Abstract
Hypoxia-induced disruption of the blood-brain barrier (BBB) is the result of many different mechanisms, including alterations to the cytoskeleton. In this study, we identified actin-binding proteins involved in cytoskeletal dynamics with quantitative proteomics and assessed changes in subcellular localization of two proteins involved in actin polymerization [vasodilator-stimulated phosphoprotein (VASP)] and cytoskeleton-plasma membrane cross-linking (moesin). We found significant redistribution of both VASP and moesin to the cytoskeletal and membrane fractions of BBB endothelial cells after 1-h hypoxic stress. We also investigated activation of actin-myosin contraction through assessment of phosphorylated myosin light chain (pMLC) with confocal microscopy. Hypoxia caused a rapid and transient increase in pMLC. Blocking MLC phosphorylation through inhibition of myosin light chain kinase (MLCK) with ML-7 prevented hypoxia-induced BBB disruption and relocalization of the tight junction protein ZO-1. Finally, we implicate the transient receptor potential (TRP)C family of channels in mediating these events since blockade of TRPC channels and the associated calcium influx with SKF-96365 prevents hypoxia-induced permeability changes and the phosphorylation of MLC needed for actin-myosin contraction. These data suggest that hypoxic stress triggers alterations to cytoskeletal structure that contribute to BBB disruption and that calcium influx through TRPC channels contributes to these events.
Keywords: cation channels, endothelial cell, stroke, blood-brain barrier, calcium, transient receptor potential C channels
breakdown of the blood-brain barrier (BBB) contributes to edema formation, infarct size, and brain damage following ischemic stroke (3, 10, 36, 39). There are many factors contributing to BBB disruption in ischemia, including generation of oxygen radicals (24, 56, 58), nitric oxide (22, 42), production of vascular endothelial growth factor (67, 69), and changes in intracellular calcium (5, 26, 35). Under normal conditions, the integrity of the BBB is maintained by tight junction complexes between adjacent brain capillary endothelial cells (2). Changes in BBB permeability are correlated with changes in tight junction structure (33, 45, 51, 59). After hypoxia, this increased permeability is associated with disruptions in the subcellular localization of the tight junction proteins zonula occludens-1 (ZO-1) (43) and occludin (5).
A major contributing factor to disruption of the BBB after hypoxia is contraction of the actin-myosin cytoskeleton (23). A recent study demonstrated that inhibition of actin-myosin contraction protected the BBB after hypoxic stress (34); hypoxia-induced BBB disruption was prevented by inhibition of myosin light chain kinase (MLCK), by inhibition of NADPH-oxidase, or by chelation of intracellular calcium. These protective effects were correlated with a decrease in the amount of phosphorylated myosin light chain (pMLC) detected by immunofluorescence. However, this study did not investigate the effects of these protective treatments on tight junction structure, which is critical to BBB functional integrity. Furthermore, the reported increases in permeability were detected by measuring changes in transendothelial electrical resistance, which may represent a very small change in barrier tightness and not translate to increased permeability of solutes larger than ions.
Activation of MLCK requires an increase in intracellular calcium and subsequent binding of calcium to calmodulin (50). Pathways for calcium entry in BBB endothelial cells have not been well characterized but may include various transporters (30, 38) and members of the transient receptor potential (TRP)C family of cation-permeable channels. There are seven families of TRP channels, three of which have been found in BBB endothelial cells (4). Of these families, the TRPC family is the best candidate for mediating calcium influx following hypoxic stress; the Drosophila TRPC homolog trp is activated by anoxia (1), and hypoxic stress can increase the expression of the TRPC4 channel isoform in human pulmonary artery endothelial cells (14). Furthermore, lung endothelial cells from TRPC4-knockout mice show blunted responses to disrupting stimuli, implicating TRPC-mediated calcium influx in barrier disruption (60).
We hypothesized that calcium influx through TRPC channels on BBB endothelial cells contributes to MLCK activation, MLC phosphorylation, and alterations in BBB permeability following hypoxic stress. We utilized quantitative proteomics to investigate relative expression of cytoskeleton-associated proteins after hypoxia, including MLCK, MLC, actin, moesin, and vasodilator-stimulated phosphoprotein (VASP). We investigated changes in the subcellular localization of actin, moesin, and VASP as well as the effect of MLCK inhibition on MLC phosphorylation, BBB permeability, and tight junction structure after hypoxic stress. Finally, we used SKF-96365, an inhibitor of the cation-permeable TRPC channels, to block calcium influx through these channels and to prevent MLC phosphorylation and BBB disruption after hypoxia.
MATERIALS AND METHODS
Chemicals and antibodies.
DMEM, fetal bovine serum, penicillin-streptomycin, TRIzol, tetramethylrhodamine-wheat germ agglutinin (Molecular Probes), and mouse anti-GAPDH were from Invitrogen (Carlsbad, CA). Mouse anti-actin was from Sigma (St. Louis, MO). The iTRAQ isobaric tag system was from Applied Biosystems (Foster City, CA). Complete MINI EDTA-free protease inhibitors were from Roche Applied Science (Indianapolis, IN). Rabbit anti-VASP, anti-moesin, and anti-pMLC were from Cell Signaling Technologies (Danvers, MA). Anti-ZO-1 was from Zymed Laboratories (San Francisco, CA). Odyssey blocking buffer and IRDye-conjugated goat anti-rabbit and anti-mouse secondary antibodies were from Li-Cor Biosciences (Lincoln, NE). Donkey serum and Cy2-conjugated donkey anti-rabbit IgG were from Jackson ImmunoResearch (West Grove, PA). [14C]sucrose was from GE Healthcare (Piscataway, NJ). 1-(5-Iodonaphthalene-1-sulfonyl)homopiperazine (ML-7) and 1-[β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole (SKF-96365) were from EMD Chemicals (Gibbstown, NJ).
Cell culture.
bEnd3 is a commercially available immortalized mouse brain endothelial cell line (49); we have previously characterized this cell line as a BBB model system (8). bEnd3 cells (American Type Culture Collection, Manassas, VA) were grown according to the supplier's instructions in DMEM with 4.5 g/l glucose, 3.7 g/l sodium bicarbonate, 4 mM glutamine, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained in a humidified cell culture incubator at 37°C and 10% CO2-90% room air as instructed by the manufacturer. For all experiments, cells were trypsinized and seeded at a density of 0.5–1.0 × 104 cells/cm2 (52) onto uncoated, tissue culture-treated plasticware or Transwell permeable supports. Cells seeded onto Transwells were grown to confluence (within 6–7 days), and the medium in the lower chamber was removed and replaced with serum-free DMEM as previously described (8). After a further 3–7 days in culture, permeability assays and protein analysis were performed as described below.
Proteomics.
Differential expression of proteins in cells exposed to hypoxia (1% O2, 1, 3, or 6 h) was determined by mass spectrometry (MS) using the iTRAQ system of isotopic labeling for the identification and quantitation of proteins (20, 21, 68). In brief, 100-μg protein mixtures from each treatment group (control, 1, 3, and 6 h hypoxia) were precipitated with acetone. The precipitate was denatured and the disulfides reduced by incubation in the presence of 0.1% SDS and 5 mM tris-(2-carboxyethyl)phosphine. Cysteine residues were blocked with methyl methane-thiosulfonate, and trypsin was added to the mixture to a protein-to-trypsin ratio of 10:1 to generate a protein digest. The mixture was incubated overnight at 37°C, and the protein digests were labeled by mixing with the appropriate iTRAQ reagent. Desalted and concentrated peptide mixtures were quantified and identified by nano-LC tandem MS (MS/MS) on a QSTAR Elite mass spectrometer (ABS Sciex Instruments) operating in positive ion mode. Peptides were loaded on a 75-μm × 10-cm, 3-μm fused silica C18 capillary column, followed by mobile phase elution: buffer A (0.1% formic acid in 2% acetonitrile, 98% Milli-Q water) and buffer B (0.1% formic acid in 98% acetonitrile, 2% Milli-Q water). The peptides were eluted in a gradient from 2% buffer B to 30% buffer B over 180 min at a flow rate of 250 nl/min. The LC eluent was directed to a NanoES source for electrospray ionization (ESI)/MS/MS analysis. With information-dependent acquisition, peptides were selected for collision-induced dissociation by alternating between an MS (1 s) survey scan and MS/MS (3 s) scans. Accumulated MS/MS spectra were analyzed by ProteinPilot software (Applied Biosystems, Foster City, CA) with the SwissProt fasta database for protein identification. The ProGroup reports were generated with a 95% confidence level for protein identification. Proteins that were consistently altered from experiment to experiment were investigated further.
Subcellular fractionation and immunoblotting.
Differential detergent fractionation was used to isolate enriched fractions of cytoplasm, membranes, and cytoskeleton (11) from cells exposed to control conditions (normoxia) or hypoxic stress (1% O2) for the indicated periods of time. In brief, after being washed in ice-cold PBS, confluent monolayers of bEnd3 cells were incubated with digitonin-EDTA extraction buffer (in mM: 10 PIPES pH 6.8, 300 sucrose, 100 NaCl, 3.0 MgCl2, and 5.0 EDTA, with 0.01% digitonin) for 10 min on ice with gentle agitation. The supernatant, containing cytoplasmic proteins, was removed and spun to pellet any detached cells. Resulting supernatant was stored at −80°C (cytosolic fraction). The remaining cellular material in the dish was then extracted with Triton X-100-EDTA extraction buffer (in mM: 10 PIPES pH 7.4, 300 sucrose, 100 NaCl, 3.0 MgCl2, and 3.0 EDTA, with 0.5% Triton X-100) for 10 min on ice with agitation. The supernatant containing the membrane/organelle fraction was removed, and any detached cells were pelleted and removed. Supernatant was stored at −80°C until further use (membrane fraction).
Cellular material still remaining on the plate after the digitonin-EDTA extraction were then extracted with Tween 40-deoxycholate extraction buffer (in mM: 10 PIPES pH 7.4, 10 NaCl, and 1.0 MgCl2, with 1.0% Tween 40 and 0.5% deoxycholate) to isolate nuclear proteins. Plates were incubated with buffer for 10 min on ice with vigorous agitation, the supernatant was removed and spun, and the resulting cell-free supernatant was stored at −80°C (nuclear fraction). Finally, the insoluble cytoskeletal fraction was extracted by scraping the remaining cellular debris into 6 M urea buffer (in mM: 6,000 urea, 10 Tris, 1 DTT, 5 MgCl2, 5 EGTA, and 150 NaCl pH 8.0). All fractions were suspended in 6 M urea buffer before protein quantification. The relative proportion of proteins in each enriched fraction was ∼35–40% cytosolic, 30–35% membrane, 5–10% nuclear, and 15–25% cytoskeletal. Protease inhibitors were included in all extraction buffers and final 6 M urea buffer used for resuspending protein pellets.
Protein samples (10–20 μg) were separated by electrophoresis on 4–20% gels at 125 V for 75–90 min. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes and were incubated with primary antibody in Odyssey blocking buffer overnight at 4°C. IRDye-conjugated secondary antibody (1:2,000–1:10,000) was applied for 30 min–1 h at room temperature. Protein bands were visualized with the Odyssey Infrared Imaging System (Li-Cor Biosciences). Quantification of band density was performed with Scion Image software [National Institutes of Health (NIH), Bethesda, MD].
Western blotting.
We used both conventional and in-cell (ICWB) Western blotting to determine changes in protein expression. For ICWB, cells were grown in 96-well plates and processed according to the manufacturer's instructions (Li-Cor Biosciences). In brief, after exposure to hypoxic stress (1% O2) for the indicated periods of time, cells were fixed in 3.7% paraformaldehyde for 10 min at room temperature. Cells were washed and permeabilized before blocking and incubation with primary antibodies (anti-VASP, anti-moesin, anti-MLC, 1:500–1:1,000 dilution; anti-actin and anti-GAPDH, 1:1,000) in Odyssey blocking buffer overnight at 4°C. IRDye-conjugated secondary antibody (1:2,000–1:10,000) was applied for 1 h at room temperature. Plates were scanned with the Odyssey Infrared Imaging System. Fluorescent intensities for two channels (1 for protein of interest, 1 for GAPDH normalization) were corrected for background (wells with antibody but no cells), and fluorescence for proteins of interested was normalized using GAPDH fluorescence as a measure of cell density.
For conventional Western blotting, protein samples were run on 4–20% SDS-PAGE gels, transferred to PVDF membrane, and blocked with Odyssey blocking buffer. Primary antibody (1:500–1:1,000 dilution) was incubated with the membranes overnight at 4°C, and IRDye-conjugated secondary antibody (1:2,000–1:10,000) was applied for 1 h at room temperature. Membranes were scanned and band intensities were quantified with Scion Image software (NIH).
Confocal microscopy.
bEnd3 cells were grown to confluence on permeable Transwell filters or glass slides. After treatment, cells were incubated in tetramethylrhodamine-wheat germ agglutinin (1:200) for 30–40 min at 4°C to label cell membranes. Cells were then fixed in 3.7% paraformaldehyde for 10 min at room temperature. After permeabilization with 0.1% Triton X-100, cells were incubated with anti-ZO-1 (1:200) in PBS with 10% donkey serum for blocking. Cells were washed, incubated with Cy2-labeled donkey anti-rabbit or anti-mouse secondary antibody, and mounted in antifade medium containing DAPI nuclear counterstain. Fixed cells were imaged with a Zeiss LSM 510 META confocal microscope with a ×63 oil immersion objective in the multiscanning mode. Excitation wavelengths were set at 488 nm (argon laser) and 543 nm (HeNe laser), and emission wavelengths were 505–530 nm and >560 nm for Cy2 and tetramethylrhodamine, respectively. pMLC confocal images were analyzed with Adobe Photoshop. Pixel intensities in the green (pMLC) and red (tetramethylrhodamine-wheat germ agglutinin, membrane) channels were determined for each individual slice in each stack. The total green channel intensity per stack was normalized to the total red channel intensity, and three stacks were averaged for each treatment.
Permeability.
Paracellular solute permeability studies were performed with [14C]sucrose to determine paracellular diffusion across confluent bEnd3 monolayers. [14C]sucrose is a relatively impermeant marker at the BBB because of its size (mol wt 342); it is largely impermeant across the tight junctions between endothelial cells under normal conditions but will diffuse across the BBB when cell-cell junctions are disrupted. Apical-to-basolateral diffusion was determined by dividing the picomoles of radioactive marker appearing in the receiver chamber by the time in minutes (6, 7). The apparent permeability coefficient was calculated with the equation Pm (cm/min) = {[volume/(SA × CD)] × (CR/time)}, where volume is the volume of medium in the receiving chamber, SA is the specific activity of the radioactive marker, CD is the initial donor concentration of radioactive marker, and CR is the concentration of the radioactive marker in the receiving chamber at a specific time.
Statistics.
All data are expressed as means ± SE. Statistical analysis was performed with Sigma Stat 2.03 (SPSS, Chicago, IL), with a significance level set at P < 0.05. Data were analyzed with one-way analysis of variance (ANOVA) or Student's t-test, with post hoc tests as appropriate.
RESULTS
Global changes in BBB proteome following hypoxic stress.
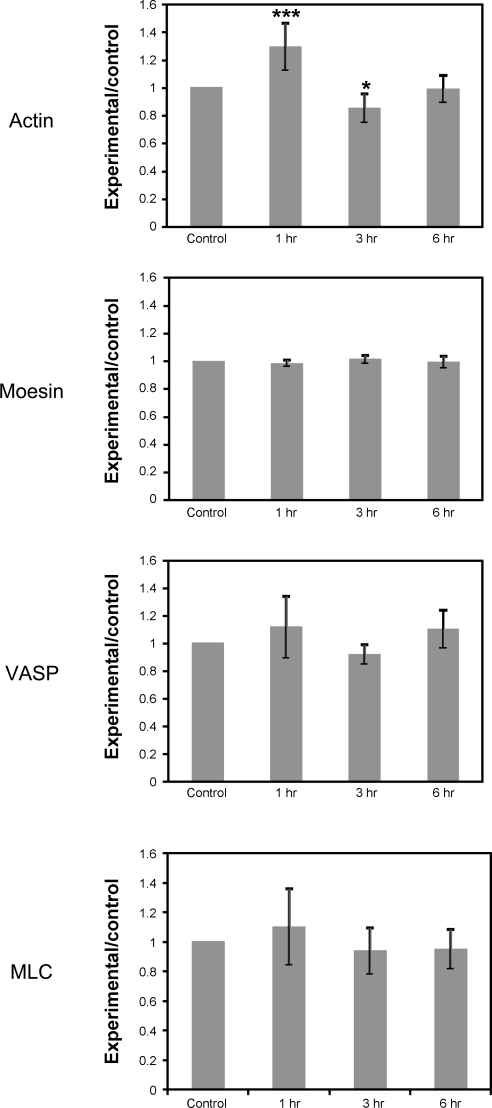
To identify broad changes in BBB protein expression after hypoxic stress, we used iTRAQ labeling and MS/MS to identify proteins up- or downregulated after 1, 3, or 6 h of hypoxic stress (1% O2). This experiment was performed three times; 367 proteins were affected in all three experiments, and the resulting fold changes were averaged. Those proteins that were actin-binding or cytoskeleton-associated proteins were compiled (Table 1). We found that, in particular, proteins involved in cytoskeletal contraction [Ras homolog gene family A (RhoA), Rho kinase (ROCK), myosin], anchoring of the cytoskeleton to the plasma membrane (moesin, α-actinin), and actin filament polymerization (VASP, profilin, cofilin-1) were altered by hypoxic stress. However, given the variability between separate experiments, the only protein that was significantly affected was actin, which was significantly upregulated at 1 h (P < 0.001) and significantly downregulated after 3 h of hypoxia (P < 0.05). This suggested that dynamic regulation of the cytoskeleton could be important in mediating BBB function after hypoxic stress. We chose to focus on one protein from each of the three groups described above (myosin, moesin, and VASP) as well as actin in further studies (Fig. 1).
Table 1.
Expression of proteins involved in cytoskeletal contraction and/or actin remodeling following hypoxic stress
| Protein | Accession No. | Average Expression (1 h) | Average Expression (3 h) | Average Expression (6 h) |
|---|---|---|---|---|
| α1-Actin | P62737 | 1.45 ± 0.11† | 0.74 ± 0.03* | 0.93 ± 0.04 |
| α1-Actinin | Q7TPR4 | 1.25 ± 0.28 | 0.89 ± 0.05 | 1.25 ± 0.28 |
| α4-Actinin | P57780 | 0.90 ± 0.04 | 0.90 ± 0.09 | 0.90 ± 0.03 |
| ARP3 | Q99JY9 | 1.33 ± 0.34 | 1.09 ± 0.36 | 1.03 ± 0.35 |
| Cofilin-1 | P168760 | 0.95 ± 0.03 | 0.99 ± 0.07 | 0.93 ± 0.05 |
| Moesin | P26041 | 0.98 ± 0.03 | 1.01 ± 0.03 | 0.99 ± 0.04 |
| MHC9 | Q8VDD5 | 1.08 ± 0.27 | 0.94 ± 0.13 | 0.94 ± 0.17 |
| MHC10 | Q61879 | 0.97 ± 0.34 | 0.79 ± 0.08 | 0.84 ± 0.18 |
| MLC6 | Q60605 | 1.10 ± 0.26 | 0.94 ± 0.16 | 0.95 ± 0.13 |
| MLC9 | Q9CQ19 | 1.85 ± 0.66 | 1.30 ± 0.36 | |
| Paxillin | Q8VI36 | 0.97 ± 0.03 | 0.77 ± 0.05 | 0.94 ± 0.29 |
| Profilin-1 | P62962 | 1.17 ± 0.23 | 1.20 ± 0.16 | 1.11 ± 0.20 |
| RhoA | Q9QUI0 | 0.89 ± 0.07 | 0.88 ± 0.05 | 0.88 ± 0.07 |
| ROCK | P70336 | 0.88 ± 0.04 | 0.82 ± 0.16 | 0.84 ± 0.14 |
| α-Spectrin | P16546 | 1.14 ± 0.13 | 1.12 ± 0.08 | 1.40 ± 0.31 |
| β-Spectrin | Q62261 | 0.92 ± 0.08 | 0.95 ± 0.05 | 1.05 ± 0.11 |
| Talin-1 | P26039 | 0.94 ± 0.08 | 0.93 ± 0.07 | 0.94 ± 0.07 |
| Thymosin-β4 | P20065 | 1.48 ± 0.49 | 1.42 ± 0.37 | 1.14 ± 0.22 |
| Tropomyosin 4 | Q6IRU2 | 0.94 ± 0.06 | 0.98 ± 0.03 | 0.98 ± 0.02 |
| VASP | P70460 | 1.12 ± 0.23 | 0.96 ± 0.09 | 1.17 ± 0.11 |
| Vinculin | Q64727 | 0.98 ± 0.01 | 0.98 ± 0.02 | 0.95 ± 0.004 |
| Zyxin | Q62523 | 0.87 ± 0.07 | 0.86 ± 0.14 | 0.87 ± 0.10 |
Data are means ± SE for 3 separate labeling experiments. Protein extracts were labeled with isobaric tags (iTRAQ system), and relative expression (normalized to control expression) was determined. ARP3, actin-related protein 3; MHC, myosin heavy chain; MLC, myosin light chain; RhoA, Ras homolog gene family A; ROCK, Rho kinase; VASP, vasodilator-stimulated phosphoprotein.
P < 0.05,
P < 0.001 vs. control.
Fig. 1.
iTRAQ proteomics confirmation of protein changes following hypoxic treatment. We found that our proteomic analysis, while identifying a similar set of proteins each time it was performed, was quite variable in the degree of change seen between data sets. Therefore we also used in-cell Western blot (ICWB) analysis to confirm changes seen in our proteomic data sets (data not shown). Actin levels as detected by iTRAQ were slightly elevated after 1 h of hypoxia, then fell at 3 and 6 h. Moesin, vasodilator-stimulated phosphoprotein (VASP), and myosin light chain (MLC) were unchanged in iTRAQ analysis. Data are presented as means ± SE for 3 iTRAQ experiments. A significant change was only found for actin levels after 1 h of hypoxia by one-way analysis of variance. *P < 0.05, ***P < 0.001 vs. control.
While the iTRAQ procedure was useful for identifying potential proteins of interest, substantial variability in the degree of change in protein expression was seen from experiment to experiment. We utilized ICWB analysis (13) to confirm the lack of changes in protein expression seen for VASP, moesin, MLC, and actin after hypoxic stress (data not shown). Overall no significant differences were found with either approach in the expression of actin (iTRAQ: P = 0.914209, ICWB: P = 0.137590), moesin (iTRAQ: P = 0.859688, ICWB: P = 0.234690), VASP (iTRAQ: P = 0.979841, ICWB: P = 0.803693), or MLC (iTRAQ: P = 0.988782, ICWB: P = 0.229830) in our treatment groups.
Changes in subcellular distribution of moesin and VASP.
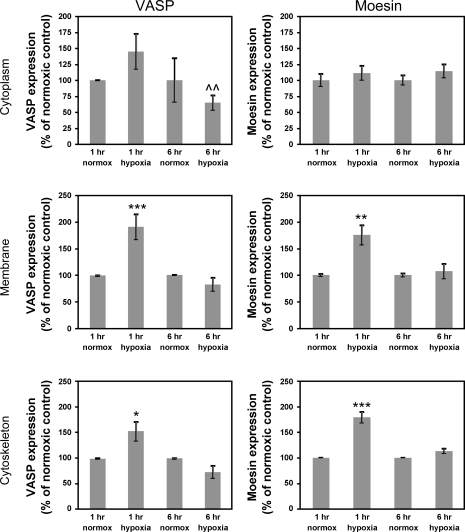
Since there was no significant change in total protein expression of moesin or VASP, we used sequential differential detergent fractionation (11) to isolate subcellular fractions from normoxic and hypoxic monolayers to determine whether there were any alterations in the subcellular localization of these proteins. Isolated protein fractions were subjected to conventional Western blot analysis. We utilized 1, 3, and 6 h of hypoxic stress in these studies. The 6 h time point is the condition under which we have found the most consistent and reproducible degree of barrier disruption, but we chose earlier time points to investigate changes that might occur before the functional end point of increased sucrose permeability. There was no significant change in VASP levels in the cytoplasm after hypoxic stress. However, both the membrane and cytoskeletal fractions had increased amounts of VASP after 1 h of hypoxia (Fig. 2, P < 0.001 and P < 0.05, respectively); this returned to control levels after 6 h of hypoxic stress. The only change seen after 6 h of hypoxia was a significantly lower distribution of VASP in the cytoplasm in cells exposed to 6-h hypoxic stress compared with those exposed to 1-h hypoxia (Fig. 2, P < 0.01 vs. 1-h hypoxic stress).
Fig. 2.
Subcellular localization of moesin and VASP is altered after exposure to hypoxic stress. We used differential detergent fractionation and conventional immunoblotting to assess the distribution of VASP and moesin after exposure to hypoxia (see materials and methods). VASP levels in the cytoplasm were not significantly altered from normoxic levels after either 1- or 6-h hypoxia, although there was a significant decrease in cytoplasmic VASP between the 1-h and 6-h hypoxia groups (^^P < 0.01 vs. 1-h hypoxia). VASP associated with the plasma membrane and the insoluble cytoskeletal fraction was significantly increased after 1-h hypoxia (***P < 0.001 and *P < 0.05, respectively) and dropped back to control levels at 6 h. Similarly, cytoplasmic levels of moesin were not altered, but levels of moesin associated with the membrane and cytoskeleton were significantly increased at 1 h (**P < 0.01 and ***P < 0.001, respectively). The 6-h levels were similar to control values. These data suggest an early and robust alteration in blood-brain barrier (BBB) endothelial cell cytoskeletal dynamics and structure that may contribute to barrier disruption. Data are presented as means ± SE for 3 fractionation studies.
The cytoplasmic distribution of moesin was not significantly increased after 1 h of hypoxic stress but was significantly increased at this time point in both the membrane and insoluble cytoskeletal fractions (Fig. 2, P < 0.01 and P < 0.001 vs. normoxic control, respectively). There was no significant alteration in the total protein levels of either VASP or moesin by iTRAQ or ICWB analysis. This therefore suggests subcellular redistribution of these proteins in response to hypoxia within the first hour of hypoxic stress. Taken together, the data in Figs. 2 and 3 suggest an involvement of the actin cytoskeletal polymerization machinery early in hypoxic stress responses; however, these changes have been resolved by the time there is measurable, reproducible disruption of barrier function.
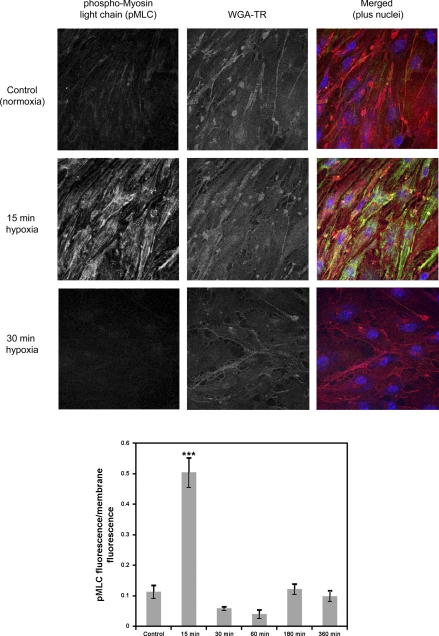
Fig. 3.
Hypoxic stress causes rapid, transient phosphorylation of MLC. A: we used confocal microscopy (×63 objective) to assess MLC phosphorylation over the time course of hypoxic stress. WGA-TR, wheat germ agglutinin-tetramethylrhodamine. B: hypoxia caused a dramatic and significant increase in phosphorylated MLC (pMLC) within 15 min (***P < 0.001) that was quickly restored to basal levels. Levels of pMLC remained similar to control (normoxic) for the duration of the hypoxic stress (6 h). Data are presented as means ± SE for 3 confocal stacks.
Phosphorylation of myosin light chain after hypoxia.
Contraction of the actin-myosin cytoskeleton has been implicated in regulation of BBB function (23). We assessed MLC phosphorylation after hypoxic stress for 15–360 min in our system, using immunofluorescence and confocal microscopy. Confocal microscopy analysis revealed a significant increase in MLC phosphorylation [F5,17 = 48.976, P < 0.001, 1-way ANOVA] after 15 min of hypoxia (Fig. 3A). This phosphorylation disappeared at 30 min of hypoxia and did not increase significantly for the duration of the hypoxic stress period (Fig. 3B). This indicates a rapid and transient activation of endothelial cell contraction, potentially contributing to later BBB disruption.
Inhibition of actin-myosin contraction protects BBB function after hypoxic stress.
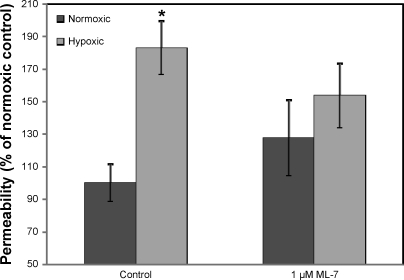
To determine the role of actin-myosin contraction in BBB disruption following hypoxia, we treated bEnd3 cells with 1 μM ML-7, a specific MLCK inhibitor, and assessed paracellular permeability, using [14C]sucrose. We found that 6 h of hypoxic stress increased BBB permeability in bEnd3 cells (Fig. 4, P < 0.05), similar to the disruption seen previously in primary cultures of BBB endothelial cells (7). This disruption was significantly inhibited by treatment with 1 μM ML-7 (1-way ANOVA, F3,51 = 3.601, P = 0.020), while ML-7 under normoxic conditions had no significant effect on barrier function. These results indicated that actin-myosin contraction plays a role in BBB disruption after hypoxic stress.
Fig. 4.
Inhibition of myosin light chain kinase (MLCK) protects against hypoxia-induced BBB disruption. Cells were incubated with and without the MLCK inhibitor ML-7 (1 μM) and exposed to 6-h hypoxia, after which paracellular permeability was assessed as described in text. Hypoxia caused a significant increase in paracellular permeability (*P < 0.05 vs. normoxic control) that was blocked by incubation with ML-7. There was no significant effect of ML-7 on basal permeability. Data are presented as means ± SE for 10–14 measurements.
Inhibition of actin-myosin contraction protects tight junction structure.
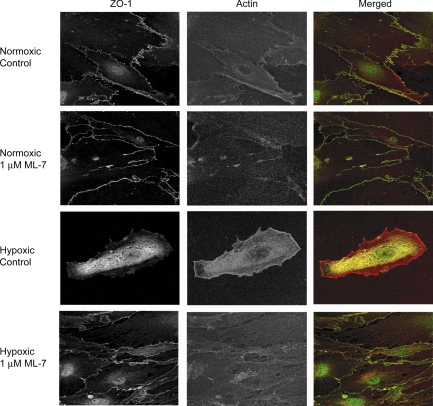
If hypoxia disrupts paracellular BBB permeability, there should be concomitant changes in tight junction structure. We assessed the subcellular localization of ZO-1, as an indicator of tight junction integrity, in bEnd3 cells exposed to hypoxic stress with and without MLCK inhibition. Hypoxia disrupted ZO-1 distribution, leading to largely cytoplasmic staining (Fig. 5) and an apparent decrease in cell-cell contact, reflective of cytoskeletal contraction. This redistribution was completely prevented by MLCK inhibition. These results indicate that actin-myosin contraction contributes to disruption of the tight junction, potentially by removing ZO-1 from the tight junction via its interaction with actin.
Fig. 5.
Inhibition of actin-myosin contraction protects tight junction structure. Zonula occludens 1 (ZO-1) is a tight junction accessory protein that links actin filaments to the plasma membrane at tight junctions (61). After 6-h hypoxic stress, ZO-1 immunofluorescence moves away from the cell-cell border into the cytoplasm, indicating disruption of the BBB. However, in cells treated with 1 μM ML-7, a MLCK inhibitor, ZO-1 localization at the plasma membrane is preserved after 6-h hypoxia. Representative fluorescent images are shown (×63 objective).
Inhibition of TRPC channels prevents barrier disruption and pMLC phosphorylation after hypoxia.
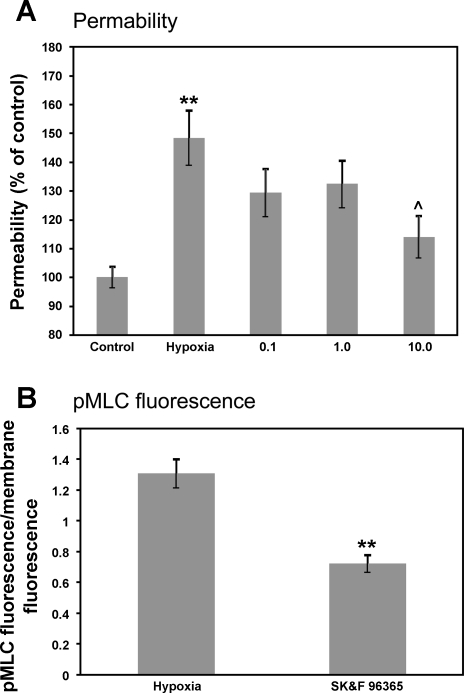
Activation of MLCK is dependent on Ca2+-calmodulin binding to the enzyme (62). In previous studies we have shown (4) the presence of functional TRPC and TRPV cation-permeable channels in brain endothelial cells, and we hypothesize that hypoxia-induced calcium influx through these channels contributes to actin-myosin contraction and BBB disruption. Permeability experiments indicate that treatment with a TRPC channel inhibitor, SKF-96365, prevents BBB disruption following 6-h hypoxia in a dose-dependent manner (Fig. 6A, P < 0.01 vs. normoxic control, P < 0.05 vs. hypoxia). The protection of barrier function was correlated with a decrease in pMLC at the point of peak pMLC fluorescence in control cells (Fig. 6B, P < 0.01 vs. hypoxic control) in cells treated with 1 μM SKF-96365. This indicates that blockade of TRPC channels can prevent both MLC phosphorylation and barrier disruption, presumably by blocking the Ca2+ influx needed to trigger Ca2+-calmodulin-mediated activation of MLCK (19) and cytoskeletal contraction.
Fig. 6.
Inhibition of transient receptor (TRP)C channels prevents BBB disruption and MLC phosphorylation following hypoxic stress. We assessed barrier function (permeability assay) and MLC phosphorylation (confocal microscopy) after hypoxia. A: treatment with SKF-96365 (0.1, 1.0, and 10 μM), an inhibitor of TRPC channels, dose-dependently blocked BBB disruption following 6-h hypoxia (**P < 0.01 vs. control, ^P < 0.05 vs. hypoxia). B: treatment with 1 μM SKF-96365 also significantly reduced pMLC immunofluorescence following hypoxia (**P < 0.01 vs. hypoxia), indicating prevention of MLCK activation and/or RhoA/ROCK mediated inhibition of MLC phosphatase (MLCP). Data are presented as means ± SE for 8–10 measurements (A) or 3 confocal stacks (B).
DISCUSSION
Stroke causes a disruption of the BBB that contributes to neuronal damage via the development of edema and swelling (28, 57). This barrier disruption is due to the activation of numerous cellular events, including hypoxia-inducible factor-1α (HIF-1α) (67), production of nitric oxide (42), and calcium influx (6, 26). In this study, we investigated global changes in the BBB endothelial cell proteome after exposure to hypoxic stress to identify proteins potentially contributing to BBB disruption following stroke, using quantitative analysis of a pool of total proteins. This enabled us to identify proteins that were potentially important in mediating BBB endothelial cell responses to hypoxic stress. Our data indicated that hypoxia activates signaling pathways involved in dynamic remodeling of the cytoskeleton, leading to alterations in cell morphology, tight junction structure, and BBB function. We found that two proteins, particularly involved in cytoskeletal dynamics and morphology, were affected by hypoxic stress: moesin and VASP.
Moesin is a member of the ezrin/radixin/moesin (ERM) family of actin-binding proteins and links actin filaments to the plasma membrane (41). Inactive (unphosphorylated) moesin is present in the cytoplasm but translocates to the plasma membrane upon activation by phosphorylation (66). Moesin can be phosphorylated by multiple kinases, including PKC-θ (54), p38 MAPK (31), and RhoA (44). Our data showed that the overall expression of moesin did not change during 6 h of hypoxic stress, but subcellular localization of the protein shifted toward the membrane and cytoskeletal fractions. This suggested activation, an increase in binding to F-actin, and an increase in membrane-cytoskeletal linkage. The exact signaling cascade responsible for the putative phosphorylation of moesin in BBB endothelial cells is unknown, but previous studies found activation of p38 MAPK (29, 53) and PKC-θ (16). RhoA is also activated during periods of acute hypoxia, leading to stress fiber formation and increased tight junction permeability (27, 65).
VASP is a member of the Ena/VASP family of proteins and is involved in actin polymerization (32), as well as in the maintenance of endothelial barriers (17). There are several domains within the protein that mediate its role in actin filament formation: the Ena/VASP homology (EVH)1 domain targets VASP to specific sites within the cell, including focal adhesions and tight junctions; the central proline-rich domain binds profilin, another actin-binding protein; and the EVH2 domain binds both G- and F-actin. When VASP is phosphorylated at Ser157 by PKA and/or PKG (12, 64), it localizes to cell-cell junctions in endothelial cells and can be colocalized with ZO-1, a tight junction accessory protein (12, 37). Our data indicated that VASP levels increased in the membrane and cytoskeletal fractions after 1 h of hypoxia, supporting the idea that there is an early dynamic change in the actin cytoskeleton. This mobilization is likely to be mediated through multiple pathways, including PKG (15, 63), MLCK (34), and RhoA/ROCK (27).
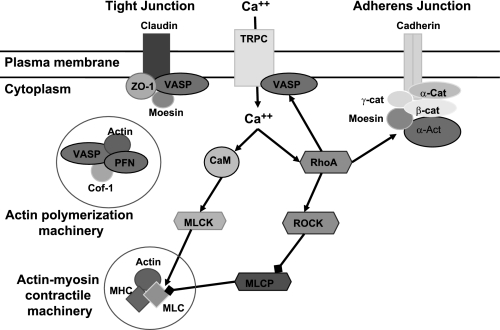
Hypoxia can also trigger cytoskeletal contraction, which is controlled by the phosphorylation state of MLC. This phosphorylation state is controlled by the calcium-calmodulin-dependent MLCK and by MLC phosphatase (MLCP) working in opposition (Fig. 7). MLCP, in turn, can be inhibited by activation of RhoA and ROCK, leading to an increase in MLC phosphorylation and actin-myosin contraction. In our study, confocal microscopy indicated peak phosphorylation of MLC after 15 min of hypoxia, which returned rapidly to baseline levels. Phosphorylation of MLC and actin-myosin contraction leads to changes in cell morphology and, in BBB endothelial cells, disruption of barrier function (18). Blocking MLC phosphorylation by inhibiting MLCK protected barrier function and tight junction structure after hypoxia, implicating cytoskeletal contraction in the disruption of the tight junction (see Fig. 7). It should be noted, however, that the studies presented here utilized bEnd3 cells, an immortalized mouse brain endothelial cell line that may not respond in the same fashion as primary cultures of BBB endothelial cells. Future experiments with primary cell cultures will confirm the involvement of the proteins we have identified in this study.
Fig. 7.
Pathways implicated in cytoskeletal regulation are altered after hypoxia. Isobaric protein labeling followed by LC tandem mass spectrometry (MS/MS) identified a number of proteins that were up- or downregulated by hypoxia in BBB endothelial cells. We are particularly interested in proteins involved in actin filament polymerization (VASP, profiling, cofilin-1), actin-myosin contraction [MLC, MLCK, Ras homolog gene family A (RhoA)/Rho kinase (ROCK)], and anchoring of actin filaments to the plasma membrane (moesin, α-actinin). Arrowheads indicate activation/phosphorylation, while blocked arrows indicate inhibition. α-Act, α-actinin; α-cat, α-catenin; β-cat, β-catenin; Ca++, calcium; CaM, calmodulin; Cof-1, cofilin-1; γ-cat, γ-catenin; MHC, myosin heavy chain; PFN, profilin.
While activation of the actin cytoskeleton contractile machinery is a reasonable hypothesis for a mechanism contributing to BBB disruption after hypoxia, presumably via calcium-calmodulin activation of MLCK, the proteins mediating calcium influx after hypoxia are not well understood. We hypothesized that members of the TRPC family might be involved. TRPC channels are expressed in BBB endothelial cells (4) and can be activated by hypoxic stress (14, 40). Furthermore, inhibiting TRPC channels prevents both phosphorylation of MLC and barrier disruption (Fig. 6). This directly links activation of TRPC channels, subsequent calcium influx, and barrier disruption through an actin-myosin contraction mechanism that contributes to morphological changes in the cells and pulls apart cell-cell junctions. This calcium influx may also contribute to changes in moesin and VASP via the p38 MAPK (9) or RhoA (27) pathways and PKG activation (53), respectively.
This is, as far as we know, the first direct link from TRPC channels to BBB functional integrity in a stroke model. The involvement of moesin and VASP in this scenario is not clear as yet, although TRPC4 has been shown to interact with EBP50, another member of the ERM family of proteins that contains moesin (48), and with VASP itself (64). We can hypothesize a system in which activation of TRPC channels under conditions of hypoxic stress leads to calcium influx and activation of MLCK. MLCK then phosphorylates pMLC, leading to cytoskeletal contraction and stress on cell-cell junctions. In addition, this calcium influx can activate RhoA, leading to phosphorylation of moesin and its translocation from the cytoplasm to the cell membrane, linking actin filaments to membrane domains. RhoA may also feed back onto TRPC channels (47) by enhancing inositol 1,4,5-trisphosphate receptor (IP3R)-TRPC interactions at the plasma membrane. Phosphorylated VASP is present at the tight junction itself, in association with ZO-1 (12, 37), and could serve a role in local actin polymerization at the site of the tight junction, while moesin may be involved at other membrane sites, such as the adherens junction (55).
In conclusion, we have identified and characterized actin-binding proteins that are altered under conditions of hypoxic stress. MLC is rapidly phosphorylated, leading to actin-myosin contraction and presumably changes in cell morphology. This event is dependent on an increase in intracellular calcium, and we demonstrated that blocking calcium influx through TRPC channels prevents both MLC phosphorylation and barrier disruption. At the same time, calcium can increase phosphorylation of moesin and enhance its ability to link the actin cytoskeleton to the plasma membrane. This tighter linkage, coupled with cytoskeletal contraction, may contribute to the disruption of tight junction structure seen in this study (Fig. 4) and many others (5, 33, 43, 46) after hypoxic stress.
Increased intracellular calcium may also increase VASP phosphorylation via the regulation of PKG by nitric oxide synthase (25, 63). In our hands, VASP bound more strongly to the membrane and cytoskeleton after 1-h exposure to hypoxia, indicating perhaps increased actin polymerization in those locations. Given the interactions of VASP with ZO-1 (12, 37) and TRPC channels (64), this opens up a very exciting scenario in which calcium influx through BBB endothelial cell TRPC channels can act to regulate cytoskeletal contraction, actin-plasma membrane interactions, and tight junction structure in a localized domain. This provides excellent targets for modulating these interactions and modifying the dynamic process of BBB disruption in stroke.
GRANTS
This work was supported by American Heart Association Scientist Development Grant 0635066N to R. C. Brown and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-70950 to R. G. O'Neil.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of W. S. Dubinsky: CCTS Proteomics Core, Dept. of Diagnostic Sciences, University of Texas Dental Branch at Houston, Sarofim Research Building, 1825 Pressler St., Houston, TX 77030.
REFERENCES
- 1.Agam K, von Campenhausen M, Levy S, Ben-Ami H, Cook B, Kirschfeld K, Minke B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci 20: 5748–5755, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16: 1–13, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bright R, Steinberg GK, Mochly-Rosen D. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res 1144: 146–155, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown R, Wu L, Hicks K, O'Neil RG. Regulation of blood-brain barrier permeability by transient receptor potential type C and type V calcium-permeable channels. Microcirculation 15: 359–371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun 327: 1114–1123, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Brown RC, Mark KS, Egleton RD, Davis TP. Protection against hypoxia-induced blood-brain barrier disruption: changes in intracellular calcium. Am J Physiol Cell Physiol 286: C1045–C1052, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs A, Davis TP. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NFkappaB. J Cell Sci 116: 693–700, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Brown RC, Morris AP, O'Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res 1130: 17–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H, Liu D, Garcia JG. CaM kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc Res 77: 30–34, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem 100: 1108–1120, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chiang E, Lim M, Patton W, Shepro D. NFkappaB translocation in human microvessel endothelial cells using a four-compartment subcellular protein redistribution assay. J Biochem Biophys Methods 46: 53–68, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 16: 583–585, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Danjo K, Robinson PA, Crabtree JE. In-cell Western analysis of Helicobacter pylori-induced phosphorylation of extracellular-signal related kinase via the transactivation of the epidermal growth factor receptor. Microbes Infect 9: 838–846, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1233–L1245, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J Cell Physiol 198: 359–369, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Fleegal M, Hom S, Borg L, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol 289: H2012–H2019, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Furman C, Sieminski A, Kwiatkowski A, Rubinson D, Vasile E, Bronson R, Fassler R, Gertler F. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol 179: 761–775, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia J, Davis H, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Geguchadze R, Zhi G, Lau K, Isotani E, Persechini A, Kamm K, Stull J. Quantitative measurements of Ca2+/calmodulin binding and activation of myosin light chain kinase in cells. FEBS Lett 557: 121–124, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gu S, Liu Z, Pan S, Jiang Z, Lu H, Amit O, Bradbury EM, Hu CA, Chen X. Global investigation of p53-induced apoptosis through quantitative proteomic profiling using comparative amino acid-coded tagging. Mol Cell Proteomics 3: 998–1008, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Gygi SP, Aebersold R. Mass spectrometry and proteomics. Curr Opin Chem Biol 4: 489–494, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Han F, Shirasaki Y, Fukunaga K. Microsphere embolism-induced endothelial nitric oxide synthase expression mediates disruption of the blood-brain barrier in rat brain. J Neurochem 99: 97–106, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res 29: 999–1009, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem 101: 566–576, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol Heart Circ Physiol 273: H2442–H2451, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda K, Nagashima T, Wu S, Yamaguchi M, Tamaki N. The role of calcium ion in anoxia/reoxygenation damage of cultured brain capillary endothelial cells. Acta Neurochir Suppl 70: 4–7, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Jin HG, Yamashita H, Nagano Y, Fukuba H, Hiji M, Ohtsuki T, Takahashi T, Kohriyama T, Kaibuchi K, Matsumoto M. Hypoxia-induced upregulation of endothelial small G protein RhoA and Rho-kinase/ROCK2 inhibits eNOS expression. Neurosci Lett 408: 62–67, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 24: 257–265, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Kayyali U, Pennella C, Trujillo C, Villa O, Gaestel M, Hassoun P. Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J Biol Chem 277: 42596–42602, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kiedrowski L. NCX and NCKX operation in ischemic neurons. Ann NY Acad Sci 1099: 383–395, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Koss M, Pfeiffer G, Wang Y, Thomas S, Yerukhimovich M, Gaarde W, Doerschuk C, Wang Q. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol 176: 1218–1227, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Krause M, Dent E, Bear J, Loureiro J, Gertler F. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol 19: 541–564, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmari E, Traweger A, Wejksza K, Bauer HC. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol 25: 129–139, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhlmann CR, Tamaki R, Gamerdinger M, Lessmann V, Behl C, Kempski OS, Luhmann HJ. Inhibition of the myosin light chain kinase prevents hypoxia-induced blood-brain barrier disruption. J Neurochem 102: 501–507, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kuhlmann CR, Zehendner CM, Gerigk M, Closhen D, Bender B, Friedl P, Luhmann HJ. MK801 blocks hypoxic blood-brain-barrier disruption and leukocyte adhesion. Neurosci Lett 449: 168–172, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Lai DM, Li H, Lee CC, Tzeng YS, Hsieh YH, Hsu WM, Hsieh FJ, Cheng JT, Tu YK. Angiopoietin-like protein 1 decreases blood brain barrier damage and edema following focal cerebral ischemia in mice. Neurochem Int 52: 470–477, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol 282: C1235–C1245, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Lehotsky J, Kaplan P, Murin R, Raeymaekers L. The role of plasma membrane Ca2+ pumps (PMCAs) in pathologies of mammalian cells. Front Biosci 7: d53–d84, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Lennmyr F, Ericsson A, Gerwins P, Ahlstrom H, Terent A. Increased brain injury and vascular leakage after pretreatment with p38-inhibitor SB203580 in transient ischemia. Acta Neurol Scand 108: 339–345, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Lin M, Leung G, Zhang W, Yang X, Yip K, Tse C, Sham J. Chronic hypoxia-induced upregulation of store-operated and receptor operated Ca2+ channels in pulmonary artery smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell 92: 305–316, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Mark KS, Burroughs A, Brown RC, Huber JD, Davis TP. Nitric oxide mediates hypoxia-induced changes in paracellular permeability of cerebral microvasculature. Am J Physiol Heart Circ Physiol 286: H174–H180, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 282: H1485–H1494, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui T, Maeda M, Doi Y, Yonemura S, Amano MK, K , Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis T. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem 106: 2395–2409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaffrey G, Willis C, Staatz W, Nametz N, Quigley CH, SA , Lochhead JJ, Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem 110: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem 278: 33492–33500, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Mery L, Strauss B, Dufour J, Krause K, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 115: 3497–3508, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Montesano R, Pepper M, Mohle-Steinlein U, Risau W, Wagner E, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell 62: 435–445, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Nairn A, Picciotto M. Calcium/calmodulin-dependent kinases. Semin Cancer Biol 5: 295–303, 1994 [PubMed] [Google Scholar]
- 51.Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol 14: 186–195, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake transport studies. Brain Res 990: 95–112, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Park JH, Okayama N, Gute D, Krsmanovic A, Battarbee H, Alexander JS. Hypoxia/aglycemia increases endothelial permeability: role of second messengers and cytoskeleton. Am J Physiol Cell Physiol 277: C1066–C1074, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Pietromonaco SS, PC, Altman A, Elias L. Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J Biol Chem 273: 7594–7603, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature 442: 580–584, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Pun P, Lu JM, S Involvement of ROS in BBB dysfunction. Free Radic Res 43: 348–364, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32: 200–219, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21: 3666–3676, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Shimojima N, Eckman C, McKinney M, Sevlever D, Yamamoto S, Lin W, Dickson D, Nguyen J. Altered expression of zonula occludens-2 precedes increased blood-brain barrier permeability in a murine model of fulminant hepatic failure. J Invest Surg 21: 101–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiruppathi C, Freichel M, Vogel SM, Paria B, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4–/– mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Ueno M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr Med Chem 14: 1199–1206, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Vetter SW, Leclerc E. Novel aspects of calmodulin target recognition and activation. Eur J Biochem 270: 404–414, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Vogel C, Bauer A, Wiesnet M, Preissner KT, Schaper W, Marti HH, Fischer S. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol 212: 236–243, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Pluznick JL, Settles DC, Sansom SC. Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. Am J Physiol Renal Physiol 293: F1768–F1776, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Woodward A, Crouch D. Cellular distribution of the ERM proteins in MDCK epithelial cells: regulation by growth and cytoskeletal integrity. Cell Biol Int 25: 205–212, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol 72: 440–449, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Goodlett DR, Peskind ER, Quinn JF, Zhou Y, Wang Q, Pan C, Yi E, Eng J, Aebersold RH, Montine TJ. Quantitative proteomic analysis of age-related changes in human cerebrospinal fluid. Neurobiol Aging 26: 207–227, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106: 829–838, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]