Abstract
Although African Americans (AA) smoke fewer average cigarettes per day (CPD) than European Americans (EA), they carry a disproportionate tobacco related morbidity and mortality burden.
Objective
To evaluate ethnic differences in markers of nicotine addiction, including rates of lifetime nicotine dependence (ND) symptoms, current smoking and smoking during pregnancy across different levels of peak lifetime cigarette consumption.
Methods
Data from 237 EA (N=118) and AA (N=119) mothers participating in the Missouri Family Study (2003-2005), an ethnically diverse family study of offspring outcomes in high and low risk families, were used to contrast prevalence of ND symptoms and other smoking behaviors between EA and AA women at low (1-10 CPD), moderate (11-19 CPD), and high (≥ 20 CPD) levels of lifetime peak daily cigarette consumption.
Results
Compared with EA smokers, AAs had lower lifetime prevalence of DSM-IV ND (68% v. 54%, p<.05), consumed fewer CPD during their heaviest lifetime consumption (18% EA v. 58% AA smoked ≤ 10 CPD, p<.0001), but did not differ in overall rates of smoking during pregnancy or current smoking. However, stratifying by categories of peak lifetime daily cigarette use, AA mothers who smoked ≤ 10 CPD reported greater lifetime ND symptoms and current smoking than their EA counterparts. In addition, nearly two-thirds of AA mothers in this smoking category smoked during pregnancy and 30% smoked throughout an entire pregnancy. The respective prevalence estimates in EA mothers were 38% and 0%.
Conclusions
Stratifying the sample into categories of lifetime peak daily cigarette use revealed significant ethnic/racial differences in smoking prevalence during pregnancy that were obscured in overall analysis. Substantial public health risks warranting clinical attention exist among light smokers, particularly AA women.
Keywords: nicotine dependence, light smoker, African American, women, pregnancy
Introduction
Historically, the majority of cigarette-smoking behavior research and tobacco-control efforts has focused on non-Hispanic white heavy smokers to the exclusion of minority and “light” smokers (defined here—and consistent with other literature—as smoking ≤ 10 cigarettes per day; CPD).1, 2 However, research in low-quantity (or “light,” as the term is used here) smoking populations, including adolescents, women, and ethnic minorities, has documented substantial prevalence of nicotine dependence (ND) symptoms.3-6 Additionally, “non-dependent” and light smokers remain at risk for ND symptomatology and smoking-related health consequences, indicating these groups, despite being clinically understudied, have significant public health relevance.
Despite higher rates of light smoking (average ≤ 10 CPD) and later onset of smoking initiation, African American (AA) men and women suffer greater smoking-related morbidity and mortality than other US ethnic groups.7, 8 Suggested reasons for these health disparities include preference for high-tar/nicotine and mentholated cigarettes, deeper tobacco inhalation, and slower nicotine metabolism in AA smokers compared with other ethnic groups.9-12 There may also be a failure by healthcare professionals to identify light smoking as a serious problem. Supporting this idea, researchers found light smokers verbalize high levels of motivation to quit, yet are less likely than heavy smokers to be asked by physicians about smoking or referred for cessation programs.13-15 This practice would disproportionately affect AA smokers.
Another group with high prevalence of light smoking and particular vulnerability to smoking’s negative health consequences include female smokers of childbearing age.16 Even with substantial public health efforts to educate women about the hazards to them and their offspring of smoking during pregnancy,17-22 over 11% of all women giving birth continue to smoke.16 Cigarette smoking is considered the largest modifiable risk factor for perinatal morbidity and mortality in the US and other developed countries and most clinicians agree that continued smoking during pregnancy may demonstrate greater dependence severity.1, 23, 24 The degree to which smoking during pregnancy is captured by ND definitions as a marker of addiction, however, may not be consistent across ethnic groups or cigarette consumption. For instance, AA and European American (EA) low-income pregnant smokers report comparable quit rates despite AA women having shorter smoking duration, lower smoking quantities, and less ND as measured by the Fagerström Test on Nicotine Dependence (FTND)25 compared to EA.26 The FTND, relying in part on quantity smoked, may underestimate ND in AA, calling into question the validity of ND measurements among light smokers and minorities.5 Thus, the extent to which current ND constructs, including DSM-IV diagnosis, adequately assess problematic cigarette use in light smokers or across racial/ethnic groups remains unclear.
Our aim in this population-based study, designed specifically to be informative about differences between AA and EA families, was to examine covariation of smoking-related problems, including ND symptoms and smoking during pregnancy, across different levels of peak daily cigarette consumption with particular focus on light smokers, and the degree to which this pattern might differ between AA and EA women.
Methods
Study design
The Missouri Family Study (MOFAM) is a longitudinal general population ethnically diverse family study of offspring outcomes in low risk and high risk families classified based on the absence or presence, respectively, of paternal alcohol problems, variously defined by maternal report or record evidence of DUI’s. State birth record data were used to select families with an index child born in birth years to be aged 13, 15, 17 or 19 at the baseline interview, plus at least one other child born to the same parents. Telephone screening interview with mothers confirmed the offspring were full siblings and determined the family’s risk status. Between 2003 and 2005, 79% of mothers contacted were successfully screened and of these, 71% of families who were invited to participate were enrolled. Risk status was not associated with rate of participation. AA families were oversampled.
To date, 472 families have been enrolled in MOFAM: 217 EA families (66 low risk and 151 high risk) and 255 AA families (126 low risk and 129 high risk). Recruitment is ongoing with a total goal of 750 families. This report is a secondary analysis of data currently collected. This study was approved by the Washington University School of Medicine HRPO and informed consent was obtained from all participants. Only data from mothers were analyzed for this report.
Sample
Of the 472 mothers, 1 was excluded due to incomplete data, 105 had never tried cigarettes, and 129 did not meet the conventional definition of smoker27 of having smoked 100 or more cigarettes across the lifetime, leaving data from 237 (118 EA, 86.4% from high risk families; 119 AA, 56.3% from high risk families) mothers for analysis.
Study assessments
Each mother was administered a comprehensive structured diagnostic interview adapted for telephone use from the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA).28, 29
Questions about smoking behaviors during pregnancy were derived from the perinatal section of the parent version of the Child-SSAGA based on the Diagnostic Interview for Children and Adolescents (DICA).30, 31 Smoking behaviors during pregnancy were obtained independent of the smoking section.
Measurements and variables
ND was defined using the two most common operationalizations: (1) diagnostic lifetime DSM-IV dependence defined as reporting at least 3 of 7 dependence symptoms clustering together within any 12-month period; and (2) a modification of the Heaviness of Smoking Index (HSI),32 a 2-item subscale of the FTND that measures time in minutes to first cigarette after awakening (≤ 5, 6-30, 31-60, > 60 minutes) and the quantity of cigarettes smoked per day (CPD 1-5, 6-10, 11-19, ≥ 20; this CPD measure was modified from original HSI to allow greater characterization of low-level smokers) during the heaviest lifetime smoking period. Tolerance, one of 7 DSM-IV criteria, was operationalized as either smoking 20+ cigarettes daily in lifetime or reporting a need to smoke more or stronger types to feel satisfied. Individuals met the tolerance criterion by endorsing either. Other smoking behaviors associated with risk for persistence in the smoking habit and with difficulty quitting smoking included: (1) lifetime daily smoking, defined as smoking “every day”; and (2) current smoking, defined as any smoking in the past month.
We investigated smoking behaviors during each pregnancy (up to 3). “Any smoking in pregnancy” was defined as reporting ever smoking in any pregnancy, including before knowledge of the pregnancy (N=178; 75.1% of smokers). A mother also reported during which trimester(s) she smoked. Nested within the “any smoking” group, a mother who reported smoking throughout all 3 trimesters of any pregnancy (N=108; 60.7% reporting “any smoking”) was categorized as “smoking throughout pregnancy.” Thus, though mothers reported on their smoking behaviors during up to three pregnancies, these datapoints were considered in aggregate, with the highest risk behavior identified in any one pregnancy determining group classification. 59 mothers (24.9%) reported no smoking during any pregnancy.
Our analysis also included additional sample descriptors: age, family risk type (low or high), current household income, level of education (less than high school v. high school or greater), receipt of prenatal care (in all pregnancies v. not), and maternal DSM-IV major depression, alcohol dependence and childhood conduct disorder, psychiatric disorders that are highly comorbid with ND.33-35
Statistical analyses
Demographics, smoking characteristics, and other descriptors were compared between AA and EA mothers using χ2 tests for categorical variables, one-way ANOVAs for continuous measures, and the Wilcoxon-Mann-Whitney test for three ordinal measures (current household income, time in minutes to first cigarette after awakening during lifetime heaviest smoking period, CPD during lifetime heaviest smoking period) using SAS 9.1.3 for Windows.36 An ordered logistic regression using CPD as the outcome variable was performed in STATA37 to examine main and interaction effects of race and family-risk type.
To examine ethnic differences in smoking behaviors while controlling for daily consumption, we compared the lifetime prevalence of smoking behaviors within 3 levels of CPD (1-10, 11-19, ≥ 20) based on sample size considerations and commonly used definitions of light (≤ 10 CPD) and heavy (≥ 20 CPD) smoking. Where sample size was sufficient, differences in the lowest ascertained group of cigarette consumption (1-5 CPD) were also investigated.
Results
Demographics
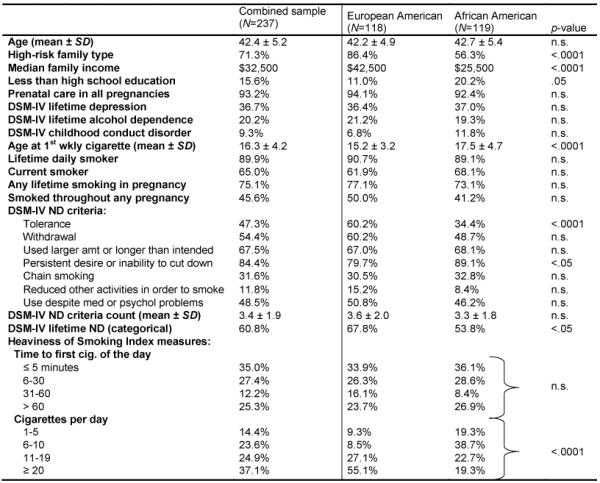
Table 1 displays demographics and frequencies of smoking behaviors for EA (N=118) and AA (N=119) mothers. The high rates of lifetime depression, alcohol dependence and childhood conduct disorder are expected given the high-risk sample design. AA women reported significantly lower income and an older age at initiation of weekly smoking, while EA women were more likely to be from a high-risk family type (Table 1). We examined main and interaction effects of race and family risk type on CPD during lifetime heaviest smoking period: being either EA (OR 4.2, CI 2.5-7.0; p<.001) or from high risk families (OR 2.0; CI 1.2-3.5, p<.01) both increased the likelihood of heavier smoking without an interaction (results not shown in table).
Table 1.
Demographic and smoking characteristics of mothers who have smoked 100 or more cigarettes lifetime, using Missouri Family Study data (2003-2005)
|
Note. SD, standard deviation; n.s., not statistically significant; ND, nicotine dependence.
Nicotine dependence symptoms and smoking-related behaviors
Lifetime prevalence of DSM-IV ND was lower among AA women and there were sharp differences in CPD (Table 1). Tolerance, consistent with its reliance upon quantity smoked, was significantly lower in AA compared with EA women. Despite consuming fewer cigarettes and having lower prevalence of DSM-IV ND than EA women, however, AA women were more likely to report wanting to cut down or having difficulty doing so, and were equally likely to have a short time to first cigarette after waking up, to be lifetime daily smokers or current smokers, and to report any smoking in pregnancy as well as smoking throughout pregnancy.
Effects of race and quantity smoked upon smoking behaviors
The patterns of ethnic/racial differences in ND measures and other smoking behaviors were different when the sample was stratified into smoking categories based on daily cigarette consumption during the heaviest smoking period in one’s life. At the lowest level of CPD (1-10), AA women were significantly more likely than their EA counterparts to report daily or current smoking, shorter time to the first cigarette after waking up, and engaging in any smoking or smoking throughout a pregnancy (Table 2). No differences were observed in the overall analysis presented in Table 1 or at the heaviest CPD smoking category. As previously mentioned, the tolerance criterion was operationalized to include both smoking quantity (history of 20+ CPD) and reports of needing to smoke more or stronger types of cigarettes. Thus, once controlling for CPD, Table 2 demonstrates a greater proportion of AA women smoking 1-10 CPD reported tolerance compared to EA women in the same CPD category, a reversal of the effect observed in the overall analysis. No racial/ethnic differences in the prevalence of tolerance were seen in the moderate and heavy CPD categories. Notably, in both EA and AA mothers who smoked at the lowest levels of CPD, rates of current and daily smoking, ND and ND symptoms, and smoking during pregnancy were substantial. Further analysis (data not shown in table) of a very low cigarette consumption category (1-5 CPD) revealed 57% of AA women (18% EA) smoked during any pregnancy, 30% smoked throughout pregnancy (0% EA) and 70% (27% EA) were still smoking at interview (all p<.05).
Table 2.
Lifetime prevalence of nicotine dependence symptoms and other related smoking behaviors in European American and African American mothers (all who have smoked ≥100 cigarettes lifetime) by average number of cigarettes smoked per day at peak lifetime use (N=237), using Missouri Family Study data (2003-2005)
| 1-10 CPD | 11-19 CPD | ≥ 20 CPD | ||||
|---|---|---|---|---|---|---|
| EA | AA | EA | AA | EA | AA | |
| Smoking behaviors | N=21 | N=69 | N=3 | N=27 | N=65 | N=23 |
| Lifetime daily smoker | 57% | 81% * | 94% | 100% | 100% | 100% |
| Current smoker | 38% | 64% * | 50% | 78% * | 75% | 70% |
| Any lifetime smoking in pregnancy | 38% | 64% * | 75% | 85% | 91% | 87% |
| Smoked throughout any pregnancy | 0% | 30% ** | 41% | 55% | 71% | 57% |
| DSM-IV ND criteria: | ||||||
| Tolerance | 0% | 19% * | 19% | 18% | 34% | 26% |
| Withdrawal | 38% | 43% | 53% | 59% | 71% | 52% |
| Used larger amt or longer than intended | 33% | 56% | 56% | 81% * | 83% | 87% |
| Persistent desire or inability to cut down | 57% | 85% ** | 72% | 96% * | 91% | 91% |
| Chain smoking | 10% | 17% | 22% | 48% * | 42% | 61% |
| Reduced other activities in order to smoke | 0% | 9% | 9% | 7% | 23% | 9% |
| Use despite med or psychol problems | 28% | 46% | 47% | 48% | 60% | 43% |
| DSM-IV ND criteria count (mean ± SD) | 1.7 ± 1.5 | 2.8 ± 1.8 * | 2.8 ± 1.8 | 3.6 ± 1.5 | 4.7 ± 1. | 4.4 ± 1.6 |
| Lifetime DSM-IV ND (categorical) | 28% | 42% | 53% | 59% | 88% | 83% |
| Time to first cig. of day ≤ 30 minutes | 10% | 51% ** | 53% | 81% * | 80% | 87% |
Note. CPD, cigarettes per day; EA, European American; AA, African American; ND, nicotine dependence; HSI, Heaviness of Smoking Index. Boldface: AA significantly different from EA.
(p<.05)
(p<.01).
In the heaviest smoking category, no differences were observed between EA and AA women on any smoking measure.
Discussion
This study highlights significant at-risk smoking-related problems and behaviors among females and, in particular, African American (AA) women who smoke 10 or fewer cigarettes per day (CPD), with difficulty cutting down, current smoking, and smoking during pregnancy most prominent. Our finding of greater low-quantity smoking among AA women is consistent with other reports.3-5 However, there has been less consistency in documented findings regarding rates of lifetime and current nicotine dependence (ND) with some studies3, 4, 26 but not all9 reporting lower rates of ND in AAs. Such inconsistencies likely reflect differences in assessment. For example, measurements using cotinine levels may differ from those relying on reports of quantity smoked (e.g., the Heaviness of Smoking Index (HSI), DSM-IV tolerance criterion). In a multiethnic adolescent sample, AAs’ lower rates of meeting criteria for ND compared to European American (EA) youth stemmed from smoking less, rather than reporting fewer symptoms at the same quantity smoked.3 Similarly, our results in an adult female sample suggest the overall lower probability of AA mothers meeting ND criteria is attributable to differences in quantity smoked. Indeed, as Table 1 illustrates, the only DSM-IV ND symptom varying by ethnicity in the appropriate direction was the tolerance measure (largely influenced by quantity smoked), yet the mean number of DSM-IV symptoms did not differ across groups. The HSI likewise relies upon cigarette quantity as a marker of addiction. Yet, once stratified on the basis of quantity, ethnic differences in prevalence rates of smoking behaviors were unmasked. In the lowest level of smoking (1-10 CPD during lifetime heaviest smoking period) where 58% of AAs were grouped, AA women reported significantly more dependence problems and had higher mean number of DSM-IV ND symptoms. Thus, our results highlight the serious limitations of current ND criteria that rely on quantity of cigarettes smoked. Notably, however, among both AA and EA women smoking 1-10 CPD, rates of some ND symptoms were substantial as were rates of current smoking and any smoking during pregnancy.
Our results also suggest that, irrespective of ND diagnosis, female light smokers remain at risk for serious problems related to tobacco use, with 57% EA and 85% AA reporting difficulty quitting, 38% EA and 64% AA still smoking at the time of interview, and 38% EA and 64% AA with any smoking during pregnancy. This last finding is especially striking: low levels of peak lifetime consumption did not preclude the high-risk behavior of smoking during pregnancy or smoking throughout pregnancy, chiefly among AAs. AAs are, in general, at increased risk for poor perinatal outcomes, including low birth weight, prematurity, and infant mortality, even controlling for age, income and education.38 And, while evidence appears strong for a dose-response relationship between smoking and certain perinatal outcomes,18 it is plausible that this relationship differs quantitatively in AAs, who metabolize nicotine more slowly and maintain higher cotinine levels per cigarette smoked.11, 12 Research has highlighted the disparity of higher rates of smoking-related problems per level smoked, lower rates of identification/referral by healthcare providers, and higher overall smoking-related health burden despite lower levels of smoking in AAs.1, 39 Our results add further evidence supporting increased research in these understudied groups. Furthermore, given the extremely high rates of prenatal care received in our sample, the potential for prevention and intervention appears obvious.
Study Limitations
There are several limitations to our study. First, our study was limited to middle aged Midwest mothers and may not be generalizable to all female smokers. The high-risk ascertainment strategy may also not generalize to the general population and findings for AA families may not apply to other light smoking minority populations. Second, data collection is currently ongoing and results may vary somewhat in the full sample. Third, our data relied on retrospective reporting of pregnancy-related behaviors, some occurring many years earlier. While under-reporting of this stigmatized behavior might be expected, over 75% of smokers in our sample admitted to any smoking during pregnancy, suggesting that under-reporting is unlikely to be substantial. Further data from twins who were asked to report on both their own and their co-twin’s smoking behaviors during pregnancies occurring one to two decades earlier revealed good reliability and minimal under-reporting.40 Fourth, our study may have excluded potential confounders to explain the higher rates of smoking in pregnancy despite lower cigarette consumption in AA women. For example, we did not assess perceptions of smoking risk during pregnancy. Women with lower perceived risk to the pregnancy have less motivation for and greater difficulty with smoking cessation.41 If such attitudes were more prevalent among the AA women, it may account for our findings, yet the extremely high rates of wanting/attempting to cut down that we found in AA light smokers suggest low identification of smoking as a serious problem was not common.
Conclusions
This study highlights problems associated with light smoking and finds these problems augmented in African American mothers. Given the known health risks of in-utero nicotine exposure and subsequent second hand smoke exposure in children, our findings bolster the entreaty for identification, prevention, and treatment of tobacco use and smoking-related morbidity among light smoking and minority populations. While many light smokers do not meet criteria for DSM-IV nicotine dependence, this should not diminish the call for better understanding of the addiction process associated with light smoking and its differential impact across ethnic groups or the amount of clinical attention from which such women (and their children) might benefit. Finally, our results suggest that future diagnostic criteria may need to take into consideration variations among subgroups, including adolescents, elderly individuals and, as indicated here, racial/ethnic groups.
Acknowledgments
Support for this study, provided by the National Institutes of Health grants: AA12640 (Bucholz), DA019951 (Pergadia), training grant T32MH17104 (Edens) and center grant AA11998, as well as the Barnes-Jewish Hospital Foundation (Lessov-Schlaggar), is gratefully acknowledged. Neither the NIH nor the Barnes-Jewish Hospital Foundation had any further role in the study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Funding for this study was provided by the National Institutes of Health grants: AA12640 (Bucholz), DA019951 (Pergadia), training grant T32MH17104 (Edens) and center grant AA11998, as well as the Barnes-Jewish Hospital Foundation (Lessov-Schlaggar). The views expressed in this study are solely those of the authors and do not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okuyemi KS, Harris KJ, Scheibmeir M, Choi WS, Powell J, Ahluwalia JS. Light smokers: issues and recommendations. Nicotine Tob Res. 2002;4(Suppl 2):S103–112. doi: 10.1080/1462220021000032726. [DOI] [PubMed] [Google Scholar]
- 2.Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007 Dec;102(12):1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 3.Kandel D, Schaffran C, Griesler P, Samuolis J, Davies M, Galanti R. On the measurement of nicotine dependence in adolescence: comparisons of the mFTQ and a DSM-IV-based scale. J Pediatr Psychol. 2005 Jun;30(4):319–332. doi: 10.1093/jpepsy/jsi027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991-1993. Nicotine Tob Res. 2000 Aug;2(3):263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JR, Moolchan ET. Ethnic differences among adolescents seeking smoking cessation treatment: a structural analysis of responses on the Fagerstrom Test for Nicotine Dependence. Nicotine Tob Res. 2007 Jan;9(1):137–145. doi: 10.1080/14622200601078400. [DOI] [PubMed] [Google Scholar]
- 6.DiFranza JR, Savageau JA, Fletcher K, et al. Symptoms of tobacco dependence after brief intermittent use: the Development and Assessment of Nicotine Dependence in Youth-2 study. Arch Pediatr Adolesc Med. 2007 Jul;161(7):704–710. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005 Jan-Feb;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006 Jan 26;354(4):333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 9.Ahijevych K, Gillespie J. Nicotine dependence and smoking topography among black and white women. Res Nurs Health. 1997 Dec;20(6):505–514. doi: 10.1002/(sici)1098-240x(199712)20:6<505::aid-nur5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Caraballo RS, Giovino GA, Pechacek TF, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988-1991. Jama. 1998 Jul 8;280(2):135–139. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. Jama. 1998 Jul 8;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 12.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999 Dec;291(3):1196–1203. [PubMed] [Google Scholar]
- 13.Owen N, Kent P, Wakefield M, Roberts L. Low-rate smokers. Prev Med. 1995 Jan;24(1):80–84. doi: 10.1006/pmed.1995.1011. [DOI] [PubMed] [Google Scholar]
- 14.Okuyemi KS, Ahluwalia JS, Banks R, et al. Differences in smoking and quitting experiences by levels of smoking among African Americans. Ethn Dis. 2004 Winter;14(1):127–133. [PubMed] [Google Scholar]
- 15.Okuyemi KS, Ahluwalia JS, Richter KP, Mayo MS, Resnicow K. Differences among African American light, moderate, and heavy smokers. Nicotine Tob Res. 2001 Feb;3(1):45–50. doi: 10.1080/14622200020032097. [DOI] [PubMed] [Google Scholar]
- 16.CDC Smoking during pregnancy--United States, 1990-2002. MMWR Morb. Mortal. Wkly. Rep. 2004;53:911–915. [PubMed] [Google Scholar]
- 17.Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998 Oct;27(3):352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- 18.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004 Apr;6(Suppl 2):S125–140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 19.Knopik VS, Heath AC, Jacob T, et al. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med. 2006 Oct;36(10):1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- 20.Monuteaux MC, Blacker D, Biederman J, Fitzmaurice G, Buka SL. Maternal smoking during pregnancy and offspring overt and covert conduct problems: a longitudinal study. J Child Psychol Psychiatry. 2006 Sep;47(9):883–890. doi: 10.1111/j.1469-7610.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 21.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007 Nov;83(11):713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 2007 Mar;46(3):362–369. doi: 10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Dempsey DA. Pharmacotherapy for smoking cessation during pregnancy. Nicotine Tob Res. 2004;6:S198–202. doi: 10.1080/14622200410001669169. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal A, Knopik VS, Pergadia ML, et al. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine Tob Res. 2008 Apr;10(4):567–578. doi: 10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- 25.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 26.Ward KD, Vander Weg MW, Sell MA, Scarinci IC, Read MC. Characteristics and correlates of quitting among black and white low-income pregnant smokers. Am J Health Behav. 2006 Nov-Dec;30(6):651–662. doi: 10.5555/ajhb.2006.30.6.651. [DOI] [PubMed] [Google Scholar]
- 27.CDC Cigarette smoking among adults--United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 28.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994 Mar;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 29.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999 Sep;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 30.Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000 Jan;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Reich W, Herjanic B, Welner Z, Gandhy PR. Development of a structured psychiatric interview for children: agreement on diagnosis comparing child and parent interviews. J Abnorm Child Psychol. 1982 Sep;10(3):325–336. doi: 10.1007/BF00912325. [DOI] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989 Jul;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 33.Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004 Jan 1;55(1):69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- 34.Bierut LJ, Dinwiddie SH, Begleiter H, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998 Nov;55(11):982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- 35.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004 Nov;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 36.SAS statistical software [computer program]. Version. Cary, NC: 2002. [Google Scholar]
- 37.Stata statistical software [computer program]. Version. College Station, TX: 2007. [Google Scholar]
- 38.CDC Infant mortality and low birth weight among black and white infants--United States, 1980-2000. MMWR Morb. Mortal. Wkly. Rep. 2002;51:589–592. [PubMed] [Google Scholar]
- 39.Moolchan ET, Fagan P, Fernander AF, et al. Addressing tobacco-related health disparities. Addiction. 2007 Oct;102(Suppl 2):30–42. doi: 10.1111/j.1360-0443.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- 40.Heath AC, Knopik VS, Madden PA, et al. Accuracy of mothers’ retrospective reports of smoking during pregnancy: comparison with twin sister informant ratings. Twin Res. 2003 Aug;6(4):297–301. doi: 10.1375/136905203322296656. [DOI] [PubMed] [Google Scholar]
- 41.Ockene JK, Ma Y, Zapka JG, Pbert LA, Goins KV, Stoddard AM. Spontaneous cessation of smoking and alcohol use among low-income pregnant women. Am J Prev Med. 2002;23:150–159. doi: 10.1016/s0749-3797(02)00492-0. [DOI] [PubMed] [Google Scholar]


