Abstract
For more than half a decade, lithium has been successfully used to treat bipolar disorder. Worldwide, it is considered the first-line mood stabilizer. Apart from its proven antimanic and prophylactic effects, considerable evidence also suggests an antisuicidal effect in affective disorders. Lithium is also effectively used to augment antidepressant drugs in the treatment of refractory major depressive episodes and prevent relapses in recurrent unipolar depression. In contrast to many psychiatric drugs, lithium has outlasted various pharmacotherapeutic ‘fashions’, and remains an indispensable element in contemporary psychopharmacology. Nevertheless, data from pharmacogenetic studies of lithium are comparatively sparse, and these studies are generally characterized by small sample sizes and varying definitions of response. Here, we present an international effort to elucidate the genetic underpinnings of lithium response in bipolar disorder. Following an initiative by the International Group for the Study of Lithium-Treated Patients (www.IGSLI.org) and the Unit on the Genetic Basis of Mood and Anxiety Disorders at the National Institute of Mental Health, lithium researchers from around the world have formed the Consortium on Lithium Genetics (www.ConLiGen.org) to establish the largest sample to date for genome-wide studies of lithium response in bipolar disorder, currently comprising more than 1,200 patients characterized for response to lithium treatment. A stringent phenotype definition of response is one of the hallmarks of this collaboration. ConLiGen invites all lithium researchers to join its efforts.
Key Words: Manic-depressive illness, Schizoaffective disorder, Mood stabilizer, Antidepressants, Suicidal behavior, Genome-wide association study, Neurogenesis, Neuroplasticity
Background
The articles in this special issue of Neuropsychobiology comprehensively review the use of lithium as a mood stabilizer in bipolar and unipolar affective disorders. They show that 60 years after Cade's discovery, lithium is still a first-line choice for prophylaxis in bipolar disorder. They furthermore discuss the evidence regarding lithium's antisuicidal effects, its use as an augmentation strategy in the treatment of unipolar depression, and provide novel insights into its neurobiological mechanisms of action. Finally, current pharmacogenetic knowledge about lithium treatment is reviewed. Taken together, however, these articles also highlight that, despite decades of lithium use in psychiatry and despite the current emphasis on the study of psychiatric genetics in modern biological psychiatry, pharmacogenetic data regarding lithium treatment have a tendency to be circumstantial and inconclusive.
Pharmacogenetics is a rapidly growing field that holds considerable promise for the development of medications that are more personalized and effective than those currently available. In all areas of medicine, pharmacogenetic studies of outcomes such as treatment response or characteristic side effects are on the rise; based on these findings, more and more pharmacogenetic tests are being offered and approved by the US Food and Drug Administration [1]. Pretreatment genetic testing has now even been added to the prescribing information for the anticoagulant warfarin [2]. Similarly, the Food and Drug Administration updated labeling for carbamazepine, recommending that patients of Asian ancestry be screened for the presence of the HLA allele B*1502 that has been implicated in carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese people [3].
Sufficiently large, well-characterized samples as well as effective and efficient collaboration between academia and the pharmaceutical industry are among the critical prerequisites for success in the field of pharmacogenetics [4,5]. Pharmacogenetic research in psychiatry has long been characterized by single lab efforts and small sample sizes. Only recently has our field witnessed large collaborative studies such as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (http://www.edc.pitt.edu/stard/) [6] in the United States, or the Genome-Based Therapeutic Drugs for Depression (GENDEP) project (http://gendep.iop.kcl.ac.uk) [7] in Europe, both of which study the pharmacogenetics of major depression. Indeed, the STAR*D and GENDEP projects have already generated several intriguing findings concerning the genetics of treatment response and side effects [8,9,10,11,12,13,14]. It is hoped that genome-wide association studies (GWAS) conducted in these and other samples will significantly increase our ability to guide the pharmacological treatment of psychiatric patients through the identification of genetic markers.
Notably, despite lithium's proven efficacy [15], to date there has been only one GWAS examining this ‘pharmacological workhorse’ of psychiatry [16]. In two cohorts encompassing more than 800 lithium-treated patients, multiple regions of interest were identified but none met the threshold for genome-wide significance. While intriguing, no adequately powered cohort yet exists to replicate and extend these findings. Here, we present a worldwide effort to address this situation: the international Consortium on Lithium Genetics (ConLiGen), spearheaded by researchers from the International Group for the Study of Lithium-Treated Patients (IGSLI) and the National Institute of Mental Health (NIMH).
The International Group for the Study of Lithium-Treated Patients
The IGSLI is an international group of scientists dedicated to lithium-related research, and its use in mental illness and mood disorders in particular. Founded in 1988 by Mogens Schou (Risskov/Aarhus, Denmark), Bruno Müller-Oerlinghausen (Berlin, Germany), and Paul Grof (Ottawa, Canada), the IGSLI has significantly contributed to lithium research over the past 20 years (www.igsli.org). Other scientists and centers have since joined the group, which currently comprises 35 members from Austria, Canada, the Czech Republic, Denmark, Germany, Poland, Switzerland, and the United States. The main goal of this group has been to conduct systematic work on those key questions regarding lithium treatment that can only be resolved by joint international effort. Unified designs have been created and scientific data from the IGSLI member centers have been linked for the purpose of shared analysis. This approach allows investigators to work with large numbers of prospectively followed patients – something that could only be accomplished via a multicenter approach. Overall, IGSLI research is based on shared, standardized, computer-based documentation of patients’ diagnoses, family histories, course of illness before and during treatment, and on comparable modalities of treatment. The group meets regularly at research conferences to plan and discuss joint projects and to prepare publications.
At the 21st IGSLI meeting, which took place in late September 2007 in Dresden, Germany, the group discussed the results from the first, newly released GWAS of bipolar disorder, performed by researchers from the NIMH and Germany [17]. The strongest findings identified and replicated in this study were those encoding diacylglycerol kinase eta, a key protein in the lithium-sensitive phosphatidylinositol pathway and several genes in the Wnt-signaling cascade. Given the absence of a hypothesis-driven selection of single nucleotide polymorphisms in GWAS – a method more typical of candidate gene association studies – the observation that these findings implicated pathways relevant to lithium's mechanism of action was particularly intriguing. Spurred on by these findings, the IGSLI researchers concluded that studying these genes in samples that included data on patient response to lithium treatment could improve our understanding of how these genes determine response to lithium treatment and impact susceptibility to bipolar disorder. The IGSLIcollaborators thus agreed to explore a framework that would allow researchers to engage in genetic studies of lithium response that were sufficiently powered. It was stated that such an endeavor should allow for participation by all bona fide lithium researchers within and beyond the IGSLI, while maintaining the highest possible level of stringency regarding phenotype definition.
May 6, 2008: The Consortium on Lithium Genetics Is Born
Following an invitation by IGSLI member Thomas G. Schulze and Francis J. McMahon, both from the NIMH's Unit on the Genetic Basis of Mood and Anxiety Disorders, prominent scientists in the field of lithium and bipolar genetic research met at the NIMH to discuss the possibility of creating an international consortium dedicated to the study of lithium pharmacogenetics. In attendance were (in alphabetical order): Martin Alda (Halifax, N.S., Canada), Michael Bauer (Dresden, Germany), Maria Del Zompo (via phone from Cagliari, Italy), Gonzalo Laje (Bethesda, Md., USA), Francis J. McMahon (Bethesda, Md., USA), Mirko Manchia (Cagliari, Italy), Roy H. Perlis (Boston, Mass., USA), Janusz K. Rybakowski (Poznan, Poland), Thomas G. Schulze (Bethesda, Md., USA), Johannes Schumacher (Bethesda, Md., USA), and Jordan W. Smoller (Boston, Mass., USA).
Reviewing evidence from the literature, and based on their own observations, the group emphasized the evident familiality in lithium treatment response, raising the possibility that genetic variation may contribute to interindividual differences in treatment response. If such differences could be identified, they might facilitate the development of novel treatments for bipolar disorder, or allow for better matching between patients and treatments. Over the last decade, the quest for a ‘personalized medicine’ approach in psychiatry has propelled a host of pharmacogenetic studies. Because of the lengthy trial-and-error process that currently characterizes the search for the most optimal treatment, pharmacogenetic studies in psychiatry have traditionally focused on treatment response or adverse effects associated with antidepressants or antipsychotic medications [18,19,20,21,22]. While initially limited by small sample sizes, pharmacogenetic studies in psychiatry have increasingly come to rely on large-scale collaborative efforts, such as STAR*D, GENDEP, or the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project. While some pharmacogenetic studies performed with these collaborative samples have produced intriguing results, difficulties in defining stringent target phenotypes across the various subsamples remain an important challenge [23,24].
The researchers gathered at the NIMH on May 6, 2008 noted that, despite considerable and well-documented worldwide experience with lithium as an effective antimanic agent, mood stabilizer, and putative antisuicidal agent, there is a surprising dearth of large-scale pharmacogenetic studies of lithium treatment. We thus decided to create an international initiative whose goal would be to facilitate high-quality, well-powered analyses of lithium treatment response data that would ultimately allow for robust conclusions. The Consortium on Lithium Genetics, hereafter referred to as ConLiGen, was born.
ConLiGen's Scientific Goals
ConLiGen aims to identify genetic determinants of response to lithium treatment in bipolar disorder, as well as genetic determinants of adverse events emerging during lithium treatment. In the long run, ConLiGen may also study response to lithium treatment in general (e.g. lithium augmentation in the treatment of major depression).
Membership in ConLiGen
Any bona fide researcher or research group with access to samples of lithium-treated patients for whom DNA is available can join ConLiGen. Any new admission request is voted upon by ConLiGen members.
Communication between the ConLiGen Members
To ensure a constant exchange of ideas between members and allow for a straightforward realization of ConLiGen's goals, a monthly conference call is conducted. Furthermore, members meet once or twice a year at international meetings of various biological psychiatric organizations.
ConLiGen Advisory Board
An Advisory Board comprising international experts in the field of mood disorders research, and lithium research in particular, was established to offer ConLiGen an outside perspective as well as guidance on broad scientific directions, to serve as a liaison to nonacademic communities such as funding institutions, or industry, and finally, to act as one of ConLiGen's publicly visible faces. Currently, the following researchers are members of the Advisory Board (in alphabetical order): Robert H. Belmaker (Division of Psychiatry, Ben Gurion University of the Negev, Beersheva, Israel), Gian Luigi Gessa (Department of Neuroscience ‘B.B. Brodie’, University of Cagliari, Cagliari, Italy), Paul Greengard (Laboratory of Molecular and Cellular Neuroscience, Rockefeller University, New York, N.Y., USA), Kay R. Jamison (Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Md., USA), Richard S. Jope (Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, Birmingham, Ala., USA), Husseini K. Manji (CNS & Pain, Johnson and Johnson Pharmaceutical Research and Development, Titusville, N.J., USA), and Leon E. Rosenberg (Department of Molecular Biology and the Woodrow Wilson School of Public and International Affairs, Princeton University, Princeton, N.J., USA).
Phenotype Definition of Lithium Response: A Major Prerequisite for Pharmacogenetic Studies of Lithium
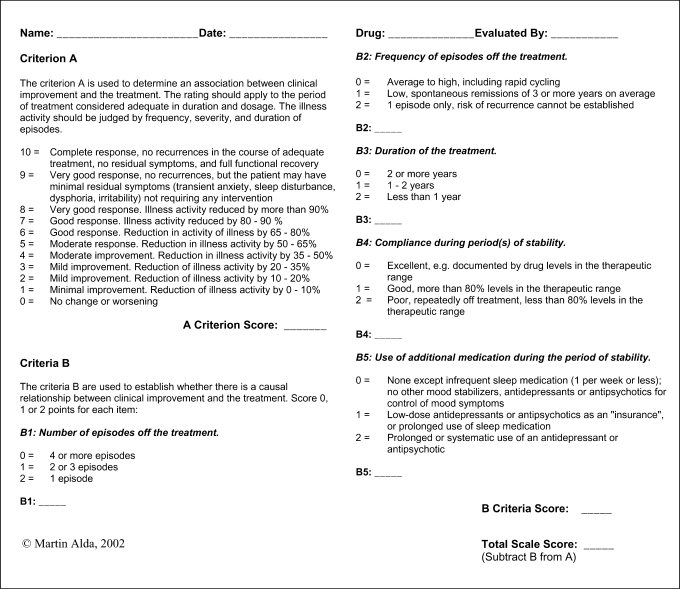
ConLiGen's first and most crucial goal is to define the phenotype of lithium response. Treatment response is a complex construct that requires researchers to make judgments about adequacy of treatment and tolerability as well as assess changes in episode frequency or symptom severity. In many cases this information must be assessed retrospectively, with the inherent limitations associated with recall bias, missing information, or the fact that the treatment has not followed a strict research protocol. One scale that incorporates such data is an 11-point scale developed by Martin Alda and colleagues [25] (fig. 1); other approaches include longitudinal outcome measures that consider time to recurrence or symptom burden during treatment [16,26].
Fig. 1.
Retrospective criteria of long-term treatment response in research subjects with bipolar disorder.
The 11-point scale measures the extent of improvement during long-term treatment. The scale's A score is a composite measure of change in frequency, duration, and severity of illness episodes in the course of lithium treatment. It is weighted by factors that influence the degree to which the observed clinical change is considered to be due to lithium (B1–B5 scores in the scale). The scale has been developed in the context of a study assessing response to treatment in subjects not followed according to a research protocol, namely relatives of probands in our genetic studies [25]. Subsequently, it has been widely used in several other studies at IGSLI centers [27,28,29] and at other centers involved in lithium research [pers. commun. from John Kelsoe, San Diego, Calif., USA and Maria del Zompo, Cagliari, Italy], which imparts face validity. Within ConLiGen, phenotypic assessment will be based on any available information including life charts when available and quantified using the scale; interrater reliability meetings will be organized, facilitated by ConLiGen member Martin Alda, and case vignettes will also be reviewed to establish between-center reliability.
Variables describing treatment tolerability or side effects may be studied in subsequent projects. Because the issue of ‘best response phenotype’ is far from trivial, ConLiGen will strive to continuously weigh evidence from future clinical and biological studies of lithium in an effort to refine the definition of phenotype response. Evaluating response to long-term treatment in an illness with a highly variable natural course presents a challenge. Many patients with bipolar disorder experience spontaneous remissions of variable timing and duration. Moreover, in a pharmacogenetic study we need to evaluate the quality of response not for groups of subjects as in clinical trials but individually for each patient. While prospective studies will be able to implement more precise measures, our approach is a practical way to assess the quality of response in a variety of patients treated in diverse settings.
ConLiGen's Current Project and Long-Term Mission
ConLiGen is poised to assess all aspects of the pharmacogenetics of lithium treatment in psychiatric disorders, including the study of genetic susceptibility to potential treatment-emergent adverse events (e.g. weight gain, hypothyroidism, tremor). As its first project, ConLiGen intends to conduct a GWAS of stringently defined response to lithium treatment in bipolar disorder. ConLiGen members and the various research centers which they are affiliated with are joining their samples for a centralized genotyping effort to be performed at the Unit on the Genetic Basis of Mood and Anxiety Disorders of the NIMH and the Department of Genomics of the Life and Brain Center at the University of Bonn, Germany. For the primary projects, a previously validated scale will be used to define response to lithium treatment, as described above. Individuals scoring between 7 and 10 will be considered lithium ‘responders’, while individuals with scores between 0 and 6 will be considered ‘nonresponders’. Presently, the total sample comprises more than 1,200 bipolar patients for whom response to lithium treatment has been or is currently being assessed by means of the scale. From preliminary analyses conducted in select IGSLI samples (data not shown), we can assume that about 35–40% of patients will qualify as responders. Previous studies [8,9] suggest larger genetic effect sizes (e.g. allelic odds ratios between 1.5 and 2) for a narrowly defined pharmacogenetic phenotype than for a categorically defined clinical diagnosis. Thus, assuming a minor allele frequency of 0.3 and genotype relative risks of 1.4 for individuals heterozygous, and of 1.96 for individuals homozygous for the risk allele, the combined ConLiGen sample will have a power of 83% to detect an effect at a significance level of 1 × 10−8 [30].
Although the combined ConLiGen sample will be the largest sample to date to investigate lithium response on a genome-wide scale, we are aware that any finding, regardless of whether it reaches levels of genome-wide significance, will ultimately have to be confirmed in independent samples. Thus, ConLiGen's mission will not be finished after the completion of its GWAS. On the contrary, ConLiGenwill continue to invite researchers to join its efforts in order to increase the available sample size of patients adequately characterized for lithium response. In collaboration with both IGSLI centers and large, long-standing multicenter projects such as the NIMH Bipolar Disorder Genetics Initiative, ConLiGen will be actively engaged in supporting and organizing urgently needed prospective studies of lithium response in bipolar disorder and other conditions.
Since Cade discovered lithium's beneficial effects in the treatment of bipolar disorder 60 years ago, this agent has become almost synonymous with the treatment of bipolar disorder worldwide [15]. Yet, little is known about the genetic underpinnings of lithium response or the development of side effects associated with its use. In a scientific environment characterized by calls for personalized medicine and the growth of large-scale pharmacogenetic studies in many fields of medicine, ConLiGen's goal is to put lithium at the forefront of pharmacogenetic studies in psychiatry.
Acknowledgements
This research was in part supported by the Intramural Program of the NIMH. Dr. Thomas G. Schulze's work is furthermore supported by NARSAD. Dr. Martin Alda's work on the phenotype of lithium response has been supported by grants from the Canadian Institutes of Health Research (CIHR, grant 64410) and by NARSAD. Dr. Smoller's work is supported in part by a grant from the NIMH (MH079799). Dr. Reif is supported by the Deutsche Forschungsgemeinschaft (DFG) (KFO 125 and SFB TRR 58) and the German Ministry for Education and Research (BMBF) (IZKF N-4). The work of the Cagliari group was partly supported by the Regional Councillorship of Health, ‘Regione Autonoma della Sardegna’.
References
- 1.Swen JJ, Huizinga TW, Gelderblom H, de Vries EG, Assendelft WJ, Kirchheiner J, Guchelaar HJ. Translating pharmacogenomics: challenges on the road to the clinic. PLoS Med. 2007;4:e209. doi: 10.1371/journal.pmed.0040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadelius M, Sörlin K, Wallerman O, Karlsson J, Yue QY, Magnusson PK, Wadelius C, Melhus H. Warfarin sensitivity related to CYP2C9, CYP3A5, ABCB1 (MDR1) and other factors. Pharmacogenomics J. 2004;4:40–48. doi: 10.1038/sj.tpj.6500220. [DOI] [PubMed] [Google Scholar]
- 3.Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 4.Penny MA, McHale D. Pharmacogenomics and the drug discovery pipeline: when should it be implemented? Am J Pharmacogenomics. 2005;5:53–62. doi: 10.2165/00129785-200505010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Roses AD. Pharmacogenetics in drug discovery and development: a translational perspective. Nat Rev Drug Discov. 2008;7:807–817. doi: 10.1038/nrd2593. [DOI] [PubMed] [Google Scholar]
- 6.Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 7.Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, Mors O, Elkin A, Williamson RJ, Schmael C, Henigsberg N, Perez J, Mendlewicz J, Janzing JG, Zobel A, Skibinska M, Kozel D, Stamp AS, Bajs M, Placentino A, Barreto M, McGuffin P, Aitchison KJ. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008;38:289–300. doi: 10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- 8.McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, Trivedi MH, Wisniewski SR, Manji H. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 10.Perlis RH, Purcell S, Fava M, Fagerness J, Rush AJ, Trivedi MH, Smoller JW. Association between treatment-emergent suicidal ideation with citalopram and polymorphisms near cyclic adenosine monophosphate response element binding protein in the STAR*D study. Arch Gen Psychiatry. 2007;64:689–697. doi: 10.1001/archpsyc.64.6.689. [DOI] [PubMed] [Google Scholar]
- 11.Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ. Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry. 2007;164:1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 12.Perlis RH, Moorjani P, Fagerness J, Purcell S, Trivedi MH, Fava M, Rush AJ, Smoller JW. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S. The FKBP5-gene in depression and treatment response – an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uher R, Huezo-Diaz P, Perroud N, Smith R, Rietschel M, Mors O, Hauser J, Maier W, Kozel D, Henigsberg N, Barreto M, Placentino A, Dernovsek MZ, Schulze TG, Kalember P, Zobel A, Czerski PM, Larsen ER, Sourey D, Giovannini C, Gray JM, Lewis CM, Farmer A, Aitchison KJ, McGuffin P, Craig I. Genetic predictors of response to antidepressants in the GENDEP project. Pharmacogenomics J. 2009;9:225–233. doi: 10.1038/tpj.2009.12. [DOI] [PubMed] [Google Scholar]
- 15.Bauer M, Grof P, Müller-Oerlinghausen B, editors. Lithium in Neuropsychiatry: The Comprehensive Guide. London: Informa Healthcare; 2006. [Google Scholar]
- 16.Perlis RH, Smoller JW, Ferreira MAR, McQuillin A, Bass N, Chir MB, Lawrence J, Sachs GS, Nimgaonkar V, Scolnick EM, Gurling H, Sklar P, Purcell S. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009;166:718–725. doi: 10.1176/appi.ajp.2009.08111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nöthen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Höfels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rietschel M, Kennedy JL, Macciardi F, Meltzer HY. Application of pharmacogenetics to psychotic disorders: the first consensus conference. The Consensus Group for Outcome Measures in Psychoses for Pharmacological Studies. Schizophr Res. 1999;37:191–196. doi: 10.1016/s0920-9964(98)00153-4. [DOI] [PubMed] [Google Scholar]
- 19.Lerer B, Segman RH, Fangerau H, Daly AK, Basile VS, Cavallaro R, Aschauer HN, McCreadie RG, Ohlraun S, Ferrier N, Masellis M, Verga M, Scharfetter J, Rietschel M, Lovlie R, Levy UH, Meltzer HY, Kennedy JL, Steen VM, Macciardi F. Pharmacogenetics of tardive dyskinesia: combined analysis of 780 patients supports association with dopamine D3 receptor gene Ser9Gly polymorphism. Neuropsychopharmacology. 2002;27:105–119. doi: 10.1016/S0893-133X(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra AK, Murphy GM, Jr, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161:780–796. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 21.Correll CU, Malhotra AK. Pharmacogenetics of antipsychotic-induced weight gain. Psychopharmacology (Berl) 2004:477–489. doi: 10.1007/s00213-004-1949-9. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra AK, Lencz T, Correll CU, Kane JM. Genomics and the future of pharmacotherapy in psychiatry. Int Rev Psychiatry. 2007;19:523–530. doi: 10.1080/09540260701563460. [DOI] [PubMed] [Google Scholar]
- 23.van den Oord EJ, Adkins DE, McClay J, Lieberman J, Sullivan PF. A systematic method for estimating individual responses to treatment with antipsychotics in CATIE. Schizophr Res. 2009;107:13–21. doi: 10.1016/j.schres.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Need AC, Keefe RS, Ge D, Grossman I, Dickson S, McEvoy JP, Goldstein DB. Pharmacogenetics of antipsychotic response in the CATIE trial: a candidate gene analysis. Eur J Hum Genet. 2009;17:946–957. doi: 10.1038/ejhg.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O'Donovan C, Alda M. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63:942–947. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- 26.Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 27.Garnham J, Munro A, Slaney C, Macdougall M, Passmore M, Duffy A, O'Donovan C, Teehan A, Alda M. Prophylactic treatment response in bipolar disorder: results of a naturalistic observation study. J Affect Disord. 2007;104:185–190. doi: 10.1016/j.jad.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Passmore MJ, Garnham J, Duffy A, MacDougall M, Munro A, Slaney C, Teehan A, Alda M. Phenotypic spectra of bipolar disorder in responders to lithium versus lamotrigine. Bipolar Disord. 2003;5:110–114. doi: 10.1034/j.1399-5618.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Hajek T, Hahn M, Slaney C, Garnham J, Green J, Ruzickova M, Zvolsky P, Alda M. Rapid cycling bipolar disorders in primary and tertiary-care treated patients. Bipolar Disord. 2008;10:495–502. doi: 10.1111/j.1399-5618.2008.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]