Abstract
Intervention to increase exercise in drug dependent patients represents a potentially useful yet unexplored strategy for preventing relapse. However, there are currently no established exercise interventions for use with this population. The purpose of this pilot study was to examine the feasibility of aerobic exercise as an adjunct to substance abuse treatment among drug dependent patients. Participants included 16 (31% female, 38.3 years old) drug dependent patients who participated in a 12-week, moderate-intensity aerobic exercise intervention. Participants attended a mean of 8.6 sessions (out of 12). Participants demonstrated a significant increase in percent days abstinent for both alcohol and drugs at the end of treatment, and those who attended at least 75% of the exercise sessions had significantly better substance use outcomes than those who did not. In addition, participants showed a significant increase in their cardiorespiratory fitness by the end of treatment. While preliminary, this study is one of the first to demonstrate the feasibility of incorporating aerobic exercise during drug abuse treatment. Future randomized control trials are a necessary next step to test the efficacy of a moderate-intensity aerobic exercise intervention as an adjunct to drug abuse treatment in this patient population.
Keywords: Exercise, Drug Dependence, Substance Abuse Treatment, Physical Activity
INTRODUCTION
Drug dependence is a major public health problem (McCrady & Epstein, 1999; Rotgers, Keller, & Morgenstern, 1996) and the associated costs of drug use disorders to society are considerable (Andlin-Sobocki, 2004; Langenbucker, McCrady, Brick, & Esterly, 1993; ONDCP, 2004). Results from the U.S. National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) point toward high rates of substance use disorders (SUD) with 9.5% of the population meeting DSM-IV criteria for any SUD and 4.07% meeting criteria for any substance dependence within the last 12 months (Grant et al., 2004). Epidemiological data from 27 community studies in European countries reveal a 12-month prevalence rate for any substance dependence of 3.4% of the adult population (Wittchen & Jacobi, 2005). Spontaneous remission rates from drug diagnoses are very low (Finney, Moos, & Timko, 1999). Relapse represents a major problem in substance use disorders, with relapse rates in the first year following treatment ranging from 60 – 90% (Brownell, Marlatt, Lichtenstein, & Wilson, 1986; Hunt, Barnett, & Branch, 1971; Xie, McHugo, Fox, & Drake, 2005). Therefore, it is crucial that accessible, affordable and efficacious primary and adjunctive drug dependence treatments be developed to address this chronic, relapsing condition.
The role of increasing lifestyle balance has been incorporated in existing relapse prevention models (Marlatt & Witkiewitz, 2005; Witkiewitz & Marlatt, 2004). Indeed, in his chapter on “Lifestyle Modification,” Marlatt cites exercise as “a highly recommended lifestyle change activity” (Marlatt, 1985, p. 309) and discusses the advantages of physical activity as a relapse prevention strategy. Other writers have agreed that lifestyle-enhancing factors such as exercise and fitness may play an important role in the prevention and treatment of addictive disorders (Brown, et al., 2009; Tkachuk & Martin, 1999). Larimer and colleagues (Larimer, Palmer, & Marlatt, 1999) describe the importance of helping the client develop “positive addictions” such as increased physical activity and meditation. Although lifestyle modification was one of the main components in Marlatt’s relapse prevention model [see Marlatt & Donovan (2005) for more details], the treatment outcome literature suggests that this component has received the least emphasis in relapse prevention programs for drug dependence. Despite this lack of attention in the empirical literature, methods that attempt to foster healthy lifestyle changes may contribute to long-term maintenance of recovery, and interventions targeting physical activity in particular, may be especially valuable as an adjunct to substance abuse treatment.
Exercise may benefit drug dependent patients attempting recovery from substance problems through a number of different mechanisms of action. First, engaging in exercise may offer drug dependent patients the ability to experience positive mood states without the use of drugs. For example, due to the potential for reductions in dopamine production and dopamine receptors availability, drug dependent patients may have an impaired capacity to experience pleasure during early recovery (Adinoff, 2004; Bressan & Crippa, 2005). On the other hand, exercise has been shown to result in acute improvements in positive-activated affect (e.g., (Reed & Ones, 2006) and alleviate mood disturbance and withdrawal symptoms in women attempting to quit smoking (e.g., Bock, Marcus, King, Borrelli, & Roberts, 1999). These positive reinforcing properties may be mediated in part by exercise effects on the endogenous opioid system and potentiation of dopaminergic systems linked importantly to the experience of enhanced mood and experienced pleasure (c.f., (Meeusen, 2005), although the application of neuroscience techniques to exercise psychology is complex and will require strong research designs (Dishman & O’Connor, 2009). Second, studies in recent years have found an association between depressive symptomatology and poor treatment outcome among patients with substance use disorders (R. A. Brown, et al., 1998; Nunes & Levin, 2004; Ouimette, Gima, Moos, & Finney, 1999; Poling, Kosten, & Sofuoglu, 2007). Engaging in exercise has been consistently associated with reductions in depressive symptoms (Craft & Landers, 1998; Dunn, Trivedi, & O’Neal, 2001; Lawlor & Hopker, 2001; Mead, et al., 2009) and thus exercise may reduce risk for relapse by reducing depressive symptoms. Third, exercise has been found to alleviate sleep disturbances (Youngstedt, 2005) and improve cognitive functioning (Kramer & Erickson, 2007) – both of which have been identified as disrupted in early drug recovery and predictive of relapse (Drummond, Gillin, Smith, & DeModena, 1998; Ersche & Sahakian, 2007; Gruber, Silveri, & Yurgelun-Todd, 2007; Jovanovski, Erb, & Zakzanis, 2005; Liu, Xiaoping, Wei, & Zeng, 2000; Rogers & Robbins, 2001; Scott, et al., 2007). Lastly, increases in self-efficacy (McAuley, Courneya, & Lettunich, 1991) and decreases in stress-reactivity (Hobson & Rejeski, 1993; Keller, 1980) associated with exercise engagement may also contribute to lower the risk of relapse among drug dependent patients. Among problem drinkers, exercise led to psychological improvement in physical self-worth and physical self-perceptions of condition and strength (Donaghy & Mutrie, 1998). Exercise has been proposed as an effective relapse prevention intervention specifically due to the potential number of positive physiological and psychological benefits. Improved mood, regulated reward systems, reduced depressive symptoms, improved sleep and cognitive function all may serve to reduce risk for relapse and efforts to illuminate mechanisms of effectiveness will be an important focus of future work in this area.
Thus far, few studies have examined the efficacy of aerobic exercise as adjunct to substance abuse treatment. Among addictive disorders, nicotine dependence has received the most attention with respect to the role of physical activity. Recent studies have demonstrated the acute effects of exercise on decreased craving and nicotine withdrawal (see Taylor, Ussher, & Faulkner, 2007) and as a means of facilitating smoking cessation (Marcus, et al., 1999; Marcus, et al., 2005; Prapavessis, et al., 2007) have been demonstrated.
The role of exercise as an adjunct to alcohol treatment has been explored by Sinyor and colleagues (Sinyor, Brown, Rostant, & Seraganian, 1982) who reported on 58 participants receiving inpatient alcohol rehabilitation treatment. Participants engaged in six weeks of “tailored” exercise, consisting of progressively more rigorous physical exercise including stretching, calisthenics and walking/running. Results revealed that these participants demonstrated better abstinence outcomes post-treatment than did non-exercising participants from two other small comparison groups. In addition, in a previous study, we developed and pilot tested a 12-week moderate-intensity aerobic exercise intervention for alcohol dependent patients (Brown et al., 2009). Results from this study suggest that, compared to the mean pretreatment percent days abstinent (PDA), significant increases in PDA were observed at the end of the 12-week exercise intervention and at the 3-month post-intervention follow-up.
Similar to alcohol treatment, there exists a lack of studies examining the role of exercise during drug abuse treatment (Donaghy & Ussher, 2005). In one small, uncontrolled pilot study of an exercise intervention conducted with substance abusing offenders in outpatient treatment, (Williams, 2000) conducted a 12-week intervention consisting of once weekly strength training groups plus recommendations for aerobic exercise during the rest of the week. While substance abuse outcomes were not presented in the results, the authors reported that the 11 out of 20 participants who completed the intervention reported that exercise was helpful in maintaining abstinence. In addition, exercise interventions have been applied with adolescent substance abusers. Collingwood and colleagues (Collingwood, Reynolds, Kohl, Smith, & Sloan, 1991) conducted a clinical trial of an 8–9 week structured fitness program with adolescent substance abusers. Adolescent participants showed improved physical fitness, reduced polysubstance use, and increased abstinence rates. Overall, there exists a lack of available studies attempting to evaluate the potential of exercise to benefit adults in treatment for drug use disorders. Stronger studies are needed to establish that exercise interventions should be more broadly implemented in practice.
The purpose of this study was to pilot test a 12-week moderate-intensity aerobic exercise intervention as an adjunct to treatment for drug dependent outpatients, as a preliminary step in a program of research. In this study, we examine the feasibility and exercise adherence among drug dependent patients. In addition, we examine drug use and cardiorespiratory fitness outcomes at the end of the 12-week intervention and at the 3-month follow-up.
METHOD
Participants
Participants were recruited from an intensive alcohol and drug treatment partial-hospitalization program at a psychiatric hospital in the Northeast USA as well as through study advertisements in the local newspaper. Eligible participants: (a) were between 18 and 65 years of age, (b) met current DSM-IV criteria for drug dependence as assessed by the Structured Diagnostic Interview for DSM-IV (SCID-P), (c) were sedentary; i.e., have not participated regularly in aerobic physical exercise (for at least 20 minutes per day, three days per week) for the past six months, and (d) were currently engaged in outpatient substance abuse treatment.
Exclusion criteria included: (a) anorexia or bulimia nervosa, (b) bipolar disorder, (c) a history of psychotic disorder or current psychotic symptoms, (d) current suicidality or homicidality, (e) marked organic impairment, (f) physical disabilities or medical problems or use of medications that would prevent or hinder participation in a program of moderate-intensity exercise, and (g) current pregnancy or intent to become pregnant during the next 12 weeks.
From the alcohol and drug partial hospital treatment program, 208 charts of patients with drug dependence were screened. In addition, 108 calls were received in response to newspaper advertisements for the research study. Of these 316 potential participants, rule outs included: 58 (18%) for a medical problem that would prohibit safely participating in exercise, 35 (11%) were not sedentary, 40 (13%) refused participation in the study (applicable only to patients from the partial hospital treatment program), 37 (12%) for one of the psychiatric exclusion criteria, and 47 (15%) were not involved in ongoing substance abuse treatment or were in a methadone maintenance program (applicable only to those calling in response to the newspaper advertisement). In addition, during the recruitment process, 64 (20%) of potential participants either expressed loss of interest in the study, dropped out of addiction treatment, were unable to attend group nights, or we lost contact with them.
As a result, the remaining 35 participants were scheduled for baseline assessments. Of these, 6 were excluded because of a SCID-diagnosed psychiatric rule out and 10 decided to no longer participate during the baseline assessment phase. Therefore, 19 participants were eligible to participate in the 12-week aerobic exercise intervention. Of these, 3 participants did not attend the intervention and were not able to be contacted further. Thus, the study sample was comprised of 16 participants who initiated the exercise intervention.
The sample of 16 participants included 11 (69%) males and 5 (31%) females. The mean age of participants was 38.3 (SD = 10.1) years. The sample was primarily Caucasian (13 of 16; 81.3%) with a minority of African-American (13%) and Hispanic (6%) individuals. Participants had an average of 12.5 (SD = 1.4) years of education. Seven participants (43.8%) were married, 6 (37.5%) were never married, and 3 (18.7%) were either divorced or separated. In addition, nine participants (56.2%) were employed full-time, while 4 (25%) were working part-time and three (18.6%) were currently unemployed. All participants were engaged in ongoing substance abuse treatment for drug dependence. In the 3 months prior to beginning the intervention, 81.3% reported using alcohol, 31.3% used cocaine, 31.3% used marijuana, 12.5% used opiates, and 6.3% reported sedative use. In the one month prior to the intervention, 63.7% of participants used two or more substances. In our clinical experience, it is common for drug dependent patients to also drink alcohol and to believe they can continue to drink while remaining abstinent from drugs.
Accordingly, while alcohol use was common in the sample, all participants were recruited if they met criteria for a current (i.e., in the last year per DSM-IV) drug dependence diagnosis and were engaged in substance abuse treatment irrespective of when the addiction treatment began. Therefore, participants began the exercise intervention with varying degrees of addiction treatment engagement. However, in the 3 months prior to study recruitment, most participants were engaged in both substance abuse and mental health treatment. Addiction focused treatment included: inpatient (31%), partial hospital day program (75%), individual sessions with a psychiatrist (37.5%), individual sessions with a counselor (50%), group therapy (25%). In addition, 56% of the sample had attended 12-step meetings in the last 3 months. Mental health treatment included: individual sessions with a psychiatrist (50%) and counselor (31.2%).
In addition, participants continued to engage in concurrent substance abuse and mental health treatment during the 12-week exercise intervention. Addiction focused treatment included: inpatient (7.1%), partial hospital day program (14.3%), individual sessions with a psychiatrist (30.8%), individual sessions with a counselor (60%), group therapy (28.6%). In addition, 71.4% of the sample had attended 12-step meetings during the 3-month intervention. Mental health treatment included: partial hospital day program (7%), individual sessions with a psychiatrist (28.6%), individual sessions with a counselor (40%), and group therapy (7%).
Exercise Intervention
There are three components to the exercise intervention: 1) moderate-intensity aerobic exercise, 2) group behavioral training component, and 3) an incentive system. The exercise intervention was also tested with a sample of alcohol dependent patients and has been published elsewhere (R.A. Brown, et al., 2009). We refer readers to this publication for detailed descriptions of each exercise component.
Aerobic Exercise Component of the Exercise Intervention
Participants attended supervised (by an exercise physiologist) aerobic exercise group sessions once a week (for 12 weeks) at the study fitness facility. At each exercise intervention session, the exercise physiologist guided participants on the intensity and duration of the exercise to be performed. Exercise sessions began at 20 minutes per session and gradually progressed to 40 minutes per session by week 12. Participants exercised at a rate that achieved 55 – 69% (moderate-intensity) of age-predicted maximal heart rate. This exercise regimen is consistent with the guidelines offered by the American College of Sports Medicine (ACSM; (American College of Sports Medicine, 2000). Heart rate and blood pressure were monitored before, during, and after exercise. Each workout session also included a 5-minute warm-up and a 10-minute cool-down to ensure safe exercise procedures. Several types of exercise equipment were available to study participants, including treadmills, recumbent bicycles, and elliptical machines. Participants in the study were also given “prescriptions” from the exercise physiologist (tailored to their level of fitness) to engage in moderate-intensity aerobic exercise on a minimum of two to three other occasions during the week for the duration of the 12- week exercise program. These other exercise occasions took place outside of the study fitness facility (e.g., in their home or through community resources). In addition, participants were required to self-monitor their exercise by filling out a weekly exercise log with the various exercise activities they engaged in during the week, the duration of each activity, and their self-reported rate of perceived exertion (RPE) for each activity.
Weekly Group Behavioral Training Component of the Exercise Intervention
Given the demonstrated efficacy of behavioral modification strategies in increasing physical activity (Woodard & Berry, 2001), this weekly, brief (15–20 minutes) group intervention based on cognitive and behavioral techniques was incorporated as a component of the exercise intervention. Through these weekly groups, co-led by both an exercise physiologist and psychologist, participants were guided as to how to increase overall fitness through behavioral changes in their daily lives. Each brief group session was focused on a certain topic designed to increase overall motivation resulting in improved exercise adherence and maintenance.
Incentive Component of the Exercise Intervention
In order to maximize adherence to the exercise program and self-monitoring of daily exercise activities, participants earned incentives for various levels of adherence to the exercise program. The incentive plan consisted of providing participants with $5 for attending each weekly combined group/exercise session and an additional $5 for returning their completed exercise self-monitoring form (from the prior week) at that session. In addition, participants earned the opportunity to draw a prize from a fish bowl (value ranging from $10–$50) at each weekly session, if they had consecutive attendance (i.e., had attended the prior week’s group + exercise session).
Procedure
Recruitment
Participants were recruited from two sources. First, the medical records of all patients entering the intensive alcohol and drug treatment partial-hospitalization program at a psychiatric hospital in the Northeast were screened for the psychiatric and medical inclusion and exclusion criteria detailed above. Second, interested participants responding to study advertisements in the local newspaper called the study center. In each case, participants were initially screened to determine potential eligibility based upon the sedentary criterion. For individuals meeting the sedentary eligibility criterion, informed consent for study enrollment was obtained and potential participants were evaluated using the diagnostic and assessment measures and a medical history review and graded exercise test under the supervision of the study physician to confirm eligibility.
The study physician reviewed the patient’s medical history. A resting electrocardiograph (ECG) was then obtained. Unless contraindicated by the resting ECG, the physician then had the patient complete a submaximal graded exercise test on a treadmill, with continuous ECG monitoring using a modified, Balke-Ware protocol (American College of Sports Medicine, 2000) that was terminated at 85% of the participant’s age-predicted maximal heart rate (see below for a more detailed description of the submaximal graded treadmill test). The physician reviewed the results of the graded exercise test and made the final determination regarding study eligibility.
Follow-up Assessments
Participants completed follow-up interviews 3 (end of treatment) and 6 months following recruitment into the study. To reduce attrition, participants were paid $40 and $50 for completion of the 3- and 6-month follow-up interviews, respectively. All payments were made in gift certificates to a local shopping mall or supermarket.
Measures
Physical Activity Screen
To determine whether patients were sedentary participants were asked the frequency and duration of at least moderate-intensity exercise (described as the equivalent of a brisk walk) over the last six months. Participants were considered sedentary if their responses suggested they were exercising less than 3 times a week for at least 20 minutes per day over the entire previous six months.
Structured Clinical Interview for DSM-IV (SCID-P; First, Spitzer, Gibbon, & Williams, 1995). Psychiatric disorders, both current and lifetime, for establishing inclusion/exclusion criteria were determined by the relevant sections of the SCID-P (First, et al., 1995).
Time-Line-Follow-Back (TLFB; Sobell & Sobell, 1996). The TLFB interview (Sobell et al., 1980) was utilized to assess alcohol and drug use at baseline and during the follow-up intervals. At baseline, it was administered for the 90 days prior to admission and at each follow-up interval for the period since its last administration. The TLFB interview is a calendar-assisted structured interview that provides a way to cue memory so that accurate recall is enhanced. A structured interview of patients’ drinking behavior has been found to be the most reliable and valid method of assessing prior alcohol use (Sobell & Sobell, 1979, 1980). In particular, the TLFB interview has excellent reliability (Sobell, Maisto, Sobell, & Cooper, 1979) and validity (Sobell & Sobell, 1980). Data from the TLFB were summarized by month to yield the primary outcome variables: percent days abstinent (i.e., number of abstinent days divided by number of days not in a restricted environment) for 1) alcohol and 2) drugs.
Cardiorespiratory Fitness was assessed using a submaximal graded exercise protocol on a motorized treadmill at baseline and follow-up evaluations. For the graded exercise test, the speed of the treadmill was constant at 3.5 mph with the initial grade set at 0% and progressed by 1% at 1-minute intervals. During testing, heart rate was assessed each minute and at the point of test termination using a 12-lead electrocardiogram. Blood pressure was measured at 2-minute intervals during the test. Further, ratings of perceived exertion (Borg 6–20 scale) were obtained at one-minute intervals during the test (Borg, 1998). This submaximal exercise test was terminated at 85% of the participant’s age predicted maximal heart rate (age-predicted maximal heart rate = 220 – age of participant). In addition, test termination guidelines outlined by the American College of Sports Medicine (American College of Sports Medicine, 2000) were followed.
For the purpose of data analysis, cardiorespiratory fitness was defined in two ways. First, equations published by the American College of Sports Medicine (American College of Sports Medicine, 2000) were used to estimate the metabolic equivalents (MET) level at which the participant achieved 85% of their age-predicted maximal heart rate. In addition, the time point during the exercise test at which the participant achieved 85% of their age-predicted maximal heart rate was used for data analysis. Increases in MET level and/or duration of the exercise test are representative of improvements in cardiorespiratory fitness.
Body Composition was evaluated across multiple domains. Weight was measured using a calibrated medical scale. Following procedures outlined by the ACSM’s Guidelines for Exercise Testing and Prescription (American College of Sports Medicine, 2000) and used in our smoking cessation studies of smokers (Marcus et al., 1999, 2005), body fat percentage was assessed using skinfold thickness measured on the right side at the triceps, suprailiac crest, and thigh using a Lange caliper. Also consistent with ACSM guidelines, body mass index (BMI) was calculated by dividing body weight in kilograms by height in meters squared.
Intervention Feedback Questionnaire
Throughout the intervention (at weeks 6 and 12), participants filled out anonymous, 20-item feedback questionnaires in which they rated (on a scale from 1=strongly disagree to 7=strongly agree) their perceptions of staff knowledge, helpfulness, availability, the extent to which exercise will help with abstinence from drugs, attainability of goals of the study, and barriers to participating in the study. The questionnaire also included open-ended questions about the strengths and weakness of the intervention.
RESULTS
Treatment Adherence
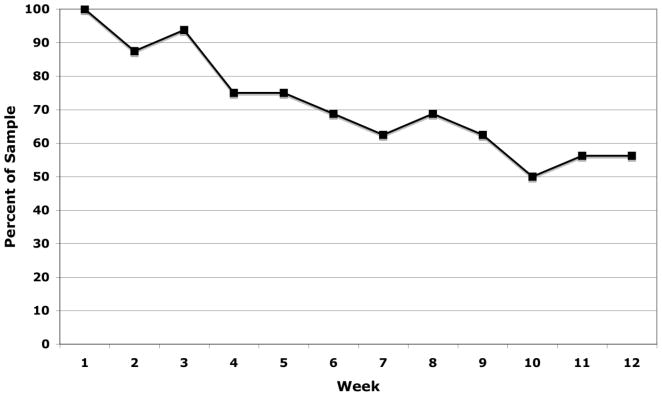
Attendance for each week of the exercise intervention is displayed in Figure 1. During the 12-week intervention, participants attended an average of 8.6 (SD = 3.9) weekly exercise sessions. Over the course of the 12-week intervention, participants exercised an average of 3.9 (SD =1.1) days per week. In addition, participants engaged in an average of 209 (SD =180) minutes of physical activity per week with 147 (SD =100) of these minutes being at an exertion level of at least moderate intensity. Further, participants earned an average of $86 (SD = 39) for weekly incentives for session attendance and completion of self-monitoring forms, and $128 (SD = 89) from the fishbowl drawing for attending exercise sessions on consecutive weeks.
Figure 1.
Weekly Attendance
Drinking and Drug Outcomes
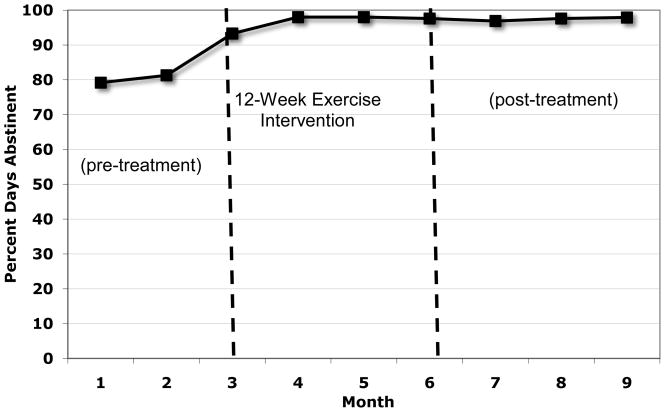
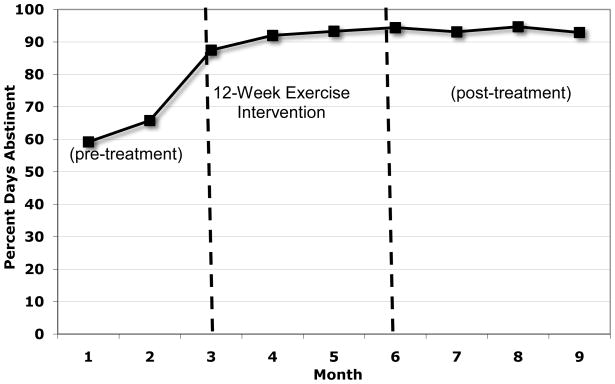
Figure 2 displays the mean percent days alcohol abstinent (PDA) for pretreatment (3 months prior to beginning the exercise intervention), during the 12-week exercise intervention, and the 3 months following the end of the intervention. Similarly, Figure 3 displays the mean percent days of drug use abstinence at each of these time points.
Figure 2.
Percent Days Abstinent from Alcohol
Figure 3.
Percent Days Abstinent from Drugs
To examine changes in alcohol and drug use at follow-ups, paired sample t-tests were conducted comparing baseline to end of treatment and 3-months post intervention on measures of alcohol and drug use PDA. These results suggest that, compared to the mean pretreatment percent days abstinent (PDA) for alcohol, significant increases in PDA were observed throughout active treatment, a difference that was significant statistically at the end of the 12-week exercise intervention (t=2.22, df=14, p<.05) and a trend toward increased PDA at the 3-month post-intervention follow-up (t=2.0, df=13, p=.07). In terms of percent days abstinence for drug use, there was a significant increase in PDA (t=2.96, df=14, p<.05) at the end of treatment and a trend toward increased PDA 3-months post-intervention (t= 2.14, df=13, p=.05).
While a comparison group is not available for analyzing differences in relapse curves, we examined substance use relapse (defined as any drug or alcohol use) and continuous abstinence during the 12-week intervention. At the end of the exercise intervention, 66.7% of the sample did not relapse and had been continuously abstinent. When examining the entire follow-up period, the median days until first use across the entire sample was 91 days.
Relationship between Participant Attendance and Treatment Outcomes
We also examined differences in substance use outcomes between participants who attended at least 75% of all sessions (i.e., 8 out of 12 weeks, n=10; Attenders) and those who attended less than 75% of sessions (non-Attenders). A log-rank analysis (Harrington & Fleming, 1982) was conducted comparing the risk for relapse among Attenders and non-Attenders. Non-Attenders were significantly (χ2 = 11.5, df=1, p<.001) more likely to relapse than Attenders (80% vs. 20%, respectively).
Cardiorespiratory Outcomes
Table 1 displays the means and standard deviations of each of the cardiorespiratory and body composition measures at baseline, end of treatment, and the 3-month post-intervention follow-up. Paired sample t-test comparisons were conducted to determine whether there were statistically significant differences between each baseline measure of cardiorespiratory fitness and body composition and their corresponding measure at the end of treatment and 3-month assessment timepoints. Compared to baseline, there was a trend toward participants improving on the duration of the submaximal treadmill test at end of treatment (t=1.93, df=12, p<.08) and significant improvement in cardiorespiratory fitness at the 3-month post-intervention follow-up (t=2.56, df=11, p<.05). In addition, compared to baseline, participants were able to reach 85% of their maximal heart rate at higher MET levels at the 3-month follow-up (t=2.62, df=11, p<.05). No significant differences were observed between baseline and follow-ups on body composition measures.
Table 1.
Changes in Cardiorespiratory Fitness and Body Composition over Time
| Baseline | End of Treatment | 3-Month Follow-up | |
|---|---|---|---|
| Metabolic Equivalents (METs)* | 8.6(2.2) | 9.1(3.3) | 9.1(2.4) |
| Duration of Exercise Test (secs)* | 696.2(271.5) | 744.9(377.6) | 743.8(324.3) |
| Body Mass Index (BMI) | 29.1(10.7) | 30.7(11.2) | 30.9(11.3) |
| % Body Fat | |||
| Women | 34.9(4.1) | 35.2(4.8) | 37.3(.93) |
| Men | 19.4(10.1) | 19.1(6.6) | 21.1(8.9) |
| Waist-to-Hip Ratio | .89(.05) | .92(.07) | .89(.07) |
Significant differences between baseline and 3-Month Follow-up.
Numbers in parentheses reflect standard deviations of the means.
Intervention Feedback
At week 6 and again at the end of the 12-week intervention, participants filled out an anonymous feedback questionnaire. On questions related to positive staff characteristics, participants reported strongly agreeing (mean = 6.75, SD =.35) with statements. Similarly, regarding their perceptions that participating in the SAE intervention would help their recovery, participants also strongly agreed (mean = 6.73, SD=.80). On open-ended questions regarding the strengths and weaknesses of the program, participants identified positive reinforcement, increased motivation, supportive atmosphere, obtaining more knowledge about exercise, and incentives as strengths. On the other hand, participants identified lack of a strength training and nutritional information component as weaknesses.
DISCUSSION
Relapse continues to pose a major problem to the substance abuse treatment field as a whole and to individuals attempting recovery from drug use disorders. Studies evaluating strategies to enhance maintenance of treatment gains have devoted relatively little attention to lifestyle modification, and research in the area of engaging in physical activity and recovery from drug use disorders is still in its infancy. For over two decades, researchers have called for studies examining the role of physical activity in recovery from substance use disorders, yet to date, very little research has been conducted to specifically examine the application of exercise interventions in adults with drug dependence. However, many patients in substance abuse programs express interest in incorporating exercise into their recovery (Read, et al., 2001).
Therefore, to address this existing gap in the literature, we developed an aerobic exercise intervention as an adjunct to addiction treatment for drug dependent patients. In this pilot study, sedentary drug dependent patients engaged in a 12-week individually-tailored moderate-intensity aerobic exercise intervention. The preliminary outcomes of this pilot study revealed good adherence to the exercise intervention with demonstrated benefits in cardiorespiratory fitness by the end of the 12-week intervention. In addition, compared to baseline, there were significant increases in percent days abstinence of alcohol and drug use at follow-up timepoints and participants who attended at least 75% of the exercise sessions had significantly better substance use outcomes than those who did not.
While we observed promising outcomes among participants engaged in this intervention, we also noted various challenges in participant recruitment and enrollment. It is very likely that our inclusion and exclusion criteria were overly stringent. Given the supervised, structured exercise program, including exercise prescriptions for moderate-intensity exercise, we excluded certain medical and psychiatric conditions, and were most likely overly conservative in so doing. The strict inclusion/exclusion criteria represent a study limitation that could affect the generalizability of our findings. Alternatively, we might have done well to seek approval for participation from patients’ primary care physician or we might have offered a less intensive intervention that required fewer restrictions, such as a program focused on walking and incorporating pedometers. A second, potentially more important factor is that despite good intentions and a desire to improve fitness and remain abstinent, a limited number of participants were unable to meet the time and physical demands of such a program or may have preferred other types of exercise. Research focused on better understanding the expectations of this population along with identifying their specific exercise preferences may be very informative in the refinement of future exercise interventions for drug dependent patients in early recovery. These recruitment challenges may also point to the need for multisite trials to increase sample sizes for study participation.
Despite these recruitment challenges, a physical activity intervention with demonstrated generalizability and the potential for dissemination would have important clinical and public health implications for the treatment of drug dependence and provide some definite advantages. Exercise offers the potential for improved health and wellness, as the physiological (e.g., (USDHHS, 1996) and psychological (e.g., Stathopoulou, Powers, Berry, Smits, & Otto, 2006) benefits of exercise have been well documented. Exercise also has the potential to be cost-effective, flexible and accessible; many forms of exercise (e.g., running, fitness videotapes, swimming) may be conducted independently, either at home or outdoors, and associated costs are likely to be minimal. Finally, exercise has minimal side effects compared to pharmacological treatment (cf., Broocks, et al., 1998). With the use of proper precautions for prevention of injuries (American College of Sports Medicine, 2000), exercise carries with it far less risk of adverse events than does the use of psychotropic medication.
Limitations of this pilot study include the lack of a control group, a small sample size and a lack of diversity of the study sample who were primarily Caucasian and largely well-educated. Indeed, this work is primarily developmental in nature, and the lack of a control group limits the extent to which one can meaningfully interpret or make definitive statements about the efficacy of the exercise intervention. There is also limited ability to examine mediating mechanisms whereby participation in aerobic exercise may have led to reduced alcohol and drug use outcomes. In addition, future studies should attempt to identify demographic and personal characteristics that could limit patient involvement in structured exercise programs in order to develop physical activity interventions that are generalizable to substance abusing patients as a whole. However, the current study has demonstrated the exercise intervention to be feasible and to generate promising levels of adherence in a drug dependent population. The efficacy of the exercise intervention warrants further investigation in future randomized clinical trials. If the efficacy of this moderate-intensity aerobic exercise intervention can be demonstrated, drug dependent patients may be provided with a valuable adjunct to traditional substance abuse treatment. Furthermore, future studies may contribute much-needed knowledge about the role of aerobic exercise in reducing alcohol and drug use and increasing fitness in drug dependent patients.
Acknowledgments
Supported in part by grant DA14599 from the National Institute on Drug Abuse to Dr. Richard A. Brown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Richard A. Brown, Alpert Medical School of Brown University/Butler Hospital.
Ana M. Abrantes, Alpert Medical School of Brown University/Butler Hospital
Jennifer P. Read, University at Buffalo, The State University of New York
Bess H. Marcus, Program in Public Health, Brown University
John Jakicic, University of Pittsburgh
David R. Strong, Alpert Medical School of Brown University/Butler Hospital
Julie R. Oakley, The Westerly Hospital, Westerly, Rhode Island
Susan E. Ramsey, Alpert Medical School of Brown University/Rhode Island Hospital
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Brown University
Gregory G. Stuart, Alpert Medical School of Brown University/Butler Hospital
Mary Ella Dubreuil, Butler Hospital.
Alan A. Gordon, Butler Hospital
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard Review of Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. Guidelines for exercise testing and prescription. New York: Lippincott, Williams and Wilkins; 2000. [Google Scholar]
- Andlin-Sobocki P. Economic evidence in addiction: A review. The European Journal of Health Economics. 2004;5(Supplement 1):S5–S12. doi: 10.1007/s10198-005-0282-5. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Borg C. Borg’s perceived exertion and pain scales. Stockholm: Human Kinetics; 1998. [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta Psychiatrica Scandinavica Supplement. 2005;427:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, et al. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. American Journal of Psychiatry. 1998;155:603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behavior Modification. 2009;33:220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Monti PM, Myers MG, Martin RA, Rivinus T, Dubreuil ME, et al. Depression among cocaine abusers in treatment: relation to cocaine and alcohol use and treatment outcome. American Journal of Psychiatry. 1998;155:220–225. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. American Psychologist. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. Journal of Drug Education. 1991;21:73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: A meta-analysis. Journal of Sport and Exercise Psychology. 1998;20:339–357. [Google Scholar]
- Dishman RK, O’Connor PJ. Lessons in exercise neurobiology: The case of endorphins. Mental Health and Physical Activity. 2009;2:4–9. [Google Scholar]
- Donaghy M, Mutrie N. A randomized controlled study to investigate the effect of exercise on the physical self-perceptions of problem drinkers. Physiotherapy. 1998;84:169. [Google Scholar]
- Donaghy ME, Ussher MH. Exercise interventions in drug and alcohol rehabilitation. In: Faulkner G, Taylor AH, editors. Exercise, Health, and Mental Health: Emerging Relationships. London, UK: Routledge; 2005. pp. 48–69. [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Medicine & Science in Sports & Exercise. 2001;33(6 Supplement):S587–597. doi: 10.1097/00005768-200106001-00027. discussion 609–510. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychology Review. 2007;17:317–336. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JW, Moos RH, Timko C. The course of treated and untreated substance use disorders: Remission and resolution, relapse and mortality. In: McCrady BSE, EE, editors. Addictions: A Comprehensive Guidebook. New York: Oxford University Press; 1999. pp. 30–49. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychology Review. 2007;17:299–315. doi: 10.1007/s11065-007-9041-y. [DOI] [PubMed] [Google Scholar]
- Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- Hobson ML, Rejeski WJ. Does the dose of acute exercise mediate psychophysiological responses to mental stress? Journal of Sport Psychology. 1993;15:77–87. [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. Journal of Clinical Psychology. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. Journal of Clinical and Experimental Neuropsychology. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Keller SM. Physical itness hastens recovery from psychological stress. Medicine & Science in Sports & Exercise. 1980;12:118–119. [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends in Cognitive Sciences. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Langenbucker JW, McCrady BS, Brick J, Esterly R. President’s Commission on Model State Drug Laws. Washington: President’s Commission on Model State Drug Laws; 1993. Socioeconomic evaluations of addictions treatment. [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Research & Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. British Medical Journal. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xiaoping W, Wei H, Zeng W. Frequency of withdrawal symptoms of natural detoxification in heroin addicts. Chinese Mental Health Journal. 2000;14:114–116. [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Archives of Intern Medicine. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine and Tobocco Research. 2005;7:871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Lifestyle modification. In: Marlatt GA, Gordon JR, editors. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. [Google Scholar]
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. 2. New York: Guildford Press; 2005. [Google Scholar]
- Marlatt GA, Witkiewitz K. Relapse prevention for alcohol and drug problems. In: Marlatt GA, Donovan DM, editors. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. 2. New York: Guildford Press; 2005. pp. 1–44. [Google Scholar]
- McAuley E, Courneya KS, Lettunich J. Effects of acute and long-term exercise on self-efficacy responses in sedentary, middle-aged males and females. Gerontologist. 1991;31:534–542. doi: 10.1093/geront/31.4.534. [DOI] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE. Inroduction. In: McCrady BS, Epstein EE, editors. Addictions: A Comprehensive Guide Book. New York: Oxford University Press; 1999. pp. 3–5. [Google Scholar]
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo MET, Lawlor DA. Exercise for depression. Mental Health and Physical Activity. 2009;2:95–96. [Google Scholar]
- Meeusen R. Exercise and the brain: insight in new therapeutic modalities. Annals of Transplantion. 2005;10:49–51. [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. Journal of the American Medical Association. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- ONDCP. The Economic Costs of Drug Abuse in the United States 1192–2002. Washington, D.C: Executive Office of the President; 2004. (Publication No. 207303) [Google Scholar]
- Ouimette PC, Gima K, Moos RH, Finney JW. A comparative evaluation of substance abuse treatment IV. The effect of comorbid psychiatric diagnoses on amount of treatment, continuing care, and 1-year outcomes. Alcoholism: Clinical and Experimental Research. 1999;23:552–557. [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. The American Journal of Drug and Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, et al. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addictive Behaviors. 2007;32:1416–1432. doi: 10.1016/j.addbeh.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Read JP, Brown RA, Marcus BH, Kahler CW, Ramsey SE, Dubreuil ME, et al. Exercise attitudes and behaviors among persons in treatment for alcohol use disorders. Journal of Substance Abuse Treatment. 2001;21:199–206. doi: 10.1016/s0740-5472(01)00203-3. [DOI] [PubMed] [Google Scholar]
- Reed J, Ones DS. The effect of acute aerobic exercise on positive activated affect: A meta-analysis. Psychology of Sport and Exercise. 2006;7:477–514. [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rotgers F, Keller DS, Morgenstern J. Introduction. In: Rotgers F, Keller DS, Morgenstern J, editors. Treating Substance Abuse: Theory and Technique. New York: Guilford Press; 1996. pp. 1–12. [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. Journal of Studies on Alcohol. 1982;43:380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behavior Research & Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Validity of self-reports in three populations of alcoholics. Journal of Consulting and Clinical Psychology. 1979;46:901–907. doi: 10.1037//0022-006x.46.5.901. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: An approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. New York: Pergamon Press; 1980. pp. 177–183. [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Toronto, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper TC, Sanders B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. New York: Pergamon Press; 1980. [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits JA, Otto MW. Exercise interventions for mental health: A quantitative and qualitative review. Clinical Psychology: Science and Practice. 2006;13:179–193. [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: Research and clinical implications. Professional Psychology: Research and Practice. 1999;30:275–282. [Google Scholar]
- USDHHS. Physical activity and health: A report of te Surgeon General. Atlanta, GA: Centers for Disease Control; 1996. [Google Scholar]
- Williams DJ. Exercise and substance abuse treatment: Predicting program completion using logistic regression. Corrections Compendium. 2000;25:4–7. [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. American Psychology. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe--a critical review and appraisal of 27 studies. European Neuropsychopharmacology. 2005;15:357–376. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Woodard CM, Berry MJ. Enhancing adherence to prescribed exercise: structured behavioral interventions in clinical exercise programs. Journal of Cardiopulmonary Rehabilitation. 2001;21:201–209. doi: 10.1097/00008483-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Xie H, McHugo GJ, Fox MB, Drake RE. Substance abuse relapse in a ten-year prospective follow-up of clients with mental and substance use disorders. Psychiatric Services. 2005;56:1282–1287. doi: 10.1176/appi.ps.56.10.1282. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD. Effects of exercise on sleep. Clinical Journal of Sport Medicine. 2005;24:355–365. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]