Abstract
Breathing-disordered states, such as in obstructive sleep apnea, which are cyclical in nature, have been postulated to induce neurocognitive morbidity in both pediatric and adult populations. The oscillatory nature of intermittent hypoxia, especially when chronic, may mimic the paradigm of ischemia-reperfusion in that tissues and cells are exposed to episodes of low and high O2 and this may lead to oxidant stress. Therefore, we decided to explore the potential contribution of oxidant stress in our intermittent hypoxia/hypercapnia animal model and the role that mitochondria might play in this stress. Neonatal mice were exposed to intermittent hypoxia/hypercapnia for 10 days and 2 wk. Combined intermittent hypoxia/hypercapnia led to a marked increase in apoptotic cell death in the cerebral cortex. Oxygen consumption studies in isolated mitochondria from intermittent hypoxia/hypercapnia-exposed brains demonstrated significant reductions in both state 4 and state 3 respiratory activities by ∼60% and 75%, respectively. Electron paramagnetic resonance spectroscopy registered a significant increase in superoxide production during nonphosphorylating state 4 by 37%, although superoxide leakage during state 3 did not increase upon treatment. Neuronal superoxide-specific dihydroethidium oxidation was also greater in exposed animals. These studies indicate that intermittent hypoxia/hypercapnia leads to oxidative stress due to mitochondrial response within the mouse central nervous system.
Keywords: cyclical oxygen deprivation, mouse, central nervous system, apoptosis, superoxide
hypoxia often results in deleterious effects on central nervous system (CNS) function and viability. The length, extent, and pattern of hypoxic exposure, as well as age at the time of exposure, determine the severity of the clinical outcome (32). Intermittent hypoxia (IH) occurs during apnea of prematurity, obstructive sleep apnea (OSA), and sickle cell disease. OSA, as the prototypical sleep-disordered breathing state, is characterized by periodic breathing, episodic hypoxemia, and repeated arousal from sleep. OSA and other sleep-disordered breathing states are associated in children with neurocognitive abnormalities including attention deficit and poor school performance (18). Neonatal animal studies have demonstrated that IH leads to executive dysfunction, increased responsiveness to novelty, and locomotor hyperactivity that persists into adulthood (11, 36). Additionally, patients who suffer from sleep-disordered breathing states demonstrate increased systemic markers of oxidative stress, and free radical generation has been reported to be increased in OSA patients (29, 38). Combined intermittent hypoxia and hypercapnia (CIH/CIC) more closely approximates breathing-disordered states than IH alone and may also mimic the paradigm of ischemia-reperfusion in that tissues and cells are exposed to episodes of low and high O2 and this may lead to oxidant stress. Therefore, our recent studies have focused on determining the participation of reactive oxygen species (ROS) in intermittent hypoxia- and hypercapnia-induced injury or cell death.
IH induces a series of cellular, molecular, and pathophysiological responses that result in either adaptation and survival or injury and cell death. IH leads to oxidative stress and induces lipid peroxidation, increased production of stress-responsive proteins, and neuronal apoptosis (19, 20, 37, 47). Even though it is still a controversial issue, it is important to realize from recent evidence that ROS and reactive nitrogen species are generated not only from conditions of increased O2 such as in hyperoxia but also from hypoxia (9, 23). Whether most ROS that are produced in cells as a result of OSA are derived from mitochondria is not clear at present. Hypoxic stress may also lead to impairment of antioxidant defense mechanisms by alterations in the activities of glutathione reductase and glutathione peroxidase, which can impair mitochondrial activity at complexes I and III (42).
The very fact that mitochondria participate in amplification of suicide signals in cells has stimulated interest in the mechanism of this and related phenomena. It seems probable that mitochondria possess an autonomic system that allows them to commit suicide as a mechanism for ridding the cell of malfunctioning mitochondria, e.g., those overproducing ROS (43). This mitoptosis or mitochondrial apoptosis is mediated by ROS, causing opening of the permeability transition pores in the inner mitochondrial membrane. This is followed by collapse of the mitochondrial membrane potential, interruption of the import of vital mitochondrial protein precursors, and eventually disintegration of the organelle (44). Therefore, mitoptosis can serve to purify the mitochondrial population of a cell from ROS-overproducing mitochondria. Mitoptosis can purify the mitochondrial population in a cell from the ROS-overproducing organelles, which could avoid catastrophic cell death if ROS levels are left unchecked. Large-scale apoptosis also can be used by organisms to eliminate some organs during ontogenesis (organoptosis). It is therefore conceivable that mitochondria tightly regulate apoptosis through ROS-mediated control of mitoptosis. Under severe hypoxia, mitochondria permeabilization of the outer membrane and release of cytochrome c are crucial events marking cell death.
We therefore hypothesized that exposure to CIH/CIC would lead to enhanced neuronal death within the CNS and would occur in response to the excessive generation of ROS. We therefore examined neonatal mice that were exposed to CIH/CIC for markers of cell death and oxidant stress, as well as for the production of superoxide.
MATERIALS AND METHODS
Animals
Pregnant CD-1 mice were obtained from Charles River (Wilmington, DE). Care was exercised in the handling of these animals, and the minimal number of animals that was absolutely required was used in this study. This study was conducted in conformity with the American Physiological Society's “Guiding Principles for Research Involving Animals and Human Beings” and was approved by the University of California, San Diego Institutional Animal Care and Use Committee. Animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 80-23, revised 1996).
Intermittent Hypoxia/Hypercapnia Exposure
Postnatal day 2 (P2) CD-1 mouse pups were housed with their dams in isobaric Plexiglas chambers. Litters were culled to eight to ensure adequate nutrition of the pups. Food and water were provided ad libitum within the chamber. The protocols used to expose mice to hypoxia and hypercapnia have been described previously (13, 15, 27). In brief, a combination of nitrogen and oxygen (O2) and carbon dioxide (CO2) was injected into the chambers through a network of tubing to achieve selected concentrations of O2 and CO2. This was controlled by the Oxycycler hydraulic system (model A44x0, BioSpherix, Redfield, NY) and ANA-Win2 software (version 2.4.17, Watlow Anafaze). For CIH/CIC, every cycle consisted of a 4-min period during which O2 and CO2 concentrations were maintained at 11 ± 0.1% and 8 ± 0.1%, respectively, followed by a 4-min period at 21 ± 0.1% and ∼0.0 ± 0.1%, respectively. The ramp time between the two levels was <1–2 min. Normal control mice were kept in the same room and were exposed to the same level of noise and light for the duration of each experiment. Litters of mice, with their respective controls, were exposed to CIH/CIC for 10 days or 2 wk, 24 h/day, starting from P2. We selected these exposure durations of 2 wk on the basis of previous studies demonstrating that peak apoptosis in response to IH alone occurred at 10 days of exposure (20) and that robust changes in the expression of acid-base transporters occurred at 2 wk of exposure in response to IH (13) and constant hypercapnia (26).
Pulse Oximetry
Respiratory rates, oxygen saturation, and pulse rates were recorded on unrestrained, unanesthetized mice (n = 4) either in room air or in the environmental chamber with the MouseOx system (Starr Life Sciences, Oakmont, PA) per the manufacturer's instruction. Data were collected for at least 5 min and only used when no error code was given; respiratory rates were then calculated as an average.
Immunofluorescence Staining
Perfusion and fixation.
Mice were deeply anesthetized with drops of isoflurane (MWI, Meridian, ID) in a closed chamber and were rapidly perfused transcardially with 0.9% saline in 0.1 M phosphate buffer (PB; pH 7.5) and 4% paraformaldehyde in 0.1 M PB (pH 7.5). The fixed tissue was dissected out and placed in fixative for 24 h, followed by cryoprotection in 20% glucose in PB for ∼48 h. Subsequently, 10-μm and 50-μm frozen sections were prepared from the dorsal aspect of the cerebral cortex from bregma (−0.94) to bregma (−3.08) according to Paxinos and Franklin (33), which overlies the entire rostro-caudal extent of the rostral hippocampal formation, with a Leica cryostat (Leica Microsystems, Bannockburn, IL).
Single-stranded DNA and cytochrome c staining for apoptosis.
Staining for apoptotic cells was performed with an antibody to single-stranded DNA (ssDNA) that is specific to apoptotic but not necrotic cells (ssDNA mouse monoclonal antibody, clone F7–26; Chemicon, Millipore) (16) and was performed according to the manufacturer's recommendations. Cryosections were incubated in 20 mg/ml proteinase K (Chemicon) at room temperature (RT) for 20 min to remove DNA-binding proteins such as histones and then rinsed with distilled, deionized water (DDW; 3 × 2 min each). Antigen retrieval was then performed by incubating cryosections in 10 mM citrate buffer, pH 6 in a microwave oven (3 cycles; 3 min each) followed by rinsing in phosphate-buffered saline (PBS; 2 × 2 min each) and DDW (1 × 2 min). Sections were then incubated in 50% formamide (Sigma-Aldrich, St. Louis, MO) in DDW at 56°C for 20 min to unravel DNA strands, after which they were transferred to ice-cold PBS for 5 min. Sections were blocked in 3% nonfat milk (Carnation, Nestle Food, Glendale, CA) in DDW for 15 min and then incubated with ssDNA primary antibody in 1% milk-PBS for 15–20 min at RT. After being rinsed in PBS three times for 5 min each, sections were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgM secondary antibody (Molecular Probes, Invitrogen Corp, Eugene, OR) and rinsed three times for 5 min each in PBS. This was followed by incubating sections with a primary antibody to NeuN (Chemicon, Temecula, CA), a neuronal marker, followed by an Alexa Fluor 546 secondary antibody (Molecular Probes) and mounted with Prolong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes).
Cell counting.
Serial sections separated by at least 30 μm were processed either on slides (10-μm sections) or as free-floating sections (50-μm sections). Cryosections were then viewed and analyzed on a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Mannheim, Germany). Positive cells were counted within a ×40 magnification high-power field (hpf) for both normoxic and hypoxic/hypercapnic tissues. A minimum of 3 sections were examined from each brain and at least 10 hpf were assayed for each section. Additionally, we probed some of the cryosections (n = 3) with antibodies to cytochrome c (Imgenex, San Diego, CA)and Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA) to determine the potential involvement of mitochondria in the observed cell death.
Dihydroethidium staining for intracellular superoxide radical.
Neonatal mice (P2) with their dams were exposed to intermittent hypoxia/hypercapnia for 10 days. Eighteen hours before tissue collection, normoxic and exposed neonates were given a series of two 27 mg/kg intraperitoneal injections, separated by 30 min, of either dihydroethidine (Molecular Probes), which readily crosses the blood-brain barrier, or PBS (sham) on day 9. Dihydroethidine is converted to the fluorescent molecules ethidium and 2-hydroxyethidium (48) upon specific oxidation by intracellular superoxide. Therefore, dihydroethidium (DHE) can be used as a specific in situ marker of superoxide production. Eighteen hours later, anesthetized mice were perfused transcardially with saline and 4% paraformaldehyde in PB (Sigma) and brains were taken for immunofluorescence. Cryosections (10 μm) were then generated and processed for routine immunofluorescence. To identify the phenotype of cells expressing DHE, double-labeling studies were performed with antibodies to glial fibrillary acidic protein (GFAP; Dako Cytomation, Glostrup, Denmark) and NeuN (Chemicon). DHE fluorescence (excitation 488 nm; emission >560 nm) was visualized on a DeltaVision deconvolution microscope (Applied Precision, Issaquah, WA).
Mitochondrial Function and Superoxide Production
As described above, mice were exposed to CIH/CIC for 2 wk starting at P2. The brains from two to four mice were removed, and mitochondria were rapidly isolated from pooled cerebral cortices by Percoll differential centrifugation, with minor modifications, within 2 h (1, 41).
Analysis of Mitochondrial Respiratory Function
Oxygen consumption was measured with a Clark-type oxygen electrode (Oxygraph, Hansatech, Norfolk, UK). Purified nonsynaptosomal mitochondria (∼1–2 mg protein) were added to the oximetry chamber in a 300-μl solution containing (in mM) 100 mM KCl, 75 mannitol, 25 sucrose, 5 H3PO4, 0.05 EDTA, and 10 Tris·HCl, pH 7.4. After 2 min of equilibration, 5 mM pyruvate and 5 mM malate were added and oxygen consumption was followed for 2 min. ADP (250 μM) was added to measure state 3 (phosphorylating) respiration. Oligomycin (2.5 μg/ml) was added 2 min later to inhibit the F0F1-ATPase and to determine state 4 (resting) respiration. The maximal uncoupled respiratory rate was obtained by adding 0.2 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) to the mixture. Oxygen utilization traces and rate determinations were obtained with Oxygraph software. Protein concentrations were quantified with a bicinchoninic acid (BCA) kit (Pierce).
EPR Measurements of Mitochondrial Superoxide Production
Mitochondrial suspensions (∼200–400 μg protein) were incubated for 10 min with 30 mM 5-(diethylphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEPMPO) and appropriate combinations of respiratory substrates. The mixture was loaded into 50-μl glass capillary and introduced into the electron paramagnetic resonance (EPR) cavity of a MiniScope MS200 Benchtop spectrometer, which allowed the temperature to be maintained at 37 ± 0.1°C with a Biotemperature control unit. We confirmed that the detected EPR signals were substrate specific and not due to redox cycling in the studied mixtures (data not shown). No EPR signals were registered when DEPMPO was mixed with combinations of substrates in the absence of mitochondria. To amplify the EPR signals, we included the superoxide dismutase (SOD) inhibitor diethyldithiocarbamic acid (DETC; 5 mM) in the final mixtures. EPR conditions were as follows: microwave power, 5 mW; modulation amplitude, 0.2 mT; modulation frequency, 100 kHz; sweep width, 15 mT centered at 334.9 mT; scan rate, 0.75 mT/s; each spectrum was the average of five scans. Assignment of the observed signals from mitochondria was confirmed through computer-assisted spectral simulation with WinSim software (http://epr.niehs.nih.gov/pest.html). Mixtures of signals due to DEPMPO-OOH and DEPMPO-OH adducts were detected, but complete removal of these signals was achieved upon the inclusion of SOD (200 U/ml), which confirmed that superoxide radical was the exclusive source of the observed EPR signals.
Statistics
Data are reported as means ± SE. Results were analyzed with the Student's unpaired t-test, and the protein nitration data were analyzed with the Wilcoxon matched pairs test (GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, CA). Differences in means were considered statistically significant when P < 0.05. Error bars indicate standard errors.
RESULTS
Effect of Intermittent Hypoxia/Hypercapnia on Body Weight and Hematocrit
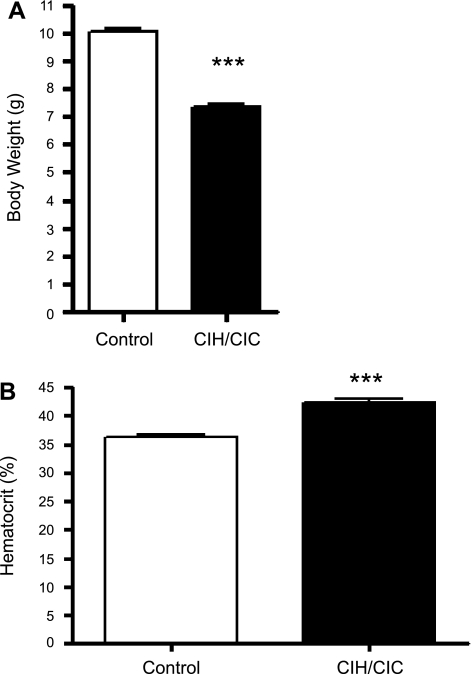
Two-day-old CD-1 mice were exposed to the hypoxic and hypercapnic exposure paradigms outlined above for 2 wk and demonstrated alterations in body weight and hematocrit (Fig. 1). Body weight and hematocrit were measured at the time of death. The data from male and female mice were not very different at this time period and therefore were combined. There were significant differences in weight gain [10.06 ± 0.12 g (n = 53) vs. 7.34 ± 0.13 g (n = 47)] and hematocrit [36.2 ± 0.58% (n = 18) vs. 42.2 ± 0.68% (n = 21)] between control and CIH/CIC-exposed mice.
Fig. 1.
Graphic representation of body weight (A) and hematocrit (B) of mice exposed to combined intermittent hypoxia/hypercapnia (CIH/CIC) for 2 wk. Significant differences between control and CIH/CIC mice: ***P < 0.0001.
Effect of Intermittent Hypoxia/Hypercapnia on Respiratory Rates
Pulse oximetry was performed on unrestrained, unanesthetized CIH/CIC-exposed mice within the chamber. There was a modest decrease in O2 saturation levels during the CIH/CIC period, which returned to control levels during the normoxic period (90.64 ± 0.799% vs. 98.84 ± 0.5372%; P = 0.003). There were no significant differences in heart rate (595.6 ± 40.97 vs. 681.7 ± 61.58 beats/min), pulse distension (221.2 ± 21.17 vs. 211.4 ± 10.68 μm), or ventilatory frequency (210.3 ± 18.97 vs. 192.1 ± 7.883 breaths/min) even though there was a trend toward increased heart rate.
Effect of Intermittent Hypoxia/Hypercapnia on Neuronal Cell Death
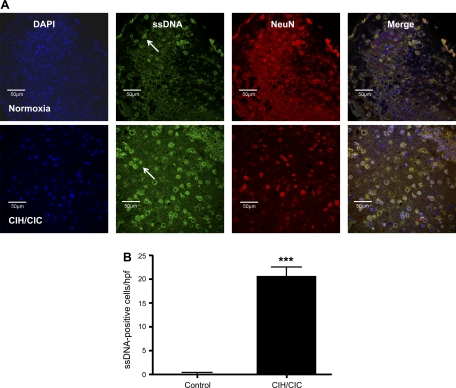
A dramatic increase in cell death as measured by immunofluorescent staining of 10-μm cryosections with an antibody to ssDNA was observed at 10 days in CNS tissues of mice exposed to CIH/CIC (Fig. 2A) compared with normoxic mice. The region of the brain that demonstrated the highest number of ssDNA-positive cells was the piriform cortex. Sparse positive ssDNA labeling was observed in the lateral cortex containing the ectorhinal, perirhinal, and lateral entorhinal cortices. No ssDNA-positive staining was observed in the superior cortex or the hippocampus. Therefore, cell counting was limited to the piriform cortex. Staining for single-stranded breaks in DNA was colocalized with NeuN and DAPI staining, and the nuclei of ssDNA-positive cells appeared condensed (Fig. 2A). Exposure of P2 mice to CIH/CIC for 10 days led to a significant increase in the number of apoptotic cells in the cerebral cortex as detected by the ssDNA antibody. Normoxic mice demonstrated very low levels of apoptosis (0.25 ± 0.14 cells/hpf; n = 3), whereas a large number of apoptotic cells were detected in the cortex of mice exposed to CIH/CIC (20.63 ± 1.9 cells/hpf; n = 3; P < 0.0001) (Fig. 2B). Whereas ∼100% of normoxic apoptotic cells were of a neuronal phenotype, 57.5% of apoptotic cells were neurons, as determined by colocalization with NeuN. A similar observation was made in that there were increased numbers of cleaved caspase 3-positive cells in CIH/CIC-exposed brains (data not shown).
Fig. 2.
A: postnatal day 2(P2) CD-1 mice were exposed to intermittent hypoxia/hypercapnia for 10 days, and brain cryosections were stained with an antibody to single-stranded DNA (ssDNA) to detect apoptotic cells. Top: coronal sections from mice exposed to normoxia. Bottom: coronal sections from mice exposed to combined CIH/CIC showing 4,′6-diamidino-2-phenylindole (DAPI) in blue, ssDNA in green, and NeuN in red. Arrows indicate ssDNA-positive cells. B: graphic representation of apoptotic cell counts from control and CIH/CIC-exposed brain cryosections (n = 3; ***P < 0.0001). hpf, High-power field.
Effect of Intermittent Hypoxia/Hypercapnia on Cytochrome c Localization
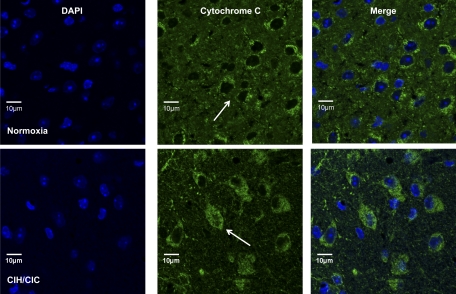
We examined the brains of normoxic and CIH/CIC-exposed mice for levels of protein expression of cytochrome c and Bcl-2. There was no change in Bcl-2 protein expression by either immunoblotting or immunohistochemistry, nor was there a change in cytochrome c protein levels by immunoblotting (data not shown). However, cytochrome c demonstrated translocation from the mitochondrial to the cytosolic pool in response to CIH/CIC (Fig. 3). Interestingly, the greatest density of cells demonstrating cytosolic cytochrome localization was in the piriform cortex, which correlates with the ssDNA data as well as the DHE data presented below.
Fig. 3.
P2 CD-1 mice were exposed to intermittent hypoxia/hypercapnia for 10 days, and brain cryosections were stained with an antibody to cytochrome c. Top: coronal sections from mice exposed to normoxia. Bottom: coronal sections from mice exposed to CIH/CIC showing DAPI in blue and cytochrome c in green. Arrows indicate cytochrome c-positive cells.
Effect of Intermittent Hypoxia/Hypercapnia on Mitochondrial Function
Mitochondrial respiratory response to CIH/CIC treatment.
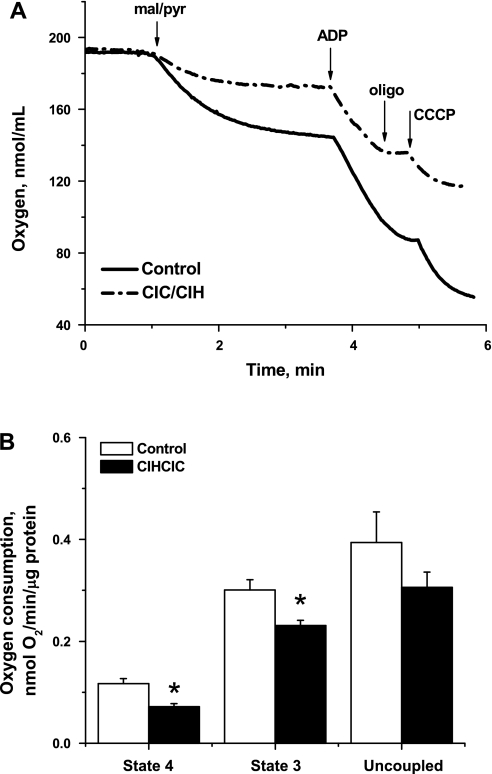
Mitochondria are generally assumed to be the major source of ROS production and have been shown to mediate somatic cellular responses to IH through ROS-activated pathways (34, 40). Because changes in mitochondrial respiratory function and coupling can directly modify ROS production, we evaluated respiration in isolated brain mitochondria from CIH/CIC-treated and untreated neonates. Respiratory steady states have been defined by Chance and Williams (8) according to a protocol for oxygraphic measurements of oxygen consumptions (d[O2]/dt) by isolated mitochondria as follows: state 1 is the d[O2]/dt in the presence of endogenous substrates with inorganic phosphate in the mitochondrial respiration medium. Addition of ADP induces a phase of initial oxygen consumption associated with effectively exhausting endogenous substrates, after which state 2 is a substrate-limited state of residual oxygen consumption (should also correspond to state 4). State 3 is then achieved by the addition of ADP in the presence of NAD+-linked substrates, which are excess malate and pyruvate in our case. Upon the depletion of ADP, respiration drops in the transition to state 4, which is an ADP-limited resting state (leak state). Significant reductions in both state 4 [resting; 61.28 ± 8.36% of control, F(1,10) = 10.236, P < 0.01; n = 5] and state 3 [phosphorylating; 76.74 ± 4.32% of control, F(1,10) = 6.41, P < 0.05; n = 5] activities were observed in the CIH/CIC-treated group (Fig. 4). Maximally uncoupled respiratory rate was moderately lower in mitochondria isolated from the treated group, indicating that CIH/CIC treatment globally reduces the activity of the respiratory enzymes. These results follow a similar trend observed under chronic constant hypoxia, although our experimental regimen reduced state 4 more drastically and state 3 more moderately (10).
Fig. 4.
CIH/CIC treatment reduces mitochondrial respiratory activities. A: representative oxygen consumption traces of isolated mitochondria from 2–4 pooled brains of neonatal mice after CIH/CIC treatment for 2 wk starting at P2 or control untreated littermates. Purified nonsynaptosomal mitochondria (∼1–2 mg protein) were added to the oximetry chamber in a 300-μl solution of the reaction buffer adjusted to pH = 7.4 and 37°C. Oxygen consumption was measured as described in materials and methods. CCCP, carbonyl cyanide m-chlorophenylhydrazone; oligo, oligomycin; mal/pyr, malate/pyruvate. B: mean ± SE rates of oxygen consumption, normalized per mitochondrial protein, during state 4, state 3, and uncoupled respirations for both control and CIH/CIC-treated animals. *Statistical significance (P < 0.05) of treatment by 1-way ANOVA followed by Tukey test. Differences in means were considered statistically significant when P < 0.05; n = 6 independent runs/group.
CIH/CIC treatment increases ROS leakage during resting but not phosphorylating respiration.
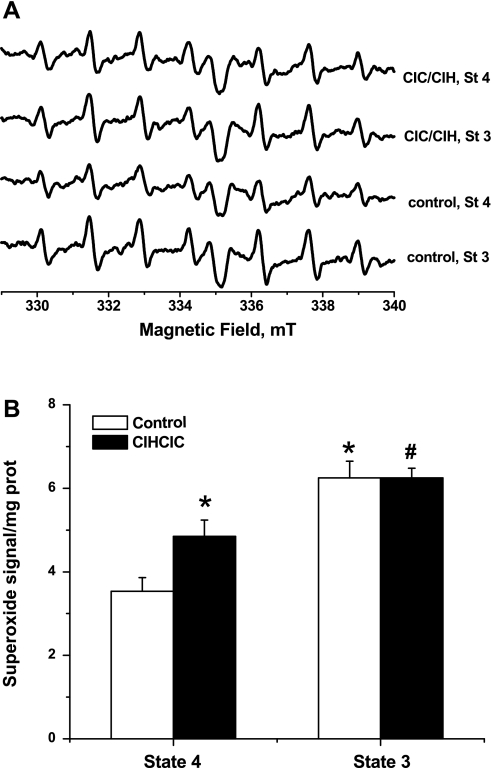
EPR spectroscopy is currently the only technique that can detect, identify, and quantify free radicals unequivocally. In spin trapping EPR experiments the short-lived radical is reacted with an exogenously added nitrone (DEPMPO in our case) to produce the much more enduring, and EPR active, nitroxide adduct. The resultant nitroxide signal can be analyzed through computer simulation to reveal the identity of the radical species involved, and the signal amplitude is proportional with its concentration. We have previously reported (1, 35) the detection of basal mitochondrial ROS leakage, i.e., without the inhibition of any of the electron transport chain (ETC) complexes. Here we used EPR spin trapping spectroscopy to follow the impact of CIH/CIC on basal ROS production during physiologically relevant respiratory states. Although it is commonly accepted that mitochondria leak ROS mostly during transition from state 3 to state 4, when the ETC is highly reduced and the ADP pool is consumed (3), our data indicate that neonatal mitochondria leak higher ROS during state 3 metabolism (Fig. 5A). This implies that ROS leakage in respiring mitochondria from neonatal brains is directly proportional to the rate of oxygen consumption. Figure 5B also shows that CIH/CIC treatment significantly increased superoxide production during resting respiration [137.32 ± 8.04% of control, F(1,10) = 6.54, P < 0.05; n = 5]. Interestingly, CIH/CIC treatment did not impact superoxide leakage during active oxidative phosphorylation (OXPHOS), when ADP is supplied.
Fig. 5.
CIH/CIC treatment increases superoxide production during resting [state 4 (St 4)] but not phosphorylating [state 3 (St 3)] mitochondrial respiration. A: electron paramagnetic resonance (EPR) spectra resulting from superoxide trapping in mitochondria isolated from brains of neonatal mice after CIH/CIC treatment for 2 wk starting at P2 or from untreated littermates (control). Mitochondria suspensions (∼200–400 μg protein) were incubated for 10 min at 37°C with 30 mM 5-(diethylphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEPMPO) and appropriate combinations of respiratory substrates to sustain the indicated respiratory states. To amplify the EPR signals, we included 5 mM diethyldithiocarbamic acid (DETC) to inhibit endogenous superoxide dismutase. EPR conditions were as follows: microwave power, 5 mW; modulation amplitude, 0.2 mT; modulation frequency, 100 kHz; sweep width, 15 mT centered at 334.9 mT; scan rate, 0.75 mT/s; each spectrum was the average of 5 scans. B: quantification of signal amplitudes, normalized by protein concentration, in both groups during resting (state 4) or phosphorylating (state 3) indicates that treatment increased reactive oxygen species during state 4 but not during state 3 respiration. *Statistical significance with respect to state 4 in control group; #statistical significance with respect to treated group. Differences in means were considered statistically significant by 1-way ANOVA when P < 0.05; n = 6 independent runs/group.
Intermittent Hypoxia/Hypercapnia Induces Superoxide Production In Situ
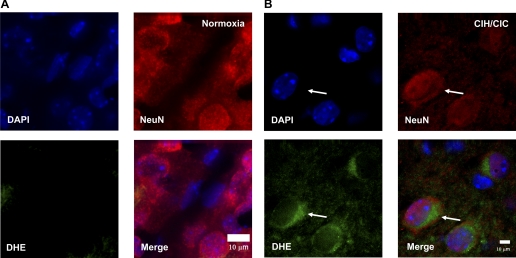
The brains of CIH/CIC-exposed mice were probed for the production of superoxide radical by examining the fluorescence signal generated by the oxidation of DHE by superoxide. As can be seen in Fig. 6B, coronal sections of CIH/CIC mouse brain (n = 3) demonstrate evidence of DHE fluorescence in cells that appear to be neurons since there is colocalization of DHE with NeuN, a neuronal marker. Similar to the results obtained with ssDNA and cytochrome c, the basolateral cortex demonstrated the highest number of DHE-positive cells. However, the number of DHE-positive cells in exposed tissue was much less than that seen with ssDNA but significantly higher than control (0–1 cells in normoxia and 7.5 ± 2.277 cells in CIH/CIC; n = 3) (Fig. 6A). Also, there was no evidence of colocalization of DHE with GFAP-positive cells, i.e., glia (data not shown). Interestingly, DHE fluorescence is seen in a perinuclear region, which is suggestive of mitochondria being the source of the observed signal even though it is also possible that these structures are endoplasmic reticulum or the Golgi apparatus. This indicates that intermittent hypoxia/hypercapnia induces superoxide production in the CNS.
Fig. 6.
P2 CD-1 mice were exposed to combined CIH/CIC for 10 days and assayed for dihydroethidium (DHE) staining. Brain cryosections were stained with DAPI and NeuN and viewed on an Applied Systems DeltaVision deconvolution microscope.: coronal sections from mice exposed to normoxia (A) and coronal sections from mice exposed to CIH/CIC (B) show DHE fluorescence (green) in the cytosol of neurons from the cerebral cortex, with DAPI in blue (arrows) and NeuN, a neuronal marker, in red.
DISCUSSION
The exposure of neonatal mice to CIH/CIC resulted in decreased body weight and increased hematocrit. CIH/CIC also resulted in increased apoptosis, translocation of cytochrome c from mitochondria to the cytosolic compartment, and increased superoxide production from mitochondria isolated from brain. This is in agreement with CIH/CIC studies performed in the piglet (31) as well as studies of IH alone (19, 20, 37, 47). For example, Gozal et al. (20) assessed apoptosis in response to an IH protocol using 10% O2 alone and found that peak apoptotic cell death occurred after 10–25 days of IH. It is noteworthy that CIH/CIC did not induce neuronal death in the hippocampus or superior cortex. It may be possible that CO2 played a neuroprotective role in these studies, and that concept will be addressed in future studies. This increased cell death may underlie the neurocognitive morbidity seen in animal models of OSA and in patients who suffer from sleep-disordered breathing.
The most significant finding in the present study is that mitochondria exposed to CIH/CIC have increased superoxide production in the presence of depressed respiratory activities. Mitochondria are involved in both apoptotic and necrotic cell death, and the outcome depends on the severity of the insult as well as the signaling pathways invoked (21). It has been suggested that oxidative stress lies upstream of mitochondria-dependent apoptosis (7). Therefore, it is possible that the increased apoptosis seen in this model is a result of increased ROS. ROS are known to be involved in cellular signaling events when produced at physiological levels (24, 45). However, various stresses can lead to the accumulation of ROS to pathological levels, which can lead to oxidative injury and cell death (5). Further evidence for the participation of ROS in neuronal injury has been provided in that N-acetylcysteine, an antioxidant, has been reported to be neuroprotective during hypoxia/ischemia (25) and other free radical quenchers and antioxidant precursors have been shown to enhance cell viability in hypoxic or ischemic stress (2).
In light of our present findings, it can be hypothesized that ROS are induced initially by alterations in the activity of the ETC caused by intermittent hypoxic/hypercapnic exposure. For example, we observed a general reduction in oxygen utilization by mitochondria isolated from CIH/CIC-treated brains, with more pronounced impact on resting respiration (state 4) and significantly higher associated ROS leakage. It has been demonstrated that hypoxia paradoxically stimulates or induces ROS release from mitochondria, and these species then can modulate transcriptional and posttranslational responses to low oxygen (14, 39). This ultimately leads to increased hydroxyl and peroxynitrite radicals (46). It is believed that ROS are generated at complex I and complex III of the mitochondrial ETC during hypoxia (4, 22). For example, it has been shown that mitochondria isolated from apoptotic cells produce superoxide by switching from normal 4-electron reduction of O2 to a 1-electron reduction upon release of cytochrome c (6). This situation would be exacerbated during state 4 respiration, when highly reduced ETC complexes tend to leak electrons to oxygen and form superoxide, instead of completely reducing O2 to water. This is expected to be associated with impeded oxygen consumption and lower state 4 respiratory rate, and more superoxide will be generated as seen in our results.
Another recent study in the chick embryo has demonstrated that hypoxia-reoxygenation leads to complex I inhibition and mitochondrial damage via an upregulation of mitochondrial nitric oxide synthase and increased nitric oxide-dependent nitration of complex I (17). We have previously demonstrated that IH alone can lead to mitochondrial dysfunction. In studies using IH-exposed mice, we observed that N-acetylaspartate/creatine (NAA/Cr) levels were decreased in hippocampus and thalamus (12). NAA/Cr levels are indicative of mitochondrial integrity because neuronal mitochondria are the site of NAA synthesis and a decrease in NAA/Cr levels implies mitochondrial dysfunction. The increased superoxide production in the present study may be indicative of impairment of ETC activity and implicates mitochondrial dysfunction as the likely mediator of neuronal cell death in this model.
Evidence accumulating in the literature suggests that oxidative stress underlies the pathophysiology of sleep apnea and other related sleep-disordered breathing states as well as in animal models of IH (30). IH also increases inducible nitric oxide synthase expression in a temporally defined manner and leads to increased peroxynitrite formation that may underlie IH-induced neuronal injury and spatial memory deficits in mice (37). Kim et al. (28) report that enhanced dopamine and acetylcholine release induced by IH in PC12 cells is due to Ca2+ and ROS. OSA patients also demonstrate enhanced release of superoxide from polymorphonuclear neutrophils (38). More recently, it has been reported that in an extended IH paradigm in which hypoxia and normoxia were alternated every 2 h in an in vitro model of primary neuronal cultures, mitochondria were the likely source of ROS (40). In that study, ROS were measured in whole cells with EPR and it was determined, with the use of blockers of the ETC and NADPH oxidases, that the source of ROS during hypoxia and the transition from normoxia to hypoxia was mitochondria.
There are some concerns with the experimental design utilized in these studies. First, we used 24 h/day exposures to CIH/CIC, which does not reflect what occurs in OSA. However, it was our intent to compare these data with those obtained with constant hypoxia previously utilized in our laboratory and others. Also, since neonatal mice tend to sleep for ∼20 h, it seemed an appropriate strategy. Additionally, there was a significant increase in hematocrit in these studies that could reflect the development of clinical polycythemia. It is postulated that OSA may lead to clinical polycythemia but only when severe, and our O2 saturation data indicated that CIH/CIC only induced modest reductions in our model. Future studies are being designed to address these issues.
In summary, CIH/CIC leads to increased cell death in the rodent brain with concomitant increases in markers of oxidative stress and reactive species such as superoxide. Therefore, despite the lack of success with antioxidant strategies in clinical trials, it is still imperative that therapeutic strategies targeted at redox signaling in OSA patients be pursued.
GRANTS
G. G. Haddad was supported by National Institutes of Health (NIH) Grant P01-HD-32573; S. S. Ali by NIH Grant 1K25-AG-026379; and L. L. Dugan by The Larry Hillblom Foundation. Imaging support was provided by the University of California, San Diego Neuroscience Microscopy Shared Facility P30-NS-047101.
DISCLOSURES
We declare that no conflicts of interest exist.
ACKNOWLEDGMENTS
We thank Shirley Reynolds and Jacinta Lucero for their excellent technical contributions to this study.
REFERENCES
- 1.Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 5: 565–574, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bailey DM, Davies B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt Med Biol 2: 21–29, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res 10: 215–224, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem 58: 79–110, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273: 11401–11404, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann NY Acad Sci 1042: 203–209, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem 217: 429–438, 1955 [PubMed] [Google Scholar]
- 9.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chavez JC, Pichiule P, Boero J, Arregui A. Reduced mitochondrial respiration in mouse cerebral cortex during chronic hypoxia. Neurosci Lett 193: 169–172, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Decker MJ, Hue GE, Caudle WM, Miller GW, Keating GL, Rye DB. Episodic neonatal hypoxia evokes executive dysfunction and regionally specific alterations in markers of dopamine signaling. Neuroscience 117: 417–425, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol 292: R1254–R1259, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na/H exchangers and HCO3-dependent transporters in mouse CNS. J Appl Physiol 95: 292–299, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619–11624, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol 43: 20–28, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Frankfurt OS. Detection of DNA damage in individual cells by flow cytometric analysis using anti-DNA monoclonal antibody. Exp Cell Res 170: 369–380, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Giusti S, Converso DP, Poderoso JJ, Fiszer de Plazas S. Hypoxia induces complex I inhibition and ultrastructural damage by increasing mitochondrial nitric oxide in developing CNS. Eur J Neurosci 27: 123–131, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 102: 616–620, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett 305: 197–201, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Green DR, Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Guzy RD, Mack MM, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal 9: 1317–1328, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91: 807–819, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med 28: 1456–1462, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Jayalakshmi K, Sairam M, Singh SB, Sharma SK, Ilavazhagan G, Banerjee PK. Neuroprotective effect of N-acetyl cysteine on hypoxia-induced oxidative stress in primary hippocampal culture. Brain Res 1046: 97–104, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kanaan A, Douglas RM, Alper SL, Boron WF, Haddad GG. Effect of chronic elevated carbon dioxide on the expression of acid-base transporters in the neonatal and adult mouse. Am J Physiol Regul Integr Comp Physiol 293: R1294–R1302, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol 290: R1105–R1114, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kim DK, Natarajan N, Prabhakar NR, Kumar GK. Facilitation of dopamine and acetylcholine release by intermittent hypoxia in PC12 cells: involvement of calcium and reactive oxygen species. J Appl Physiol 96: 1206–1215, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lavie L. Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis 51: 303–312, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Machaalani R, Waters KA. Increased neuronal cell death after intermittent hypercapnic hypoxia in the developing piglet brainstem. Brain Res 985: 127–134, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Neubauer JA. Physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol 90: 1593–1599, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates San Diego, CA: Academic, 2001, p. 160 [Google Scholar]
- 34.Prabhakar NR, Peng YJ, Yuan G, Kumar GK. Reactive oxygen species facilitate oxygen sensing. Novartis Found Symp 272: 95–99, 2006 [PubMed] [Google Scholar]
- 35.Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging 29: 117–128, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 162: 566–570, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Schumacker PT. Current paradigms in cellular oxygen sensing. Adv Exp Med Biol 543: 57–71, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Shan X, Chi L, Ke Y, Luo C, Qian S, Gozal D, Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis 28: 206–215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 55: 698–707, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Singh SN, Vats P, Kumria MM, Ranganathan S, Shyam R, Arora MP, Jain CL, Sridharan K. Effect of high altitude (7,620 m) exposure on glutathione and related metabolism in rats. Eur J Appl Physiol 84: 233–237, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 11: 473–485, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta 1363: 100–124, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol 35: 67–86, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126: 313–323, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA 102: 5727–5732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]