Abstract
Cells respond to a hyposmotic challenge by swelling and then returning toward the resting volume, a process known as the regulatory volume decrease or RVD. The sensors for this process have been proposed to include cationic mechanosensitive ion channels that are opened by membrane tension. We tested this hypothesis using a microfluidic device to measure cell volume and the peptide GsMTx4, a specific inhibitor of cationic mechanosensitive channels. GsMTx4 had no effect on RVD in primary rat astrocytes or Madin-Darby canine kidney (MDCK) cells but was able to completely inhibit RVD and the associated Ca2+ uptake in normal rat kidney (NRK-49F) cells in a dose-dependent manner. Gadolinium (Gd3+), a nonspecific blocker of many mechanosensitive channels, inhibited RVD and Ca2+ uptake in all three cell types, demonstrating the existence of at least two types of volume sensors. Single-channel stretch-activated currents are present in outside-out patches from NRK-49F, MDCK, and astrocytes, and they are reversibly inhibited by GsMTx4. While mechanosensitive channels are involved in volume regulation, their role for volume sensing is specialized. The NRK cells form a stable platform from which to screen drugs that affect volume regulation via mechanosensory channels and as a sensitive system to clone the channel.
Keywords: cytoskeleton, GsMTx4, intracellular Ca2+, regulatory volume decrease, stretch-activated channels
cell volume regulation is essential for viability since metabolism and the resulting changes in osmotic pressure are universal, and environmental challenges are commonplace (1, 21). How does a cell know if its volume has changed? One common model uses mechanosensitive ion channels (MSCs), either cation selective (8, 9, 25) or anion selective (30, 33) and activated by membrane tension in a swollen cell. Proposed effector proteins that restore volume after a perturbation include transporters of neutral osmolytes such as the KCl cotransporter (13, 40), amino acid transporters (20), paired anion/cation channels, or the cytoskeleton operating in a three-dimensional mode (34).
In the traditional interpretation of cell volume regulation as a membrane-bound process (33, 35), cell swelling causes an increase in membrane tension that activates mechanosensitive channels, allowing an influx of Ca2+ and Na+. Most cells do show an influx and elevation of intracellular Ca2+ before regulatory volume decrease (RVD), suggesting that it serves as a second messenger for effector proteins (15). Ca2+ elevation can be triggered directly by Ca2+ passing through MSCs (8) or arriving through the Na+/Ca2+ exchanger (31). Channels selective for particular ions cannot regulate volume by themselves since volume regulation requires transport of net neutral solutes. In bacteria, the nonselective MSCs protect against extreme osmotic stress (22) because they open before lysis. Despite the presumptive role of MSCs as sensors of animal cell volume, channel activation has only been occasionally observed in patch-clamp experiments (8, 25) and there is no direct evidence linking MSCs to volume regulation. The hypothesis that MSCs play a role in volume regulation began as an explanation of why MSCs are present in all cells (8). Supporting the idea of MSCs having a sensory function for RVD, the lanthanides such as Gd3+ and La3+ prove capable of inhibiting both the channels (17, 43), swelling-induced Ca2+ elevation, and volume regulation (4, 9). However, the lanthanides are nonspecific reagents and may have multiple targets.
In contrast, the cationic MSCs are specifically inhibited by GsMTx4, a 4-kDa inhibitory cysteine knot (ICK) peptide (28) found in tarantula venom (6). The peptide has unusual pharmacological properties as it functions in both the D and L enantiomeric forms (39). If these mechanosensitive channels serve as sensors for volume regulation, then RVD should be blocked by GsMTx4, and we have tested this prediction in three different cell types. Only one cell type, normal rat kidney (NRK-49F), responded to GsMTx4 with a loss of RVD and Ca2+ influx, while astrocytes and Madin-Darby canine kidney (MDCK) cells were unaffected. In contrast, Gd3+ inhibited both responses in all three cell types, consistent with its observed nonspecific interaction with MSCs and other membrane-bound proteins and lipids. The ability of GsMTx4 to inhibit RVD in NRK cells by inhibiting the channels, Ca2+ uptake, and volume regulation suggests that in this cell type the channels do in fact serve as physiological sensors of cytoplasmic osmotic stress. The MSCs in other cell types apparently serve different functions.
MATERIALS AND METHODS
Cell volume measurement.
Cell volume was measured at room temperature using an impedance-based microfluidic sensor (3) that measures the resistance of a small chamber containing the cells. As cells swell, they displace saline and thus increase the chamber resistance. The cells were grown as monolayers on coverslips, and these were inverted over the 24-μm deep sensing chamber and clamped in place. The chamber resistance was measured using a four-point resistance bridge (evaporated Pt) driven at 1 μA and 50 Hz, and the voltage drop was measured using a lock-in amplifier. A commercial version of this instrument is now available (CVC-7000, Reichert Instruments, Depew, NY). The chamber was perfused with test solutions, and the cell volume was monitored in real time. The ratio of the volume change to the resting volume was calculated as previously described (18). A fresh cell culture was used for each trace, and the data represent the first challenge since we have found that RVD rapidly disappears with repeated stimulation (3). Results are plotted as means ± SE of n traces.
Cytosolic Ca2+ measurements.
Cells were washed and incubated in isotonic solution containing 6 μM Fluo-4 AM (Invitrogen, Carlsbad, CA) at 37°C for 15 min for astrocytes, and 40 min for MDCK and NRK cells. The cells were then washed and kept in the dark at room temperature for 15 min before the experiment. The microfluidic chamber was also used for fluorescence measurements using a Zeiss upright microscope (Axio Imager M1, Zeiss), and the data were recorded using a charge-coupled device camera (Axiocam MRm, Zeiss). We used the filter set 38 HE EGFP (excitation: 470 ± 40 nm; dichroic filter: 495 nm; emission: 525 ± 50 nm, Zeiss). Time-lapse experiments were recorded with AxioVision software (Zeiss). The latencies for the Ca2+ increase were quantified as the time from solution change until reaching 10% of (peak-baseline) Ca2+.
Patch clamp.
An Axopatch 200B (Axon Instruments) was used to control patch voltage. Data were collected (National Instruments) using QUBIO software. Currents were sampled at 10 kHz and low-pass filtered at 2 kHz through a four-pole Bessel filter. All potentials are defined with respect to the extracellular surface.
Electrodes were pulled on a HEKA pipette puller (PIP5), painted with Sylgard 184 (Dow Corning), and fire polished. Electrodes were filled with potassium solution of (in mM) 140 KCl, 5 EGTA, 3 MgCl2, and 10 HEPES, pH 7.4. Electrode resistances ranged from 3 to 8 MΩ. Bath saline contained (in mM) 140 NaCl, 5 KCl, 3 MgCl2, 1 CaCl2, and 10 HEPES, pH 7.4. Pressure pulses were generated with a high-speed pressure clamp (ALA Scientific, Farmingdale, NY). GsMTx4 was perfused over the patch at the indicated concentration using an ALA Scientific perfusion system. Offline data analysis was done with free QUB software (www.qub.buffalo.edu). Data were averaged for a minimum of five pressure pulses.
Cell culture.
Cells were grown to 80–95% confluence on glass coverslips in 35-mm petri dishes. Primary astrocytes were isolated from rat brain and stored frozen as previously described (37) and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 1% penicillin and streptomycin. NRK-49F cells obtained from American Type Culture Collection were cultured in DMEM containing 5% calf serum and 1% penicillin and streptomycin. MDCK epithelial cells were grown in DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin.
Solution and reagents.
Isotonic and hypotonic solutions both contained (in mM) 75 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, and 10 HEPES titrated to pH 7.4. The isotonic solution (320 mosM) was prepared by adding mannitol to the hypotonic solution (187 mosM), keeping the ionic strength fixed. To remove extracellular Ca2+, CaCl2 was replaced with MgCl2. The conductivity of all solutions was gently titrated to equality using NaCl, and the final osmolarity was measured with an osmometer (model 303, Advanced Instrument). Gadolinium chloride (Signa-Aldrich, St. Louis, MO) was prepared as a 10 mM stock solution in water. The enantiomeric forms of GsMTx4 were made and purified according to previously published protocols (26, 39).
RESULTS
Effects of GsMTx4 on RVD.
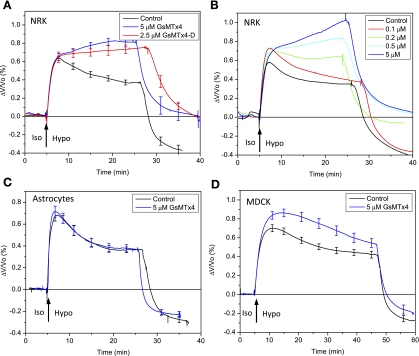
Cells were initially tested for RVD in the sensing chamber by perfusing an isotonic solution (320 mosM) followed by a hypotonic solution (187 mosM). To test the effect of GsMTx4, we perfused fresh cultures (to avoid the adaptation seen in repeated stimulation) with isotonic saline containing 5 μM GsMTx4 for 10–15 min followed by a hypotonic challenge in saline also containing 5 μM GsMTx4. In NRK cells, GsMTx4 eliminated RVD and the cells continued to swell as long as the stimulus was applied (Fig. 1A). The D enantiomer of GsMTx4 (2.5 μM) also blocked the RVD in NRK cells as shown in Fig. 1A. However, GsMTx4 had no effect on RVD in either astrocytes or MDCK cells (Fig. 1, C and D). In NRK cells, the dose-response behavior of RVD to GsMTx4 (Fig. 1B) indicated a Kd of 100–200 nM in agreement with published results on the sensitivity of MSCs to GsMTx4 in patch-clamp experiments (37). GsMTx4 had no significant effect on the steady-state volume of challenged MDCK or astrocytes, although it slightly slowed the kinetics of RVD in both cell types.
Fig. 1.
Effects of GsMTx4 on cell volume regulation of normal rat kidney (NRK-49F) cells (A and B), primary rat astrocytes (C), and Madin-Darby canine kidney (MDCK) cells (D). The chamber was perfused with isotonic solution (Iso) for 10–15 min and then switched to hypotonic solution (Hypo) at the times indicated. The ratio of the volume change to the resting cell volume, ΔV/Vo, is plotted in A–D. Both GsMTx4 L enantiomer (5 μM) and D enantiomer (2.5 μM) blocked regulatory volume decrease (RVD) in the NRK cells (control and L-form, n = 5; D-form, n = 3). The cells responded in a dose-dependent fashion (B) with an apparent Kd of 100–200 nM. In primary rat astrocytes (n = 5) and MDCK (n = 6) cells, 5 μM GsMTx4 in both isotonic and hypotonic solutions had no significant effect on RVD (C and D).
While all three cell types have cationic MSCs that are inhibited by GsMTx4 as shown later in this section, the results above suggest that cationic MSCs do not universally function as volume sensors. MSC currents that are visible in inside-out or cell-attached patch-clamp recordings appear generally unresponsive to osmotic stress in the bath, suggesting that they are well protected by the cytoskeleton (24, 34). The effect of GsMTx4 on mild slowing of the RVD kinetics in astrocytes and MDCK cells may be an effect of GsMTx4 on Ca2+-activated K+ channels (19, 32).
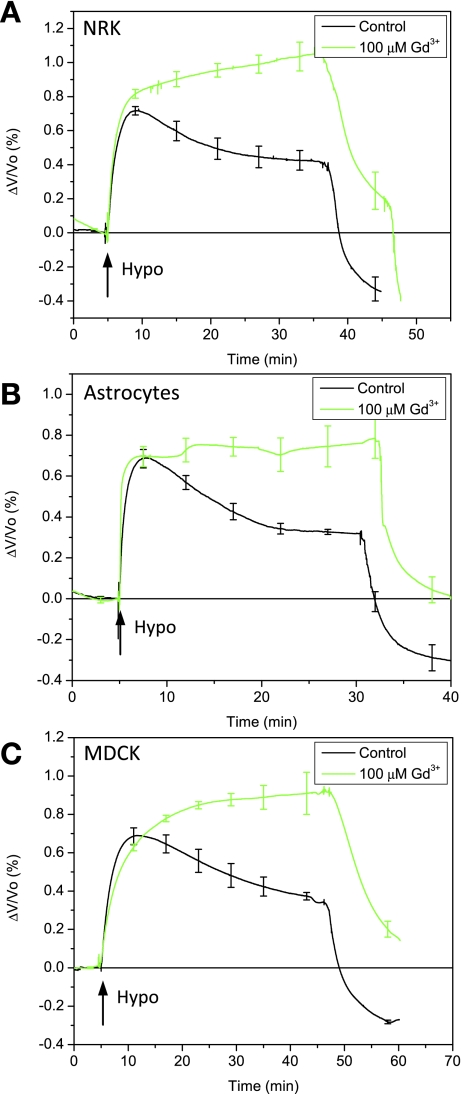
Since the lanthanides are known to inhibit RVD (9), swelling-induced Ca2+ uptake (2), and MSC activity (43), we tested the cells' sensitivity to 100 μM Gd3+. In agreement with previously published work (25), Fig. 2, A–C, shows that Gd3+ inhibited RVD and Ca2+ uptake (data not shown) in the three cell types by mechanisms that may include sensors and/or effectors.
Fig. 2.
Sensitivity of RVD to Gd3+ for NRK-49F (A), primary rat astrocytes (B), and MDCK cells (C). Gd3+ (100 μM) in isotonic and hypotonic solutions inhibited RVD in all three cell types, causing a slow, extended, increase in volume (NRK, n = 4; astrocytes, n = 3; MDCK, n = 3).
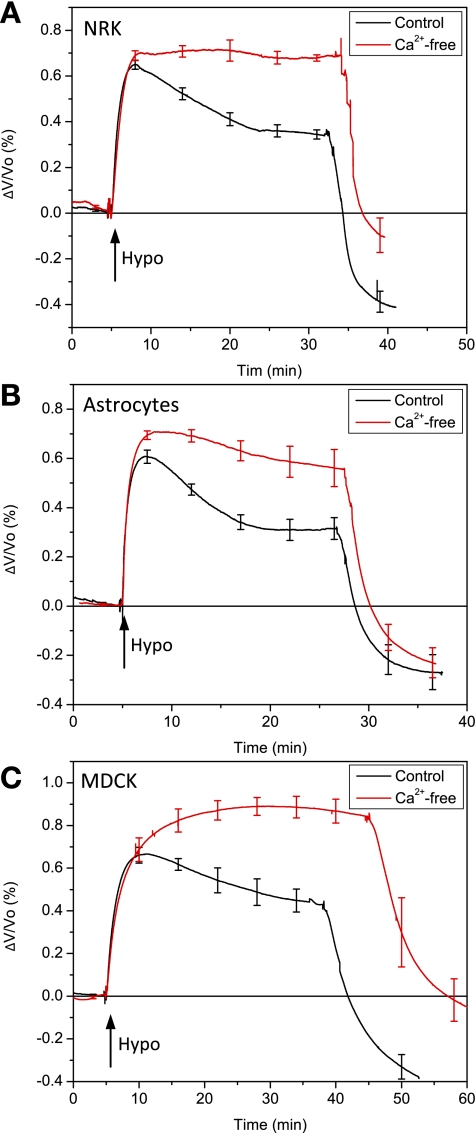
Extracellular Ca2+ entry is required for RVD.
Most cells require an influx of extracellular calcium (23) for RVD, and cationic MSCs that are permeable to Ca2+ are a likely source. We tested the effect of extracellular Ca2+ concentration on the swelling-induced Ca2+ uptake. As expected (37), replacing extracellular Ca2+ with Mg2+ suppressed the swelling-induced Ca2+ increase in all three cell types (Fig. 3, A–C). These results are consistent with previous findings for astrocytes (8) and MDCK (32). Inward Ca2+ current via L-type channels has been reported in NRK-49F cells (10), although there are no data available in the literature on RVD in NRK cells. Removing extracellular Ca2+ without chelation did not completely abolish RVD, probably resulting from contamination with 50–100 μM Ca2+ present in other salts.
Fig. 3.
The dependence of cell volume regulation on extracellular Ca2+ for NRK-49F (A), primary rat astrocytes (B), and MDCK cells (C). The removal of Ca2+ suppressed RVD in all three cell types (NRK, n = 4; astrocytes, n = 4; MDCK, n = 3).
MSCs are coupled to RVD via Ca2+ entry in NRK cells.
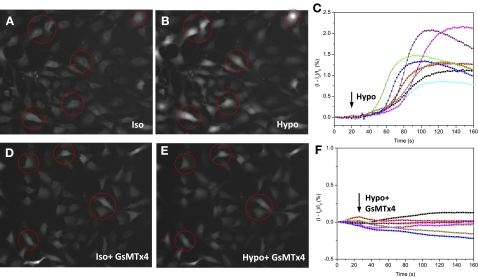
If Ca2+ entry in a hypotonic challenge occurs via MSCs, it should be blocked by GsMTx4. In NRK cells a hypotonic challenge led to an elevation of Ca2+ with a striking 30 ± 8.6-s latency (Fig. 4, A–C). The Ca2+ elevation was inhibited by 5 μM GsMTx4 (Fig. 4, D–F). Thus, for NRK cells the simple picture of cell swelling increasing membrane tension and opening cationic MSCs causing Ca2+ influx seems correct. The long latency may reflect the time required for cell swelling to cause fracturing of the cytoskeleton that normally protects the channels from osmotic stress (38).
Fig. 4.
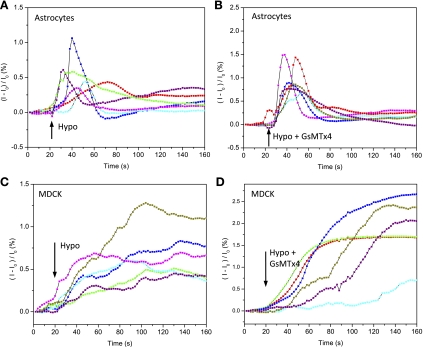
Response of intracellular Ca2+ to hypotonic challenges in NRK-49F cells. A and B: Ca2+ Fluo-4 images before (A) and after (B) hypotonic challenge in control solutions. C: typical traces of the time dependence of the Ca2+ increase from representative cells marked in A and B are plotted as the fractional change in fluorescence (I − Io)/Io. Similar responses were observed in ∼80% of cells. D–F: application of 5 μM GsMTx4 in both isotonic and hypotonic solutions eliminated the swelling-induced Ca2+ elevation (same conditions as in A–C).
In both astrocytes and MDCK cells, a hypotonic challenge increased intracellular Ca2+ (Fig. 5, A and C), but in these cells, GsMTx4 (5 μM) had no effect on swelling-induced Ca2+ elevation (Fig. 5, B and D). This observation suggests that the swelling-induced Ca2+ flux did not enter through cationic MSCs. In apparent contradiction, the latency for Ca2+ elevation in these cells was significantly shorter than for the NRK cells (P < 0.0001), as though the volume sensors in those cells were not cationic MSCs and were unprotected.
Fig. 5.
GsMTx4 has little effect on swelling-induced Ca2+ elevation in astrocytes and MDCK cells. A: time dependence of swelling-induced intracellular Ca2+ in astrocytes as taken from representative cells. B: time dependence of swelling-induced Ca2+ elevation in the presence of 5 μM GsMTx4. C and D: swelling-induced Ca2+ elevation in MDCK cells (as per A and B).
Effect of GsMTx4 on stretch-activated currents.
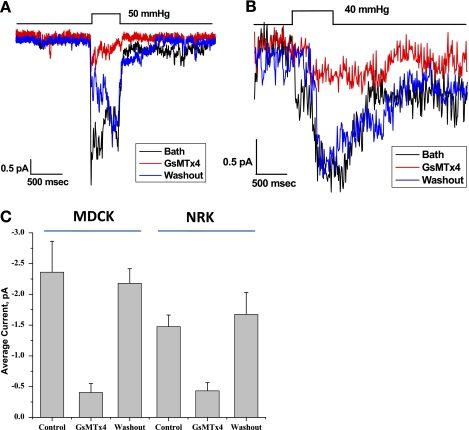
Both MDCK and NRK cells showed mechanosensitive channel activity in outside-out patch recordings, and these currents were inhibited by GsMTx4 (Fig. 6). The same effect of GsMTx4 was observed in primary astrocytes and reported previously (28). The patches were perfused with bath solution to determine the baseline activity (Fig. 6, A and B, black line). GsMTx4 (3 μM) dissolved in bath solution was perfused over the patch (Fig. 6, A and B, red line) to demonstrate inhibition. This reaction was reversible when the peptide was washed out with bath saline (Fig. 6, A and B, blue line). The channels in both cell types were nonselective cation channels reversing near 0 mV (data not shown). The response for three patches is shown in Fig. 6C, determined by averaging channel currents during the pressure pulse in the presence and absence of GsMTx4.
Fig. 6.
Inhibition of stretch-activated currents by GsMTx4. A and B: GsMTx4 at 3 μM concentration inhibited pressure-dependent channel activity for MDCK cells (A) and NRK cells (B) for outside-out patches. Outside-out patches were formed and channel activity was monitored at −50 mV with the indicated pressure pulse. Inhibition was reversed with washout with bath solution. The data were averaged for 5–10 pulses before, during, and after (washout) the application of the peptide. C: the average response over three patches, using the current amplitude during the pressure pulse.
DISCUSSION
In all three cell types, RVD required extracellular Ca2+ and was blocked by Gd3+. However, only in NRK cells did the L and D enantiomers of GsMTx4 block both Ca2+ uptake and RVD. GsMTx4 is known to inhibit cationic MSCs in a variety of cell types including rat astrocytes, chick heart, skeletal muscle, and human smooth muscle (6, 11, 37). Although the physiological function of these channels remains uncertain, the general function is not volume regulation.
The influx of Ca2+ through MSCs seems to couple channel activation to the effectors that are responsible for RVD. The effectors in NRK cells are not known, although their sensitivity to GsMTx4, RVD, and extracellular Ca2+ provides a useful tool to screen candidates. Since RVD requires an influx of Ca2+ to trigger a release from intracellular stores, the MSCs that mediate Ca2+ entry must be close to the Ca2+ release sites so the Ca2+ concentration will rise significantly above the mean.
Most work on cell volume regulation has focused on membrane-based models that utilize transport mechanisms across membranes to sense and determine cells volume, consistent with a process for the early transient stage of volume regulation before the involvement of three-dimensional cytoskeleton. However, attached cells under osmotic stress are not spheres so that the stress in the cytoskeleton dominates the response. The role of the cytoskeleton in determining the steady-state cell volume appears to have been underestimated (12) and this can account for the continued swelling when volume regulation is inhibited. When highly swelled cells were returned to normal media there was undershoot in the volume, suggesting that the cells did lose diffusible osmolytes during the RVD (control data in Figs. 1–3). However, the NRK cells treated with GsMTx4 (Fig. 1A) and cells treated with Gd3+ (Fig. 2, A–C) returned to the resting volume, suggesting no net gain or loss of osmolytes during the challenge.
There is a 1:1 correlation between cytoskeletal volume and cell volume. The response to a hypotonic challenge begins with an influx of water, with the cell volume lagging as it increases only with the integral of the flux. Recent atomic force microscopy experiments show that most of the osmotic stress of attached cells is borne by the cytoskeleton (34). Swelling of the cytoskeleton may lead to breakage of cross-links and subsequent reformation in a new pattern of interaction (7) with a lower osmotic pressure. The osmotic pressure of a gel is inversely related to the cross-link density (41). The swelling-induced Ca2+ elevation could have a significant effect on cytoskeleton cross-linking (14), so that the cytoskeleton itself may serve as one of the effectors. During the initial phase of swelling, cross-links in the cortex can break, transferring stress to the bilayer and activating MSCs. The physiological function of MSCs may be to detect weakness in the local cytoskeleton.
The pronounced latency of the Ca2+ increase with NRK cells (Fig. 4C), also observed with cells in cultures of astrocytes and MDCK cells (cf. Fig. 5), is a signature of multiple reactions preceding Ca2+ entry. Some of the delay will arise from the fact that the cell volume is the integral of the flux, but that will not explain the differences in latency between cell types. We hypothesize that the latency reflects the time required for delamination of the membrane from the cytoskeleton as described for blebs (7), and this will vary with cell type, shape and metabolic status. The short latency of astrocytes and MDCK cells to a hypotonic challenge implies that if they use membrane-bound sensors, they are partly delaminated at rest. The cell volume sensors employed in astrocytes and MDCK cells are clearly different from the MSCs observed in patch-clamp recordings since the channels in the patch experiments are blocked by GsMTx4 (Fig. 6A). This subtle variability in the sensor of cell volume may explain why cloning of the endogenous sensors has proven so difficult (16). While cationic MSCs are likely to be members of the transient receptor potential superfamily, there are clearly many variations (29).
The ready characterization of MSCs in NRK cells using only cell volume and/or intracellular Ca2+ probes creates a screen for pharmacological agents that are active on that class of MSCs. The MSCs have been implicated as drug targets for the treatment of muscular dystrophy (36), atrial fibrillation (5), glial tumors (27), and other diseases that may involve defects in the distribution of mechanical stress. The NRK screen could be exploited to clone the GsMTx4-sensitive channels by using randomized RNAi libraries (42).
GRANTS
This work was supported by National Institutes of Health Grants DK-77302 (to S. Z. Hua) and HL-054887 (to F. Sachs) and by New York State Office of Science, Technology & Academic Research (NYSTAR).
DISCLOSURES
P. A. Gottlieb and F. Sachs are stockholders in Rose Pharmaceuticals.
ACKNOWLEDGMENTS
We thank Dr. Stephen Besch for assistance on the instrumentation for volume measurements.
REFERENCES
- 1.Acher R. Water homeostasis in the living: molecular organization, osmoregulatory reflexes and evolution. Ann Endocrinol (Paris) 63: 197–218, 2002 [PubMed] [Google Scholar]
- 2.Arniges M, Vazquez E, Fernandez-Fernandez JM, Valverde MA. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem 279: 54062–54068, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ateya DA, Sachs F, Gottlieb PA, Besch S, Hua SZ. Volume cytometry: microfluidic sensor for high-throughput screening in real time. Anal Chem 77: 1290–1294, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 118: 2435–2440, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature 409: 35–36, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49: 249–270, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435: 365–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature 330: 66–68, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Clemo HF, Baumgarten CM. Swelling-activated Gd3+-sensitive cation current and cell volume regulation in rabbit ventricular myocytes. J Gen Physiol 110: 297–312, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Roos A, Willems PH, van Zoelen EJ, Theuvenet AP. Synchronized Ca2+ signaling by intercellular propagation of Ca2+ action potentials in NRK fibroblasts. Am J Physiol Cell Physiol 273: C1900–C1907, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol 575: 913–924, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebner HL, Cordas A, Pafundo DE, Schwarzbaum PJ, Pelster B, Krumschnabel G. Importance of cytoskeletal elements in volume regulatory responses of trout hepatocytes. Am J Physiol Regul Integr Comp Physiol 289: R877–R890, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol 288: C1451–C1460, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa R, Maselli A, Thomson SAM, Lim RWL, Stokes JV, Fechheimer M. Calcium regulation of actin crosslinking is important for function of the actin cytoskeleton in Dictyostelium. J Cell Sci 116: 187–196, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Galizia L, Flamenco MP, Rivarola V, Capurro C, Ford P. Role of AQP2 in activation of calcium entry by hypotonicity: implications in cell volume regulation. Am J Physiol Renal Physiol 294: F582–F590, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev 48: 231–252, 1996 [PubMed] [Google Scholar]
- 18.Heo J, Meng FJ, Sachs F, Hua SZ. Dynamic effects of Hg2+-induced changes in cell volume. Cell Biochem Biophys 51: 21–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert IH. Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem Res 29: 27–63, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247–306, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18: 1730–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarty NA, O'Neil RG. Calcium signaling in cell-volume regulation. Physiol Rev 72: 1037–1061, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Morris CE. Mechanoprotection of the plasma membrane in neurons and other non-erythroid cells by the spectrin-based membrane skeleton. Cell Mol Biol Lett 6: 703–720, 2001 [PubMed] [Google Scholar]
- 25.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292: C460–C467, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ostrow KL, Mammoser A, Suchyna T, Sachs F, Oswald R, Kubo S, Chino N, Gottlieb PA. cDNA sequence and in vitro folding of GsMTx4, a specific peptide inhibitor of mechanosensitive channels. Toxicon 42: 263–274, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Ostrow L, Sachs F. Mechanosensation and endothelin in astrocytes-hypothetical roles in CNS pathophysiology. Brain Res Rev 48: 488–508, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Oswald RE, Suchyna TM, McFeeters R, Gottlieb P, Sachs F. Solution structure of peptide toxins that block mechanosensitive ion channels. J Biol Chem 277: 34443–34450, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol 428: 183–207, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Petrunkina AM, Harrison RA, Ekhlasi-Hundrieser M, Topfer-Petersen E. Role of volume-stimulated osmolyte and anion channels in volume regulation by mammalian sperm. Mol Hum Reprod 10: 815–823, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Reeves JP, Abdellatif M, Condrescu M. The sodium-calcium exchanger is a mechanosensitive transporter. J Physiol 586: 1549–1563, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothstein A, Mack E. Volume-activated calcium uptake: its role in cell volume regulation of Madin-Darby canine kidney cells. Am J Physiol Cell Physiol 262: C339–C347, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Sabirov RZ, Okada Y. ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn J Physiol 54: 7–14, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Spagnoli C, Beyder A, Besch S, Sachs F. Atomic force microscopy analysis of cell volume regulation. Phys Rev E Stat Nonlin Soft Matter Phys 78: 031916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stutzin A, Hoffmann EK. Swelling-activated ion channels: functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol (Oxf) 187: 27–42, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys J 973: 738–747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suchyna T, Sachs F. Dynamic regulation of mechanosensitive channels; capacitance used to monitor patch tension in real time. Phys Biol 1: 1–18, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys J 97: 738–747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suchyna TM, Tape SE, Koeppe RE, 2nd, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430: 235–240, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Taouil K, Hannaert P. Evidence for the involvement of K+ channels and K(+)-Cl− cotransport in the regulatory volume decrease of newborn rat cardiomyocytes. Pflügers Arch 439: 56–66, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Trombetta G, Di Bona C, Grazi E. The transition of polymers into a network of polymers alters per se the water activity. Int J Biol Macromol 35: 15–18, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Wang YE, Cotticelli MG, Wilson RB. A random shRNA-encoding library for phenotypic selection and hit-optimization. PLoS ONE 3: e3171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243: 1068–1071, 1989. [DOI] [PubMed] [Google Scholar]