Abstract
The cytokine interleukin-7 (IL-7) has essential growth activities that maintain the homeostatic balance of the immune system. Little is known of the mechanism by which IL-7 signaling regulates metabolic activity in support of its vital function in lymphocytes. We observed that IL-7 deprivation caused a rapid decline in the metabolism of glucose that was attributable to loss of intracellular glucose retention. To identify the transducer of the IL-7 metabolic signal, we examined the expression of three important regulators of glucose metabolism, the glucose transporter GLUT-1 and two glycolytic enzymes, hexokinase II (HXKII) and phosphofructokinase-1 (PFK-1), using an IL-7-dependent T-cell line and primary lymphocytes. We found that in lymphocytes deprived of IL-7 loss of glucose uptake correlated with decreased expression of HXKII. Readdition of IL-7 to cytokine-deprived lymphocytes restored the transcription of the HXKII gene within 2 h, but not that of GLUT-1 or PFK-1. IL-7-mediated increases in HXKII, but not GLUT-1 or PFK-1, were also observed at the protein level. Inhibition of HXKII with 3-bromopyruvate or specific small-interfering RNA decreased glucose utilization, as well as ATP levels, in the presence of IL-7, whereas overexpression of HXKII, but not GLUT-1, restored glucose retention and increased ATP levels in the absence of IL-7. We conclude that IL-7 controls glucose utilization by regulating the gene expression of HXKII, suggesting a mechanism by which IL-7 supports bioenergetics that control cell fate decisions in lymphocytes.
Keywords: cytokine, metabolism, signaling, ATP
maintenance of lymphocyte survival and proliferation is dependent on the ability of immune cells to acquire sufficient nutrients to support cellular metabolism. Growth factors enable this process by transducing signals that promote metabolic activity. Of these, the cytokine interleukin-7 (IL-7), first identified as a B-cell and later T-cell growth factor (36, 41, 52), is a potent agent for immunoreconstitution through its diverse activities supporting survival and proliferation. IL-7 is generally considered a product of stromal or accessory cells (reviewed in Refs. 3, 17, 35). The receptor for IL-7 is expressed on lymphocytes and consists of the unique IL-7Rα chain (IL-7R) and the common cytokine γ chain (γc) that is also shared by the receptors for IL-2, IL-4, IL-9, IL-15, and IL-21 (26). Upon IL-7 binding, the two receptor chains heterodimerize (58), which leads to the activation of the receptor-associated Janus kinases, JAK1 and JAK3 (46). The activated JAK proteins in turn phosphorylate specific residues on the IL-7R, creating docking sites for signaling molecules that have Src homology 2 (SH2) domains such as the transcription factor, STAT5 (signal transducers and activators of transcription 5) (29), and to a lesser extent STAT1 and STAT3 (57). Multiple gene products are produced from engagement of the IL-7R, which include antiapoptotic proteins, signaling molecules, growth factors, and receptors (22). Although mice deficient in IL-7 (52), the IL-7 receptor (IL-7R) (41), or components of its signaling pathway, such as JAK3 (38) or STAT5 (56), have reduced thymic cell counts and impaired T-cell development, adoptive transfer experiments with IL-7-deficient mice proved that this cytokine is also a mediator of the homeostatic mechanisms that maintain stable numbers of naive and memory T cells in the peripheral immune system (45, 49).
One of the ways by which IL-7 supports T-cell development and homeostasis is through the maintenance of survival by upregulating the expression of antiapoptotic members of the BCL-2 family, such as BCL-2 and BCL-XL (1, 22, 34), or downregulating proapoptotic BCL-2 family members, such as BAX (20, 21) or BIM (40). In addition to survival, IL-7 also has a proliferative function, promoting the replication of T cells by preventing the degradation of the cell cycle-activating phosphatase, Cdc25A (18), or repressing the cell cycle inhibitor, p27Kip1 (30). Yet the activities of survival and proliferation mediated by IL-7 are dependent on a T cell's ability to uptake nutrients, such as glucose or glutamine, from the environment to support metabolic needs (19, 23, 31). Despite their importance, the essential metabolic targets of an IL-7 signal transduction pathway have not been characterized.
Actively dividing lymphocytes have an increased energetic demand, which is mostly met through the metabolism of glucose. Resting T cells, in contrast, have minimal energetic requirements (7, 13, 14). T cells can satisfy their needs for ATP from glucose metabolism through the processes of glycolysis or oxidative phosphorylation (tricarboxylic acid cycle). Glycolysis is a rapid process that can take place in the cytosol independent of oxygen consumption. Through glycolysis, one molecule of glucose generates two molecules of ATP. Oxidative phosphorylation is a longer process that generates more ATP (30 ATP) from glucose and pyruvate and takes place in mitochondria. It is generally accepted that quiescent T cells utilize oxidative phosphorylation to generate ATP, whereas proliferating T cells utilize glycolysis, especially when the extracellular environment is rich in glucose (10, 27).
One mechanism through which cytokines, such as IL-7, could control glucose metabolism in T cells is by increased trafficking of the glucose transporter GLUT-1 to the cell surface (54). GLUT-1 is the main glucose transporter expressed in T cells (42). However, one study showed that during T-cell activation glucose uptake increased well before GLUT-1 expression was observed (14). Moreover, IL-7 itself was able to increase glucose uptake in resting T cells without affecting the levels of GLUT-1 protein (54), suggesting that the activity of other factors in the glucose metabolic pathway could be targets for IL-7 regulation. Possible targets are the hexokinase (HXK) enzymes or phosphofructokinase 1 (PFK-1). Hexokinase I (HXKI) and II (HXKII) convert glucose to glucose-6-phosphate, retaining the hexose inside the cell. In a subsequent step, PFK-1 catalyzes the conversion of fructose-6-phosphate to fructose-1-6-bis-phosphate, a process that consumes ATP. Thus both HXK and PFK mediate two key steps in glycolysis: the retention of intracellular glucose after transport and the irreversible transfer of a phosphate from ATP to fructose-6-phosphate. Evidence that cytokines support glycolytic activity comes from studies with the B-cell line, FL5–12, in which IL-3 increased the activities of HXK and PFK (6). HXK may also have antiapoptotic activities as well, since others have reported that the transfer of HXK to mitochondria may protect the cells from death induced by cytokine withdrawal (43). This idea is offset by the fact that increased ATP, as generated from glycolysis, can promotes the process of apoptosis (12).
Glucose metabolism is thus a multistep process that could be regulated by IL-7 at different points. To characterize the critical transducers of an IL-7 metabolic signal, we used a unique IL-7-dependent T-cell line, D1, as well as primary murine T cells to show that IL-7 induces glucose uptake by controlling the gene expression of HXKII. We found that HXKII protein levels followed IL-7-mediated increase in HXKII mRNA and that inhibition or forced expression of HXKII in T cells could control glucose uptake and ATP levels independently of IL-7. Our results demonstrate that HXKII is a key regulatory target in the metabolic pathway controlled by IL-7 whose activity is decisive in determining the ultimate fate of a lymphocyte.
MATERIALS AND METHODS
Cell lines, mice, and T-cell purification.
The IL-7-dependent T-cell line D1 was established from pro-T cells isolated from a p53−/− mouse as previously described (22). D1 T cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1% penicillin (5,000 U/ml)-streptomycin (5,000 μg/ml), 50 μM β-mercaptoethanol, and 50 ng/ml recombinant human IL-7 (Peprotech, CA). Primary lymph node (LN) T cells from 8- to 12-wk-old C57BL/6 mice were isolated by mechanical teasing and cultured (5 × 106 cells/ml) in the presence of 150 ng/ml of IL-7 (Peprotech). To enrich for IL-7-dependent cells, as needed for experiments, we used our published method of ex vivo expansion and cultured LN T cells with 150 ng/ml of IL-7 for 7 days (24). Animal use was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Central Florida.
Plasmids and nucleofection for transient gene expression.
Plasmids used for transient expression of HXKII and GLUT1 were pcDNA-HXKII (a kind gift from Dr. Wilson, and Dr. De Xon, Michigan State University) and prGT3-GLUT-1 (a kind gift from Dr. Birnbaum, Howard Hughes Medical Institute, Chevy Chase, MD). To transiently express the plasmids, D1 and primary T cells were “nucleofected” by use of the Murine T-cell Nucleofection kit (Amaxa), following the manufacturer's protocol. Briefly, 1 × 106 T cells were incubated with (4 μg) plasmid DNA in 100 μl of the mouse T-cell solution, placed in a cuvette within the Nucleofector device, and electroporated with the specific program optimized for mouse T cells. Nucleofected T cells were incubated in the supplemented media with or without IL-7 for 4–8 h prior to analysis. In D1 T cells nucleofection efficiency averaged ∼40–50% expression of the target gene with viabilities ranging 60 to 80%.
Glucose uptake, ATP assay, and apoptosis assay.
D1 or primary T cells were incubated in glucose-free, serum-free RPMI (Mediatech, Manassas, VA) supplemented with or without IL-7 for 1 h. 2-Deoxy-d-[3H] glucose (2 μCi/reaction) (Sigma) was added for 3 min or 3-O-methyl-[3H]-glucose (2 μCi/reaction) (GE Healthcare) was added for 1 min. Reactions were stopped by adding 250 μl of ice-cold 0.3 mM phloretin (Sigma). Cells were then centrifuged through a cushion of 10% bovine serum albumin, and lysed with 0.1% Triton X-100. Radioactivity was measured with scintillation counter (LS6500, Beckman Coulter). To measure glucose uptake by the alternative method of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2-NBDG) incorporation, IL-7-dependent T cells were resuspended in glucose-free, serum-free RPMI 1640 for 4 h. 2-NBDG (30 μM) was then added to the cells for 5 min and analyzed by flow cytometry using a FACSCalibur (BD Biosciences). Signals were acquired through the FL2 channel at an emission wavelength of 550 nm. Flow cytometry data was analyzed via the software FCS express (De Novo). Statistical analysis was done with Prism software (GraphPad). ATP levels were quantitated by using the ENLITEN rLuciferase/Luciferin Reagent (Promega) following manufacturer's guidelines. Briefly, lysates were prepared from 2 × 106 cells, to which the reconstituted rLuciferase/Luciferin reagent was added and immediately read in a luminometer (Perkin Elmer). Apoptosis was measured by using the FITC Annexin V Apoptosis Detection Kit (BD Biosciences) following manufacturer's protocol. Staining was assessed by flow cytometry as described above.
Gene expression analysis by real-time PCR.
For RNA extraction, D1 T cells (2.0 × 106) were centrifuged and cell pellets were resuspended in 1 ml TRIzol (Invitrogen). One microgram of RNA was converted into cDNA with iScript cDNA synthesis kit (Bio-Rad). Real-time PCR reactions were performed with Fast SYBR Green Mix (Applied Biosystems) on a 7500 Real-time PCR system (Applied Biosystems). β-Actin, a housekeeping gene which is independent of IL-7 signaling, was used as endogenous control. Primer sequences for β-actin were previously published (2). Primer sequences for PFK-1 were previously published (44). GLUT-1 and HXKII primer sequences were developed with Primer 3 Software (http://frodo.wi.mit.edu/). GLUT1 primers: forward 5′GCCTGAGACCAGT TGGAAGCAC3′, reverse 3′CTGCTTAGGTAAA GTTACAGGAG5′. HXKII primers: forward 5′CACTGGGTACTAAGGCTCAA3′, reverse 3′CGGAGTTGTTCTGCTTTGGA5′. Relative expression or real-time quantitative (RQ) values were calculated by the following formulas: ΔCt = target gene (GLUT-1, HXKII, or PFK-1) − endogenous gene (β-actin); ΔΔCt = ΔCt − calibrator gene (2- or 18-h time points for each experiment); RQ = 2−ΔΔCt, where Ct is cycle threshold. Statistical analysis was done with Prism software (GraphPad).
Immunoblots for HXKII, GLUT1, and PFK-1.
D1 T cells (2 × 107) were lysed by using a whole cell lysis buffer [20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Nonidet P-40, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin]. Cell lysates were separated on 8% SDS-PAGE gels, then transferred onto nitrocellulose membrane and immunoblotted for HXKII, GLUT1, PFK-1, and p38 MAPK (as a loading control) using monoclonal antibodies against HXKII (C-14, Santa Cruz), GLUT1 (15309, AbCam), PFK-1 (L684, AbGen) and p38 MAPK (C-20, Santa Cruz) respectively. Appropriate secondary antibodies used were horseradish peroxidase conjugated. Signal was developed by enhanced chemiluminescence (Pierce) and visualized on BioMax ML film (Kodak).
Measurement of GLUT-1 surface and intracellular expression.
Levels of cell surface GLUT-1 was assessed by using the EGFP-GLUT-1 ligand (ABCYS Biologie, Paris, France) with flow cytometry. D1 T cells were incubated with the EGFP-GLUT-1 ligand for 30 min. The signal for the EGFP-GLUT-1 ligand was acquired through the FL-1 channel at an emission of 520 nm using a C6 flow cytometer (Accuri). To visualize GLUT-1 surface and intracellular protein levels, we used confocal microscopy. Briefly, D1 T cells (106 cell/ml) were incubated with 100 μl of the EGFP-GLUT-1 ligand as described above. Washed cells were fixed with 4% paraformaldehyde and attached to slides by cytospin. Images were acquired with Zeiss confocal microscope (LSM520) at ×60. Images were analyzed with Axiovision 4.7 software from Zeiss.
siRNA inhibition.
HXKII predesigned Accell SMART pool small-interfering RNA (siRNA) was purchased from Dharmacon (Thermo Scientific) and was introduced into cells according to manufacturer's protocol. Briefly, SMART pool siRNA combines four different siRNAs to reduce off-target effects. The Accell siRNA is also designed for optimal delivery to hard-to-transfect cells and no transfection reagents are required to introduce the siRNAs. HXKII predesigned Accell SMART pool siRNA was introduced to murine LN T cells or D1 T cells in the Accell delivery medium and incubated for 72 h prior to assay for glucose uptake. Delivery efficiency and siRNA specificity were tested by using Accell green (FITC) nontargeting control siRNA, GAPDH-specific siRNA, and an Accell nontargeting control siRNA (Dharmacon).
RESULTS
IL-7 regulates glucose utilization in T cells.
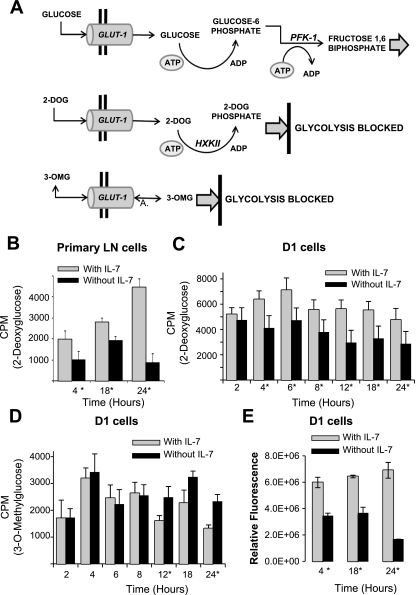
T cells maintain their survival and proliferative activities by acquiring sufficient nutrients to support cellular metabolism, a process dependent on cytokine signals (23). In lymphocytes, glucose is transported into the cell through the transporter, GLUT-1, and intracellular retention occurs upon the phosphorylation of glucose by HXKII, producing glucose-6-phosphate, the substrate for the next steps of glycolysis. This process is shown in Fig. 1A. To measure glucose uptake and retention, we used two different glucose analogs: 1) [3H]2-deoxyglucose (2-DOG), a glucose molecule that cannot be metabolized but is phosphorylated by HXKII, making it an indicator of HXKII activity and glucose use, and 2) [3H]3-O-methylglucose (3-OMG), a nonmetabolizable glucose analog that is not phosphorylated by HXKII but can be used to assess glucose transport (Fig. 1A). To determine the mechanism through which IL-7 regulates glucose uptake and metabolism in T cells, we first assessed glucose uptake in primary LN T cells isolated from wild-type C57BL-6 mice. We and others have shown that freshly isolated T cells from lymphoid organs are mostly naive and require both cytokine and T-cell receptor (TCR) signaling for maximal stimulation (9, 24, 42). To enrich for the small number of IL-7-responsive cells found in lymphoid organs (mainly memory CD8 T cells), we used our published method of ex vivo expansion and cultured LN T cells with 150 ng/ml of IL-7 for 7 days (24). This dose of IL-7 was chosen after examining the effect of low and high concentrations of IL-7 and determining that expansion of IL-7-dependent cells was best achieved using high doses of the cytokine (Kittipatarin C. et al., unpublished observations). After 7 days, LN T cells were incubated with or without 150 ng/ml of IL-7 for 4, 18, and 24 h, and glucose use was measured through incorporation of 2-DOG, the phosphorylatable glucose analog. We observed that the levels of 2-DOG in LN T cells incubated with IL-7 were significantly increased compared with LN T cells that were deprived of IL-7 (Fig. 1B), indicating that an IL-7 signal is required to support glucose uptake in T cells.
Fig. 1.
IL-7 signaling controls glucose retention. A: model depicts the pathway by which glucose is imported through the GLUT-1 transporter and metabolized in the first steps of glycolysis by the enzymes hexokinase II (HXKII) and phosphofructokinase-1 (PFK-1). The uptake and metabolism of 2 glucose analogs, [3H]2-deoxyglucose (2-DOG), a glucose molecule that cannot be metabolized but is phosphorylated by HXKII, and [3H]3-O-methlyglucose (3-OMG), a nonmetabolizable glucose molecule that is not phosphorylated by HXKII, are shown. B–D: IL-7-cultured murine lymph node (LN) T- cells (B) were incubated with or without 150 ng/ml IL-7 and IL-7 dependent D1 T-cell line (C and D) were incubated with or without IL-7 (50 ng/ml) for the specified periods of time shown in the figures and cells assayed for glucose use with radiolabeled 2-DOG (B and C) or glucose import with radiolabeled 3-OMG (D) as described in materials and methods. E: D1 T cells were cultured with or without IL-7 (50 ng/ml) for 4, 18, and 24 h and total cellular ATP measured using the rLuciferase/Luciferin reagent (Promega) in a luminometer as described in materials and methods. Shown are the values for relative fluorescence that correlate with ATP concentrations. Results are representative of 3 independent experiments performed in triplicate (values are average ± SD). *P < 0.05 compared with w/IL-7 in each time point pair. CPM, counts per minute.
Next, we examined glucose use in response to IL-7 using an IL-7-dependent T-cell line, D1. D1 T cells were originally generated in the laboratory of Dr. Scott Durum [National Cancer Institute (NCI)-Frederick] from p53−/− mice and depend on IL-7 at an optimal concentration of 50 ng/ml for survival and proliferation; if the concentration of IL-7 is decreased, D1 T cells stop dividing (18) and die after 48 h (22). Use of this cell line enabled us to dissect the mechanism by which IL-7 controls glucose metabolism in a manner not possible with primary T cells. D1 T cells were cultured with or without 50 ng/ml of IL-7 for the indicated periods of time. Results shown in Fig. 1C, using D1 T cells, validated results with LN T cells (Fig. 1B) that glucose use, measured by 2-DOG incorporation, was consistently higher in the presence of IL-7 at all time points examined. Statistically significant differences in 2-DOG uptake in D1 T cells cultured with or without IL-7 were observable from 4 h through 24 h of culture (Fig. 1C). At the 2-h time point, increased 2-DOG import in the presence of IL-7 was observed but differences were not significant, likely because of handling effects that are resolved after this time point. Earlier time points, 0.5–2 h, were previously examined, and glucose uptake was decreased in both IL-7-containing and IL-7-deprived cells owing to handling stress resulting from centrifugation and media changes (19).
We examined the process of glucose import in D1 T cells, as distinct from intracellular retention, by using the nonphosphorylatable glucose analog, 3-OMG. Results shown in Fig. 1D revealed that glucose import is not dependent on IL-7 (50 ng/ml) and is in fact likely independent of IL-7. This was revealed by the apparent inconsistent results at each time point in which 3-OMG uptake was either slightly increased (6 and 8 h) or decreased (12, 18, and 24 h) in the presence of IL-7. This is in contrast to the results observed with the uptake 2-DOG, which was always increased in cells cultured with IL-7 at all time points examined (Fig. 1C).
An expected, a consequence of the loss of glucose uptake would be reduced metabolism that can be indirectly assessed by measuring ATP levels. Using a luciferin-luciferase detection system, we measured total ATP levels in D1 T cells deprived of 50 ng/ml of IL-7 for 4, 18, and 24 h and observed significant decreases in ATP over time of IL-7 withdrawal (Fig. 1E). We concluded, therefore, that as part of its growth activity, IL-7 had a necessary function in maintaining cellular energetics.
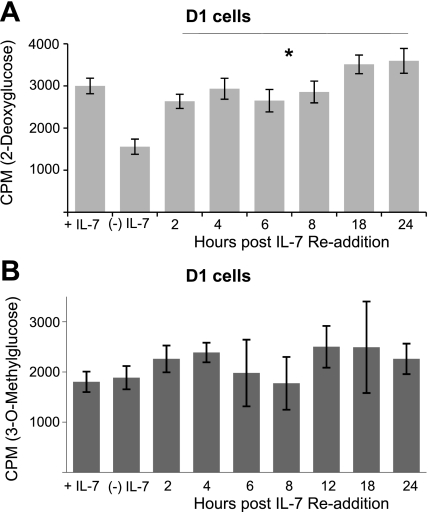
To establish conclusively that an IL-7 signal is required to promote glucose, we deprived IL-7-dependent D1 T cells from IL-7 for 18 h then pulsed them with 50 ng/ml of IL-7. By depriving cells of IL-7 and readding the cytokine, we can show that observed effects are solely due to the cytokine signal and not other environmental factors. Note that, under these conditions, D1 T cells, although growth arrested, are still viable (data not shown) (22). After deprivation, we readded IL-7 and measured glucose use at time points from 2 through 24 h, using radiolabeled 2-DOG (Fig. 2A). 2-DOG uptake, indicative of glucose use, was rapidly restored after 2 h of IL-7 readdition and this increase was sustained through 24 h, restoring glucose uptake to the levels observed in the presence of the cytokine (Fig. 2A). We also noted that addition of IL-7 to deprived D1 T cells for 30 min to 1 h caused minimal increases in 2-DOG import (data not shown), suggesting that de novo synthesis could be required for IL-7 to enable the utilization of glucose. When the same experiment was repeated, measuring glucose import with the nonphosphorylatable, 3-OMG (Fig. 2B), we did not observe any significant differences upon readdition of IL-7 to cells deprived of IL-7. Collectively our findings indicate that a signal through the IL-7 receptor controls glucose utilization in a manner that is dependent, not on import, but on the phosphorylation and retention of the hexose.
Fig. 2.
Restoration of glucose uptake upon IL-7 readdition is dependent on phosphorylation of the hexose. IL-7-dependent D1 T cells were incubated without cytokine for 18 h and IL-7 (50 ng/ml) readded at the time points specified in the figures. Controls included were D1 T cells grown continuously in IL-7 (+IL-7) and D1 T cells deprived of IL-7 for 18 h (−IL-7). D1 T cells were assayed for glucose use with 2-DOG (A) or glucose import with 3-OMG (B) as described in materials and methods. Results are representative of 3 independent experiments performed in triplicate (values are average ± SD). *P < 0.05 compared with cells cultured −IL-7.
IL-7 regulates the expression of genes involved in glucose metabolism.
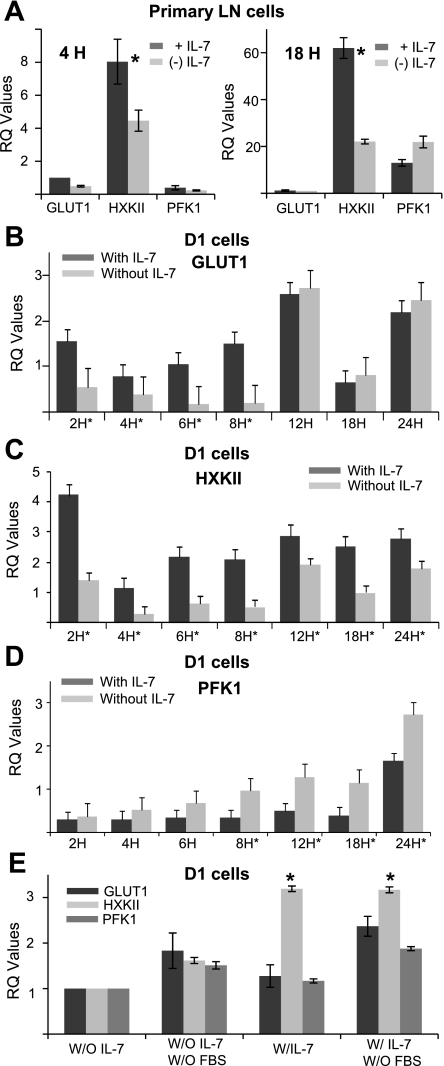
The metabolism of glucose involves a number of enzymes, some of which are key regulators in the process of generating ATP. Of these, GLUT-1, HXKII, and PFK-1 are the essential first mediators of glucose import and metabolism (Fig. 1A). To investigate the role of each of the three proteins, we first examined the gene expression of GLUT-1, HXKII, and PFK-1, using real-time quantitative PCR (qPCR), in LN T cells either freshly isolated and pulsed with 150 ng/ml of IL-7 for 4 h or cultured with 150 ng/ml of IL-7 for 7 days (as described for Fig. 1D) and pulsed with or without cytokine for 18 h. Results are shown as relative gene expression through calculations of RQ values. In Fig. 3A, we observed that LN T cells displayed elevated levels of HXKII transcripts, but not GLUT-1 or PFK-1, when stimulated with 150 ng/ml of IL-7.
Fig. 3.
IL-7 regulates gene expression of glycolytic enzymes involved in glucose metabolism. LN T cells or D1 T cells were incubated with or without IL-7 for various time points (as indicated in the figures) and total RNA was extracted and transcribed to cDNA as described in materials and methods. Quantitative PCR (qPCR) was performed to measure the gene expression of GLUT-1, HXKII, and PFK-1. Real-time quantitative (RQ) values were calculated from qPCR data to show relative gene expression as explained in materials and methods. A: murine T cells, isolated from lymph nodes of C57BL/6 mice, were pulsed with (+IL-7) or without (−IL-7) 150 ng/ml of IL-7 for 4 h (left) or cultured with 150 ng/ml of IL-7 for 7 days (+IL-7) and the cytokine withdrawn for 18 h (−IL-7) (right) and RNA extracted for qPCR to measure transcription of GLUT-1, HXKII, and PFK-1 as described above. B–D: D1 T cells were cultured with or without 50 ng/ml of IL-7 and RNA extracted for qPCR to measure transcription of GLUT-1 (B), HXKII (C), and PFK-1 (D) as described above. E: D1 T cells were incubated with or without 50 ng/ml of IL-7 in serum-containing or serum-free media for 18 h and gene expression changes for GLUT-1, HXKII and PFK-1 measured as described for B–D. Results are representative of at least 3 or more independent experiments. The calibrator sample chosen to determine RQ values was the 18-h time point (B–E) without IL-7. The exception to this was A, in which the GLUT-1 gene, 2 h without IL-7, was used as calibrator. *P < 0.05 compared with without IL-7.
Next, we incubated D1 T cells with or without 50 ng/ml of IL-7 for 0–24 h and measured the gene expression of GLUT-1, HXKII, and PFK-1 using qPCR as described above. We observed that, during the first 8 h of culture without IL-7, GLUT-1 gene expression decreased compared with D1 T cells grown with IL-7 (Fig. 3B). However, after 12 h of IL-7 withdrawal, GLUT1 levels increased compared with D1 T cells cultured with IL-7 (Fig. 3B), indicating that other factors, in addition to IL-7, may be involved in regulating GLUT-1 gene expression, as will be further discussed. On the basis of this result, we would expect that glucose import in IL-7-deprived cells would increase beyond 12 h, giving time for protein synthesis to take place. This was observed in Fig. 1D in which increased uptake of 3-OMG occurred at the 18-h time point in cells cultured without IL-7 compared with cells grown with IL-7.
We then measured gene expression of HXKII, a critical enzyme in the glycolytic pathway in D1 T cells. HXKII generates glucose-6-phosphate from glucose, which prevents glucose from being transported out of cells. Results shown in Fig. 3C demonstrated that IL-7 regulates the transcription of the HXKII gene. HXKII expression was decreased in D1 T cells incubated without IL-7 at every time point analyzed, with results being statistically significant (P > 0.05). These results correlated well with the observation that IL-7 loss caused decreased 2-DOG uptake (Fig. 1C). IL-7 induced HXKII gene expression increased an average of three- to fourfold compared with IL-7-deprived conditions. The results for HXKII (Fig. 3C) were strikingly different from those that were obtained for GLUT-1 (Fig. 3B), suggesting that HXKII gene transcription may primarily be controlled by IL-7.
A different outcome was observed with the detection of PFK-1 transcripts in D1 T cells. In contrast to the IL-7-mediated upregulation of HXKII gene expression, we observed that PFK-1 gene expression was downregulated in the presence but not absence of 50 ng/ml of IL-7 (Fig. 3D), perhaps as a result of a negative feedback mechanism through the amount of glucose-6-phosphate formed and ATP produced: more IL-7, more ATP made (Fig. 1E), less PFK-1 is needed. The decrease in PFK-1 gene expression in IL-7-containing D1 T cells was statistically significant (P < 0.05) at every time point, other than at 24 h, usually by a margin of one- to twofold (Fig. 3D). These results demonstrated that IL-7 directly or indirectly regulates the gene expression of all three glycolytic factors but clearly has the most direct effect on the gene expression of HXKII.
To address the issue of whether GLUT-1 gene expression can be regulated by other factors in addition to IL-7, we incubated D1 T cells with or without IL-7 (50 ng/ml) in serum-free media for 18 h. We found that in the absence of FBS, the gene expression of GLUT1 and PFK-1 were slightly higher and that this increase was independent of IL-7 (Fig. 3E), suggesting that PFK-1 and GLUT1 expression may be controlled by additional factors present in the serum. In contrast HXKII gene expression was similar in IL-7-containing D1 T cells incubated in serum-free media or media with 10% FBS (Fig. 3E), which led us to infer that gene expression of HXKII is likely under the control of IL-7 signaling, whereas GLUT-1 and PFK-1 may be under the indirect control of IL-7 in combination with other factors.
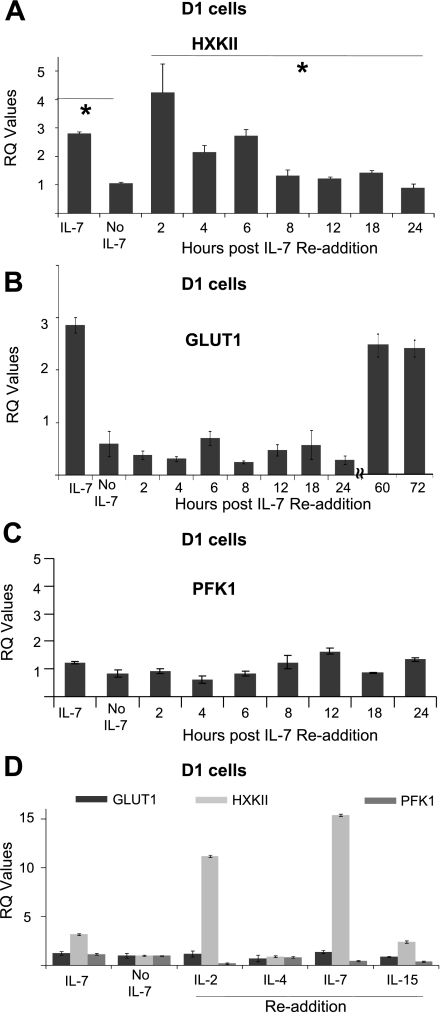
To demonstrate conclusively that IL-7 directly regulates the transcription of HXKII, we examined the gene expression of GLUT-1, HXKII, and PFK-1 during a readdition experiment. D1 T cells were deprived of the cytokine for 18 h and then incubated with IL-7 (50 ng/ml) for specified periods of time through 24 h. Expression of HXKII (Fig. 4A), GLUT-1 (Fig. 4B), and PFK-1 (Fig. 4C) was measured by qPCR. We found that only the gene expression of HXKII was restored immediately after addition of IL-7 (Fig. 4A). Transcription of HXKII, after 2 h of IL-7 readdition, exceeded that of cells grown continuously in the cytokine (IL-7). Gene expression of GLUT-1 did eventually recover but only after 60 h of IL-7 readdition (Fig. 4B), indicating that HXKII is an early gene response to an IL-7 signal, whereas GLUT-1 is a late gene response. The gene expression of PFK-1 showed no statistically significant differences after IL-7 readdition (Fig. 4C). Our results thus establish the gene for HXKII as an important regulatory target for IL-7 signal transduction in dependent T cells due to its rapid induction upon IL-7 readdition.
Fig. 4.
Readdition of IL-7 restores gene expression of HXKII. A–C: D1 T cells were deprived of IL-7 for 18 h and washed, and then IL-7 (50 ng/ml) was readded for the periods of time specified in the figures. Total RNA was isolated and transcribed to cDNA as described in materials and methods. qPCR was performed to analyze the gene expression of HXKII (A), GLUT-1 (B), and PFK-1 (C). RQ values were calculated from qPCR data to show relative gene expression as explained in materials and methods. D: D1 T cells were deprived of IL-7 for 18 h then incubated with IL-2, IL-4, IL-7, and IL-15 at an optimal concentration (50 ng/ml) for 2 h. Total RNA was isolated and transcribed to cDNA, and gene expression was analyzed by qPCR as described above. RQ values were calculated from qPCR data to show relative gene expression as explained in materials and methods. The calibrator sample chosen to determine RQ values was the 18-h time point without IL-7. Results are representative of at least 3 independent experiments and values represent average ± SD. *P < 0.05 compared with without IL-7.
Our studies raised the question whether the IL-7-mediated regulation of HXKII gene expression was specific to IL-7 or was a general function of other gamma-c (γc) cytokines. To answer this, we deprived D1 T cells of IL-7 for 18 h then incubated the cells with different murine γc cytokines: IL-2, IL-4, IL-7, and IL-15 at the optimal dose of 50 ng/ml. After 2 h of readdition, we measured the gene expression of GLUT1, HXKII, and PFK-1 by qPCR. We found that only IL-2 and IL-7 were immediately able to increase the transcription of the HXKII gene (Fig. 4D) but not GLUT-1 or PFK-1 genes. Readdition with IL-4 for 2 h had no effect. Surprisingly, readdition of IL-15, although slightly increasing the gene expression of HXKII, did so to a much lesser extent than IL-2 or IL-7 (Fig. 4D). Note that D1 T cells bear receptors capable of responding to the tested murine cytokines (22). Similar experiments have been performed by others but focusing on much later time points (37). These results suggested that cytokines capable of inducing T-cell proliferation (IL-2 or IL-7) but not differentiation (IL-4) can also drive glucose utilization.
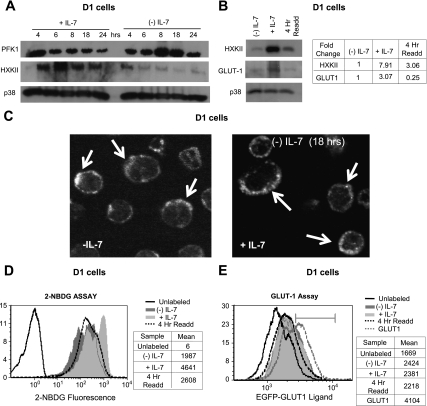
Using D1 T cells, we next examined the effect of IL-7 upon protein expression of the two cytosolic proteins, HXKII and PFK-1, to identify any posttranslational regulatory mechanisms. Consistent with the changes in gene expression shown by qPCR (Figs. 3 and 4), protein levels of PFK-1 increased and protein levels of HXKII decreased in the absence of IL-7 (Fig. 5A). These results were validated by performing IL-7 readdition experiments in which the protein levels of HXKII were restored after 4 h of incubation with the cytokine (Fig. 5B).
Fig. 5.
Protein levels of HXKII increase in response to an IL-7 signal. A: D1 T cells were incubated with or without IL-7 (50 ng/ml) for the time points specified in the figure and lysed, and cell lysates were immunoblotted with antibodies against HXKII and PFK-1. p38 MAPK was assayed as control for equal loading. B: IL-7-deprived D1 T cells were incubated with IL-7 (50 ng/ml) for 4 h and then lysed, and protein lysates were immunoblotted for HXKII and GLUT-1 with use of specific antibodies. p38 MAPK was measured to show equal loading. Bands were quantitated using ImageJ software (http://rsbweb.nih.gov/ij/). D1 T cells incubated with or without IL-7 (50 ng/ml) for 18 h were included for comparison. C: D1 T cells were incubated with or without IL-7 (50 ng/ml) for 18 h, washed, then incubated with EGFP-GLUT-1 ligand for 30 min; cells fixed with 4% formaldehyde and attached to slides by cytospin. Results were visualized by confocal microscopy using a Zeiss confocal microscope (LSM520) at ×60. D–E: IL-7-deprived D1 T cells were cultured for 4 h with IL-7 (50 ng/ml), washed, and incubated in glucose-free medium and tested for glucose uptake by flow cytometry with fluorescent 2-NBDG (Cflow software, C6 flow cytometer, Accuri) (D), or incubated for 30 min in glucose-free medium with the EGFP-GLUT-1 ligand and tested for GLUT-1 surface expression by flow cytometry (CFlow software, C6 flow cytometer, Accuri) (dotted black lines) (E). Cells cultured with (silver) or without IL-7 (gray) for 18 h are shown as comparison. For the 2-NBDG assay and the EGFP-GLUT-1 ligand data, controls shown are unlabeled, unstimulated cells (solid black lines). As a positive control for measurement of GLUT-1 surface expression, D1-T cells, nucleofected as described in materials and methods with the cDNA for GLUT-1, are also shown (dotted gray line). Marker indicates the percentage of cells transiently expressing high levels of GLUT-1. Data in the tables are the mean peak positions for each sample in the histograms. Controls shown are unlabeled, unstimulated cells (black lines in figures). Data in the tables are the mean peak positions for each sample in the histograms. Shown are representative results of 3 independent experiments performed.
GLUT-1 is a large, glycosylated 12-membrane-spanning protein that we found in primary lymphocytes to be transcribed at levels that are much lower than HXKII (Fig. 3A), potentially making protein detection more difficult. Immunoblotting GLUT-1 from lysates isolated from D1 T cells resulted in detection of a faint band (Fig. 5B) that suggested that the protein could be detected more so in the presence than in the absence of IL-7. We detected 3-fold more total GLUT-1 in the IL-7-containing D1 T cells and yet observed that, unlike HXKII, rapid protein induction of GLUT-1 was not detected after 4 h of IL-7 readdition (Fig. 5B), supporting the conclusion from the gene expression analysis (Fig. 4) that GLUT-1 was a late gene product of IL-7.
To improve the detection of GLUT-1 protein over that achieved by immunoblotting, we used a commercially available product, the EGFP-GLUT-1 ligand, which binds specifically to GLUT-1. The ligand is based in the sequence of human T cell leukemia virus (HTLV) envelope glycoprotein (HRBD) shown to bind to GLUT-1 (32). The EGFP-GLUT1 ligand binds the carboxy-terminal extracellular loop of GLUT-1, enabling detection of membrane-associated GLUT-1 by flow cytometry or fluorescence staining. Because we observed that total protein levels for GLUT-1 were elevated in the presence of IL-7 (Fig. 5B), but we did not observe any IL-7-dependent increases in glucose import using 3-OMG (Fig. 1D), it was possible that GLUT-1 could be expressed on the cell surface independently of IL-7. To address this, we examined GLUT-1 surface expression on D1 T cells, grown with or without 50 ng/ml of IL-7 for 18 h, by confocal microscopy. As shown in Fig. 5C, we were able to detect significant membrane-associated GLUT-1 in D1 T cells regardless of the addition of IL-7, indicating that the transporter was present and that glucose transport was possible in IL-7-deprived cells.
We confirmed our microscopic observations by measuring glucose uptake with a fluorescent glucose analog, 2-NBDG, in the same D1 T cells and under the same conditions that we assessed GLUT-1 surface expression with EGFP-GLUT-1 ligand by flow cytometry. The data in Fig. 5D show that D1 T cells incubated with IL-7 increased glucose uptake in the presence of the cytokine and decreased glucose uptake in the absence of the cytokine, but at the same time they evidenced little difference in the levels of GLUT-1 surface expression (Fig. 5E). Readdition of IL-7 (50 ng/ml) to cytokine-deprived D1 T cells after 4 h cells also resulted in increased glucose uptake (Fig. 5D) that was not accompanied by an increase in the surface expression of GLUT-1 (Fig. 5E). We concluded, therefore, that IL-7 promotes the synthesis of GLUT-1 as a late gene product (Figs. 4B and 5B), but that trafficking of GLUT-1 to surface can occur in the presence as well as the absence of IL-7 (Fig. 5, C and E). As a control for GLUT-1 surface expression we transiently expressed GLUT-1 in D1 T cells by the process of nucleofection (Amaxa) and detected elevated levels of the transporter in ∼50% of the cells, which correlated well with transfection efficiency (Fig. 5E).
HXKII regulates glucose metabolism in IL-7-dependent T cells.
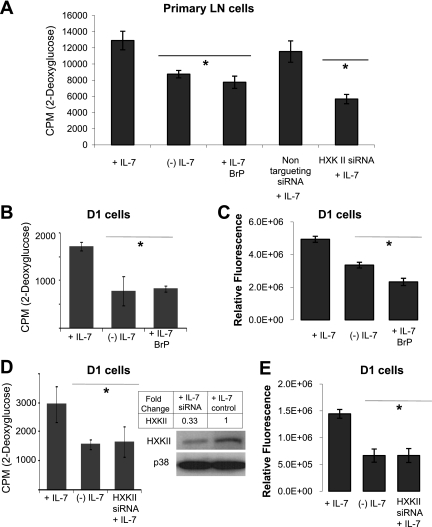
Having demonstrated that IL-7 controls the expression of HXKII, we next determined whether loss of HXKII would alter glucose use in IL-7-dependent T cells. We inhibited the enzymatic activity of HXKII using 3-bromopyruvate (3BrPA), a synthetic brominated derivative of pyruvic acid (25). Previously, we determined that 10 μM was the least toxic dose of 3BrPA that could inhibit glucose uptake and found that, in the absence of IL-7, BrPA had minimal toxic effects (data not shown). To specifically inhibit HXKII, we also knocked down gene expression using a commercially tested product composed of four pooled siRNAs specifically predesigned to target HXKII (Smart Pool, Dharmacon) and optimized for delivery to T cells using supplied delivery media (Accel, Dharmacon). Using a nontargeting FITC-labeled control siRNA and GAPDH siRNA, we previously determined siRNA delivery efficiency to T cells to be ∼50%. We assayed glucose use by 2-DOG incorporation and ATP production in the presence or absence of IL-7 and 3BrPA or HXKII siRNAs.
In Fig. 6A, we examined the effect of HXKII inhibition upon primary LN T cells that were isolated and cultured with 150 ng/ml of IL-7 for 7 days. Results observed showed that 2-DOG uptake was decreased in the absence of IL-7 as well as upon treatment with 3BrPA. LN T cells incubated in the presence of HXK Smart Pool siRNAs also significantly decreased the uptake of 2-DOG compared with a nontargeting siRNA control. These results suggested that HXKII is an important mediator of glucose metabolism in primary LN T cells.
Fig. 6.
Inhibition of HXKII decreases glucose uptake and ATP levels independently of IL-7. A: LN T cells were cultured with 150 ng/ml of IL-7 for 7 days and then incubated with or without IL-7 (150 ng/ml) for 18 h and treated with either 3-bromopyruvate (BrP) or the Smart Pool HXKII small interfering RNA (siRNA; Accell) described below. A nontargeting siRNA was used as control. Glucose use was measured by assaying the uptake of 2-DOG as described in materials and methods. B: D1 T cells were incubated with IL-7 (50 ng/ml) or without IL-7 and BrP for 18 h, then assayed for glucose use with 2-DOG as described in materials and methods. C: D1 T cells were incubated with IL-7 (50 ng/ml) or without IL-7 and BrP as in B, and total cellular ATP was measured by using the rLuciferase/Luciferin reagent (Promega) in a luminometer as described in materials and methods. Shown are the values for relative fluorescence that correlate with ATP concentrations. D–E: HXKII gene expression was inhibited in D1 T cells using Smart Pool HXKII siRNA (Accell). Knockout of HXKII gene expression with specific HXKII siRNA is shown in the immunoblot for HXKII (D). p38 is included as a loading control. Relative densities of bands in the blot were determined by using ImageJ software. To assess glucose use upon HXKII inhibition, 2-DOG uptake was measured as described in materials and methods. E: total intracellular ATP was measured as described in C. Results are representative of 3 independent experiments performed in triplicate (values are average ± SD). *P < 0.05 compared with cells cultured + IL-7.
Findings obtained with LN T cells were further investigated using D1 T cells. We observed that in the presence of IL-7 (50 ng/ml), 3BrPA reduced glucose use in D1 T cells by almost twofold, decreasing 2-DOG uptake to levels detected in the absence of the cytokine (Fig. 6B). As a consequence of reduce glycolytic activity, treatment of D1 T cells with 3BrPA also resulted in decreased levels of ATP in the presence of IL-7 (50 ng/ml) (Fig. 6C). To confirm these results, we treated D1 T cells with HXKII Smart Pool siRNAs. HXKII knockdown, by ∼67%, was confirmed by immunoblot (Fig. 6D, inset). As shown in Fig. 6, D and E, specific inhibition of HXKII was able to reduce 2-DOG uptake in the presence of IL-7 (50 ng/ml) to levels comparable to those observed in IL-7-deprived D1 T cells and also to significantly lower total intracellular ATP. These results convincingly show the essential role that HXKII plays in the metabolism of glucose in response to an IL-7 signal.
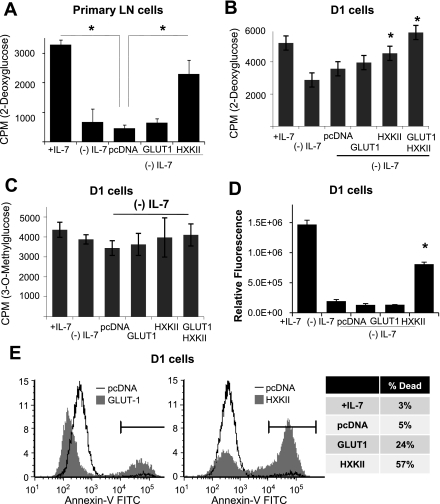
We then addressed whether expression of HXKII could replace the IL-7 signal and promote glucose use in the absence of the cytokine. To express HXKII, we used the method of nucleofection to transiently express the HXKII cDNA in the nucleus of T cells. In T cells, gene expression efficiencies ranged as high as 50%, eliminating the need for a selectable marker. As comparison, we also introduced by nucleofection the empty vector (pcDNA) and a plasmid encoding GLUT-1. In Fig. 5E, we showed that over 50% of the nucleofected D1 T cells expressed high levels of GLUT-1, confirming the efficiency of the gene expression system.
Using freshly isolated LN T cells (optimized for nucleofection efficiency), we expressed either the empty vector (pcDNA) or the cDNAs for HXKII or GLUT-1 in IL-7-deprived LN T cells and assayed for glucose uptake using 2-DOG. The results shown in Fig. 7A demonstrated that the expression of HXKII was able to rescue 2-DOG uptake in LN T cells cultured without IL-7. This is in contrast to the minimal effect of GLUT1 overexpression (Fig. 7A). These results were followed by a similar experiment using D1 T cells. HXKII or GLUT-1 was expressed in D1 T cells grown with or without IL-7; we measured glucose use and HXKII activity by incorporation of 2-DOG (Fig. 7B) or glucose import by incorporation of 3-OMG (Fig. 7C) and compared results with cells receiving the empty vector, pcDNA. We observed that nucleofection of HXKII cDNA was able to significantly increase 2-DOG uptake over that observed with pcDNA (Fig. 7B). Nucleofection of GLUT-1 also slightly increased 2-DOG import, but results were not statistically different from pcDNA (Fig. 7B). Nucleofection of both GLUT-1 and HXKII together resulted in the largest increase in 2-DOG uptake (Fig. 7B), as would be anticipated by increasing the levels of the transporter and the kinase in the absence of the cytokine. In contrast, little effect of GLUT-1 or HXKII expression was observed upon 3-OMG import, confirming the need for phosphorylation of the hexose to promote glucose uptake and retention (Fig. 7C). Note that the same outcome was obtained with primary T cells (p53+/+) in Fig. 7A and D1 T cells (p53−/−) in Fig. 7B, suggesting that p53 did not contribute to our results. Because we detected only minimal effects of GLUT-1 expression upon glucose import, we confirmed that GLUT-1 was being made by measuring GLUT-1 mRNA in nucleofected D1 T cells and found that GLUT-1 mRNA levels increased more than 1,800-fold in D1 T cells nucleofected with the GLUT-1 plasmid (data not shown), demonstrating that GLUT-1 was being made in these cells but having modest effects upon the utilization of glucose. Thus it is the phosphorylation of glucose, mediated by HXKII, that promotes glucose retention after import by the transporter that is the critical regulatory event in the metabolic response controlled by IL-7.
Fig. 7.
Overexpression of HXKII increases glucose uptake and ATP levels independently of IL-7. A: freshly isolated murine T cells from lymph nodes were nucleofected with either empty vector (pcDNA) or the cDNAs for GLUT-1 or HXKII and glucose use by 2-DOG uptake in the absence of IL-7 measured as described in materials and methods. Cells cultured with IL-7 (+Il-7) are shown as controls. B–C: D1 T cells were deprived from IL-7 for 18 h, nucleofected (Amaxa) with cDNAs for GLUT-1, HXKII, or empty vector (pcDNA), then incubated with or without IL-7 (50 ng/ml) for 4–8 h and assayed for glucose use with 2-DOG (A) or glucose import with 3-OMG (B) as described in materials and methods. D: D1 T cells were incubated with IL-7 or without IL-7, and deprived cells were nucleofected with plasmids as described in B. Total cellular ATP was measured by using the rLuciferase/Luciferin reagent (Promega) in a luminometer as described in materials and methods. Shown are the values for relative fluorescence that correlate with ATP concentrations. E: D1 T cells were incubated with or without IL-7 (50 ng/ml) and IL-7-deprived cells were nucleofected with plasmids as described in B. Cell death was assessed by staining with annexin-V-FITC and read by flow cytometry as described in materials and methods. Results are representative of 3 or more independent experiments performed in triplicate (values are average ± SD). *P < 0.05 compared with cells cultured without (−) IL-7.
To further investigate our findings that HXKII promotes glucose uptake in the absence of IL-7, we examined the levels of ATP as an indirect assessment of glucose metabolic activity. We had observed that inhibition of HXKII activity and expression resulted in decreased intracellular ATP (Fig. 6, C and E). In Fig. 7D, we found that D1 T cells, deprived of IL-7 and nucleofected with HXKII for 4 h, had increased total ATP approaching those levels found in IL-7 cultured cells. Increased ATP was not detected as a consequence of GLUT-1 expression (Fig. 7D). The question remained whether increased ATP as a consequence of HXKII expression would be protective or induce cell death in cells deprived of IL-7. We discovered that increased expression of HXKII for more than 8 h in cytokine-withdrawn D1 T cells accelerated the death process (data not shown). Moreover, in D1 T cells maintained in IL-7 the elevated levels of ATP mediated by overexpression of HXKII were ultimately toxic as seen by the increase in annexin-V staining. (Fig. 7E). Therefore, we concluded that, as a result of HXKII activity, ATP is produced that provides for the energetic needs of a cell but, in excess, can promote apoptosis.
In summary, our data show that IL-7 regulates glucose utilization by transcriptionally controlling the expression of the HXKII gene and that this process results in increased glucose metabolism that supports the growth activity of IL-7 in lymphocytes.
DISCUSSION
In support of its growth activities, we report that IL-7 controls glucose use in T cells by regulating the initial phosphorylation and subsequent intracellular retention of the hexose. Addition of IL-7 to cytokine-deprived T cells restored the uptake of a phosphorylatable glucose analog but not a nonphosphorylatable glucose analog within 2–4 h. This restoration correlated with an increase in the gene expression of HXKII, but not GLUT-1 or PFK-1, in response to an IL-7 signal. Moreover, overexpression of HXKII restored glucose uptake and ATP levels in cytokine-deprived IL-7-dependent T cells, whereas inhibition of HXKII decreased glucose uptake and ATP in the presence of IL-7. Our results therefore suggest that IL-7 promotes glucose use by directly regulating the transcription and therefore activity of HXKII.
Others have shown that incubation with IL-7 increased glucose uptake in naive T cells as well as activated T cells (54). These findings are consistent with our own in which we show, with both a T-cell line and primary T cells, that an IL-7 signal modulates glucose uptake. To address the mechanism underlying glucose transport in T cells, we found that only HXKII gene expression was restored in cytokine-deprived cells upon readdition of IL-7 and that only expression of HXKII could rescue glucose uptake during IL-7 withdrawal. We did not observe that IL-7-deprived cells expressing GLUT-1 could retain significant amounts of glucose, nor did we find that GLUT-1 gene expression was induced rapidly within a few hours of IL-7 readdition. We did note, however, that after 60 h of IL-7 readdition, the levels of GLUT-1 mRNA did increase. Others have reported a similar trend with detection of increased GLUT-1 surface expression after 72 h of incubation with IL-7 (47). This suggests that IL-7 may induce the production of GLUT-1 as a late gene product but that the trafficking of GLUT-1 to the cell surface may also occur by an IL-7-independent mechanism. In support, others have shown that IL-7 induced glucose uptake without an accompanying increase in GLUT-1 protein levels (54) and that high glucose in the extracellular milieu inhibited glucose phosphorylation and glucose uptake without affecting glucose transport (51). Given that the activity of HXKII retains glucose within the cells, once the hexose is transported through GLUT-1, and that this is a critical step in the glycolytic pathway, it is likely that transcriptional control of HXKII is the most effective mechanism by which IL-7 can rapidly modulate glucose use in T cells to supply energy as needed.
Further support of our findings comes from studies in which the regulation of HXKII activity was found to be subject to growth factor regulation (15). However, reports that growth factors like IL-3 increased GLUT-1 protein trafficking to the cell surface (53), and that IL-7 induced GLUT-1 expression and glucose uptake in proliferating thymocytes, T-Acute Lymphoblastic Leukemia (ALL) cells, and recent thymic emigrants (RTEs) seem to contradict our results (4, 33, 48). In similar studies, the phosphoinositide 3-kinase (PI3K) signaling pathway was implicated in the synthesis and trafficking of GLUT-1 (5, 8). Our results do not rule out that IL-7 could control GLUT-1 expression, perhaps through PI3K signaling. We put forward the concept that regulation of GLUT-1 activity by IL-7 is not the principal mechanism by which IL-7 promotes glucose utilization, but rather it is through the rapid induction of HXKII synthesis that IL-7 enables glucose metabolism in response to the energetic needs of T cells.
We also observed that IL-7 negatively regulated PFK-1. The most probable explanation for this observation is that a negative feedback mechanism, perhaps through accumulation of ATP, which is dependent on IL-7, exists. Hence the expression of PFK is downregulated when glucose is being transported and metabolized in response to IL-7 to control the production of ATP. We observed that overexpression of HXKII was not protective but did result in elevated levels of ATP that proved detrimental to cells, inducing apoptosis even in the presence of IL-7. There is support for the concept that ATP levels control the induction of apoptosis. Others have reported that increasing glucose concentrations, thereby increasing ATP, resulting in a switch from necrosis to apoptosis, with higher amounts of ATP driving the apoptotic process (28, 50). Such studies also showed that lowering ATP prevented apoptosis and that lowering ATP followed by recovery of ATP caused cell death (16).
Our result that IL-7 controls glucose uptake through the transcription of the HXKII gene is significant given the cytokine's critical role in T-cell development and homeostasis but also has implications for the therapeutic use of the cytokine. IL-7 has been listed among the top five immunotherapy compounds by the NCI (11). IL-7 is typically given to patients in superphysiological concentrations. Our findings suggest that excessive glycolytic activity could result from receiving a large dose of IL-7, mimicking the tumorogenic activation of glycolysis. Tumor cells tend to generate their energy through glycolysis instead of oxidative phosphorylation, a phenomenon known as the Warburg effect (55). Tumor cells are also known to overexpress HXKI and HXKII (39). By showing that the increased synthesis of HXKII by T cells is a direct result of engagement of the IL-7R, we have revealed a previously unknown activity of the cytokine that has implications for the development of IL-7 in the treatment of cancer, immunodeficiencies, and other disorders of the hematopoietic system.
GRANTS
This study was supported by an RO1 research grant, CA109524 (A. Khaled), and CA109524 supplement (M. Chehtane), from the NCI at the National Institutes of Health.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
Editorial assistance was provided by Dr. Kathleen Nemec, Rebecca Boohaker, Shannon Ruppert, and Christina Kittipatarin (University of Central Florida).
REFERENCES
- 1.Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol 26: 294–299, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291: H1411–H1420, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barata JT, Cardoso AA, Boussiotis VA. Interleukin-7 in T-cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk Lymphoma 46: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med 200: 659–669, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem 274: 20281–20286, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J 18: 1303–1305, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bental M, Deutsch C. Metabolic changes in activated T cells: an NMR study of human peripheral blood lymphocytes. Magn Reson Med 29: 317–326, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Bentley J, Itchayanan D, Barnes K, McIntosh E, Tang X, Downes CP, Holman GD, Whetton AD, Owen-Lynch PJ, Baldwin SA. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem 278: 39337–39348, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol 162: 3795–3801, 1999 [PubMed] [Google Scholar]
- 10.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J 11: 388–395, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 222: 357–368, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res 57: 1835–1840, 1997 [PubMed] [Google Scholar]
- 13.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5: 844–852, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol 172: 4661–4665, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15: 1406–1418, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izyumov DS, Avetisyan AV, Pletjushkina OY, Sakharov DV, Wirtz KW, Chernyak BV, Skulachev VP. “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim Biophys Acta 1658: 141–147, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev 16: 513–533, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Khaled AR, Bulavin DV, Kittipatarin C, Li WQ, Alvarez M, Kim K, Young HA, Fornace AJ, Durum SK. Cytokine-driven cell cycling is mediated through Cdc25A. J Cell Biol 169: 755–763, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaled AR, Durum SK. Death and Baxes: mechanisms of lymphotrophic cytokines. Immunol Rev 193: 48–57, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA 96: 14476–14481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaled AR, Li WQ, Huang J, Fry TJ, Khaled AS, Mackall CL, Muegge K, Young HA, Durum SK. Bax deficiency partially corrects IL-7 receptor alpha deficiency. Immunity 17: 561–573, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Khaled AR, Reynolds D, Young HA, Lee CK, Durum SK. Characterization of an interleukin-7-dependent thymic cell line derived from a p53(−/−) mouse. J Immunol Methods 274: 177–184, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine 39: 75–83, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kittipatarin C, Khaled AR. Ex vivo expansion of memory CD8 T cells from lymph nodes or spleen through in vitro culture with interleukin-7. J Immunol Methods 344: 45–57, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett 173: 83–91, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev 202: 67–83, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Krauss S, Brand MD, Buttgereit F. Signaling takes a breath—new quantitative perspectives on bioenergetics and signal transduction. Immunity 15: 497–502, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185: 1481–1486, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard WJ. Role of Jak kinases and STATs in cytokine signal transduction. Int J Hematol 73: 271–277, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med 203: 573–582, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 84: 949–957, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manel N, Battini JL, Sitbon M. Human T cell leukemia virus envelope binding and virus entry are mediated by distinct domains of the glucose transporter GLUT1. J Biol Chem 280: 29025–29029, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115: 449–459, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89: 1011–1019, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol 7: 144–154, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 333: 571–573, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol 8: 1049–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3: 771–782, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta 1555: 14–20, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med 200: 1189–1195, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 180: 1955–1960, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol 167: 6869–6876, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23: 7315–7328, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richard AM, Webb DL, Goodman JM, Schultz V, Flanagan JN, Getty-Kaushik L, Deeney JT, Yaney GC, Dunaway GA, Berggren PO, Tornheim K. Tissue-dependent loss of phosphofructokinase-M in mice with interrupted activity of the distal promoter: impairment in insulin secretion. Am J Physiol Endocrinol Metab 293: E794–E801, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1: 426–432, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I. Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: comparative analysis of gamma(c), Jak3, and gamma(c) and Jak3 double-deficient mice. Int Immunol 12: 123–132, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Swainson L, Kinet S, Manel N, Battini JL, Sitbon M, Taylor N. Glucose transporter 1 expression identifies a population of cycling CD4+ CD8+ human thymocytes with high CXCR4-induced chemotaxis. Proc Natl Acad Sci USA 102: 12867–12872, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 109: 1034–1042, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA 98: 8732–8737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tatsumi T, Shiraishi J, Keira N, Akashi K, Mano A, Yamanaka S, Matoba S, Fushiki S, Fliss H, Nakagawa M. Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovasc Res 59: 428–440, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Vinals F, Gross A, Testar X, Palacin M, Rosen P, Zorzano A. High glucose concentrations inhibit glucose phosphorylation, but not glucose transport, in human endothelial cells. Biochim Biophys Acta 1450: 119–129, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Von-Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 181: 1519–1526, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18: 1437–1446, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111: 2101–2111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292: C125–C136, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, Hennighausen L, O'Shea JJ. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA 103: 1000–1005, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu CR, Young HA, Ortaldo JR. Characterization of cytokine differential induction of STAT complexes in primary human T and NK cells. J Leukoc Biol 64: 245–258, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Ziegler SE, Morella KK, Anderson D, Kumaki N, Leonard WJ, Cosman D, Baumann H. Reconstitution of a functional interleukin (IL)-7 receptor demonstrates that the IL-2 receptor gamma chain is required for IL-7 signal transduction. Eur J Immunol 25: 399–404, 1995 [DOI] [PubMed] [Google Scholar]