Abstract
The endocannabinoid system is rapidly emerging as a potential drug target for a variety of immune-mediated central nervous system diseases. There is a growing body of evidence suggesting that endocannabinoid interventions may have particular relevance to Alzheimer's disease. Here we present a review of endocannabinoid physiology, the evidence that underscores its utility as a potential target for intervention in Alzheimer's disease, and suggest future pathways of research.
Keywords: Alzheimer's disease, CB1, CB2, dementia, endocannabinoids
INTRODUCTION
While the understanding of the pathophysiologic cascade of events in Alzheimer's disease (AD) is rapidly evolving, effective treatments interrupting this cascade are lacking. The endocannabinoid (eCB) system consists of lipid signaling molecules that bind to at least two G-protein-coupled receptors (GPCRs) and impact both immunity and cognition. As AD is a neurodegenerative cognitive disorder with an inflammatory component, agents influencing immunity or cognition may have relevance in AD treatment. GPCRs have historically made for `drugable' targets (α- and β-adrenergic receptors, muscarinic acetylcholine receptors), and the ability to manipulate eCB tone and act directly at cannabinoid receptors-demonstrated with a variety of agents in both cell culture and animal models-indicate the potential for a novel drug class in AD treatment. Several recent experiments suggest a direct role for the eCB system in AD pathophysiology and are thus encouraging in terms of the potential for cannabinoid interventions. The direction of those interventions (whether to augment or attenuate eCB tone) depends on the confluence of a clear understanding of the relationship between immunity and disease progression with a clear understanding of the impact of cannabinoids on immunity and cognition. In an effort to provide a context in the midst of a great deal of complexity and uncertainty regarding immunity and AD, and to point the way to future research in eCBs in AD, here we review: 1) eCB physiology; 2) immunity and AD; and 3) the studies establishing the relevance of the eCB system to AD and cognition. We follow this with suggestions for future investigations that would move this nascent yet promising field forward.
COMPONENTS OF ENDOCANNABINOID PHYSIOLOGY
The discovery of a dedicated cannabinoid receptor, CB1, came as a surprise to the research community as the only known ligand for the receptor at the time of its discovery was the active component of marijuana, Δ9-tetrahydrocannabinol. This eventually led investigators to the identification of endogenous ligands; a second cannabinoid receptor, CB2; eCB synthesizing and metabolizing enzymes; and a reuptake transport system [9, 53,56,63]. The endogenous cannabinoids are signaling biolipids, and include N -arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG), the most widely studied [66]. Both AEA and 2-AG are synthesized from lipid precursors derived from the enzymatic cleavage of cell membrane components in both immune competent cells and neurons, in response to immune cell activation or neuronal membrane depolarization; they are then released where they act at CB1 and CB2 receptors. The intracellular enzymes fatty acid amide hydrolase (FAAH) and monoacylglyerol lipase (MAGL) degrade AEA and 2-AG, respectively [12].
While the CB1 and CB2 receptors are both GPCRs with significant homology, they diverge both in their function and in their specificity of cellular expression [36]. The CB1 receptor is expressed in brain, especially in cerebral cortex, hippocampus, basal ganglia, and cerebellum; and in peripheral neural tissue, both on sensory nerve fibers and in the autonomic nervous system [68]. Activation of the presynaptic CB1 receptor leads to attenuation of calcium currents and subsequent inhibition of release of neurotransmitters (GABA, glutamate, and serotonin), opening of potassium channels, and stimulation of several protein kinases [4, 66]. There is evidence that cannabinoids are able to inhibit the activity of nigrostriatal dopaminergic neurons, perhaps explaining their ability to produce catalepsy at high doses [2,71]. The CB1 receptor participates in a variety of cell maintenance functions, in addition to a specific role in memory consolidation, reviewed in more detail below [4,37].
The CB2 receptor is expressed in a variety of immune cells, including B lymphocytes, natural killer cells, monocytes/macrophages, polymorphonuclear neutrophils, and T cells, a fact which has contributed to its reputation as a “peripheral receptor;” however, it is also densely expressed by activated microglia in the central nervous system (CNS) [36,50]. Although not true under all conditions, a wealth of evidence suggests that stimulation of immune cells with cannabinoids generally has an immunosuppressive effect [17, 44]. Stimulation of cultured microglial cells with lipopolysaccharide and anti-CD40 antibodies induces increased expression of the CB2 receptor, suggesting a feedback inhibition function for CB2 [45]. Specifically, cannabinoids have been demonstrated to suppress the production of a variety of pro-inflammatory cytokines in both human cell cultures and animal models, mediated by CB2 receptors [46]. The function of the CB2 receptor as a feedback-inhibitor of immune responsiveness in the CNS, analogous to its function in the periphery, was demonstrated in a culture of human fetal astrocytes, in which a cannabinoid was shown to decrease production of tumor necrosis factor-α (TNF-α) and several chemokines following interleukin-1β (IL-1β) stimulation [78]. This effect was reversed by a CB2 antagonist. The effect of cannabinoids on cytokines was confirmed in two studies in which marijuana smoking was associated with inhibition of cytokine production and antimicrobial activity of pulmonary alveolar macrophages [6,77]. In cultured microglial cells, CB2 receptor stimulation has been shown to suppress TNF-α and nitric oxide production [23]. However, in activated microglia, the innate immune cells of the central nervous system which respond to neuronal damage, CB2 receptors have been localized to the leading edge of lamellipodia and have been shown to regulate cell migration triggered by production of 2-AG [89]. In fact, experiments have provided evidence of the capacity of 2-AG to induce migration, putatively via the CB2 receptor, in a variety of cell types including B cells, dendritic cells, and eiosinophils, establishing its function as a chemotactic agent [40,46]. Cannabinoids, acting via CB2 receptor stimulation, also have a proliferative impact on a variety of cells, including microglia [18,79]. Taken together, this suggests that eCBs function in a complex rather than a simple anti- or pro-inflammatory manner by both directing and attenuating the immune response.
The endogenous cannabinoids anandamide and 2-AG have affinity for both the CB1 and CB2 receptors [36]. There is evidence of low levels of constitutive production of AEA in the periphery and the CNS, while the evidence of constitutive production of 2-AG is primarily in the CNS [29,75,84]. In general, production of eCBs is “on demand,” either stimulated by membrane depolarization in neurons or immune cell activation [66]. Concentrations of eCBs are increased in response to traumatic and otherwise pathogenic events, leading most to speculate on regulatory or compensatory mechanism in their production [4]. Of particular importance to immune modulation, the production of eCB is stimulated by activation of immune cells including macrophages, and dendritic cells, and stimulated immune cells have reduced expression of eCB degrading enzymes [46]. Since the discovery and partial functional elucidation of receptor subtypes within the eCB system, a variety of agents targeting this system have been developed. These include receptor agonists and antagonists of varying level of receptor specificity, as well as molecules that block eCB reuptake or increase local eCB tone through inhibition of enzymatic degradation [90]. These molecules may form the basis for new drugs aimed at altering eCB physiology in diseases of immunity or cognition.
The eCB system interventions have demonstrated treatment potential in rodent models of a variety of neurological disorders in neuroprotective, anti-inflammatory, and anti-nociceptive capacities. Mice subjected to closed head injury developed elevated concentrations of 2-AG in traumatized hemispheres; the administration of 2-AG to mice after closed head injury reduced brain edema, infarct volume, and hippocampal cell death compared with uninjured mice, suggesting a neuroprotective role of eCBSs in the CNS [67]. In experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), a basal 1.3–2 fold increase in anandamide and a 1.4–1.8 fold increase in 2-AG was demonstrated in the CNS [5]. Cannabinomimetic compounds have been demonstrated to reduce spasticity and tremor in these mice [5]. In a spinal cord model of inflammatory pain in rats, a cannabinoid reuptake inhibitor reduced pain-related behaviors and reduced c-fos, a marker of pain-related activation in neurons [14].
The exact role(s) of eCBs in the CNS is not yet clear. In humans, elevated cerebrospinal fluid concentrations of eCBs have been found following seizures, prompting speculation that CNS pathology activates cannabinoid production mechanisms in a non-disease-specific manner, and may represent a compensatory defense against excitotoxicity [4,52]. Perturbation of eCBs have also been found in the CSF of patients with schizophrenia, migraine headaches, and multiple sclerosis [19,30,73]. More research is required to elucidate the function of eCBs in both health and disease.
THE COMPLEXITY OF IMMUNITY IN ALZHEIMER'S DISEASE
There is evidence of a wide range of immunologic response in AD pathology, both innate and adaptive, central and peripheral [59]. The innate response includes the local production of pro-inflammatory cytokines (IL-1β, TNF-α); chemokines (MIP-1α, MIP-1β, MCP-1); nitric oxide; and the activation of the complement cascade, mediated by microglia [59]. Though once conceptualized as immunologically privileged, there is clear evidence that activated T cells do make it into the CNS, and there is evidence of their association with the neuritic plaque of AD [8,55,86]. Additionally, a peripheral amyloid-β (Aβ)-reactive T cell response has been demonstrated in the elderly and, more robustly, in AD patients [60]. However, it is becoming increasingly evident that the participation of the immune system in AD pathogenesis is not monolithic; while a large body of evidence supports the contention that AD is an inflammatory illness, mobilizing rather than paralyzing immune response is becoming an increasingly popular approach to the development of interventions in arresting disease progression.
Though far from conclusive, a large body of evidence points to the abnormal parenchymal deposition of Aβ peptide as a critical event in the pathogenesis of AD; the Aβ-associated histologic hallmark of neuroinflammation in AD – and the cell most responsible for the “neurotoxicity” of immunologic response - is the activated microglia [55]. Though not conclusive, data suggests that microglia are bone marrow-derived cells believed to migrate and differentiate during early postnatal development from blood monocyte precursors and are replenished from that source; some recent evidence challenges this notion and suggests local maintenance and expansion of microglial within the CNS rather than recruitment from the peripheral circulation [1,57,64]. Evidence suggests that microglia, following activation in response to Aβ, differentiate into phagocytic cells which then ingest Aβ and secret proinflammatory cytokines, chemokines, and nitric oxide [59]. Transformation into the phagocytic macrophage-like cell is associated with the locally cytotoxic respiratory burst; with the assembly of the NADPH enzyme in the cell membrane, superoxide ions are generated from O2 and subsequently hydrogen peroxide is generated from superoxide dismutase [55,64]. Additionally, microglia act as antigen presenting cells for Aβ peptide-reactive T cells, which can then themselves stimulate microglia activation [59]. In this model, Aβ peptide and microglia may cooperate in the inflammatory toxicity of AD in at least two ways. First, following activation of microglia with Aβ peptide, and through the action of reactive oxygen intermediates and proteases, there is direct tissue damage to the local brain environment [55]. Second, Aβ peptide itself may direct blood-borne cells to amyloid plaques, further potentiating local inflammatory cascades and toxicity [64]. Activated microglia are usually associated with neuritic Aβ deposits rather than diffuse amyloid (without dystrophic neurites), suggesting that it is the microglial secretions which are cytotoxic [41]. As further evidence of their pathogenic predisposition in the CNS, few activated microglia are seen in control brains [55]. Additionally, there is ample evidence of free radical damage in AD brain, including proteins modified by glycation, low molecular weight compounds that have been oxidized, and lipids that have been peroxidated [55,87]. In vivo evidence for a toxic model of immune participation in AD initially came from studies showing reduced risk of disease following exposure to non-steroidal anti-inflammatory drugs (NSAIDs) [3,83]. There has been generous support from over 20 studies for the notion that higher levels of NSAIDs usage reduce the prevalence of AD [38, 55]. In a postmortem examination of non-demented elderly, non-users of NSAIDs were found to have three times the number of activated microglia as users of NSAIDs, though there is some suggestion that this is a result of direct impact on amyloid-β protein precursor (AβPP) processing, reducing production of Aβ1–42, rather than an immune-mediated effect [43,48]. Additional evidence has come from studies investigating the association of polymorphisms in the genes encoding pro-inflammatory cytokines and AD [72]. However, surprisingly, clinical trials of antioxidants and NSAIDs to reduce inflammatory toxicity have been generally discouraging, with little effect on progression of disease, suggesting that the benefits of NSAIDs may be primarily in disease prevention [43].
There is a growing body of evidence which calls into question the “neurotoxic” theory of activated microglia in AD, and actually suggests that activation of microglia may be a reasonable goal of preventative intervention. Results from a passive immunization trial in mice transgenic for a disease-linked mutant form of human amyloid-β protein precursor (TgAPP) suggest that the administration of antibodies to Aβ reduces plaque burden specifically by inducing microglial phagocytosis [7]. Additionally, active immunization of TgAPP mice with Aβ stimulated microglial phagocytosis of plaque [35,74]. Consistent with this, TgAPP mice injected intrahippocampally with lipopolysaccharide showed reduced Aβ burden seven days after administration; this outcome was prevented by co-treatment with dexamethasone [32]. Further, deficiency of CCR2, a chemokine receptor that mediates the trafficking of mononuclear cells to sites of infection, accelerated disease progression in TgAPP mice through attenuation of microglial accumulation [24]. When mice transgenic for both AβPP and presenilin 1 disease-linked mutations (AβPP/PS1) were crossed with mice overexpressing IL-1β, Aβ deposits were reduced [76]. Finally, overproduction of transforming growth factor β1 (TGF-β1), a cytokine that mediates inflammatory response in the CNS especially following injury, in TgAPP mice stimulated microglial activation and reduced Aβ burden in the hippocampus and neocortex by nearly 50%, and substantially decreased the number of dystrophic neurites [92]. The exception to the trend suggesting that improvement in Aβ burden follows pro-inflammatory modulation of transgenic AD mice is α1-anti-chymotrypsin. This acute-phase inflammatory protein, when coexpressed in TgAPP mice, enhances Aβ deposition [62,65]. Human studies suggest that in AD there is a pronounced defect in innate immunity, impairing the ability of cells of monocytic origin to phagocytose Aβ, perhaps contributing to plaque formation [27]. Taken together, these studies suggest that while the products of microgliosis may be locally toxic, stimulation of microglial phagocytosis of Aβ may be an important component of any potential immune therapy in AD. What may be required in the immune therapy of AD is balanced immune-modulation rather than all or nothing anti- or pro-inflammatory therapies. eCB interventions have an excellent profile in this capacity.
EXPERIMENTAL EVIDENCE RELATING THE eCB SYSTEM TO AD PATHOLOGY AND TREATMENT
There is an emerging interest in the relevance of the eCB system to AD. A plethora of cell culture and animal model studies support this interest. For a recent review, see Benito et al. 2007 [11]. As a consequence of insights that the studies have provided, the CB2 receptor system has become a specific focus of investigation. Benito and colleagues have demonstrated the abundant and selective microglial expression of CB2 receptors in AD brain, presumably a function of cellular immune activation [10]. Consistent with this, polyclonal antibodies to CB2 receptors revealed dense CB2 expression in areas populated with microglia associated with neuritic plaque in entorhinal cortex and parahippocampus. No CB2 receptor expression was detected in similar areas from healthy individuals. Additionally, increased fatty acid amide hyrdolase activity was selectively demonstrated in regions of Aβ-enriched neuritic plaque, suggesting increased eCB production with subsequent enzymatic degradation. Ramirez and colleagues replicated this finding, demonstrating selective expression of CB2 receptors localized in regions of neuritic plaque and dystrophic neurites and co-localized with markers of activated microglia in AD brain [69]. These experiments provide concrete evidence that the eCB system, particularly the CB2 receptor system, is activated in the pathology of AD.
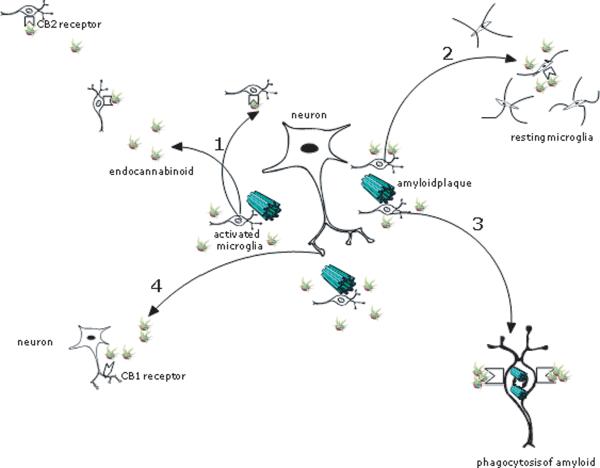
The selective expression of CB2 receptors in regions of neuritic plaque suggest that the CB2 receptor plays a role in the inflammation associated with AD pathology. Specifically, CB2 receptor expression may be a response to excessive inflammation induced in regions of Aβ deposition; by downregulating microglial cell activation and cytokine production, there may be amerlio-ration of immune-mediated neurotoxicity which may be beneficial in AD. In line with this theory, Ramirez and colleagues investigated the utility of CB2 receptor stimulation in preventing Aβ induced microglial activation and related cognitive impairment in an animal model of AD [69]. First, in a cell culture model, a CB2-selective agonist counteracted fibrillar Aβ induced microglial activation, reversing rod-like (activated) morphology to oval (resting) morphology, demonstrating that the CB2 receptor exerts and influence over microglial activation. Next, intracerebroventricular injections of Aβ or control peptide were administered to mice with and without a cannabinoid. As expected, resting microglia were observed in the control peptide population, and activated microglia were observed in the cortex of Aβ treated mice. As hypothesized, resting microglia were observed in mice treated with both Aβ and cannabinoid, evidence that cannabinoids prevent microglial activation in vivo, although this was not demonstrated to be CB2 receptor dependent. Further, while Aβ and control peptide treated-mice manifested very typical AD-model impairments of spatial navigation, the cannabinoid prevented Aβ-induced cognitive impairment. Interestingly, the role of the CB2 receptor may well go beyond compensatory modulation of inflammation. In a recent cell culture experiment, CB2 receptor stimulation was shown to promote, through attenuation of CD-40 mediated inhibition, successful phagocytosis of Aβ1→42 peptide [23]. This, together with the evidence suggesting chemokinetic and proliferative effects of eCBs on microglia, suggests that local production of eCBs in AD may promote clearance of Aβ through balanced immunomodulation (Fig. 1). Further focused research is required to determine to what degree and under what conditions eCBs influence such a diverse set of cellular functions, proliferation, migration, phagocytosis, and activation, and to what extent these processes are mutually exclusive.
Fig. 1.
Potential impact of endocannabioid production in regions of neuritic plaque: 1) Increased proliferation of microglia and chemotaxis to site of injury via CB2 receptor; 2) Attenuation of microglial activation and reduction of pro-inflammatory cytokines via CB2 receptor; 3) Enhanced phagocytosis of Aβ via CB2 receptor; 4) Stimulation of CB1 receptor with reduction of neurotransmitter production and uncertain effect on cognition.
Non-receptor-specific cannabinoid compounds may also have utility in the management of AD. Cannabidiol is a non-pyschoactive constituent of marijuana and non-receptor-specific agonist of cannabinoid receptors. It is the active component of Sativex, used in Canada to treat neuropathic pain of multiple sclerosis. Cannabidiol has been shown to protect PC12 neuronal cells from Aβ-induced toxicity [39]. More recently, cannabidiol was shown to inhibit Aβ-induced tau hyperphosphorylation in P12 cells, an effect likely to reduce tau protein pathology [26]. This inhibition was associated with a reduction in activity of GSK-3β, which has been linked to neurofibrillary tangle formation in brains of patients with AD [82]. There was no reported investigation of receptor specificity in this study (CB1/CB2), and it is possible that the effect was non-receptor mediated. Animal model studies are needed to evaluate the value of this compound in AD.
THE POTENTIAL IMPACT OF eCBS ON AD-RELATED COGNITION
The negative effects of marijuana on learning and memory have been well established, and are believed to be secondary to CB1 receptor stimulation [13,20,58]. Acute and chronic use of marijuana impair immediate recall, short-term memory, and memory retrieval [21]. Consistent with this, CB1 receptor localization has revealed abundant expression in hippocampus, cerebral cortex, cerebellum, and basal ganglia [33]. The memory impairment induced by marijuana mimics that seen in AD, a disease of hippocampal degeneration, and has stimulated investigations into the function of eCB physiology in normative hippocampal functioning [21]. In the human cortex, density of receptor expression is highest in temporal and frontal lobes, and asymmetric, with increased receptor expression in the left hemisphere suggesting a relationship with verbal language and memory systems [31]. That Δ9–THC, the psychoactive component of marijuana, impacts memory has been confirmed in both rodents and monkeys employing a variety of outcome measures including radial maze, instrumental discrimination tasks, and the Morris water maze [66]. Additionally, administration of low dose Δ9–THC has been shown to impair spatial memory in young healthy adults [49].
While the effects of Δ9–THC (acting at the CB1 receptor) on verbal learning and memory are well established, the relevance of endogenous cannabinoids to learning and memory is not clear [49]. Both AEA and 2-AG have been established as natural CB1 ligands in the brain [22,84]. In vitro experiments suggest that endogenous cannabinoids promote changes in neural activities related to memory, with a potential role in long-term synaptic plasticity; this suggests that eCBs, in contradistinction to Δ9–THC, have a potentially positive effect on cognition [28]. In vivo experiments with mice have been equivocal, with reports of both enhanced and impaired memory performance in null mutant CB1−/−mice [47,70]. In humans, a triplet repeat polymorphism in the CB1 gene has been associated with hebephrenia, a cognitively impaired phenotype of schizophrenia [88]. CB1 receptors in hippocampus and neocortex are distinctly expressed by GABAergic interneurons, and interact with eCBs produced in post-synaptic neurons in a retrograde manner, with resultant depolarization-induced suppression of inhibition [91]. As modulation of inhibition generally has effects on long-term potentiation (LTP) at excitatory synapses, and as the hippocampus plays such a crucial role in the anatomy of memory, this physiology argues for eCB mediated enhancement of cognition. However, in a recent stress model of depression in mice, reductions in hippocampal 2-AG levels were associated with deficits in behavioral flexibility (suggested to be related to the inability to forget), implicating hippocampal eCB signaling in the “pruning” of normative memory systems [34]. Additionally, in a mouse model of AD, a CB1 receptor antagonist prevented the amnesia induced by Aβ (i.c.v) peptides, suggesting that local production of eCBs in AD contributes to cognitive impairment [54]. The effects of eCBs on cognition are of particular interest in AD where there is already cognitive impairment loosely associated with synapse loss but not absolutely linked to any distinct neuropathology. There is a potential, not yet elucidated, for local concentrations of eCBs to explain some of the phenomenology of memory impairment in AD. More research is required to determine what role eCBs play in normative cognition and AD (Fig. 1).
CONCLUSION
Inflammation and oxidative stress are generally accepted as a critical risk factor for the development of AD, and interventions such as cannabinoids that attenuate these risks without arresting microglial activity and have innate neuroprotective benefits are attractive as potential preventative treatments for AD. Breakthroughs in the engineering of gene knockout and transgenic mice furnish the capability to observe very directly the impact of eCBs and the relevant receptor systems on AD. Null mutant CB2−/− mice have been generated in which the last 341 bp of the CB2-coding exon is replaced by the neomycin gene, resulting in a receptor lacking intracellular loop 3 and rendering the receptor non-functional [15]. When EAE was induced in wild-type and CB2−/− mice, it was found that disease was induced more frequently in CB2−/− mice, and that these mice had more severe disease and a slower recovery [51]. Encephalitogenic T cells isolated from these mice induced EAE in wild-type mice, which had higher mortality rates and increased mononuclear cell infiltration than EAE wild-types that were induced without adoptive transfer. Additionally, encephalitogenic T cells isolated from CB2−/− mice secreted more pro-inflammatory cytokines than encephalitogenic T cells isolated from wild-type mice. It follows that CB2−/− mice could be bred with transgenic human mutant AβPP (TghAPP) mice in order to produce a strain of CB2−/− TghAPP mice. This would help to explicate the role of CB2 receptors in the microgliosis and plaque formation of AD. Cannabinoid agonists, antagonists, eCB reuptake inhibitors, and non-selective or non-receptor mediated compounds such as cannabidiol could be administered to CB2+/+ TghAPP mice in order to evaluate the effects of cannabinoid modulation on Aβ deposition and plaque formation. From a cognitive standpoint, commercially available CB1 −/− mice bred with TgAPP mice to produce a strain of TgAPP CB1−/− and studied for cognitive performance would help to resolve questions about the nature of the impact of the CB1 receptor on cognition and neuroprotection.
Several human studies crucial to reconciling the relevance of the eCB system to AD and its general impact on inflammation and cognition are missing from the literature. Polymorphisms in genes encoding the eCB receptors and related enzymes have been associated with osteoporosis, obesity, substance abuse, and autoimmune disease [42,61,80,81]. No published studies, positive or negative, have investigated the potential associations of these polymorphisms with AD. As neuropathological studies have suggested overproduction of eCBs in regions of AD pathology, simple case-control studies of potential perturbations of concentrations (peripheral and central) of eCBs in AD are required. It has been established that elevation in inflammatory plasma proteins can herald the onset of clinical dementia [25,85]. As eCBs seem to be regulators of inflammatory protein production, it would be interesting to see in a longitudinal study of those at risk for dementia whether perturbations in plasma eCB concentrations predict the onset or progression of dementia. In terms of cognition, it remains unclear what influence eCBs and the CB1 receptor have in non-pathological cognitive function, let alone AD. There is a potential for the development of CB1 interventions, whether agonists or antagonists, with applications for a variety of cognitive disorders including neurodegenerative disorders and schizophrenia. The recent discovery of a CB1 receptor Positron Emission Tomography tracer for clinical use may provide the opportunity to evaluate the impact of the regional distribution of CB1 receptors in brain on domain-specific cognitive performance (memory, executive function, praxis) in healthy individuals [16]. Additionally, if AD is a disease of overproduction of eCBs, this may be visualized in case-control CB1 receptor binding studies.
The emerging data suggest that the eCB system is a potential target for immune and/or cognitive intervention in AD. A wealth of available chemical compounds capable of intervening in the eCB system at a variety of levels and the success with which these compounds have been used in animal models suggest the potential for human drug development. What is missing is a clear direction for that development based on a concise conceptualization of eCB system function in both health and in neurodegenerative and inflammatory conditions such as AD. Focused experiments are now required to move the field forward.
Footnotes
DISCLOSURE STATEMENT Dr. Davies is a paid consultant to, and equity owner in, Applied Neurosolutions, Inc.
References
- [1].Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- [2].Anderson JJ, Kask AM, Chase TN. Effects of cannabinoid receptor stimulation and blockade on catalepsy produced by dopamine receptor antagonists. Eur J Pharmacol. 1996;295:163–168. doi: 10.1016/0014-2999(95)00661-3. [DOI] [PubMed] [Google Scholar]
- [3].Anthony JC, Breitner JC, Zandi PP, Meyer MR, Jurasova I, Norton MC, Stone SV. Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology. 2000;54:2066–2071. doi: 10.1212/wnl.54.11.2066. [DOI] [PubMed] [Google Scholar]
- [4].Bahr BA, Karanian DA, Makanji SS, Makriyannis A. Targeting the endocannabinoid system in treating brain disorders. Expert Opin Investig Drugs. 2006;15:351–365. doi: 10.1517/13543784.15.4.351. [DOI] [PubMed] [Google Scholar]
- [5].Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- [6].Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156:1606–1613. doi: 10.1164/ajrccm.156.5.9704146. [DOI] [PubMed] [Google Scholar]
- [7].Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- [8].Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- [9].Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- [10].Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Benito C, Nunez E, Pazos MR, Tolon RM, Romero J. The endocannabinoid system and Alzheimer's disease. Mol Neurobiol. 2007;36:75–81. doi: 10.1007/s12035-007-8006-8. [DOI] [PubMed] [Google Scholar]
- [12].Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Block RI, Ghoneim MM. Effects of chronic marijuana use on human cognition. Psychopharmacology (Berl) 1993;110:219–228. doi: 10.1007/BF02246977. [DOI] [PubMed] [Google Scholar]
- [14].Borsani E, Labanca M, Bianchi R, Rodella LF. AM404 decreases Fos-immunoreactivity in the spinal cord in a model of inflammatory pain. Brain Res. 2007;1152:87–94. doi: 10.1016/j.brainres.2007.03.071. [DOI] [PubMed] [Google Scholar]
- [15].Buckley NE. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol. 2008;153:309–318. doi: 10.1038/sj.bjp.0707527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, Lin LS, Liu P, Goulet MT, Gottesdiener K, Wagner JA, de Hoon J, Mortelmans L, Fong TM, Hargreaves RJ. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci USA. 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. 1998;83:116–123. doi: 10.1016/s0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- [18].Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- [19].Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, Chiara VD, Battistini L, Bernardi G, Bernardini S, Martino G, Maccarrone M. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain. 2007;130:2543–2553. doi: 10.1093/brain/awm160. [DOI] [PubMed] [Google Scholar]
- [20].Darley CF, Tinklenberg JR, Roth WT, Atkinson RC. The nature of storage deficits and state-dependent retrieval under marihuana. Psychopharmacologia. 1974;37:139–149. doi: 10.1007/BF00437420. [DOI] [PubMed] [Google Scholar]
- [21].Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42:993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- [22].Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- [23].Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- [25].Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- [26].Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med. 2006;84:253–258. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- [27].Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2005;7:221–232. doi: 10.3233/jad-2005-7304. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- [28].Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- [30].Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- [31].Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- [32].Herber DL, Mercer M, Roth LM, Symmonds K, Maloney J, Wilson N, Freeman MJ, Morgan D, Gordon MN. Microglial activation is required for Abeta clearance after intracranial injection of lipopolysaccharide in APP transgenic mice. J Neuroimmune Pharmacol. 2007;2:222–231. doi: 10.1007/s11481-007-9069-z. [DOI] [PubMed] [Google Scholar]
- [33].Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- [35].Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- [36].Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- [37].Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- [38].in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- [39].Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- [40].Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- [41].Kalaria RN, Cohen DL, Premkumar DR. Cellular aspects of the inflammatory response in Alzheimer's disease. Neurodegeneration. 1996;5:497–503. doi: 10.1006/neur.1996.0069. [DOI] [PubMed] [Google Scholar]
- [42].Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005;14:3389–3396. doi: 10.1093/hmg/ddi370. [DOI] [PubMed] [Google Scholar]
- [43].Kitazawa M, Yamasaki TR, LaFerla FM. Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann N Y Acad Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- [44].Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–115. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- [45].Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- [46].Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- [47].Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- [48].Mackenzie IR, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50:986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- [49].Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31:462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- [50].Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- [51].Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- [52].Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- [53].Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- [54].Mazzola C, Micale V, Drago F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol. 2003;477:219–225. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- [55].McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- [56].Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- [57].Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- [58].Miller LL, Branconnier RJ. Cannabis: effects on memory and the cholinergic limbic system. Psychol Bull. 1983;93:441–456. [PubMed] [Google Scholar]
- [59].Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer's disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- [60].Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Monteleone P, Tortorella A, Martiadis V, Di Filippo C, Canestrelli B, Maj M. The cDNA 385C to A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) is associated with overweight/obesity but not with binge eating disorder in overweight/obese women. Psychoneuroendocrinology. 2008;33:546–550. doi: 10.1016/j.psyneuen.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [62].Mucke L, Yu GQ, McConlogue L, Rockenstein EM, Abraham CR, Masliah E. Astroglial expression of human alpha(1)-antichymotrypsin enhances Alzheimer-like pathology in amyloid protein precursor transgenic mice. Am J Pathol. 2000;157:2003–2010. doi: 10.1016/s0002-9440(10)64839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- [64].Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- [65].Nilsson LN, Bales KR, DiCarlo G, Gordon MN, Morgan D, Paul SM, Potter H. Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Onaivi ES, Suguira T, Di Marzo V. Endocannabinoids: The Brain and Body's Marijuana and Beyond. Taylor and Francis; Boca Raton: 2006. [Google Scholar]
- [67].Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- [68].Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- [69].Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharmacol. 1999;379:R1–R2. doi: 10.1016/s0014-2999(99)00496-3. [DOI] [PubMed] [Google Scholar]
- [71].Romero J, Garcia L, Cebeira M, Zadrozny D, Fernandez-Ruiz JJ, Ramos JA. The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: role of nigrostriatal dopaminergic neurons. Life Sci. 1995;56:2033–2040. doi: 10.1016/0024-3205(95)00186-a. [DOI] [PubMed] [Google Scholar]
- [72].Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- [73].Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, Calabresi P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32:1384–1390. doi: 10.1038/sj.npp.1301246. [DOI] [PubMed] [Google Scholar]
- [74].Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- [75].Schmid PC, Krebsbach RJ, Perry SR, Dettmer TM, Maasson JL, Schmid HH. Occurrence and postmortem generation of anandamide and other long-chain N-acylethanolamines in mammalian brain. FEBS Lett. 1995;375:117–120. doi: 10.1016/0014-5793(95)01194-j. [DOI] [PubMed] [Google Scholar]
- [76].Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shay AH, Choi R, Whittaker K, Salehi K, Kitchen CM, Tashkin DP, Roth MD, Baldwin GC. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J Infect Dis. 2003;187:700–704. doi: 10.1086/368370. [DOI] [PubMed] [Google Scholar]
- [78].Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49:211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- [79].Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- [80].Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol. 2005;78:231–238. doi: 10.1189/jlb.0205111. [DOI] [PubMed] [Google Scholar]
- [82].Sperber BR, Leight S, Goedert M, Lee VM. Glycogen synthase kinase-3 beta phosphorylates tau protein at multiple sites in intact cells. Neurosci Lett. 1995;197:149–153. doi: 10.1016/0304-3940(95)11902-9. [DOI] [PubMed] [Google Scholar]
- [83].Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- [84].Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- [85].Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- [86].Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- [87].Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [88].Ujike H, Takaki M, Nakata K, Tanaka Y, Takeda T, Kodama M, Fujiwara Y, Sakai A, Kuroda S. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry. 2002;7:515–518. doi: 10.1038/sj.mp.4001029. [DOI] [PubMed] [Google Scholar]
- [89].Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- [92].Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]