Abstract
Aims:
This study aims to assess the feeding inhibition and repellency effect of three brands of mosquito coils in experimental huts (East African design). Evaluated products were all pyrethroid-based mosquito coils–Kiboko®, Total® and Risasi®. Mosfly (0.1% D-allethrin) was a positive control. Indoor resting behavior, feeding inhibition and induced exophily were measured as responses of burnt coil smoke in huts.
Materials and methods:
Resting mosquitoes were collected inside the huts, in window traps and verandah traps using mechanical aspirators. Identified to species level and sex.
Results:
A total of 1460 mosquitoes were collected, 58.9% (n=860) were Anopheles gambiae s.l while 41.1% (n=600) Culex quinquefasciatus. Indoor resting mosquitoes in all treated huts were significantly reduced than in negative control (DF=4, F=18.6, P < 0.001). Species found to rest indoors were not statistical different between the positive control (Mosfly coil) and other three treated huts (DF=3, F=1.068, P=0.408). Cx.quinquefasciatus had significantly higher induced exophily in all treatments comparing to An.gambiae s./ (DF=1, F=5.34, P=0.050). Comparison between species (An.gambiae s.l and Cx. quinquefasciatus) for the feeding inhibition among treated huts was not statistically significant (DF=1, F=0.062, P=0.810).
Conclusion:
Introduction of several personal protection measures will be ideal to supplement the existing gap in reducing the man vector contacts hence lowering the disease transmission.
Keywords: Bio-efficacy, Kiboko®, Mosquito-coils, Risasi®, Total®, Tanzania
BACKGROUND
Vector-borne diseases are responsible for 17% of the estimated global burden of infectious disease.[1] Plasmodium falciparum, a mosquito transmitted malaria parasite, is responsible for 350 to 500 million clinical malaria cases and over 1.7 million deaths annually.[2,3] In Tanzania, twenty-eight million citizens are exposed to risk of stable malaria causing 16 million clinical cases and one hundred thousands child deaths with over 25% of total death.[4,5] In a state of emergence where there is no well planned program for vector control measures such as insecticides treated bed nets (ITNs) and long lasting insecticide nets (LLINs);[6,7] use of both synthetic and plant based repellents[8–10] and use of indoor residual spray,[11] mosquito coils can be deployed. Also, the house design improvements have been considered as the physical barrier tool for indoor mosquito reduction.[12] The use of mosquito coils has been gaining popularity in communities with both high and low malaria transmission intensities as a supplement for protection(meanwhile indoor but outside bed net).[13,14] But the coverage of the ITNs in community has been highly affected by social economic status and availability in rural areas.[13,15] The bio-efficacy of mosquito coils impregnated with synthetic pyrethroids against mosquito have been reported in other studies.[16,17] However, efficacy of different and new coil formulations may need to be evaluated in different settings. It was therefore the objective of this study to evaluate three brands of pyrethroid based mosquito coils in the field for its feasibility in community use against mosquito house entry and biting.
MATERIALS AND METHODS
Study area
The work was conducted between February to March 2008 using Experimental Hut at Magugu Field Station of the Tropical Pesticides Research Institute in Northern Tanzania (4' 00 S, 35' 46 E). The use of experimental huts and the biological tests involved in testing efficacy of various insecticides against anthropophilic mosquitoes is well described by Smith,[18] Smith and Webley[19]
Evaluated products
The tested products were all pyrethroid based mosquito coils–Kiboko® with 0.15% D-allethrin imported by MSK Industries Ltd of Dar es Salaam, Tanzania. The second product was Total® with 0.2% D-allethrin, imported by Total Tanzania Ltd. The third product was Risasi®, a mosquito coil which is a botanical based insecticide/repellent, with 0.2% w/v pyrethrins as its active ingredient imported and distributed by Meghji Sundries of Dar-Es-Salaam, Tanzania. Mosfly (0.1% D-allethrin) was used as a standard for comparison purpose because it was a registered mosquito coils in Tanzania.
Experiment design
Five experimental huts (East African design) were used in this study. Three huts with mosquito coil treatments and two served as negative and positive control in a 5×5 Latin square design. The three mosquito coils were randomly allocated to the huts. Two huts remained as negative and positive controls. In the experimental huts, mosquito coils were supported by their stands, placed distantly from a bed occupied by a volunteer with untreated bed net. The coils were lit at 20.00 hours and left to bum out, burning duration was recorded for each coil type. Mosquitoes from inside the huts, window and verandah traps were collected and recorded as described in other studies.[19,20] Experiments were repeated by systematic rotation of coils in huts to avoid hut positional and individual attractiveness biasness. The treatments were rotated in two days after first experimental rotation to avoid contamination in the hut due to coils effect.
Mosquito collection
From 06:30 to 07:30 hours in the morning, mosquitoes were collected from indoors, verandah and window traps of the huts. Collections were done by a pair of trained mosquito collectors using hand aspirators.[20] Mosquitoes collected were put separately in paper cups and later sorted into their species and abdominal conditions.[21] Males were ignored during collections.
Ethical approval
The study was approved by the Tropical Pesticides Research Institute, Research Ethics Committee. The oral and written informed consent was obtained from the sleepers to participate in the study before getting involved in experiments. Malaria screening were done weekly and treatments were given to any person with malaria parasite free of charge, but fortunately none of volunteer had malaria parasites during and two weeks after study.
Statistical analysis
Mosquitoes collected from experimental and control huts were compared using SPSS program version 15.0 for windows. General linear model univariate was performed to assess the effect of the factors in experiments. Days, volunteers, huts and coils were analyzed to assess the effect of each in experimental design. Significance level was considered at Probability (P) <0.05.
Indoor resting mosquitoes
The mean numbers of mosquitoes collected in the treated huts were compared with the mosquitoes from positive and negative control huts. Mosquitoes collected were compared by day, hut, treatments and volunteers.
Induced exophily (deterrence/repellency effect of coils)
The mosquitoes found in verandah and window traps were considered escaping the excito-repellency/irritant effect of the coils smoke in the hut and therefore were used to estimate repellency/deterrence effect of the coils. The mean numbers of unfed mosquitoes from verandah and window traps were compared separately with the untreated hut mosquitoes from verandah and window traps respectively. The induced exophily was calculated using Abbott formula {(Nc – Nt)/Nc} × 100% where Nt is a number of mosquitoes from verandah and window traps of treated hut while Nc is a number of mosquitoes from verandah and window traps of untreated hut.
Mosquitoes feeding inhibition
The mean number of fed mosquitoes collected indoors, verandah trap and window trap were subjected to ANOVA for statistical differences between treated and untreated huts. The percentage inhibition was also calculated using Abbott formula,[22] {(Fc – Ft)/Fc} × 100% where Fc is a number of mosquitoes found fed in untreated hut while Ft is a number of mosquitoes fed in treated hut.
RESULTS
Catches of An. gambiae s.l and Cx. quinquefasciatus
During the 25 days of experiments, a total of 1460 mosquitoes were collected in indoor, verandah and window traps of the five huts. Among those 58.9% (Number of mosquitoes (N)=860) were An.gambiae s.l while 41.1% (N=600) were Cx. quinquefasciatus.
Indoor resting mosquitoes
In analysis, other factors (day, hut, and volunteers) had no effect on experiment except mosquitoes populations caught indoor in each treatment. The total populations of indoor resting mosquitoes in all treated huts were significantly reduced in comparison to negative control (Degree of Freedom {DF}=4, F-Test result (F)=18.6, Probability (P)<0.001) as shown in Figure 1. The species found to rest indoors were not statistically different between the positive control (Mosfly coil) and other three treated huts (DF=4, F=1.068, P=0.408). (F- Indicate result of F-test which was used to compare difference between means of the collected mosquito samples)
Figure 1.
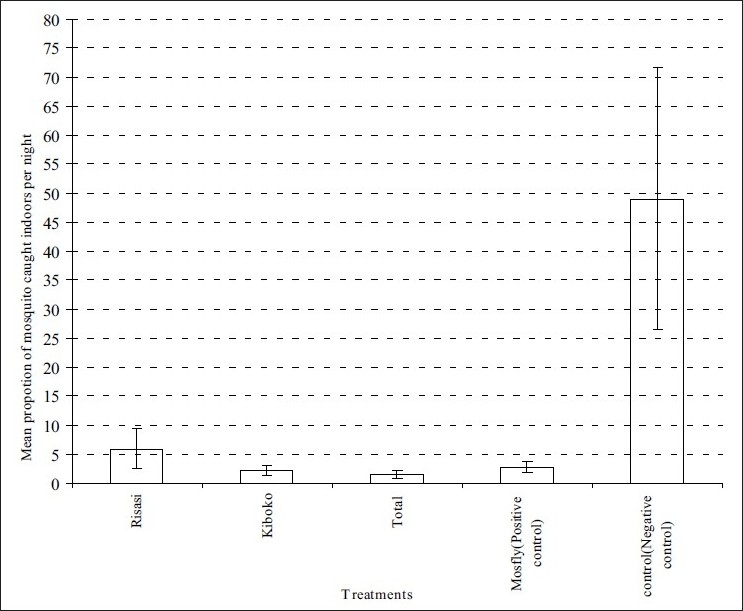
Mean proportion of mosquitoes resting indoors in each treatment
Induced exophily (deterrence/repellency effect of coils)
The induced exophily for the Cx.quinquefasciatus was 92%, 96%, 96%, 96% and 0% while for the An.gambiae s.l was 63%, 64%, 60%, 60% and 0% for Mosfly, Risasi, Total, Kiboko and negative control respectively. The statistical comparison of the two species showed that, Cx. quinquefasciatus had significantly higher induced exophily comparing to An.gambiae s.l in all treatments (DF=1, F=5.34, P=0.050).
Feeding inhibition
In all treatments, the feeding inhibition for the Cx.quinquefasciatus was 100%, 96%, 91%, 100% and 0% while for the An.gambiae s.l was 100%, 59%, 100%, 94% and 0% for Mosfly, Risasi, Total, Kiboko and negative control respectively. Comparison between species (An.gambae s.l and Cx.quinquefaciatus) for the feeding inhibition among treated huts was not statistically significant (DF=1, F=0.062, P=0.810).
Mortality
In all experimental huts, among Cx.quinquefasciatus and An.gambiae s.l collected, none died after being held for twenty-four hours of observation in provision of 10% sugar solution.
DISCUSSION
This study has proven the effectiveness of mosquito coils as personal protection tool for malaria vectors and nuisance mosquitoes based on mosquito behavior, which is in accordance with previous studies.[16] In this study, the parameters which were aimed to measure the impact of coils in reducing human vector contact such as feeding inhibition, induced repellency (deterrence) and indoor resting behavior were evaluated. In assessing feeding inhibition, all coils did better against the two species except Risasi which protected only 59% of An.gambiae s.l from feeding in eight hours time. In induced exophily for An.gambiae s.l, Total and Kiboko gave a repellency of 60% while Risasi gave 63% in An. gambiae s.l while in Cx. quinquefasciatus all evaluated coils induced exophily above 90% relative to the negative control. These observations suggest that low levels of pyrethrins cause repellency to mosquitoes, including feeding inhibition and induced exophily as concluded by other workers[16,23] hence reduction in disease transmission.[14] As found by MacIver,[23] the low level of pyrethrin have been considered to have no knockdown effect unless the concentration of pyrethrin increased. The current investigations of mosquito coils have yielded similar observations that, pyrethrins at lower dosages were more efficient in repellency as observed in other studies.[24,25] The factors evaluated suggest the possibilities of recommending these coils to be used in urban and rural areas where culicines and anophelines predominate respectively. The evaluated coils were found to have more than 75% feeding inhibition for An.gambiae s.l, the malaria vectors which are similar to results recommended for personal protection tool to be effective in reducing disease transmission in community level.[6]
From these results, we therefore recommend the use of pyrethroid based mosquito coils in complementing the existing mosquito control measures such as Indoor Residual Sprays (IRS) and Insecticide Treated Nets (ITNs), especially in rural area where the disease is endemic.[11,12,14] Malaria vectors and nuisance biting mosquitoes in most of African disease endemic areas have developed resistance against commonly used insecticides for treating bed nets.[26–28] In Tanzania insecticides resistance genes towards pyrethroids; most used insecticides is still at very low frequencies.[29] the area were these mosquitoes coils were evaluated malaria vectors are susceptible to pyrethroid insecticides used.[29,30] Resistance to available insecticides has posed a major threat in vector control and more efforts have to be done in implementation of integrated vector control management practices.
The burning duration for these coils was eight hours which was reported to be similar to other studies done in Tanzania with pyrethroid coils.[16] The burning duration covers the active mosquito biting cycles and therefore acceptable in malaria control programs in supplementing the existing control tools for community use. Moreover, the low costs of most mosquito coils, as compared to other tools, make this mosquito control tool appropriate for low income rural communities and in areas with emergence control needs such as refugee camps. There were no complaints from the volunteers in experimental huts in respect to the side effects of the coils as reported from other mosquito-coil studies.[31,32] The proper use of mosquito coils will complement the existing tools for personal protection against infective bites of malaria and nuisance mosquitoes.
Despite the great achievements of this study, the main constraints during the experiments were during mosquito-collection, which sometimes was done when other people were still on bed. The study duration was restricted by the number of mosquito coils provided by factories for evaluation.
CONCLUSION
It can be concluded from the findings of this study that, pyrethroid-based mosquito coils when properly used and coupled with other tools such as indoor residual spray and insecticides treated bed nets can have an impact in reducing indoors vector-human contact.
Acknowledgments
The authors wish to thank the volunteers who spent nights in experimental huts. We acknowledge Mzee Charles Massenga and Ester Lyatuu for technical support and the industries which distributed the coils for evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. The World Health Report 2004: Changing History. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.World Heath Organization. World Malaria Report 2005. World Health Organization; 2005. [Google Scholar]
- 3.Rowe AK, de Savigny D, Lanata CF, Victora CG. How can we achieve and maintain high-quality performance of health workers in low-resource settings? Lancet. 2005;366:1026–35. doi: 10.1016/S0140-6736(05)67028-6. [DOI] [PubMed] [Google Scholar]
- 4.Magesa SM, Lengeler C, deSavigny D, Miller JE, Njau RA, Kramer K, et al. Creating an “enabling environment” for taking insecticide treated nets to national scale: the Tanzanian experience. Malar J. 2005;4:34. doi: 10.1186/1475-2875-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health: National Malaria Medium-term Strategic Plan, July 2002–June 2006. Tanzania: Dar-es-salaam; 2002. [Google Scholar]
- 6.Curtis CF, Maxwell CA, Magesa SM, Rwegoshora RT, Wilkes TJ. Insecticide-treated bed-nets for malaria control. J Am Mosq Control Assoc. 2006;22:501–6. doi: 10.2987/8756-971X(2006)22[501:IBFMMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Binka F, Akweongo P. Prevention of malaria using ITNs: potential for achieving the millennium development goals. Current Molec Med. 2006;6:261–7. doi: 10.2174/156652406776055203. [DOI] [PubMed] [Google Scholar]
- 8.Vernede R, van Meer MM, Alpers MP. Smoke as a form of personal protection against mosquitoes, a field study in Papua New Guinea. Southeast Asian J Trop Med Public Health. 1994;25:771–5. [PubMed] [Google Scholar]
- 9.World Health Organization. Technical Report Series No. 936. Geneva: World Health Organization; 2006. Malaria Vector Control and Personal Protection: Report of a WHO Study Group. [PubMed] [Google Scholar]
- 10.Babu BV, Mishra S, Mishra S, Swain BK. Personal-protection measures against mosquitoes: a study of practices and costs in a district, in the Indian state of Orissa, where malaria and lymphatic filariasis are co-endemic. Ann Trop Med Parasitol. 2007;101:601–9. doi: 10.1179/136485907X193897. [DOI] [PubMed] [Google Scholar]
- 11.Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–56. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 12.Kweka EJ, Nkya WM, Mahande MA, Assenga C, Mosha FW, Lyatuu EE, et al. Mosquito abundance, bed net coverage and other factors associated with variations in sporozoite infectivity rates in four villages of rural Tanzania. Malar J. 2008;7:59. doi: 10.1186/1475-2875-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlwood JD, Jolley D. The coil works (against mosquitoes) in Papua New Guinea. Trans R Soc Trop Med Hyg. 1984;78:678. doi: 10.1016/0035-9203(84)90238-4. [DOI] [PubMed] [Google Scholar]
- 14.Pates HV, Lines JD, Keto AJ, Miller JE. Personal protection against mosquitoes in Dar es Salaam, Tanzania, by using a kerosene oil lamp to vaporize transfluthrin. Med Vet Entomol. 2002;16:277–84. doi: 10.1046/j.1365-2915.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 15.Goesch JN, Schwarz NG, Decker ML, Oyakhirome S, Borchert LB, Kombila UD, et al. Socio-economic status is inversely related to bed net use in Gabon. Malar J. 2008;7:60. doi: 10.1186/1475-2875-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosha FW, Njau RJ, Alfred S. Efficacy of Esbiothrin mosquito coils at community level in northern Tanzania. Med Vet Entomol. 1992;6:44–6. doi: 10.1111/j.1365-2915.1992.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 17.Yap HH, Tan HT, Yahaya AM, Baba R, Loh PY, Chong NC. Field efficacy of mosquito coil formulations containing D-allethrin and D-trans allethrin against indoor mosquitoes especially Culex quinquefasciatus Say. Southeast Asian Trop Med and Public Health. 1990;21:558–63. [PubMed] [Google Scholar]
- 18.Smith A. A verandah trap hut for studying the house frequenting habits of mosquitoes and for assessing insecticides. 1. Description of verandah trap hut and studies of the egress of Anopheles gambiae (Giles) and Mansonia uniformis (Theo) from untreated hut. Bull Entomol Res. 1965;56:161–7. doi: 10.1017/s0007485300057114. [DOI] [PubMed] [Google Scholar]
- 19.Smith A, Webley DJ. A verandah trap hut for studying the house frequenting habits of mosquitoes and for assessing insecticides. III. The effects of DDT on behaviour and mortality. Bull Entomol Res. 1969;59:33–46. doi: 10.1017/s000748530000300x. [DOI] [PubMed] [Google Scholar]
- 20.In Prepared by the WHO division of malaria and other parasitic disease. Geneva: World Health Organ; 1975. World Health Organization: Manual on practical entomology in malaria Part II. [Google Scholar]
- 21.Gillies TM, de Meillon DB. Johannesburg: Republic of South Africa. 2nd ed. Vol 54. Publication of the South African Institute for Medical Research; 1968. The Anopheles of Africa South of Sahara (Ethiopian Zoogeographic Region) [Google Scholar]
- 22.Abbott WS. A method of computing the effectiveness of the insecticide. J Econ Entomol. 1987;18:265–7. [Google Scholar]
- 23.MacIver DR. Mosquito Coils, Part II: Studies on the action of mosquito coil smoke on mosquitoes. Pyrethrum Post. 1964;7:7–14. [Google Scholar]
- 24.Chadwick PR, Lord CJ. Tests of Pyrethroid Vaporising Mats against Aedes aegypti (Diptera: Culicidae) Bull Entomol Res. 1977;67:667–74. [Google Scholar]
- 25.Amalraj DD, Kalyanasundaram M, Das PK. Evaluation of EMD vaporizers and bioallethrin vaporizing mats against mosquito vectors. Southeast Asian Trop Med Public Health. 1992;3:474–8. [PubMed] [Google Scholar]
- 26.Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994;8:71–5. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 27.Chandre F, Darriet F, Manguin S, Brengues C, Carnevale P, Guillet P. Pyrethroid cross-resistance spectrum among populations of Anopheles gambiae s.s from Côte d'Ivoire. J Am Mosq Control Ass. 1999;15:53–9. [PubMed] [Google Scholar]
- 28.Müller P, Chouaibou M, Pignatelli P, Etang J, Walker ED, Donnelly MJ, et al. Pyrethroid tolerance associated with elevated expression of antioxidants and agricultural practice in Anopheles arabiensis sampled from an area of cotton fields in Northern Cameroon. Mol Ecol. 2008;17:1145–55. doi: 10.1111/j.1365-294X.2007.03617.x. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni MA, Rowland M, Alifrangis M, Mosha FW, Matowo J, Malima R, et al. Occurrence of the Leucine-to-phenylalanine knockdown resistance (kdr) mutation in Anopheles arabiensis populations in Tanzania, detected by a simplified high-throughput SSOP-ELISA method. Malar J. 2006;5:56. doi: 10.1186/1475-2875-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni MA, Malima R, Mosha FW, Msangi S, Mrema E, Kabula B, et al. Efficacy of pyrethroid-treated nets against malaria vectors and nuisance-biting mosquitoes in Tanzania in areas with long-term insecticide-treated net use. Trop Med Intl Health. 2007;12:1061–73. doi: 10.1111/j.1365-3156.2007.01883.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu WK, Wong MH, Mui YL. Toxic effects of mosquito coil (a mosquito repellent) smoke on rats I. Properties of the mosquito coil and its smoke. Toxicol Lett. 1987;39:223–30. doi: 10.1016/0378-4274(87)90237-2. [DOI] [PubMed] [Google Scholar]
- 32.West S, Hildesheim A, Dosemeci M. Non-viral risk factors for nasopharyngeal carcinoma in the Philippines: results from a case- control study. Int J Cancer. 1993;55:722–7. doi: 10.1002/ijc.2910550504. [DOI] [PubMed] [Google Scholar]


