of cells responded to 5 mM l-phenylalanine (47.2%, n = 110).
Fig. 1.
A: addition of l-proline (Pro) or l-phenylalanine (Phe) induced an increase in intracellular Ca2+ concentration ([Ca2+]i) in STC-1 cells. Three separate experiments with l-proline, l-phenylalanine, or l-tryptophan (Trp) are overlaid. At the time marked by the downward pointing arrow, amino acid is injected into the cuvette (final concentration 5 mM, injection time + mixing time < 3 s). The concentration of Ca2+ in HBSS was 1.8 mM. B: increase in [Ca2+]i (defined as the difference between the peak [Ca2+]i of the transient response and the baseline value) after addition of l-proline (n = 13), l-phenylalanine (n = 4), or l-tryptophan (n = 5). C: dose-response curves of peak [Ca2+]i as a function of l-proline or l-phenylalanine concentration in STC-1 cells incubated in HBSS containing 1.8 mM Ca2+. D: single-cell imaging shows the majority of STC-1 cells respond to 5 mM l-proline. Top: cluster of 20–30 STC-1 cells at rest. Cells are pseudocolored to show varying levels of [Ca2+]i. Scale bar shows colors change from blue to green to red to white with increasing [Ca2+]i. Bottom: 30 s after addition of 5 mM l-proline to the HBSS bathing the cells. Virtually all cells showed increased [Ca2+]i. E: response of human embryonic kidney (HEK)-293 cells expressing human Ca2+-sensing receptor (CaR) to the same amino acids (i.e., l-proline, l-phenylalanine, or l-tryptophan), as applied to the STC-1 cells in A. Amino acids were added to the cuvette at the times marked by the downward arrow.
To compare the efficacy of l-phenylalanine, l-tryptophan, and l-proline in promoting Ca2+ signaling through the CaR under the experimental conditions used in this study with STC-1 cells, we tested the effect of these amino acids (all at 5 mM) on Ca2+ signaling in HEK-293 cells stably expressing the hCaR (50). Typical tracings, illustrated in Fig. 1E, show that l-phenylalanine and l-tryptophan stimulated elevations in [Ca2+]i that were much larger than the small response induced by l-proline in parallel cultures of these cells. These results are in stark contrast to those obtained in STC-1 cells, where l-proline produced the largest Ca2+ response, l-phenylalanine induced a much reduced response, and l-tryptophan failed to elicit any significant Ca2+ signaling.
We considered the possibility that the different l-amino sensitivity of mouse STC-1 cells compared with HEK-293 cells expressing the hCaR could be due to a difference in the specificity of the mouse CaR (mCaR), which, although highly analogous to the human receptor, could have different sensitivities to l-amino acids. To test this possibility, we cloned the mCaR and created a stable line of HEK-293 cells expressing the mCaR. The l-amino acid profile of the mCaR in HEK-293 cells was l-phenylalanine > l-tryptophan > l-proline, a profile that was mirrored by the hCaR (results not shown).
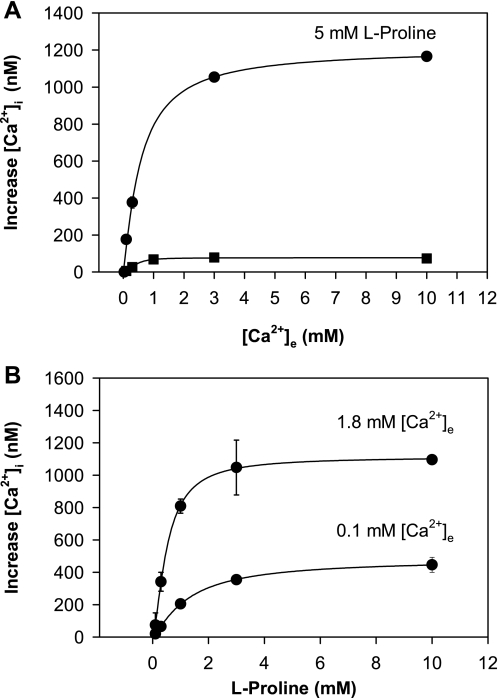
Effect of External Ca2+ Concentration on Amino Acid-Induced Ca2+ Signaling
Previous studies demonstrated that l-phenylalanine induces a leftward shift of the concentration-response curves for agonist (extracellular Ca2+)-induced intracellular Ca2+ mobilization through the CaR (9). Specifically, in the presence of this amino acid, the EC50 of extracellular Ca2+ for inducing intracellular Ca2+ signaling was shifted from 4.2 to 2.2 mM, but the maximal [Ca2+]i response, achieved at 10 mM extracellular Ca2+, was not affected (9). We also determined the effect of increasing concentrations of extracellular Ca2+ on intracellular Ca2+ signaling in STC-1 cells in the absence or presence of 5 mM l-proline. The results, illustrated in Fig. 2A, show peak [Ca2+]i as a function of extracellular Ca2+ concentration. In the absence of amino acid, an increase in the extracellular Ca2+ concentration up to 10 mM produced only a small increase in [Ca2+]i. In the presence of 5 mM l-proline, the peak [Ca2+]i was strikingly increased with only a small change in the EC50 values for extracellular Ca2+. Similar results were obtained when l-serine was added instead of l-proline (results not shown). These results in STC-1 cells are in sharp contrast to the behavior of the hCaR (9), since the addition of l-proline to STC-1 cells dramatically increased the peak [Ca2+]i in response to extracellular Ca2+ at concentrations as high as 10 mM.
Fig. 2.
A: effect of extracellular Ca2+ concentration ([Ca2+]e) on increase of [Ca2+]i in STC-1 cells in the absence or in the presence of l-proline. Each data point represents a separate assay, where the cells were placed into a cuvette in Ca2+-free HBSS, and then Ca2+ was added to reach the final concentration shown in the abscissa. Increase in [Ca2+]i is plotted against final [Ca2+]e. Top trace was obtained from 2 experiments, where Ca2+-containing solution was added in the presence of 5 mM l-proline. The bottom trace, also from 2 experiments, was in the absence of l-proline. B: effect of increasing concentrations of l-proline on [Ca2+]i in the presence of [Ca2+]e at either 0.1 or 1.8 mM. The top trace is derived from 2 separate experiments with [Ca2+]e of 1.8 mM. The bottom curve is derived from 3 separate experiments with [Ca2+]e at 0.1 mM.
Many studies demonstrated that l-amino acid-induced Ca2+ signaling in response to aromatic amino acids by the CaR requires a threshold external Ca2+ at a concentration ≥1 mM (9, 49). Indeed, amino acid addition with external Ca2+ concentration <1.0 mM produces negligible response via the CaR. We examined the effect of increasing concentrations of l-proline on [Ca2+]i in STC-1 cells incubated for 2 min in HBSS containing 0.1 mM or 1.8 mM Ca2+ before the addition of the amino acid. As shown in Fig. 2B, stimulation of STC-1 cells with l-proline induced an increase in [Ca2+]i in STC-1 cells exposed to either 0.1 mM or 1.8 mM Ca2+ in the medium. Addition of l-proline in the presence of 0.1 mM external Ca2+ induced a reduced, but measurable, [Ca2+]i response in a concentration-dependent manner. These results provided further support to the notion that STC-1 cells sense amino acids through a pathway(s) that appears not to depend on CaR function.
Expression of CaR Immunoreactivity at the Surface of STC-1 Cells
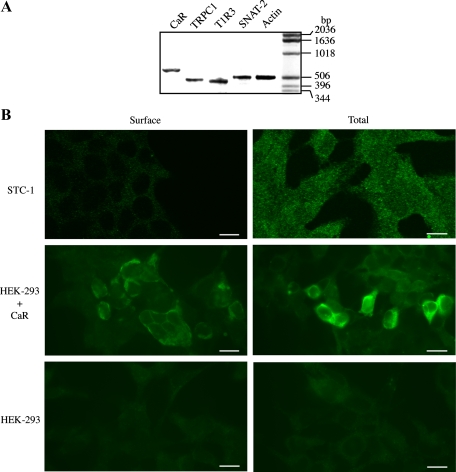
Although the functional studies presented above did not support the notion that the CaR plays a central role in amino acid sensing in STC-1 cells, these cells express transcripts encoding CaR and TRPC1, a downstream element in CaR signaling in response to aromatic amino acids (30), as shown by RT-PCR (Fig. 3A) and in agreement with the recent report of Hira et. al. (15). It is recognized that appropriate GPCR localization on the cell surface is required for access to ligands and signal transduction effectors (42). Consequently, we considered the possibility that the CaR protein might not be efficiently localized to the plasma membrane of STC-1 cells, as it occurs in certain CaR mutants that are retained intracellularly (26, 45). To test this possibility, we stained permeabilized and nonpermeabilized STC-1 cells with an antibody directed to an epitope located in the extracellular domain of the CaR. As shown in Fig. 3B, most of the CaR immunoreactivity in STC-1 cells was in intracellular compartments, since they could be detected only after permeabilization of STC-1 cells. In contrast, using an identical approach, we could readily detect CaR immunoreactivity at the surface of HEK-293 cells, shown for comparison (Fig. 3B). Thus our results indicating that CaR is not detected at the cell surface of STC-1 cells are in line with the functional studies presented above and suggest that the CaR does not appear to play a major role in mediating amino acid sensing by enteroendocrine STC-1 cells.
Fig. 3.
A: expression of CaR, transient receptor potential canonical 1 (TRPC1), T1R3 (taste receptor), and Na+-coupled neutral amino acid transporter 2 (SNAT2) in STC-1 cells. RT-PCR was performed using specific primers for each of the mRNAs encoding CaR, TRPC1, T1R3, SNAT2, and actin (see materials and methods) on 5 μg of total RNA isolated from STC-1 cells. PCR products were resolved on 2% agarose, and the gel was then stained with ethidium bromide. Products of the predicted size for the CaR (627 bp), TRPC1 (462 bp), T1R3 (423 bp), SNAT2 (506 bp), and actin (502 bp) were detected only when both antisense (A) and sense (S) primers were included during the second cDNA strand synthesis reactions. The results are representative of two independent experiments. B: intracellular distribution of the CaR in STC-1 cells. Fixed STC-1 cells, either permeabilized (total) or not (surface) using 0.3% Triton X-100, were incubated with an antibody that recognizes the NH2-terminus of the CaR extracellular domain. After extensive washes, the samples were incubated with Alexa Fluor 488-conjugated anti-rabbit and examined with a confocal microscope, as described under materials and methods. As controls, HEK-293 cells transfected (HEK-293 + CaR) or not (HEK-293) with a plasmid encoding the CaR were prepared for immunocytochemistry, as described for STC-1 cells, to detect total and surface CaR expression. The selected cells displayed in the figure were representative of 90% of the population of positive cells. Scale bars, 10 μm.
Multiple l-Amino Acids Stimulate an Increase in [Ca2+]i in STC-1 Cells
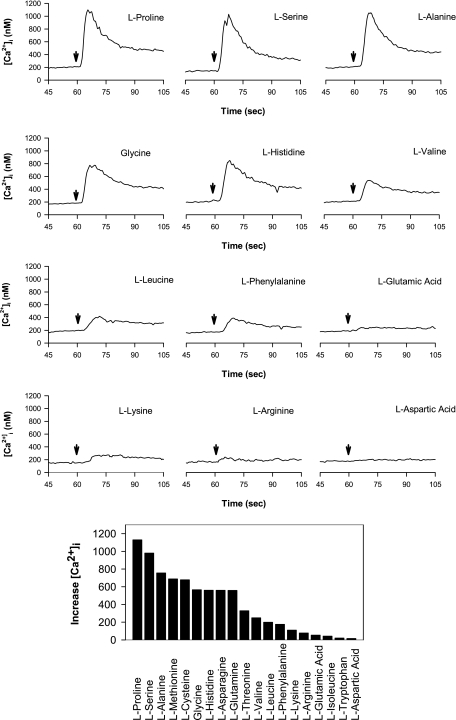
The preceding results prompted us to define the complete profile of amino acid sensing by STC-1 cells. Consequently, we examined the efficacy of 19 different l-amino acids (all at 5 mM) in increasing [Ca2+]i in STC-1 cells. As shown in Fig. 4, STC-1 cells responded to the addition of l-proline, l-serine, l-alanine, l-glycine, and l-histidine with a rapid and transient increase in [Ca2+]I, followed by a slower return to baseline. Peak [Ca2+]i values were much reduced in response to l-valine, l-leucine, and l-phenylalanine and acidic (l-glutamic and l-aspartic acid), basic (l-lysine, l-arginine), or branched (l-isoleucine) amino acids and elicited small or not detectable Ca2+ signaling in STC-1 cells.
Fig. 4.
Top: effect of addition of different l-amino acids on [Ca2+]i in STC-1 cells. Time of addition is marked by downward arrow. In each case, the final amino acid concentration was 5 mM, and the [Ca2+]e was 1.8 mM. Bottom: average increase in [Ca2+]i in response to 19 different amino acids, each at 5 mM. Responses from 2 separate slides for each amino acid were averaged together.
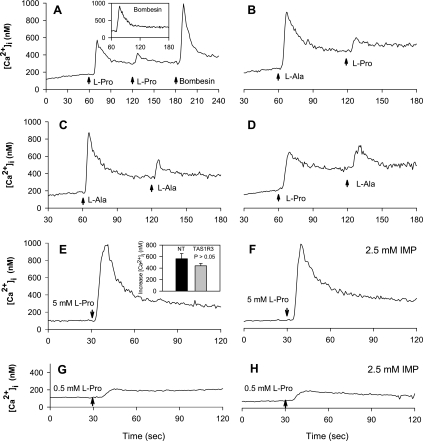
As a first step to determine whether amino acid-induced Ca2+ signaling in STC-1 cells is mediated by a single pathway, we examined the effect of sequential addition of amino acids in STC-1 cells (Fig. 5, A–D). In agreement with preceding results, addition of 1 mM l-proline induced a rapid and transient increase in [Ca2+]I, followed by a slower return to baseline. When the first l-proline addition was followed after 60 s by another pulse of 1 mM l-proline (Fig. 5A), the second [Ca2+]i response was greatly diminished (∼70% inhibition). In contrast, Ca2+ signaling in response to the subsequent addition of 10 nM bombesin was not reduced (compare to inset), indicating that sequential addition of amino acids did not interfere with Ca2+ signaling induced via a Ca2+-mobilizing GPCR agonist. It is known that bombesin, at the concentration used in Fig. 5, produces an initial fast rising transient due to mobilization of Ca2+ from internal stores, followed by a sustained increase in [Ca2+]I, resulting from entry of external Ca2+ (33), and neither of these two processes was affected by amino acid stimulation. More importantly, addition of l-proline greatly reduced the Ca2+ response induced by subsequent addition of l-alanine (Fig. 5D), and reciprocally, prior addition of l-alanine greatly diminished Ca2+ signaling in response to l-proline (∼75% inhibition, Fig. 5B). Similar results were obtained in experiments in which l-proline and l-phenylalanine or l-alanine and l-phenylalanine were added sequentially to STC-1 cells (data not shown). Taken together, these results are consistent with the notion that l-amino acids, including l-proline, l-alanine, and l-phenylalanine, act through a single pathway or site to induce [Ca2+]i responses in STC-1 cells.
Fig. 5.
A–D: amino acids inhibit the increase in [Ca2+]i induced by the subsequent addition of the same or a different amino acid. The amino acids were added (time of addition marked by arrows) at 1 mM, and the [Ca2+]e was 1.8 mM. A: addition of 1 mM of l-proline inhibits the increase in [Ca2+]i induced by a second addition of 1 mM l-proline. In contrast, the Ca2+-mobilizing effect of 10 nM bombesin added to the culture after the second stimulation with l-proline was not impaired, indicating that the pools of intracellular Ca2+ were not depleted by consecutive exposure to l-proline. Inset shows the response to the addition of 10 nM bombesin in a parallel culture without prior exposure to amino acid. B, C, and D show that addition of 1 mM l-alanine inhibits the increase in [Ca2+]i produced by the subsequent addition of either l-proline (B) or l-alanine (C), and, similarly, that l-proline inhibits the increase in [Ca2+]i induced by subsequent addition of 1 mM l-alanine (D). E and F: the response of STC-1 cells to l-proline is not potentiated by previous exposure to 2.5 mM inosine 5′-monophosphate (IMP). E: response to 5 mM l-proline added to control solution. Inset: cells transfected with small interfering RNA (siRNA) targeting TAS1R3 did not change the response to l-proline. Cells were challenged with 5 mM l-proline 48 h after transfection with either siRNA targeting TAS1R3, or nontargeting (NT) siRNA. F: pretreatment of cells with HBSS containing 2.5 mM IMP (2 min) did not change the response to 5 mM l-proline. G and H: response to lower concentration (0.5 mM) of l-proline is similarly unaffected by prior exposure to 2.5 mM IMP.
Role of T1R1/T1R3 in amino acid sensing by STC-1 cells.
The l-amino acid taste receptor expressed in taste buds of the lingual epithelium is a heterodimer comprised by T1R1 and T1R3 subunits that responds to a variety of l-amino acids with increases in [Ca2+]i (24). We verified that STC-1 cells express transcripts encoding T1R3 mRNA, as shown by RT-PCR (Fig. 3A). However, the T1R1/T1R3 receptor is responsive to both acidic and basic amino acids (44), both of which were not effective in stimulating Ca2+ signaling in STC-1 cells. These results were not consistent with a major contribution of the T1R1/T1R3 receptor in mediating amino acid sensing in STC-1 cells. Furthermore, Nelson et. al. (24) reported that the increase in [Ca2+]i induced via agonist stimulation of the T1R1/T1R3 receptor is markedly potentiated by the addition of inosine 5′-monophosphate (IMP). As shown in Fig. 5, F and H, addition of IMP did not enhance the Ca2+ responses elicited by either high (5 mM) or low (0.5 mM) concentrations of l-proline in STC-1 cells. In addition, transfection with siRNA targeting TAS1R3 did not significantly change the [Ca2+]i response of STC-1 cells to 5 mM l-proline, compared with cells transfected with nontargeting siRNA (Fig. 5E, inset). Collectively, these results imply that the taste receptor T1R1/T1R3 does not play a major role in amino acid sensing in STC-1 cells.
Role of the SNAT2 Amino Acid Transporter as Mediator of Amino Acid-induced Signaling in STC-1 Cells
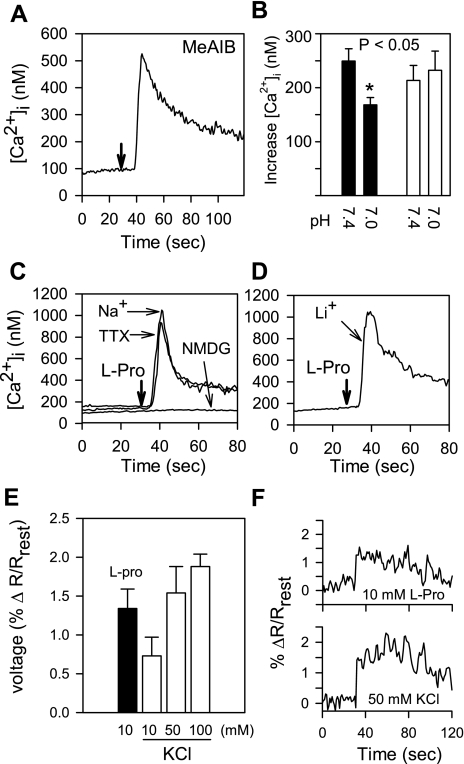
The profile of amino acid-induced Ca2+ signaling in STC-1 cells shown in Fig. 4 closely matches the transport specificity of SNAT2, a widely distributed member of the SNAT of the SLC38 gene family (20). In contrast to other members of the SLC38 family, SNAT2 has considerable preference for l-proline, one of the most effective amino acids in promoting Ca2+ signaling in STC-1 cells. Consequently, we hypothesize that the inward current of Na+ associated with the function of this transporter leads to membrane depolarization and activation of VSCCs that mediate Ca2+ influx, thereby leading to an increase in [Ca2+]i in enteroendocrine STC-1 cells. To test this hypothesis, we examined whether amino acid-induced Ca2+ signaling in STC-1 cells exhibits specific properties displayed by SNAT2, including recognition of N-methylated amino acids, dependence on Na+ in the medium, tolerance of Li+ substitution for Na+, and sensitivity to inhibition by low extracellular pH. An additional important prediction of this hypothesis is that amino acid-induced Ca2+ signaling in STC-1 cells should be prevented by specific inhibitors of L-type VSCCs.
SNAT2-mediated plasma membrane transport of metabolizable amino acids can have an impact on cellular metabolism. To dissociate transport from its possible metabolic consequences, we determined whether the nonmetabolized synthetic substrate MeAIB, which is selectively transported into the cells by SNAT1 and SNAT2, also promotes Ca2+ signaling in STC-1 cells. As shown in Fig. 6A, addition of MeAIB induced a striking increase in [Ca2+]i in STC-1 cells.
Fig. 6.
A: STC-1 cells respond to 10 mM α-methyl-amino-isobutyric acid (MeAIB) with an increase in [Ca2+]i. In this and subsequent panels, injection times are marked by downward arrows. Resting external Ca2+ was 1.8 mM, pH 7.4. B: lowering pH from 7.4 to 7.0 reduces the response of STC-1 cells to MeAIB (solid bars), but did not alter Ca2+ signaling in response to bombesin (open bars). The addition of 10 mM MeAIB to STC-1 cells was followed after 120 s with 5 nM bombesin. C: the response of STC-1 cells to 5 mM l-proline required the presence of Na+ in the medium. When Na+ was substituted by NMDG, l-proline-induced Ca2+ signaling was abolished. The response to 5 mM l-proline in Na+-containing saline was not blocked by tetrodotoxin (TTX; 1 μM). D: STC-1 cells responded to l-proline when Na+ was replaced by Li+ in the medium. E: addition of 10 mM l-proline to STC-1 cells produces membrane depolarization, as indicated by the voltage-sensitive dye di-8-aminonaphthylethenylpyridinium (di-8-ANEPPS). Depolarization is measured as percent change (Δ) in the ratio of emissions at 560 nm and 620 nm (R) over resting levels (Rrest). Solid bar, l-proline; open bars, KCl at different concentrations. F: individual traces showing responses to 10 mM l-proline and 50 mM KCl.
Previous studies showed that the activity of SNAT2 is reduced by ∼40% when the pH of the external medium is lowered from 7.4 to 7.0 (40, 48). As shown in Fig. 6B, we found that the increase in [Ca2+]i induced by MeAIB in STC-1 cells was reduced 32.5% by lowering the pH of the external medium from pH 7.4 to pH 7.0 (249.5 ± 22.8 nM, n = 5, vs. 168.5 ± 13.5 nM, n = 4; P < 0.05). For comparison, we verified that an identical reduction in the pH of the medium did not change the [Ca2+]i increase induced by 5 nM bombesin (208.8 ± 27.8 nM, n = 4, vs. 232.1 ± 35.8 nM, n = 4). These results show that MeAIB, a substrate of SNAT2 (20), induces Ca2+ signaling in STC-1 cells, in line with the hypothesis implicating this amino acid transporter in mediating the Ca2+ response in these cells.
An important property of the SNATs is their dependence on extracellular Na+ for amino acid transport (20). To determine whether amino acid-induced Ca2+ signaling in STC-1 cells also depends on extracellular Na+, we used impermeant NMDG as a replacement for NaCl. As illustrated in Fig. 6C, when external NaCl was substituted by NMDG, Ca2+ signaling in STC-1 cells in response to 5 mM l-proline was abolished. The requirement of Na+ is not dependent on tetrodotoxin (TTX)-sensitive voltage-gated Na+ channels, since l-proline-induced Ca2+ signaling was not affected by treatment of STC-1 cells with the specific Na+ channel blocker TTX (1 μM) added to cells incubated in Na+-containing medium. In contrast, when NaCl was replaced by LiCl in the medium, addition of l-proline remained effective in increasing [Ca2+]i in STC-1 cells (Fig. 6D). These results are consistent with the notion that the increase in [Ca2+]i induced by l-proline is highly dependent on entry of either Na+ or Li+ via SNAT2.
To examine whether the inward current of Na+ associated with the function of this transporter leads to membrane depolarization, STC-1 cells were loaded with the voltage-sensitive dye di-8-ANEPPS and then challenged with 10 mM l-proline. The dye reports changes in fluorescence ratio, indicating depolarization (Fig. 6, E and F). The average percent change in ratio (560 nm/620 nm) was 1.34 ± 0.23% (n = 4). This compares to an average percent change in ratio from depolarization induced by 100 mM KCl of 1.88 ± 0.16% (n = 3), that from 50 mM KCl depolarization of 1.54 ± 0.34% (n = 3), and that of 10 mM KCl depolarization of 0.73 ± 0.24% (n = 3). Our laboratory's previous results showed that addition of KCl at 10–25 mM to STC-1 cells produced a striking increase in [Ca2+]i (8).
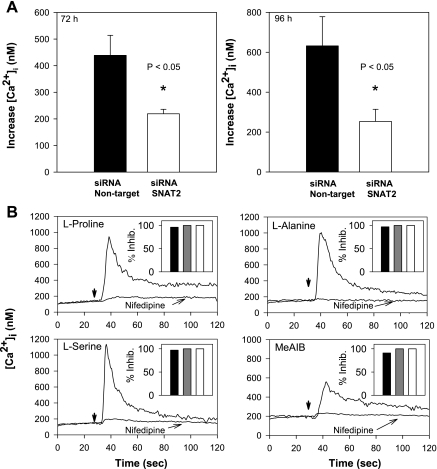
To substantiate the functional studies implicating SNAT2 in amino acid-induced increases in [Ca2+]i, we examined the effect of siRNA-mediated SNAT2 knockdown on Ca2+ signaling in response to l-proline. Cultures of STC-1 cells were transfected with siRNA targeting SNAT2 or with nontargeted siRNA. After 72 or 96 h, cells were challenged with 1 mM l-proline (Fig. 7A). At 72 h, cells transfected with siRNA to SNAT2 showed a decrease in peak [Ca2+]i in response to l-proline of 50%, compared with cells receiving nontargeted siRNA (from 438.8 ± 75.4 to 219.2 ± 16.9 nM, P < 0.05). After 96 h of transfection, there was a 67% decrease in peak [Ca2+]i in response to 1 mM l-proline in cells transfected with siRNA targeting SNAT2 compared with the nontargeted controls (from 632.0 ± 146.6 to 235.5 ± 61.0 nM, P < 0.05). Similar results were obtained when the cells were stimulated with 5 mM l-proline instead of 1 mM l-proline. These results support the notion that Ca2+ signaling in response to l-proline is mediated by SNAT2-mediated Na+-coupled amino acid transport in STC-1.
Fig. 7.
A: transfection of STC-1 cells with siRNA targeting SNAT2 for either 72 h (left) or 96 h (right) significantly reduced Ca2+ signaling in response to 1 mM l-proline, compared with cells transfected with NT siRNA. B: responses of STC-1 cells to different amino acids (added at the times marked by the downward arrows) or MeAIB are blocked by the L-type voltage-sensitive Ca2+ channel blockers, either nifedipine or nitrendipine. Traces show responses in STC-1 cells incubated in the absence or in the presence of nifedipine at 1 μM. Nifedipine or nitrendipine was added to the cells 2–5 min before the addition of amino acids at 5 mM, or MeAIB at 10 mM. Two separate experiments are superimposed in each panel. For clarity, starting [Ca2+]i were translated to align. Insets show percent inhibition of the responses to nifedipine at 1 μM (solid bars), nifedipine at 10 μM (shaded bars), and nitrendipine at 1 μM (open bars).
Role of L-type VSCC in Mediating Amino Acid-Induced Ca2+ Signaling in STC-1 Cells
L-type VSCCs mediate influx of extracellular Ca2+ into neuronal and neuroendocrine cells in response to membrane depolarization (5, 10). Using RT-PCR, our laboratory previously verified that STC-1 cells express the pore-forming α1-subunit isoforms Cav1.2 and Cav1.3 of L-type VSCCs (8). In addition, we demonstrated that the addition of depolarizing concentrations of KCl (5–25 mM) to STC-1 cells induced a robust spike in [Ca2+]i via L-type VSCC (8). Since our results indicated that amino acid stimulation of STC-1 produces membrane depolarization of a magnitude comparable to that induced by KCl at concentrations that lead to the opening of L-type VSCCs, we hypothesized that SNAT2-mediated Na+-coupled amino acid transport in STC-1 induces Ca2+ influx through the opening of L-type VSCCs. In line with this hypothesis, we demonstrated previously, in Fig. 2, that amino acid-induced Ca2+ signaling in STC-1 cells depends on extracellular Ca2+, in agreement with the notion that amino acids elevate [Ca2+]i by stimulating Ca2+ influx into STC-1 cells. An additional important prediction of the hypothesis that SNAT2-mediated amino acid transport in STC-1 leads to membrane depolarization and thereby to the opening of L-type VSCC is that amino acid-induced Ca2+ signaling in STC-1 cells should be prevented by specific inhibitors of L-type VSCCs.
The common pharmacological feature of all isoforms of L-type VSCCs is their sensitivity to dihydropyridines, e.g., nifedipine or nitrendipine. Consequently, we examined the effects of these L-type VSCC blockers on [Ca2+]i increases elicited by amino acids in STC-1 cells. As shown in Fig. 7B, the increase in [Ca2+]i induced by multiple amino acids, including l-proline, l-alanine, l-serine, and MeAIB, was inhibited by greater than 90% by cell treatment with 1–10 μM of the L-type VSCC blocker nifedipine. Furthermore, the elevation of [Ca2+]i induced by these amino acids was completely blocked by STC-1 cell exposure to 1 μM nitrendipine before stimulation.
DISCUSSION
The GI tract is a sensory organ that responds to a large array of signals originating in the lumen, including nutrient and nonnutrient chemicals, mechanical factors, and microorganisms. Molecular sensing by GI cells plays a critical role in the control of multiple fundamental functions in digestion, including secretory activity of GI glands, absorptive activity, motility, and blood supply of the intestinal tract. Furthermore, molecular sensing of luminal contents also initiates hormonal and/or neural pathways, leading to the regulation of caloric intake, pancreatic insulin secretion, and metabolism. Although these fundamental control systems have been known for considerable time, the initial molecular recognition events that sense the chemical composition of the luminal contents of the GI tract have remained elusive. Indeed, the signaling pathways that transduce the effects of many luminal molecules have not been identified or remain incompletely characterized.
The results presented here show that enteroendocrine STC-1 cells respond to a broad range of amino acids, including l-proline, l-serine, l-alanine, l-glycine, l-histidine, l-cysteine, and l-methionine, with a rapid and transient increase in [Ca2+]i, followed by a slower return to baseline. Interestingly, Ca2+ signaling in STC-1 cells was much reduced in response to l-phenylalanine and virtually not detectable in response to l-tryptophan, or acidic (l-glutamic and l-aspartic acid), basic (l-lysine, l-arginine), or branched (l-isoleucine) amino acids. These results significantly extended previous reports demonstrating that STC-1 cells, a model system for studying nutrient and nonnutrient sensing by GI endocrine cells (see Introduction for references), are responsive to stimulation with l-phenylalanine. The results of sequential addition of different amino acids (l-proline, l-alanine, and l-phenylalanine) are consistent with the notion that l-amino acids induce [Ca2+]i responses in STC-1 cells through a common pathway or site. These findings raise important questions regarding the identity of the molecular mechanism by which such a broad spectrum of neutral amino acids can initiate robust Ca2+ signaling in STC-1 cells.
It is increasingly recognized that amino acids are sensed by a variety of recently identified family C GPCRs, including CaR and T1R1/T1R3 heterodimers (44). The CaR has been implicated in sensing aromatic amino acids (9, 49) in a variety of tissues, including the GI tract (4, 12). Recently, Hira et. al. (15) concluded that the stimulatory effect of l-phenylalanine on [Ca2+]i in STC-1 cells is mediated by the CaR, but the effects were elicited at high concentrations of l-phenylalanine, and the efficacy of other l-amino acids was not examined. In contrast to the study of Hira et al., the profile of amino acid effectiveness for inducing Ca2+ signaling in STC-1 cells delineated in this study is clearly not consistent with a major role of CaR in amino acid sensing in these cells. Specifically, the sequence of efficacy of amino acid-induced Ca2+ signaling in STC-1 cells is l-proline = l-serine = l-alanine > l-histidine > l-leucine = l-phenylalanine, and no response from l-tryptophan. In stark contrast, the sequence of efficacy of amino acid-induced Ca2+ signaling via the CaR is l-phenylalanine = l-tryptophan = l-histidine > l-alanine > l-serine = l-proline = l-glutamic acid > l-aspartic acid, and no response from l-leucine or l-isoleucine. Thus, l-proline, which is in the midrange of the profile for CaR, is the most potent inducer of Ca2+ signaling in STC-1 cells. Conversely, l-tryptophan, a potent agonist of the CaR, is not effective in STC-1 cells. In this study, we confirmed that l-phenylalanine and l-tryptophan are much more effective than l-proline in promoting Ca2+ signaling via the hCaR or mCaR. In addition to these important differences in amino acid selectivity, we found deficient expression of CaR immunoreactivity at the cell surface of STC-1 cells. These results imply that the CaR is not a major amino acid-sensing pathway in these enteroendocrine cells.
The T1R1/T1R3, a broadly tuned amino acid-sensing receptor in the mouse, offered another plausible mechanism. However, amino sensing by STC-1 cells was not potentiated by IMP, and the profile of amino acid efficacy in STC-1 cells also differed from that previously reported for the murine T1R1/T1R3 (44). In addition, transfection with siRNA targeting T1R3 did not produce a significant decrease in [Ca2+]i signaling in response to l-proline. These results militate against a major role of this taste receptor in mediating Ca2+ signaling in STC-1 cells in response to neutral amino acids.
In addition to GPCR-mediated sensing mechanisms, it is increasingly recognized that Na+-coupled amino acid transporters can also operate as amino acid sensors (16, 17). The results presented here produced several lines of evidence, indicating that amino acid transport through the SNAT2 is a major pathway leading to Ca2+ signaling in STC-1 cells: 1) the amino acid efficacy profile for elevating [Ca2+]i in STC-1 cells closely matches the amino acid specificity of SNAT2, but differs from SIT1 (sodium/imino-acid transporter 1), which mediates l-proline but not l-alanine transport (41); 2) amino acid-induced Ca2+ signaling in STC-1 cells was suppressed by removing Na+ from the medium, but was not suppressed by the Na+ channel blocker TTX; 3) the nonmetabolized synthetic substrate of amino acid transport MeAIB produced a marked increase in [Ca2+]i; 4) transfection of siRNA targeting SNAT2 produced a marked decrease in Ca2+ signaling in response to l-proline in STC-1 cells; and 5) amino acid-induced increase in [Ca2+]i was abrogated by the dihydropyridines nifedipine or nitrendipine, which block L-type VSCCs. The effectiveness of the dihydropyridines in blocking amino acid-induced Ca2+ signaling also eliminates the operation of reverse mode Na+/Ca2+ exchange. Collectively, these results support the notion that SNAT2 plays a critical role in mediating amino acid-induced Ca2+ signaling in enteroendocrine STC-1 cells.
In previous studies, our laboratory verified that STC-1 cells express the pore-forming α1-subunit isoforms Cav1.2 and Cav1.3 of L-type VSCCs, and that treatment with the L-type VSCCs opener BAY K 8644 increases [Ca2+]i in these cells (8). In addition, our laboratory demonstrated that the addition of depolarizing concentrations of KCl (5–25 mM) to STC-1 cells induced a robust early spike in [Ca2+]i, followed by a sustained plateau phase (8). The results presented here showed that addition of l-proline to the medium bathing STC-1 cells produced membrane depolarization of a magnitude comparable to that induced by the addition of KCl at concentrations that lead to the opening of L-type VSCCs.
Consequently, we hypothesize that the inward current of Na+ associated with the function of SNAT2 leads to membrane depolarization and activation of L-type VSCCs that mediate Ca2+ influx, thereby leading to an increase in the [Ca2+]i in enteroendocrine STC-1 cells. This conclusion is in agreement with other studies using the GLUTag enteroendocrine cell line, showing that glutamine-induced secretion of glucagon-like peptide-1 is driven, at least in part, by membrane depolarization associated with the inward current generated by Na+-coupled glutamine transport in these cells (28). Similarly, addition of alanine to insulin-secreting BRIN-BD11 produced membrane depolarization and elevation of [Ca2+]i (23). Our results show, for the first time, that transfection of siRNAs targeting SNAT2 blunted the increase in [Ca2+]i in response to l-proline and therefore provide, not only functional, but also molecular, evidence implicating SNAT2 in triggering Ca2+ signaling via L-type VSCCs in enteroendocrine cells.
The products of protein digestion are absorbed at different rates in the GI tract, with free amino acids reaching the distal region of the small bowel. In this region, amino acids induce the release of PYY and glucagon-like peptide-1 from L cells and neurotensin from N cells. These regulatory peptides have important physiological functions, including control of food intake, release of insulin from the β-cells of the pancreas, and regulation of the proliferation of intestinal epithelial cells. Although conclusions concerning the physiological significance of results obtained with model cell lines, including STC-1 cells, should be drawn with prudence, our results raise the possibility that enteroendocrine cells located in the distal region of the small bowel and in the colon may sense sudden surges in the concentration of luminal or local neutral amino acids, at least in part, through SNAT2.
GRANTS
This work was supported by National Institutes of Health Grants R01DK55003, R01DK56930, and P30DK41301 (to E. Rozengurt); R01DK079155 (to C. Sternini); and K22 CA128883 and K22 CA128883-03S1 (to O. Rey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
E. Rozengurt is the Ronald S. Hirshberg Professor of Translational Pancreatic Cancer Research.
REFERENCES
- 1.Ballantyne G. Peptide YY(1–36) and peptide YY(3–36). I. Distribution, release and actions. Obes Surg 16: 651–658, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Beach JM, McGahren ED, Xia J, Duling BR. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am J Physiol Heart Circ Physiol 270: H2216–H2227, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Buchan AMJ, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology 120: 1128–1139, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E. International Union of Pharmacology XL compendium of voltage-gated ion channels: calcium channels. Pharmacol Rev 55: 579–581, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Chey WY, Braggins L, Coy DH, Chang TM. Pituitary adenylate cyclase-activating polypeptide stimulates cholecystokinin secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 271: G516–G523, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Chang CH, Chey WY, Sun Q, Leiter A, Chang TM. Characterization of the release of cholecystokinin from a murine neuroendocrine tumor cell line, STC-1. Biochim Biophys Acta 1221: 339–347, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Chen MC, Wu SV, Reeve JR, Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol 291: C726–C739, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Conigrave AD, Quinn SJ, Brown EM. l-Amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA 97: 4814–4819, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolphin AC. The GL Brown Prize Lecture. Voltage-dependent calcium channels and their modulation by neurotransmitters and G proteins. Exp Physiol 80: 1–36, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Furness JB, Kunze WAA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol Gastrointest Liver Physiol 277: G922–G928, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol 71: 205–217, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+ sensing receptor along the gastrointestinal tract. Annu Rev Physiol 71: 205–217, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Guilmeau S, Buyse M, Tsocas A, Laigneau JP, Bado A. Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes 52: 1664–1672, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J 275: 4620–4626, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296: E603–E613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J 373: 1–18, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liddle RA. Cholecystokinin cells. Annu Rev Physiol 59: 221–242, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Lomax RB, McLaughlin JT, Dockray GJ, Thompson DG, Warhurst G. Fatty acids stimulate cholecystokinin secretion from STC-1 enteroendocrine cells by Ca2+ influx through L-type Ca2+ channels. Journal of Physiology (Cambridge) 511P: 25P–26P, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflügers Arch 447: 784–795, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Mangel AW, Prpic V, Wong H, Basavappa S, Hurst LJ, Scott L, Garman RL, Hayes JS, Sharara AI, Snow ND, Walsh JH, Liddle RA. Phenylalanine-stimulated secretion of cholecystokinin is calcium dependent. Am J Physiol Gastrointest Liver Physiol 268: G90–G94, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Mangel AW, Scott L, Prpic V, Liddle RA. Regulation of cholecystokinin secretion in STC-1 cells by nitric oxide. Am J Physiol Gastrointest Liver Physiol 271: G650–G654, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Miguel JC, Patterson S, Abdel-Wahab YHA, Mathias PCF, Flatt PR. Time-correlation between membrane depolarization and intracellular calcium in insulin secreting BRIN-BD11 cells: studies using FLIPR. Cell Calcium 36: 43–50, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Nemoz-Gaillard E, Cordier-Bussat M, Filloux C, Cuber JC, Van Obberghen E, Chayvialle JA, Abello J. Bombesin stimulates cholecystokinin secretion through mitogen-activated protein-kinase-dependent and -independent mechanisms in the enteroendocrine STC-1 cell line. Biochem J 331: 129–135, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet 15: 2200–2209, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev 78: 1087–1108, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Reimann F, Ward PS, Gribble FM. Signaling mechanisms underlying the release of glucagon-like peptide 1. Diabetes 55: S78–S85, 2006 [Google Scholar]
- 29.Rey O, Young SH, Cantrell D, Rozengurt E. Rapid protein kinase D translocation in response to G protein-coupled receptor activation. Dependence on protein kinase C. J Biol Chem 276: 32616–32626, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Rey O, Young SH, Papazyan R, Shapiro MS, Rozengurt E. Requirement of the TRPC1 cation channel in the generation of transient Ca2+ oscillations by the calcium-sensing receptor. J Biol Chem 281: 38730–38737, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem 280: 22875–22882, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Rindi G, Grant SGN, Yiangou Y, Ghatei MA, Bloom SR, Bautch VL, Solcia E, Polak JM. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice: heterogeneity of hormone expression. Am J Pathol 136: 1349–1364, 1990 [PMC free article] [PubMed] [Google Scholar]
- 33.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213: 589–602, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and α-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291: G171–G177, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol 7: 557–562, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the α-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291: G792–G802, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Skipper M, Lewis J. Getting to the guts of enteroendocrine differentiation. Nat Genet 24: 3–4, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Snow ND, Prpic V, Mangel AW, Sharara AI, McVey DC, Hurst LJ, Vigna SR, Liddle RA. Regulation of cholecystokinin secretion by bombesin in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 267: G859–G865, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of “taste” in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes 15: 73–78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem 275: 16473–16477, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J Biol Chem 280: 8974–8984, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept 145: 12–16, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Wellendorph P, Johansen LD, Brauner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol 76: 453–465, 2009 [DOI] [PubMed] [Google Scholar]
- 45.White E, McKenna J, Cavanaugh A, Breitwieser GE. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol Endocrinol 23: 1115–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics 22: 139–149, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99: 2392–2397, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem 275: 22790–22797, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Am J Physiol Cell Physiol 282: C1414–C1422, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Young SH, Wu SV, Rozengurt E. Ca2+-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor require negative feedback by protein kinase C. J Biol Chem 277: 46871–46876, 2002. [DOI] [PubMed] [Google Scholar]