Abstract
Extracellular matrix fibers (ECM) such as collagen, elastin, and keratin provide biological and physical support for cell attachment, proliferation, migration, differentiation and ultimately cell fate. Therefore, ECM fibers are an important component in tissue and organ development and regeneration. Meanwhile, polymer nanofibers could play the same critical role in tissue regeneration process. Fibrous structures can be fabricated from a variety of materials and methods with diameters ranging throughout the size scale where cells can sense individual fibers (several nanometers to several microns). Polymer nanofiber scaffolds can be designed in a way that predictably modulates a variety of important cell behaviors towards a desired overall function. The nanofibrous topography itself, independent of the fiber material, has demonstrated the potential to modulate cell behaviors desirable in tissue engineering such as: unidirectional alignment; increased viability, attachment, and ECM production; guided migration; and controlled differentiation. The versatility of polymer nanofibers for functionalization with biomolecules opens the door to vast opportunities for the design of tissue engineering scaffolds with even greater control over cell incorporation and function. Despite the promise of polymer nanofibers as tissue engineering scaffolds there have been few clinically relevant successes because no single fabrication technique currently combines control over structural arrangement, material composition, and biofunctionalization, while maintaining reasonable cost and yield. Promising strategies are currently being investigated to allow for the fabrication of optimal polymer nanofiber tissue engineering scaffolds with the goal of treating damaged and degenerated tissues in a clinical setting.
Keywords: Polymer, Nanofiber, Scaffold, Biomaterial, Tissue engineering, Regeneration, Electrospinning, Phase Separation, Self assembly
1. Introduction
The fibrous component of the extracellular matrix (ECM) in the tissue is made up of protein fibers such as collagens, elastin, keratin, laminins, fibronectin and vitronectin. These protein fibers provide structural support to tissues and regulate many aspects of cell behavior. The building blocks of protein nanofibers are synthesized intracellularly by cells, and then secreted into the extracellular space by exocytosis. Soluble precursors are enzymatically modified within the ECM for formation into fibers. ECM fibers provide structural support and mechanical integrity to tissues as well as locations for cell adhesion and regulation of cell functions such as proliferation, shape, migration, and differentiation [1]. Tissues requiring high levels of mechanical strength such as tendons, ligaments, and bone, have high levels of organized fibers to impart mechanical strength, while some soft tissues have much higher levels of unorganized fibers. Composition and arrangement of ECM fibers in a tissue can impart fine tuned mechanical properties. For example, arteries exhibit precise mechanical behavior characterized by highly elastic behavior at low levels of distension and stiffer behaviors at high levels of distention due to a highly organized microstructure of elastin and collagen fibers [2]. ECM fibers also offer locations for cell adhesion and can regulate cell shape and migration patterns based on composition and arrangement. Structural fibrous proteins can also act as storage locations for the release of small bioactive peptides and growth factors upon release by proteolytic cleavage.
The ECM in natural tissue is contently being remodeled by proteolytic cleavage and cellular reassembly, and accelerated ECM remodeling is critical during embryonic development and during tissue regeneration. For example, assembly of a fibrillar matrix from fibronectin is crucial for embryonic development and wound healing [3] and multiple ECM fibrous proteins, such as fibronectin and laminin are required for the process of branching morphogenesis evident in the development of organs such as the kidney, lung, and prostate [4,5]. Remodeling in adult bone tissue involves an initial assembly of collagen fibers with entrapped cells, followed by calcium salt precipitation on the collagen fibers to form bone. In the peripheral nervous system after axonal degeneration following injury, regenerating axons grow back to their targets by following the laminin and fibronectin containing basal lamina tube that surrounded the original nerve fiber [6].
Tissue engineering strategies utilizing fibrous components attempt to fill the role of fibrous components in natural tissue. Fibrous components of tissue engineering scaffolds can impart mechanical strength, structure for cell attachment, and act as reservoirs for biomolecule delivery in much the same way as the natural fibrous components of the ECM. For example, in many bone tissue engineering strategies a fibrous mesh is used as a template for subsequent mineralization in a similar way to collagen fibers in natural remodeling. The fabricated matrix forms a bonelike structure and provides an attractive microenvironment for osteogenic function in resident cells. Blood vessel tissue engineering with nanofibrous scaffolds benefits from its properties of high tensile strength, good cell attachment properties for endothelium formation, and directional alignment for smooth muscle cells in the vessel wall. In vivo, injectable self-assembling nanofibrous networks introduced to injury sites have been used to provide a 3-dimensional structural environment that has been shown to increase the infiltration and retention of endogenous cells [7,8].
2. Methods of polymer nanofiber scaffold fabrication
Polymer nanofibers have been fabricated using a number of different techniques. The methods of nanofiber fabrication are varied and utilize physical, chemical, thermal, and electrostatic fabrication techniques. The methods of polymer nanofiber fabrication most commonly associated with tissue engineering scaffolds in the literature are electrospinning, self assembling peptide reactions, and phase separation [9–11]. There are however several other ways to fabricate polymer nanofibers as well. Several of these fabrication methods are briefly described in the following section and some of the advantages and disadvantages of each method are listed in Table 1.
Table 1.
Comparison of methods for fabricating polymer nanofiber scaffolds.
| Advantages | Disadvantages | |
|---|---|---|
| Electrospinning | • Easy to setup | • Poor cell infiltration into the core of the scaffolds |
| • Cost effective | • 2-Dimensional pore or microstructure arrangement |
|
| • High level of versatility allows control over fiber diameter, microstructure and arrangement |
• Toxic solvents often used | |
| • Vast materials selection | ||
| Self Assembly | • Easy incorporation of cells during fiber formation |
• Complex procedure |
| • 3-Dimensional pore arrangement | • Lack of control of fiber orientation and arrangement |
|
| • Injectable for in vivo assembly | • Limited fiber diameter ~2–30 nm and length ~10 µm |
|
| Phase Separation | • 3-Dimensional pore arrangement | • Complex procedures |
| • Lack of control of fiber arrangement | ||
| Bacterial Cellulose | • Low cost | • Limited material selection |
| • High yield | • Lack of versatility for functionalization |
|
| Templating | • Vast materials selection | • Sacrificial materials |
| • Control over fiber diameter and length |
• Limitation on fiber dimensions and arrangement |
|
| Drawing | • Vast materials selection | • Low productivity (One single fiber at a time) |
| • Simple procedure | • Difficult to form fibers with consistent diameter | |
| Extraction | • Natural materials | • Limited material selection |
| • Limited control of fiber diameter and length (a few microns) |
||
| Vapor-Phase Polymerization | • Polymer synthesized directly into nanofibers |
• Limited control of fiber diameter and length (hundreds of microns) |
| • Limited material selection | ||
| • Complicated procedures | ||
| Kinetically controlled solution synthesis | • Polymers synthesized directly into nanofibers |
• Limited control of fiber diameter and length (60 µm) |
| • Limited material selection | ||
| • Complicated procedures | ||
| Chemical Polymerization of Aniline | • Polymers synthesized directly into nanofibers |
• Limited control of fiber diameter and length |
| • Limited material selection | ||
| • Complicated procedures |
2.1. Electrospinning
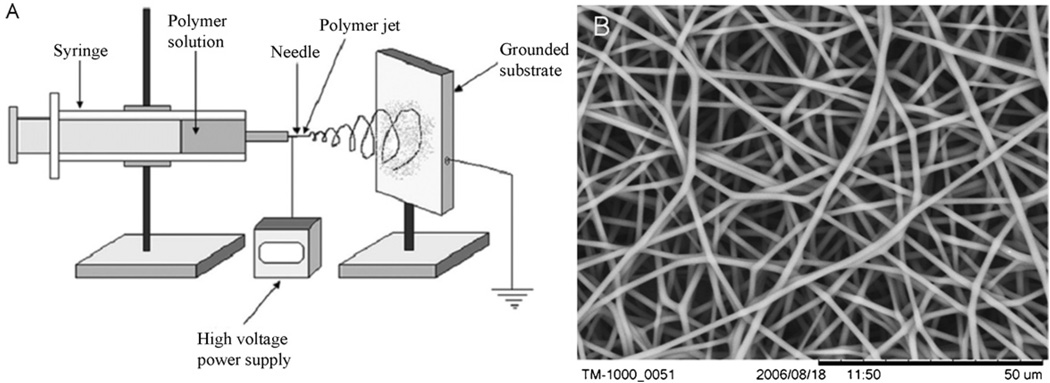
Electrospinning is an electrostatically driven method of fabricating polymer nanofibers. Nanofibers are formed from a liquid polymer solution or melt that is feed through a capillary tube into a region of high electric field. The electric field is most commonly generated by connecting a high voltage power source in the kilovolt range to the capillary tip (Fig. 1). As electrostatic forces overcome the surface tension of the liquid, a Taylor cone is formed and a thin jet is rapidly accelerated to a grounded or oppositely charged collecting target. Instabilities in this jet cause violent whipping motions that elongate and thin the jet allowing the evaporation of some of the solvent or cooling of melts to form solid nanofibers on the target site. Nanofiber size and microstructure can be controlled by several processing parameters including: solution viscosity, voltage, feed rate, solution conductivity, capillary-to-collector distance, and orifice size [12]. The electrospinning technique is very versatile and a wide range of polymer and copolymer materials with a wide range of fiber diameters (several nanometers to several microns) can be fabricated using this technique. Many different types of molecules can be easily incorporated during the electrospinning fabrication process to produce functionalized nanofibers. Electrospun nanofibers are usually collected from an electrospinning jet as non-woven randomly or uniaxially aligned sheets or arrays.
Fig. 1.
(A) schematic (A) of a standard electrospinning setup (reproduced with permission from Year 2006 Dove Medical Press Ltd. [11]) and a scanning electronmicroscope (SEM) image (B) of electrospun polyurethane nanofibers.
2.2. Self assembly
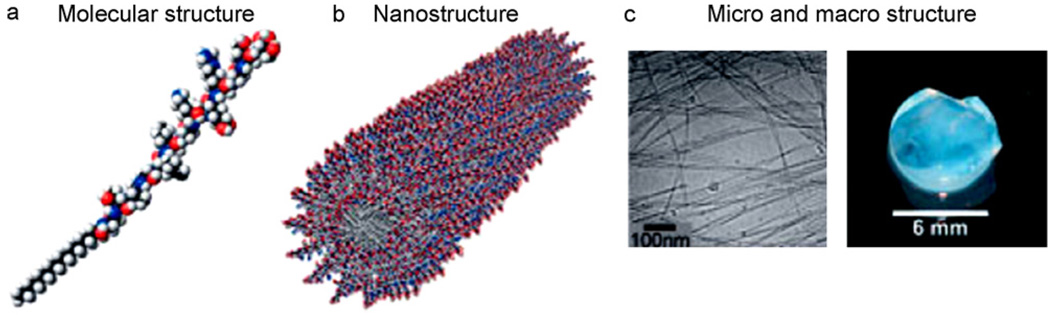
Self assembly is a process by which molecules organize and arrange themselves into patterns or structures through non-covalent forces such as hydrogen bonding, hydrophobic forces, and electrostatic reactions. Dialkyl chain amphiphiles containing peptides were developed to mimic the ECM. These peptide amphiphiles (PA), derived from a collagen ligand, allow for a self assembling system that consists of a hydrophobic tail group and a hydrophilic head group (Fig. 2) [13]. The specific composition of amino acid chains in peptide amphiphile systems determines the assembly, chemical, and biological properties of the system, and therefore PA systems can be tailored to specific applications [14,15]. Nanofibers with diameters around 5–25 nm can be formed by the self assembly process, and systems have been developed where nanofiber assembly can be induced by appropriate pH values [16,17]. Cells can be encapsulated in a nanofibrous PA structure if they are added during the self assembly process and PAs can also be injected in vivo where they subsequently self assemble into a nanofibrous network. It has been demonstrated that self assembled peptide nanofibers can spontaneously undergo reassembly back to a nanofiber scaffold after destruction by sonication, and after multiple cycles of destruction and reassembly, the peptide nanofiber scaffolds were still indistiquishable from their original structures [18]. While peptides are most commonly used in self assembly of tissue engineering structures, synthetic polymer nanofibers have also been fabricated by self assembly [19,20].
Fig. 2.
Schematics of the (A) molecular structure and (B) nanostructure, and images of the (C) micro and macro structure of a self assembling peptide-amphiphile nanofiber network (reproduced with permission from Year 2005 Wiley-VCH Verlag GmbH & Co. KGaA [21]).
2.3. Phase separation
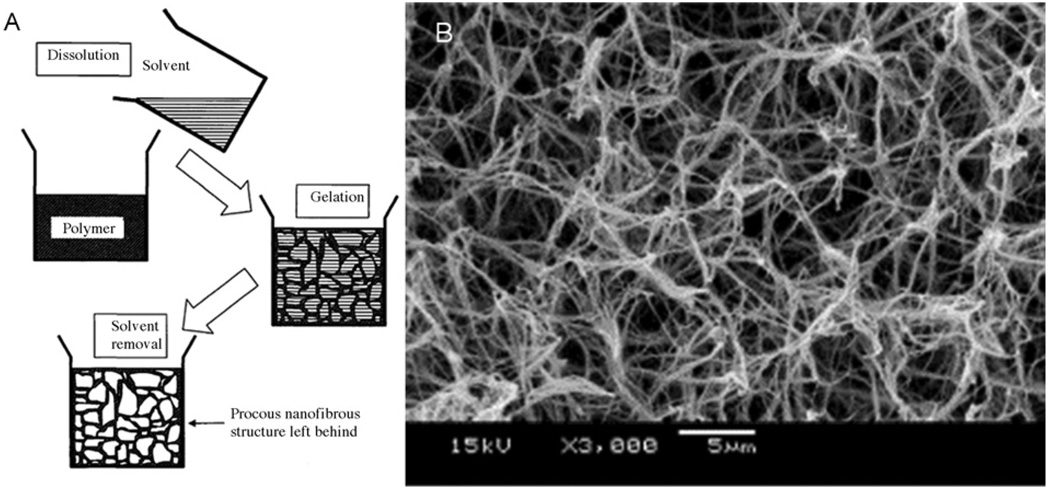
Nanofibrous foam materials have been fabricated by a technique called thermally induced liquid-liquid phase separation [22]. This fabrication procedure involves (a) the dissolution of polymer in solvent (b) phase separation and polymer gelatination in low temperature (c) solvent exchange by immersion in water and (d) freezing and freeze-drying (Fig. 3). The morphology of these structures can be controlled by fabrication parameters such as gelatination temperature and polymer concentration. Interconnected porous nanofiber networks have been formed from polymer such as, poly-L-lactide acid (PLLA), poly-lactic-co-glycolic acid (PLGA), and poly-DL-lactic acid (PDLLA) with fiber diameters from 50–500 nm, and porosities up to 98.5%.
Fig. 3.
A schematic (A) of nanofiber formation by phase separation (reproduced with permission from Year 2005 World Scientific Publishing [23]), and an SEM image (B) of nanofibrous structure fabricated by this technique[22].
2.4. Bacterial cellulose
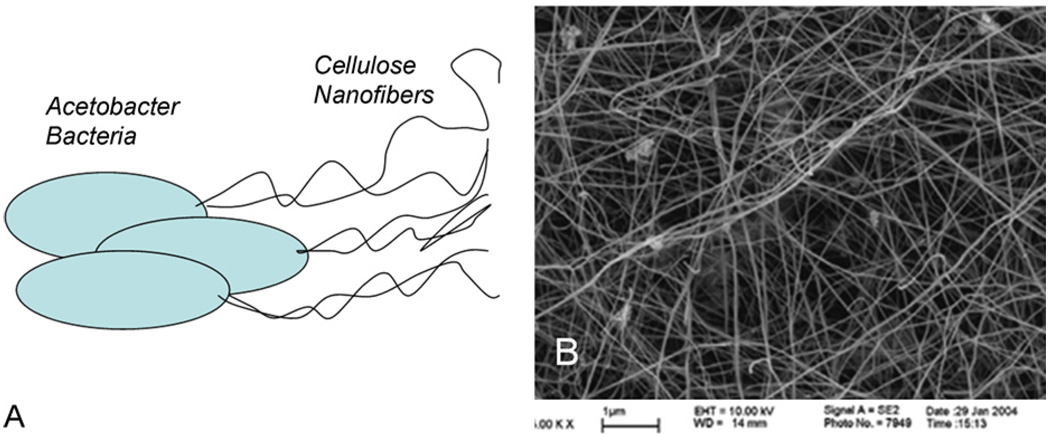
Cellulose nanofibers produced by bacteria have been long used in a variety of applications, including biomedical applications [24]. Cellulose synthesis by Acetobacter involves the polymerization of glucose residues into chains, followed by the extracellullar secretion, assembly and crystallization of the chains into hierarchically composed ribbons (Fig. 4). Networks of cellulose nanofibers with diameters less than 100 nm are readily produced, and fibers with different characteristics may be produced by different strains of bacteria [24]. Copolymers have been produced by adding polymers to the growth media of the cellulose producing bacteria [25,26].
Fig. 4.
Schematic of Acetobacter cells depositing cellulose nanofibers (A), and an SEM image of a cellulose nanofiber mesh produced by bacteria (B) (reproduced with permission from Year 2007 American Chemical Society [24]).
2.5. Templating
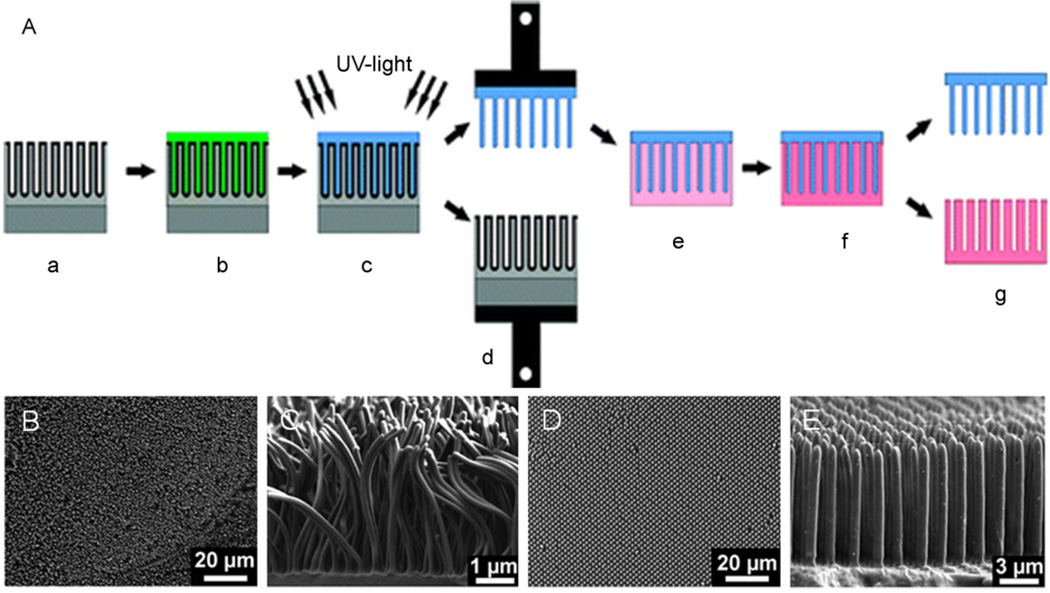
Polymer nanofibers can be fabricated using templates such as self-ordered porous alumina. Alumina networks templates with pore diameters from 25 to 400 nm, and pore depths from around 100nm to several 100 µm have been be fabricated. Polymer nanofiber arrays can be released from these molds by destruction of the molds or mechanical detachment (Fig. 5) [27,28]. The length of polycaprolactone (PCL) nanofibers fabricated from alumina templates can be controlled as a function of parameters such as melt time and temperature [29].
Fig. 5.
(A) Schematic of the fabrication of polymer nanofibers using a nondestructive templating technique (grey: alumina template, green: resin, blue: polymer nanofibers, pink: silica replica template. (B) SEM images of 120 nm (B&C) and 1 µm (D&E) polymer fibers fabricated by the above technique (reproduced with permission from Year 2008 American Chemical Society [27]).
2.6. Drawing
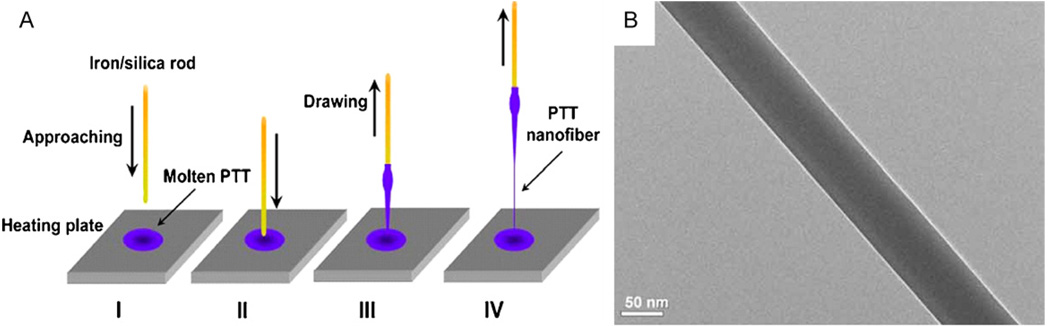
Nanofibers can be mechanically drawn from viscous polymer liquids directly [30]. In one example, nanofibers were drawn directly when a rod was placed in a polymer melt and moved up forming a thin filament that cooled to form a nanofiber (Fig. 6). This method was used to fabricate poly(trimethylene terephthalate) nanofibers with diameters as low as 60 nm, and lengths up to 500 mm [31]. An automated drawing technique utilized a pipette dispensing liquid polymer solution while intermittently contacting a substrate and moving the x–y direction across the substrate [32]. This process allowed the formation of thin suspended nanofibers connecting droplet shaped dots on the substrate. This technique was used to fabricate polystyrene nanofibers with diameters ranging from tens nanometers to several microns in highly ordered patterns.
Fig. 6.
(A) Schematic of nanofiber fabrication by the drawing technique. (B) Transmission electron microscope (TEM) image of a polymer nanofiber fabricated using the drawing technique (reproduced with permission from Year 2008 The Optical Society [31]).
2.7. Extraction
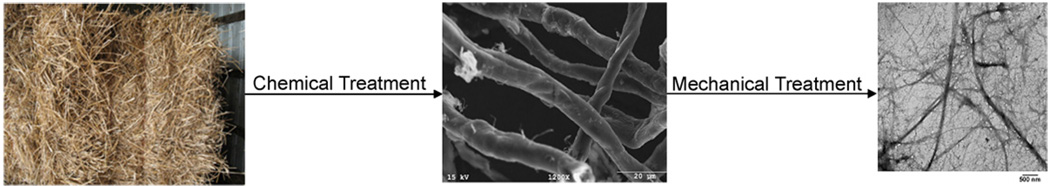
Nanofibers can be extracted from natural materials using chemical and mechanical treatments. Cellulose fibrils can be disintegrated from plant cell walls. In one example, cellulose nanofibers were extracted from wheat straw and soy hull with diameters ranging from 10 to 120nm and lengths up to a few thousand nanometers (Fig. 7) [33]. Invertebrates have also been used as a source for the extraction of nanofibers. Chitin nanofibers 3–4 nm in diameter and a few micrometers in length were extracted from squid pen and Poly-N-acetyl glucosamine nanofibers isolated from a marine diatom demonstrated prothrombotic interactions with red blood cells [34,35].
Fig. 7.
Images of natural wheat straw[36],wheat straw microfibers[33] after chemical treatment and wheat straw nanofibers [33] after chemical treatment.
2.8. Vapor-phase polymerization
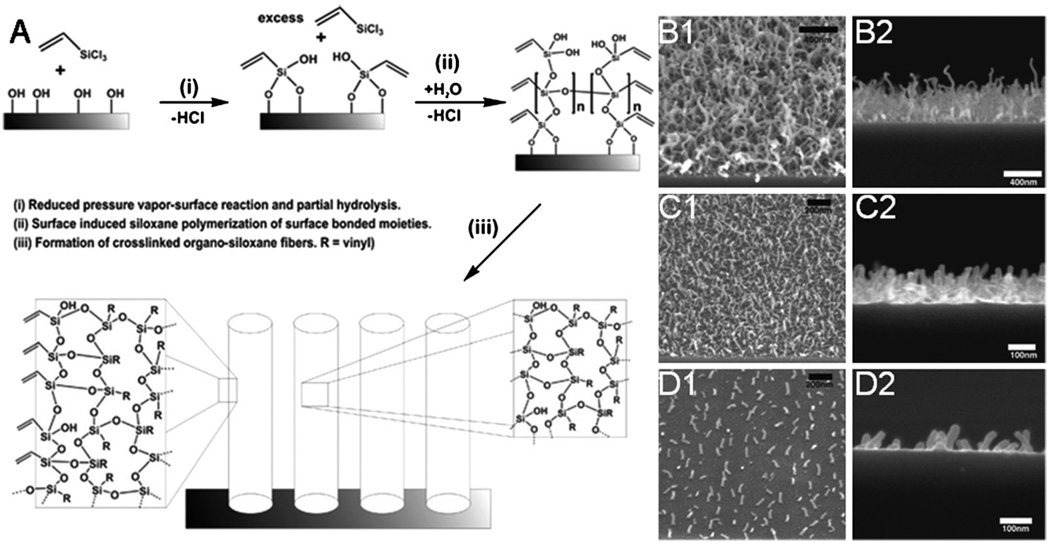
Polymer nanofibers have also been fabricated from vapor-phase polymerization. Plasma-induced polymerization of vapor phase vinyltrichlorosilane produced organosiloxane fibers of with diameters around 25 nm and typical lengths of 400–600 nm and cyanoacrylate fibers with diameters from 100 to 400 nm and lengths of hundreds of microns (Fig. 8) [37,38].
Fig. 8.
(A) Schematic describing a proposed mechanism for nanofiber formation by vapor-phase polymerization (B) Arial (1)and side views (2) of polymer nanofibers fabricated from vapor-phase polymerization at high (B), intermediate (C) and low (D) packing densities [37]. (Reproduced with permission from Year 2007 American Chemical Society [37]).
2.9. Kinetically controlled solution synthesis
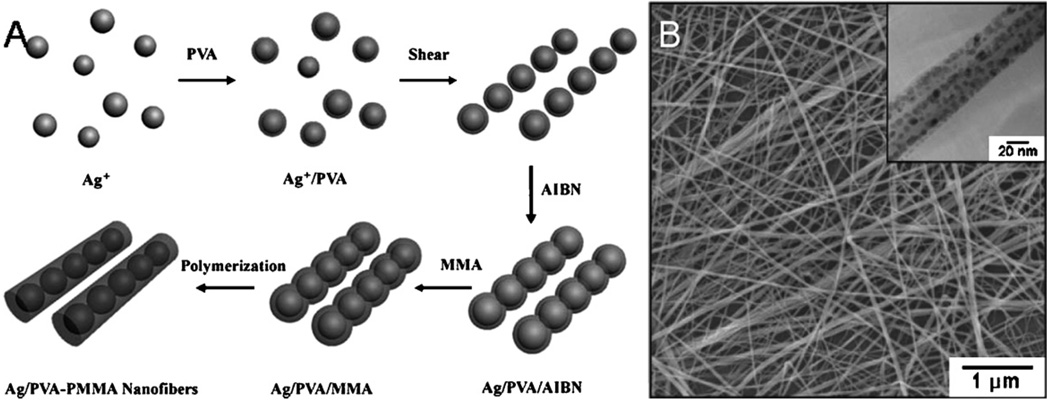
Nanofibers and nanowires have been fabricated in solution using linear aligned substrates as templating agents such as iron-cation absorbed reverse cylindrical micelles and silver nanoparticles [39]. Poly(vinyl alchohol)-poly(methyl methacrylate) nanofibers were fabricated using silver nanoparticle that were linearly aligned in solution by vigorous magnetic stirring (Fig. 9) [40]. These nanoparticle chain assemblies acted as a template for further polymerization of nanofibers with diameters from 10 to 30 nm and lengths up to 60 µm.
Fig. 9.
(A) Schematic of silver nanoparticle embedded polymer nanofibers fabrication (B) SEM and TEM images of silver nanoparticle embedded polymer nanofibers (reproduced with permission from Year 2006 Royal Society of Chemistry [40]).
2.10. Conventional chemical oxidative polymerization of aniline
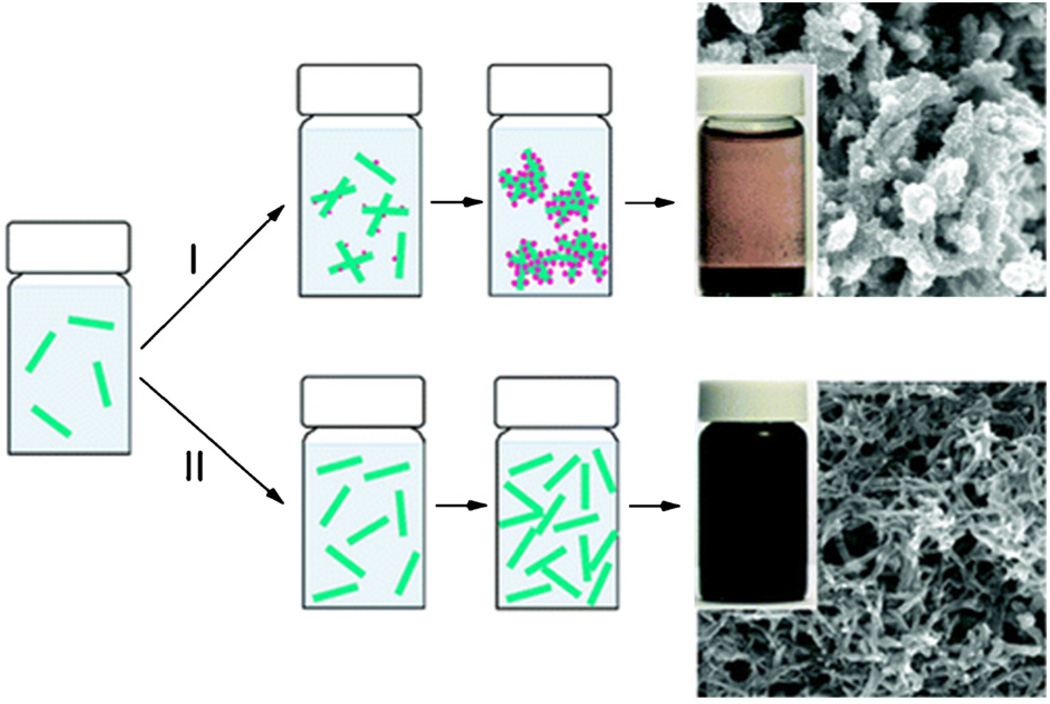
Chemical oxidative polymerization of aniline is a traditional method for synthesizing polyaniline and during the early stages of this synthesis process polyaniline nanofibers are formed (Fig. 10). Optimization of polymerization conditions such as temperature, mixing speed, and mechanical agitation allows the end stage formation of polyaniline nanofibers with diameters in the range of 30–120 nm [41,42].
Fig. 10.
Schematic showing the nucleation of polyaniline nanofibers. (I) Under non-ideal nucleation conditions aggregate formation is present. (II) When ideal nucleation conditions are predominant, well-dispersed polyaniline nanofibers are formed. Typical images of the reaction vials and microstructure are displayed next to the schematic (reproduced with permission from Year 2009 American Chemical Society [42]).
3. Biofunctionalization of polymer nanofibers
Polymer nanofibers are excellent structures for the design of tissue engineering scaffolds because of the wide variety of biocompatible polymer materials that can be formed into nanofibrous structures. Different types of biocompatible polymers demonstrate a variety of mechanical properties, degradation rates, and cell-material interactions, and new types of polymers are continuously being synthesized. In addition to the vast material selection available, one of the most favorable characteristics of polymer materials in tissue engineering scaffolds are their suitability for biofunctionalization. Biofunctional polymer nanofibers can be fabricated entirely from materials found in the ECM, and a variety of biomolecules and drugs can be incorporated into polymer nanofibers during the fabrication process or by using post processing surface modification techniques. Biofunctionalization techniques can be utilized to fabricate tissue engineering scaffolds that guide cells toward desired behavior and function.
3.1. Natural ECM molecule nanofibers
Molecules that are naturally occurring in the extracellular matrix are ideal materials for cell attachment, survival, proliferation, and differentiation. In addition, substrate interactions between cells and ECM molecules may modulate certain cell functions. Biofunctional nanofibers can be directly fabricated from natural ECM materials or synthetic polymers can be blended with natural ECM molecules to form copolymer fibers. Blended nanofibers generally benefit from improved physical properties due to the synthetic polymer component and improved bioactivity due to the natural ECM component [43,44].
3.1.1. Collagen
Pure collagen nanofibers are commonly electrospun from solutions of soluble type I collagen dissolved in organic solvents such as 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) or aqueous acids. Bead free pure collagen nanofibers have been fabricated with diameters ranging from 100 to 500 nm [45,46]. Water soluble collagen nanofibers must be crosslinked before use in cell culture with crosslinking agents, such as glutaralde-hyde vapor, 1,6-diisocyanatohexane, genipin, and so on [45,47,48]. Nanofiber blends containing collagen and synthetic polymers such as PCL are also easily fabricated by electrospinning when both materials are dissolved in the same solvents [49]. In nanofibers electrospun from single solvent solutions containing collagen and PCL, collagen was well dispersed as small spherical aggregates at low concentrations (10wt%) and as much larger irregular shapes at higher concentrations (50 wt%) [50]. Cells cultured on synthetic nanofibers blended with collagen have shown better attachment, growth, and ECM production than fibers without collagen incorporation [51,52]. Blends allow for the fabrication of collagen containing nanofibers with a greater range of mechanical properties and fiber diameters. It has been shown that electrospinning collagen from organic solvents causes extensive denaturation and it was suggested that similar bioactivity may be obtained using gelatin in these cases [53]. However, several studies directly comparing electrospun collagen and gelatin nanofibers indicate that electrospun collagen nanofibers may retain some favorable bioactivity when compared to gelatin nanofibers despite solvent denaturation [51,54,55].
3.1.2.Gelatin
Gelatin nanofibers have been electrospun by dissolution in organic solvents such as HFIP and 2,2,2-trifluoroethanol (TFE) or aqueous acids. Pure gelatin nanofibers have been electrospun with fiber diameters around 50 to 500 nm [45,56]. Gelatin nanofibers are crosslinked before use in cell culture with crosslinking agents, such as glutaraldehyde vapor, 1,6-diisocyanatohexane, genipin, and so on [45,48]. Gelatin nanofiber blends can also be electrospun by combination of gelatin and other polymers in one solution with a variety of fiber diameters. Cells attached and proliferated better on synthetic fibers when they were blended with gelatin [51,57–59]. Increases in cell attachment and proliferation have been showed to be a function of the ratio of gelatin in the fiber blends [44]. PCL fibers blended with gelatin also enhanced nerve differentiation as compared to plain PCL nanofibrous scaffolds [57].
3.1.3. Elastin
Alpha-elastin and tropoelastin fibers have been electrospun from HFIP solutions and aqueous acids with diameters ranging from 1 to several microns [45,46,48]. Electrospun elastin fibers have been observed to possess a ribbon like morphology as opposed to the uniform crosssection filament shape most common in other electrospun fibers. Elastin nanofibers must be crosslinked before use in cell culture with various crosslinking agents such as glutaraldehyde vapor, 1,6-diisocyanatohexane, and so on [45,46,48]. Elastin fiber blends with collagen and synthetic materials have also been successfully electrospun [45,60].
3.1.4. Chitosan
Pure chitosan nanofibers are difficult to electrospin due to limited solubility, ionic character and three-dimensional networks of strong hydrogen bonds [61]. High molecular weight chitosan nanofibers have however been successfully electrospun using high concentration acid solutions (diameter = 130 nm) and organic solvents (diameter = 60–330 nm) [61–63]. Chitosan can be mixed with other polymers in organic solvent solutions such as HFIP or acid solutions to more easily form blended nanofibers [43]. Chitosan nanofibers have been electrospun as blends with very low concentrations of polyethylene oxide (PEO) (10%) with diameters from 150 to 200 nm [64]. Chitosan/cellulose blends have also been produced by bacteria fabrication with chitosan concentrations of 7–10% dry weight [26]. Nanofiber scaffolds containing chitosan have demonstrated favorable properties as tissue engineering constructs [64].
3.1.5. Dextran
Dextran fibers with a wide range of fiber diameters have been electrospun from aqueous solutions and organic solvents such as dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) mixtures [65]. Dextran is highly soluble in an aqueous environment, but methacrylated dextran nanofibers photocrosslinked after electrospinning formed stable hydrogels in the aqueous environment [65]. Blended PLGA/dextran nanofibers have also been fabricated and have demonstrated favorable tissue engineering properties [66].
3.1.6. Fibrinogen
Fibrinogen nanofibers with diameters from 80 to 700 nm have been electrospun from solutions of fibrinogen dissolved in HFIP and 10× minimal essential medium (9:1) [67]. Fibrinogen nanofiber scaffolds were able to maintain their structure when hydrated without crosslinking and demonstrated good interactions with cells in vitro culture conditions [68,69]. The protease inhibitor, aprotinin can be added to cell culture media to slow down the degradation rate of fibrinogen nanofibers [68,69].
3.1.7. Laminin
Laminin purified from Engelbreth–Holm–Swarm (EHS) tumor was solubilized in HISP and electrospun into 90 to 300 nm fibers with various amounts of bead composition [70]. Bioactivity of laminin nanofibers was confirmed by the in vitro behavior of PC12 and human adipose stem cells. Laminin nanofibers were able retain their structure under hydrated conditions without crosslinking.
3.1.8. Hyaluronic acid
Hyaluronic acid nanofibers are very difficult to fabricate, but a combination procedure involving electrospinning from an acidic solution with heated air blown around the electrospining jet was developed to obtain uniform fibers [71]. Pure hyaluronic acid nanofibers (diameter = 110nm) were also fabricated by electrospinning hyaluronic acid:PEO blended nanofibers followed by extraction of the PEO component of the fibers [72,73]. Scaffolds assembled from these hyaluronic acid nanofibers demonstrated good cell interaction properties in vitro.
3.2. Surface functionalization
One of the major advantages of polymeric materials in tissue engineering scaffold design is vast versatility for surface modifications. Biomolecules can be absorbed or chemically bonded to the surface of polymer nanofibers in order to modulate specific cell functions in tissue engineering. In this review only surface functionalization techniques that have previously been carried out with polymer nanofiber structures are included, but in theory any method of polymer surface modification may be applicable to polymer nanofibers as long as some part of the process does not destroy the sometimes fragile nanofiber structures.
3.2.1. Physical absorption
The simplest method for surface biofunctionalization of polymer nanofibers with biomolecules is to incubate nanofiber meshes with solutions containing solubilized biomolecules to allow for physical absorption of the biomolecules onto the nanofiber surfaces. Biomolecules may adhere to the surface of the nanofibers because of interactions such as Van der Waals force, electrostatic forces, hydrophobic interactions, and hydrogen bonding. The efficiency of physical adsorption onto hydrophobic nanofiber scaffolds can be increased by treatment with air plasma to make them more hydrophilic and allow greater infiltration of aqueous solutions containing water soluble biomolecules. Plasma treatment can also be used to improve the hydrophilicity of nanofiber meshes for improved covalent grafting modifications explained in the following section [74]. The efficiency of plasma treatment may be improved by placing a sample at a distance downstream of the plasma source to allow surface modification while minimizing damages due to etching and ablation [75].
3.2.2. Covalent surface bonding
Covalent surface bonding can be used to chemically bond biomolecules to exposed functional groups directly on the nanofiber surface. Covalent surface bonding provides a much more efficient coating and greater long term retention of biomolecules [76]. Covalent coating of biomolecules on nanofibers usually involves at least two requirements: (1) exposed functional groups on the fiber surface, (2) covalent bonding of biomolecules to these functional groups.
3.2.2.1. Exposing functional groups
Depending on the molecular configuration of the specific polymer requiring modification, functional groups may already be present, or can be added to the surface by various chemical treatments. The functional groups most commonly used in polymer nanofiber surface modification reactions in the literature are carboxyl and amine groups. Carboxyl groups can be exposed on the surface of nanofibers made from materials, such as PLLA and PCL, by treatment with chemicals such as sodium hydroxide (NaOH) [77,78]. Amine groups were grafted on the surface of degradable polyester nanofibers by treatment with 1,6-hexanediamine/propanol solution or ethylenediamine (ED) [79,80]. When the desired functional groups cannot be easily grafted directly on the polymer nanofiber surface then a linker molecule with the desired exposed functional groups can be tethered to the fiber surface. Acrylic acid in the gas phase was grafted to various polymer nanofibers as a plasma treatment to add carboxyl groups [81]. An additional di-amino-poly(ethylene glycol) (di-NH2-PEG) was used as a linker molecule to add functional amine groups to polyester nanofibers that had previously been soaked in NaOH to expose carboxylic acid groups [82]. Exposed carboxyl groups were added to polyethersulfone (PES) nanofibers by photo polymerization grafting of poly (acrylic acid) as a linker molecule [83]. When adding linker molecules to the surface of polymer nanofibers it may be important to consider the properties of the specific linker molecules used because several research groups have shown that linker properties such as length have an effect on the cell response to functionalized polymer nanofibers [83–85]. Polymer blends and modified polymers can be also be synthesized to include desired exposed functional groups on their structure. A PCL-PEG block copolymer was synthesized with functional amine groups on the surface via PEG linkers and electrospun into nanofibers that were further function-alized [86]. A copolymer of methyl methacrylate (MMA) and acrylic acid (AA) was synthesized and electrospun into nanofibers with different carboxyl group contents by varying the ratio of MMA to AA [87]. In another case PLGA-b-PEG-NH2 di-block copolymer was directly blended with PLGA in organic solvents and electrospun to form PLGA nanofibers with exposed functional amino groups [88].
3.2.2.2. Covalently attaching biomolecules to functional groups
Covalently attaching biomolecules to polymer nanofiber surfaces may require the activation of exposed functional groups on the polymer surface, the biomolecules or both. Carboxyl groups can be activated for reaction with primary amines by treatment with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). This method of activation is commonly referenced in the literature for bonding biomolecules to polymer nanofibers [55,74,75]. The efficiency of EDC initiated bonding can be improved by adding N-hydroxysuccinimide (NHS), which converts an unstable amine-reactive intermediate formed by the EDC reaction into an amine-reactive NHS ester [55,74]. Glutaraldehyde, and 1-hydroxybenzole (HOBt) with EDC have been used to attach biomolecules to exposed amine groups on polymer nanofibers [79,86].
3.2.3. Examples of biomolecule surface functionalized nanofibers
A variety of biomolecules have been attached to polymer nanofibers to increase their bioactivities. Many research groups have used the physical absorption method to coat nanofibers with nanocrystalline apatites by immersion in simulated body fluid (SBF), which is an aqueous solution that contains ions in concentrations similar to those of human body plasma [89]. The degree of coating can be controlled by incubation time and SBF solution composition [77,78]. Hydroxyapatite coatings stimulate the expression of osteogenic genes by osteoblastic cells when compared to uncoated scaffolds [78]. The physical absorption method has also been used to coat synthetic nanofibers with biomolecules such as collagen, laminin, E-selection, and many others and successful biomolecule incorporation is reflected in enhanced cell spreading, viability, attachment, and phenotype preservation [76,90,91]. The physical absorption method has the advantage of being a very simple and gentle procedure. Modifications preformed by this method limit damage to fragile nanofiber structures and biomolecules, but binding can be relatively weak.
Many biomolecules have been covalently bonded to the surface of polymer nanofiber scaffolds as well. Covalent attachment of collagen and laminin to polymer nanofibers enhanced the suitability of the nanofibers as neural tissue engineering scaffolds [76,87]. Collagen synthesis by esophageal epithelial cells was increased by covalent bonding of fibronectin to nanofibers, and covalent attachment of an RGD peptide enhanced the attachment and proliferation of fibroblasts [79,88]. Polymer nanofibers have also been covalently functionalized with growth factors [4]. When compared to physical absorption, covalent bonding provides a more efficient and stable surface attachment of biomolecules. The major disadvantages of covalent bonding are more complex procedures and the potential use of harsh conditions that may limit the types of biomolecules that can be incorporated. For example, the organic solvents used in some covalent binding procedures may deactivate biomolecules such as growth factors.
Combinational techniques to utilize both covalent bonding and physical absorption can also be used to biofunctionalize polymer nanofiber surfaces. The physical absorption of hydroxyapatite coatings with SBF can be made more efficient if carboxyl groups are exposed by NaOH prior to incubation [77,78]. Growth factors can be bound to polymer nanofibers using a combinational technique where molecules such as heparin are covalently bonded to act as reservoirs that stabilize, store, and protect growth factors introduced by physical absorption [4,55]. This technique allows for strong, stable covalent binding of heparin to the nanofiber surface, without exposing the growth factors to harsh conditions that could reduce their biofunctionality.
3.3. Bulk biomolecule incorporation
In addition to surface functionalization, biomolecules can also be incorporated into the bulk material of nanofibers during the fabrication process. The three most common methods of bulk biomolecule incorporation into polymer nanofibers are direct mixing into a polymer solution, co-axial electrospinning, and insertion of biofunctional peptide sequences into peptide amphiphile molecules for self assembly. It has been shown that the direct incorporation technique can allow for greater amounts of biomolecule incorporation and improved bioactivity when compared to surface modification techniques [76]. In addition, biomolecules incorporated by this technique are embedded into the bulk material of the fibers and can facilitate extended release of biomolecules by diffusion through the nanofibers or release by degradation of the nanofibers in the case of biodegradable materials. The release kinetics of biomolecules contained in polymer nanofibers commonly involves an initial burst followed by steady extended release.
3.3.1. Mixing biomolecules directly into a polymer solution
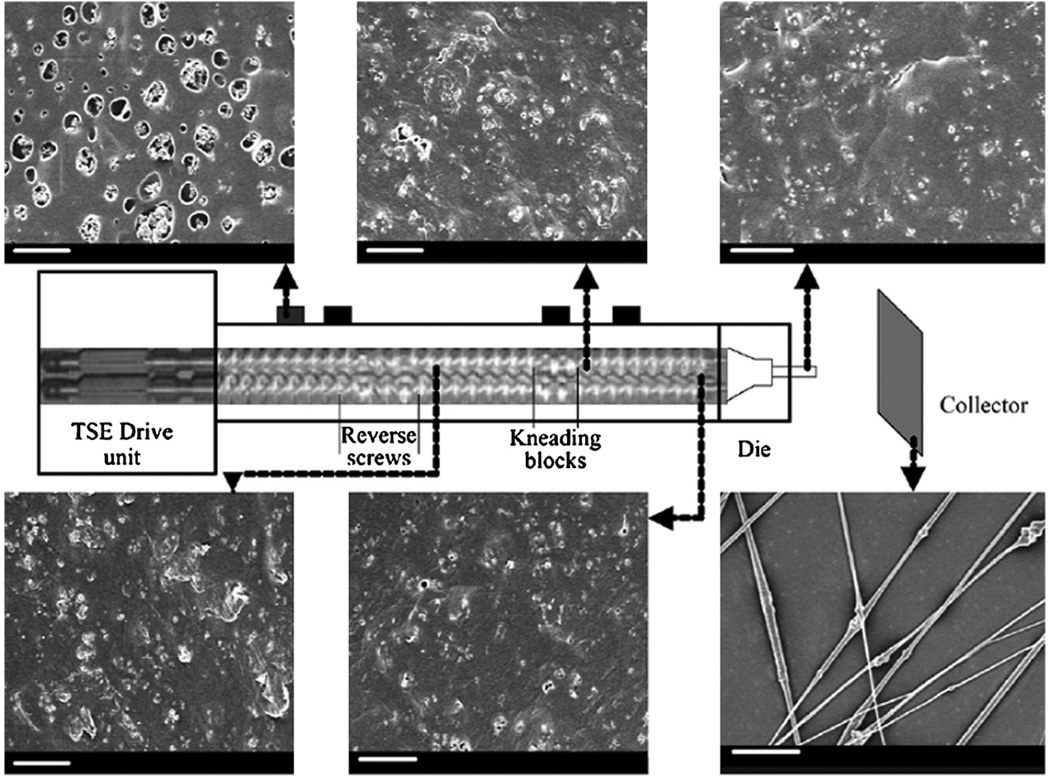
Many biomolecules can be directly incorporated into a polymer solution during fabrication. This method of biomolecule incorporation can be employed for nanofiber fabrication techniques that utilize a polymer solution such as electrospinning, phase separation, templating, drawing, and soon, most notably electrospinning. Biomolecules may be directly dissolved in a polymer solution if a common solvent is available [92], dissolved in a miscible solvent and incorporated as a suspension [92–94], or dissolved in an immiscible solvent and incorporated as an emulsion with agitation [95–97]. When two miscible solvents for a particular polymer and biomolecule solution are not available, a multi-component system may be used. For example a three component solution containing dichloromethane (DCM), methanol, and water was used to incorporate water soluble heparin into DCM soluble PCL nanofibers [98]. Heparin was dissolved in water, which is miscible in methanol, which is miscible in DCM. Electrospinning of emulsions formed by stirring, vortexing, or sonication resulted in beadlike pockets containing an aqueous phase or even a core-shell structure [99–101]. Inclusion of miscible solvents and emulsions may however disturb the electrospinning process and make fiber formation more difficult due to inclusion of multiple phases in the jet [95]. Distribution of a biomolecule in a single phase solution was found to be even thoughout the nanofiber, while biomolecule distribution was aggregated for a two phase system [92,97]. However, even biomolecule distribution has also been observed in nanofibers electrospun from miscible solvent suspensions [98]. Reported efficacy of direct biomolecule incorporation in electrospun nanofibers varies greatly. This should be expected due to the high level of variations in material selection and electrospinning parameters reported in the literature. Heparin electrospun as a miscible suspension had an efficiency of around 100% [98], laminin directly blended in organic solvent with PLLA had an efficiency of around 75% [76], and nerve growth factor (NGF) electrospun into nanofibers from an emulsion had a reported efficiency of just 2.5% [95]. Incorporation of biomolecules can have an effect on the morphology of electrospun nanofibers because biomolecule incorporation can change the solution properties such as viscosity and charge density [92,93,98]. For example, fiber diameter decreased with increasing loading levels of retinoic acid, while fiber diameter increased with increased loading levels of bovine serum albumin (BSA) [92]. Incorporation of biomolecules can also have an effect on the mechanical properties of nanofibers because of the inclusion of aggregates. Increased loading levels of BSA were observed to decrease the ductility of PCL and ethyl ethylene phosphate nanofibers, while the presence of retinoic acid increased the strength of the nanofibers independent of the fiber diameter [92]. A variety of biomolecules have been directly incorporated into electrospun nanofibers. NGF was mixed with BSA as a carrier protein and electrospun from an emulsion with sustained release up to 3 months after a 20% burst effect and retention of at least some degree of bioactivity [95]. Antibiotics have also been incorporated into electrospun polymer nanofibers with sustained release and bioactivity retention [93]. DNA has also been successfully incorporated into electrospun nanofibers with a sustained release of intact DNA capable to cellular transfection and protein encoding [94]. Vitamins were also loaded into electropsun polymer nanofibers with expended release [102]. Nanoparticles have also been loaded directly into protein solutions and electrospun as dispersions [103]. A special extruder (Fig. 11) used to feed polymer solution/nanoparticle dispersions into an electrospinning capillary helped to break up particle aggregates and maintain a uniform solution composition [103].
Fig. 11.
Deagglomeration of nanoparticles during stages of continuous processing in twin screw extrusion electrospinning apparatus (scale bar is 20 µm [103].
3.3.2. Co-axial incorporation (electrospinning)
An alternative approach to directly mixing biomolecules in an electrospinning solution is to use a special co-axial nozzle, which allows the formation of core-shell nanofibers where one material makes up a core filament that is surrounded by another material in a concentric geometry [104]. Multiple solutions are feed though a special nozzle in a concentric geometry to form a two phase liquid electrospun jet where a solution at the center is surrounded by an outer solution, forming solid core shell nanofibers upon solvent evaporation. Hollow tube nanofibers have also been electrospun using the co-axial technique [105]. Core shell nanofibers offer many attractive properties in tissue engineering applications, especially in biomolecule incorporation. Biomolecules incorporated inside of core shell nanofiber are not affected by initial burst and they do not require extended contact with any toxic solvents used in the formation of the shell materials [106]. The flow rate of each component of the nanofiber can be independently controlled to vary the overall diameter of the fibers and the core to shell volume ratio. Coaxial electrospinning can also be used to electrospin materials that cannot beelectrospun using conventional methods. For example, nanofibers were formed from poly(glycerol sebacate), a material that cannot be electrospun directly, by encapsulating its precursors in a constraining shell material that was removed after curing [107].
3.3.3. Incorporation of biofunctional peptide sequences (self assembly)
Biofunctional peptide sequences can be directly incorporated into peptide amphiphile nanofiber scaffolds. Biofunctional sequences are inserted into peptide amphiphile molecules and these biofunctional sequences are presented to cells on the nanofiber surface after self assembly. In one example, a peptide sequence called RADA16 was mixed with modified RADA16 sequences directly coupled with various bioactive motifs to promote cell adhesion and osteogenic functions [108]. Compared to pure RADA16 scaffolds, scaffolds containing bioactive motifs increased cell proliferation and markers of osteogenic differentiation. Incorporation of a designed chemokine similar to stromal cell-derived factor-1 improved cardiac function after myocardial infarction [109] and cell adhesion, differentiation and bone marrow homing motifs added to RADA16 sequences improved neural cell survival in nanofiber scaffolds [110]. A peptide amphiphile nanofiber scaffold with the incorporation of the pentapeptide epitope isolucinelysine-valine-alanine-valine (IKVAV), known to promote neurite sprouting and to direct neurite growth was designed as a tissue engineering scaffold specifically for neuronal cells [15].The effect of IKVAV incorporation in the peptide nanofibers was demonstrated by the superior bioactive properties of these scaffolds versus controls without the IKVAV sequence. The arrangement of biofunctional peptide sequences can have an effect on their bioactivity in nanofibrous scaffolds. RGD peptide sequences incorporated into self assembling peptide scaffolds had better cell attachment properties when the motif was displayed at a branched site than with linear incorporations of RDG because of increased motif availability at the nanofiber surface [111].
4. Cell behavior on polymeric nanofibers
Cells in their natural in vivo surroundings are exposed to a complex chemical and structural environment. The natural extracellular matrix is made up of structural components that are of nanoscale dimensions. Major fibrous extracellular molecules such as collagen fibers, elastin fibers, keratin fibers, etc. have nano-scale diameters. It is highly important to mimic the natural environment when culturing cells in vitro because cell behavior is determined by both genetic make up and the surrounding environmental cues. Cellular behaviors such as proliferation, differentiation, morphology, and migration are commonly controlled in culture by modulation of the chemical environment. Cells also respond to different morphological cues that can be determined by the growth substrate in vitro and in vivo. Four components may be involved in the growth, differentiation, functions and morphology of cells on biomaterial surfaces: (1) adsorption of serum components (2) extracellular matrix components secreted by the cell (3) cell adhesion molecules and (4) cytoskeleton mechanics [112]. It has been shown that the structural substrate property of surface roughness can cause selective protein absorption, and that higher surface roughness increases total protein absorption [113]. In this case, increased protein adsorption could be attributed to an increase in surface area for rough surfaces and thus could be important in relation to nanotopographical materials because these exhibit extremely high surface area. In relation to nanostructure and cell interactions, the cytoskeletal mechanics is of importance because cells cultured on substrates with nanoscale features can take on different shapes in response to the specific features that are encountered.
Nanotopography can affect cellular behavior through known mechanisms such as the regulation of cell shape and surface protein absorption properties, but it is possible that there are unknown effects associated with nanotopographies as well. Cells can react to objects as small as 5 nm [114] and it is possible that nanostructures, especially those with similar dimensions to the natural ECM, can influence cell behavior through unknown mechanisms. It has been shown that cell behavior can be highly dependent on the substrate that they are cultured on, and the understanding of cell substrate interactions with nanostructures could provide valuable information that would allow for the design of better tissue engineering scaffolds. Nanofibrous scaffolds present nanostructured features, mimic the fibrous components of natural tissue, allow three-dimensional configurations, and provide a unique mode of presentation of chemical and biological cues to cells. In order to gain a better understanding of the effect that nanofibrous architecture has on cell behavior, experimental results have been summarized according cell morphology, alignment, attachment, viability, ECM production, differentiation, and migration.
4.1. Cell morphology
Cell morphology can be influenced by the substrate that a cell is attached to. Cells many times adopt a different morphology on nanofibrous substrates compared to flat substrates, and cell morphology on nanofibrous substrates can be influenced by the fiber diameter. Cell morphology is commonly described by the projected area/degree of spreading or the aspect ratio (ratio of the long and short axis). Cell morphology is an important characteristic in tissue engineering scaffold design because of its significance in controlling cell arrangement and the translational effects that cell morphology have on other cell functions.
Cells may adopt a more rounded shape with a smaller projected area when cultured on nanofibers as opposed to flat surfaces. Osteoprogenitor cells cultured on electrospun polymer fiber meshes with diameters of 140 and 2100nm displayed significantly smaller projected areas than cells cultured on smooth surfaces [115], and fetal bovine chondrocytes cultured on nanofibrous scaffolds maintained a round or spindle-like shape in contrast to a flat well spread morphology observed on tissue culture plate [116]. Rounded morphologies may correspond with lack of organized actin fibers compared to cells with more well spread morphologies [116]. Chondrocytes grown on polymer nanofibers (500–900 nm) also adopted a rounded shape with a disorganized actin cytoskeleton in contrast to cells grown on 15 µm microfibers, which adopted a well spread shape [117]. Because fifteen microns approaches the size of a cell, they may react to these fibers in a manner similar to a flat substrate in the previous example.
Cell also may adopt a more elongated shape on nanofibers of certain diameters. Osteoprogenitor cells cultured on 140 nm fibers adopted a similar aspect ratio to those on smooth surfaces, but cells on 2100 nm fibers had a statistically higher aspect ratio [115]. In agreement with this result, dermal fibroblasts had statistically significant increase in aspect ratio for fiber diameters of 8640 and 970 nm versus flat controls, but not for fiber diameters of 160 and 650 nm [118]. Human umbilical vein endothelial cells also showed enhanced elongated cell morphology on larger fibers (1–5 µm) than smaller nanofiber scaffolds (200–1000 nm and 10–200 nm) [119]. This elongating effect could be due to cells growing along single fibers, as it would be logical that cells would be more likely to grow along single fibers in nanofiber meshes with larger diameter fibers.
4.2. Cell alignment
One of the most important cell morphologies associated with tissue engineering is elongated unidirectional cell alignment. Many tissues such as nerve, skeletal and cardiac muscle, tendon, ligament, and blood vessels contain cells oriented in a highly aligned arrangement, thus it is desirable that scaffolds designed for these tissue types are able to induce aligned cell arrangements. It is well documented that cells adopt a linear orientation on aligned substrates such as grooves and fibers. Aligned nanofibers arrays can be easily fabricated using the electrospinning method [120,121] and many studies have shown that cells align with the direction of the fibers in these scaffolds. Primary cardiac ventricular cells and smooth muscle cells aligned in the direction of 1.8 µm and 550 nm aligned nanofiber meshes respectively [122,123]. Neuron elongation and outgrowth was parallel to the direction of aligned 113 and 431 nm nanofibers and neuronal stem cells seeded on random and aligned 300 nm and 1.5 µm nanofibers turned through large angles in order to grow parallel to the fiber alignment independent of fiber diameter [57,124]. Human skeletal muscle cells grown on 300 nm nanofiber scaffolds aligned on aligned fibers scaffolds in contrast to a random orientation adopted on randomly oriented scaffolds [125]. The effect of nanofiber alignment on neurite alignment of dorsal root ganglion (DRG) was measured quantitatively for nanofibrous scaffolds with different degrees of alignment using Fourier image analysis. Neurite alignment corresponded to the degree of fiber alignment as demonstrated by full-width-half max of the intensities of the fast Fourier transform images of 38, 69, and 84% (p < 0.0001) for highly aligned, intermediate, and randomly oriented samples respectively [126].
In addition to the influence on fiber arrangement, cell alignment can have positive effects on cell growth within tissue engineering scaffolds. Myotube formed on aligned nanofiber scaffolds were more than twice the length of myotubes grown on randomly oriented fibers (p < 0.05) and neurites extending from DRG explants on highly aligned scaffolds were 16 and 20% longer than those grown on intermediate and randomly aligned scaffolds respectively [125,126].
4.3. Attachment
When designing tissue engineering scaffolds it is critically important that cells ready attach to the constructs. Many studies have confirmed that nanofibrous architectures have cell attachment properties superior to flat surfaces. Two factors that may lead to improved cell attachment on nanofibers could be the physical entrapment of cells penetrating inside of nanofiber meshes or improved focal adhesions. Many more filopia have been observed projecting from the edges of cells cultured on small diameter fibers compared to flat surfaces [118] and uniform focal adhesion distribution has been observed in cells cultured on nanofibers as compared to a distribution around the cell perimeter for cells cultured on flat surfaces [118]. An extreme example of physical entrapment was demonstrated when large hepatocyte spheroids completely enveloped nanofibers and demonstrated improved adhesion to flat controls upon agitation [127].
Relatively consistent results have been reported on the improved attachment of various cell types on nanofibers as opposed to flat surfaces. Fibroblasts, adipose stem cells, and smooth muscle cells that were allowed 15 min to 8 h to attach, adhered to nanofibers (100–500 nm) at rates 50–150% higher than cells on flat substrate controls [70,123,128]. Bone marrow-derived hematopoietic stem cells attached to nanofibrous scaffolds fabricated from various materials at levels at least four times that of tissue culture plate at time points from 10 to 60 min [91]. After long term cell culture of 10 days, 40% of total hematopoietic stem cells grown on polymer nanofiber meshes were adherent after washing, while only 25% of total cells stayed on flat substrates [129].
Fiber diameter can have an affect on the cell attachment properties of nanofibrous scaffolds, however optimum fiber size seems to vary for different types of cells and conditions. The attachment of bone marrow-derived hematopoietic stem cells was found to be around 40% greater on 1 µm diameter fibers as compared to 500 nm fibers [130], but in contrast, attachment of 3T3 fibroblasts on nanofiber scaffolds was greatest on 425 nm diameter scaffolds and decreased with increasing fiber diameter for 641 and 900 nm scaffolds [131]. On a smaller size scale, astrocytes seeded on 60,100,120, and 200 nm carbon fiber disks attached best to the largest fiber diameter disks [132].
4.4. Viability: survival/proliferation
Cell survival and proliferation must be well controlled in tissue engineering scaffolds. In most cases, maximum survival and proliferation are desired, but in some applications reductions in cell survival and proliferation are required. For example, astrocyte proliferation at an implant/tissue interface in the central nervous system causes the formation of an undesirable glial scar. It is well documented that nanofibrous architecture can have a significant effect on the survival and proliferation of cells, however the specific effects may vary for different cell types and conditions. Nanofibers have been shown to both increase and decrease cell proliferation for various cell types and conditions when compared to flat control. Fiber diameter effects on cell proliferation also vary for different cell types and conditions.
Self assembling peptide nanofiber scaffolds were found to improve the viability of transplanted islet cells after 7 days of standard culture and in hypoxia injury model [133]. Cell viability as confirmed by MTT assay was increased from 58.6% to 81.0% in standard culture and from 29.2% to 60.6% in injury model. Viable fibroblast cell numbers after 8–13 days of culture were increased up to 3 times on 300–500 nm fibers vs. flat control [128]. It was further confirmed by transwell culture that nanofibrous architecture caused cells to release soluble factors that could stimulate increased cell numbers. In contrast, hematopoietic stem cells cultured on three different types of 500 nm fibers had 10–50% fewer cells than those cultured on flat films after 10 days [129], and Schwann cells cultured on five different polymer materials for 3–5 days had decreased MTT viabilities on four types of polymer fibers (1–4 µm) compared to films and similar viability on one type of nanofiber (130 nm) when compared to film [134].
Specific fiber diameter also has a variable effect on the proliferation of cultured cells. 3T3 fibroblasts cultured on polymer nanofibers with 6 diameters from 117 to 1051 nm had similar cell counts on day 1, but on days 3–7 cell number was greatest on 428 nm scaffolds and decreased towards the ends of the range up to 40% for the smallest and largest fiber diameters [131]. In contrast, human umbilical vein endothelial cells demonstrated higher viability and cell densities on 1000 to 1500nm fibers as compared to 10–200 nm and 200–1000 nm fibers [119]. Carbon nanofibers with diameters of 60 and 100 nm resulted in osteoblast numbers nearly 3 times that of 125 and 200 nm fibers after 7 days of culture, but astrocyte cell counts were up to 66% higher on 125 and 200 nm fibers as compared to 60 and 100 nm fibers after 5 days of culture [132,135].
4.5. ECM production
The goal of many tissue engineering approaches is to provide a framework for the assembly of natural ECM secreted by resident cells in vitro or in vivo. For example, biodegradable tissue engineering scaffolds are designed to provide initial structural support that is intended to be replaced by naturally produced ECM as the original scaffold materials degrade. Thus it is important that a well designed tissue engineering scaffold promotes ECM production from resident cells. Several studies have shown that nanofibrous architecture can elicit increased ECM production from resident cells and that deposited ECM may be more highly organized. Chondrocytes seeded on 700 nm nanofiber scaffolds produced more sulfated proteoglycan rich, cartilaginous matrix than those seeded on tissue culture plate [116] and chondrocytes grown on nanofiber (500–900 nm) meshes showed a nearly 2 fold increase in glycosaminoglycan (GAG) production at 28 days as compared to microfiber (15 µm) culture [117]. Cartilage ECM markers, such as collagen II and IV, aggrecan, and cartilage proteoglycan link protein were also greater in chondrocyte cultures grown on nanofibers than microfibers as confirmed by immunostaining [117].
Fiber diameter and orientation can affect the ECM production of cells residing in nanofibrous scaffolds. Calcium production in osteoblasts from 7 to 21 days was significantly higher in cells cultured on 60 and 100nm carbon fibers compared to cells cultured on 125 and 200 nm fibers [135]. Lee et al. found that human ligament fibroblasts cultured on 650 nm nanofibers synthesized significantly more collagen on aligned fibers than on randomly oriented fibers as confirmed by collagen assay normalized by DNA content at 3 and 7 days [136].
In addition to stimulating increased ECM production, nanofibrous structure can also result in organized deposition of ECM products. Immunohistochemistry at 7 and 14 days revealed that rotator cuff fibroblasts deposited collagen matrix in an aligned orientation on aligned nanofiber scaffolds but not on randomly oriented scaffolds [137]. Baker et al. linked an increase in scaffold strength after in vitro culture of mesenchymal stem cells (MSCs) and meniscal fibrochondrocytes on aligned fiber scaffolds to organized collagen arrangement independently of collagen production. Collagen produced by both cell types oriented parallel to aligned nanofibers, and despite similar overall collagen content, aligned fiber scaffolds showed a >7 MPa increase in modulus over 10 weeks in culture as compared to an ~1 MPa increase in modulus for randomly oriented fiber scaffolds [138].
4.6. Differentiation
Many tissue engineering strategies require the selective differentiation of stem or progenitor cells into a specific lineage. It is well established that various soluble cues can be applied to certain cell types to induce them to differentiate to a specific fate. There is also evidence that the physical environment can affect cell differentiation. Nanofibrous architecture can have strong effects on cell fate versus flat culture surfaces and fiber properties, such as diameter and arrangement can affect cell differentiation. The effect of nanofibers on cell differentiation can be quite strong, but varies for different cell types and conditions. Nanofibrous architecture has also been shown to promote, prevent, and have no effect on differentiation when compared to flat surfaces.
Mouse embryonic fibroblasts cultured in self assembling peptide scaffolds were observed to undergo strong osteogenic differentiation after osteogenic induction while cells cultured on flat culture plate did not differentiate [139]. Furthermore, mouse embryonic stem cells cultured in nanofibers without osteogenic induction expressed enhanced early stage markers of osteoblast differentiation compared to tissue plate culture [139]. Primary cardiac ventricular cells cultured on 2 µm diameter fiber scaffolds also shifted to a more mature phenotype than those grown on tissue culture plate [122]. In another study, differentiated chondrocyte phenotype was maintained on 700 nm nanofiber scaffolds for 21 days of culture while chondrocyte cells seeded on flat tissue culture plate de-differentiated [116]. In contrast MSCs cultured on 50–1000nm nanofibers showed no differences in osteogenic phenotype markers compared to tissue culture plate after 12 days of osteogenic differentiation induction [140].
Nanofibrous scaffolds have also been observed to prevent differentiation and allow for the proliferation and maintenance of a pluripotent niche, which is important in vitro stem cell expansion. Hematopoietic stem cells cultured in 529 nm polymer nanofiber meshes for 10 days mediated a greater percentage of primitive progenitor cells when compared to the flat surface [129] and proliferation and self-renewal of pluripotent mouse embryonic stem cells were greatly enhanced in nanofibrillar meshes when compared to smooth culture surface [141]. While proliferation with self-renewal was allowed to continue in nanofiber topography, the cells were observed to maintain their ability to differentiate when exposed to differentiation factors [141]. In another study, a small fraction of mouse embryonic stem cells appeared to develop into small embryo body like colonies [139]. It was found that the frequency of these colonies was remarkably higher in self assembling peptide nanofiber cultures than in flat tissue plate culture.
In addition to possessing the capability to support both stem cell differentiation and self renewal, nanofibrous topography can selectively influence differentiation based on fiber diameter and alignment. Differentiation of neural stem cells cultured on aligned and random nanofiber meshes with fiber diameters of 300 and 1500 nm was observed to be highly dependent on fiber diameter [124]. The quantitative differentiation rates evaluated on the basis of shape change were approximately 80% and 40% for 300 and 1500 nm fibers respectively. Expression of ligament markers by bone marrow stromal cells was effected by both fiber diameter and alignment [142]. While expression of Col-1α1, decorin, and tenomodulin in cells cultured on 270 nm fibers was greaterthan on films, expression of all three markers was significantly repressed in cells cultured on 820 and 2300 nm fibers. In contrast, expression of the ligament marker Scleraxis increased with increasing fiber diameter and fiber alignment. Primary cardiac ventricular cells cultured on aligned fiber scaffolds also expressed more markers of a mature phenotype than those grown on randomly oriented fibers [122].
4.7. Cell migration
Cell migration is a critical process in determining the success of tissue regeneration process. In many designs, tissue scaffolds are populated by cells due to cell migration, either from cells seeded on the scaffold surface in vitro or endogenous cell migration in vivo. Tissue engineering scaffolds designed with controlled conduction of cell migration are desirable, and nanofibrous architecture can have a significant effect on cell migration properties in vitro and in vivo.
Traditional agarose droplet spreading experiment confirmed that nanofibrous surface topography can have an effect on the specific migration patterns of adult human dermal fibroblasts [118]. Migration and aggregation of hepotocyte cells appeared to be restricted by nanofibrous architecture and this resulted in the formation of smaller more uniform aggregates as compared to flat films [127]. MSCs also had reduced migration distances on three different ranges of collagen nanofibers (50–200, 200–500, and 500–1000 nm) that were quantified and found to be 37–56% of those observed on TCPS control [140].
Endogenous cell migration in vivo was increased with the addition of nanofibrous architecture. A 3D peptide nanofiber mesh assembled in the in vivo myocardium was able to recruit endothelial progenitor cells, smooth muscle cells, and myocyte progentitor cells and promote vascularization and tissue regeneration. Implantation of matrigel without fibers as control resulted in few numbers of endothelial cells and no myocyte progenitors [7]. In this case nanofibrous structure may facilitate cell migration by providing structure in 3-dimensional space for them to move along and attach to.
In addition to the structural roles of nanofibers, they may also elicit functional responses in cells to migratory and remodeling behaviors. Nanofibrous structure induced fibroblasts to increase production of collagenase that degrades the adhesive collagen between the scaffold surface and cell membrane, and this lead to the hypothesis that the nanofibrous architecture may have been encouraging migratory or remodeling behavior in comparison to a smooth surface [128].
4.8. Potential mechanisms of cell–nanostructure interactions
It is clear that nanofibrous architecture has major effects on modulating a wide variety of cell behaviors. It is not surprising that nanofibrous architecture has such an effect on cell behavior when considering the differences in local environmental conditions experienced by a cell in a nanofibrous substrate as compared to a flat culture surface. It is theorized that nutrient infiltration, surface molecule presentation, and cell shape many be mechanisms by which nanofibrous architecture modulate cell behavior. It is intuitive that cells in a 3D nanofibrous structure would be able to exchange nutrients and utilize receptors throughout their surface, while cells in flat culture conditions are limited to nutrient exchange on only one side. By limiting the useable surface area of cells, flat culture conditions may also limit their function.
The surface molecule presentation of nanofibers may be another mechanism by which nanofibers modulate cell behavior. Interest in nanofibrous architecture in biology is many times credited to mimicking the fibers of the natural ECM, but the more general property that has fuelled the study of nanofibers is their extremely high surface area to volume ratio. It has been shown that this property results in higher protein absorption and more efficient presentation of biomolecules to cells in nanofibrous architectures and that this translates to modulation of cell behavior. Leong et al. [143] found that solid poly(D,L-lactide) fibers (850 nm) absorbed 16 times as much protein as films, and they fabricated porous nanofibers that absorbed 80% more protein than the solid fibers. Cell attachment on the three scaffolds increased in agreement with the observed increases in protein absorption. Baker et al. found that polystyrene nanofiber scaffolds (200 nm) also absorbed 16 fold more serum than the equivalent flask area and hypothesized that stromal cell differentiation into smooth muscle cells was inhibited by non-specific serum protein absorption [144]. Observed increases in myoblastic mouse cell attachment, proliferation and differentiation on fibrous pressed carbon nanotubes as compared to flat pressed graphite were amplified by pre-culture incubation with 50% fetal bovine serum to increase the effect of surface protein absorption on cell behavior [145]. It has also been shown that biomolecules incorporated into nanofibers are much more efficient at modulating cell behavior than flat coated surfaces or soluble factors due to the high surface area to volume ratio of nanofibers. Three dimensional and two dimensional self-assembling peptide nanofiber meshes incorporating the IKVAV motif of laminin induced neural stem cell (NSC) differentiation into neurons at a rate of 35% after 1 day, as compared to 15% after 7 days on flat laminin coated substrates [15]. Neuronal differentiation was not increased in non-bioactive fiber meshes with the addition of soluble IKVAV and it was hypothesized that improved differentiation rates were caused by an increased efficiency in the presentation of the motif to the cells, which was estimated to be amplified by10[3] times compared to flat laminin coated surfaces.
Another pathway for modulation of cell behavior by nanofibrous architecture may be through cell shape. One proposed mechanism for the transduction of cell shape information into gene expression is by the transmission of mechanic forces directly from the actin cytoskeleton to the nucleus [146]. It has been shown that regulation of cell shape can influence the differentiation of multipotent human mesenchymal stem cells (hMSCs) into adiogenic or osteoblastic fate [147]. Human mesenchymal stem cells allowed to flatten and spread expressed osteoblastic markers, such as alkaline phosphatase, while constrained cells that remained unspread and rounded expressed adiogenic lipid production. While cytoskeletal organization is related to cell shape, the cytoskeleton can influence gene expression independently of cell shape. The inhibition of myosin-generated cytoskeletal tension in hMSCs caused decreased alkaline phosphatase activity and increased lipid production without changing cell shape [147]. In a similar study, Spiegelman and Ginty [148] found that differentiation of an adipogenic cell line could be inhibited when it was allowed to attach and spread on fibronectin coated surfaces and that the inhibitory effect on cell differentiation could be reversed by keeping the cells rounded and by chemically disrupting the actin cytoskeleton.
Nuclear shape has also been measured directly and cor-related to gene expression and protein synthesis [149,150]. Intermediate values of nuclear distention promoted maximum collagen I synthesis in primary osteogenic cells [149]. Two to nine fold increases in gene expression above baseline accompanied significant rounding of nuclei in mesenchymal stem cells [150].
Nanofibrous architecture is able to influence a variety of cell behaviors through a variety of mechanisms, therefore smart design of nanofibrous components in tissue engineering scaffolds can allow for increased control over resident cell behavior and thus improved overall function. It is however important to consider that the effect of nanofibers on cell behavior varies widely with specific cell types, fiber type and environmental conditions. For example, osteoprogenitor cells cultured on electrospun polymer fiber meshes exhibited a lower cell density than those on smooth surfaces in the absence of osteogenic factors, but when osteogenic factors were added the cell density of fiber surfaces was equal to or greater than that on smooth surfaces [115].
5. Tissue engineering applications of polymer nanofiber scaffolds
Many different applications for nanofibers in tissue engineering have been explored. Because polymer nanofibers can provide three-dimensional architecture, modulate cell behavior, and have the potential to deliver biomolecules, they are a good candidate for a wide variety of tissue engineering applications. Some of the other properties of polymer nanofiber scaffolds lead them to be well suited for more specific applications. For example, nanofibers may offer good mechanical properties for load bearing applications, directional alignment to tissues with aligned structures, and nanofiber meshes with fine pores act as membranes that allow transport of nutrients and waste, but limit cellular infiltration. Table 2 organizes several references to experiments where polymer nanofibers have been used as scaffolds for specific tissue regeneration applications. Each of these experiments is related to a specific tissue or application based on (1) The incorporation of tissue specific biomolecues (2) eliciting a desirable response from tissue specific cells in vitro (3) sharing a similar microstucture or mechanical properties to a natural tissue and (4) in vivo applications.
Table 2.
Nanofibrous scaffolds for tissue engineering applications.
| Tissue Type | Functionalization with tissue specific biomolecules |
Promotion of desired behaviors from tissue specific cells in vitro |
Favorable Structural properties for tissue specific application |
In vivo models |
|---|---|---|---|---|
| Bone | Secondary surface mineralization [14,77,78,89,152,153] Bioactive peptide incorporation [108] Hydroxyapatite particle incorporation [154–158] BMP-2 incorporation [157] |
Osteoblastic and pre-osteoblastic cells [159–161] Mesenchymal stem cells [28,162,163] |
Selective pore size [164] | Subcutaneous implantation [165–167] Bone Defect-Skull [167–169] Bone defect- Femur [170] |
| Cartilage | Chondrocytes [116,117,171,172] Meniscal fibrochondrocytes [138] Mesenchymal stem cells [138,173] |
Physiological shape [174] Suitable mechanical properties [175] |
||
| Vascular | Elastin/collagen blend [176] |
Endothelial cells [90,177] Smooth muscle & endothelial cells [178,179] |
Shape & mechanical properties [180,181] |
Epigastric vein graft [182] Abdominal aortic graft [183] |
| Ligament/Tendon | Ligament/tendon derived fibroblasts[136,137,184] |
|||
| Muscle | Skeletal muscle derived cells/myobasts [125,185] |
|||
| Neural | Bioactive peptide incorporation [15,186] Laminin surface modification [82] |
Brain derived neural stem cells [57,80,187] Dorsal root ganglion (DRG) [126] DRG & perineural membrane derived primary neurons [188] |
Shape [189] | Severed optic tract [8] Spinal cord transaction [190] Tubular sciatic nerve bridging device [191,192] |
| Wound Healing | Antibiotic incorporation [193] |
Hair follicular cells [51] Dermal fibroblasts [66,194,195] Dermal fibroblasts & keratinocytes [196,197] Human skin equivalent tissue model [198] |
Surgical wound model [47,199,200] Burn wound model [86,201] Subcutaneous implantation [202] |
|
| Intervertebral Disc | Mechanical properties [203] Shape & Structure [204] |
|||
| Islet/Hepotocyte Transplantation |
Islets [133] Hepatocytes [205,206] Stem cell derived hepatic cells[207] |
Islet transplantation [208] |
The mechanical integrity of polymer nanofibers has lead to a vast amount of research for bone tissue engineering scaffolds. Many of these studies concentrated on the functionalization of nanofibers with bone specific biomolecules such as hydroxyapatite. It has been demonstrated that nanofibrous scaffolds can support osteogenic differentiation in vitro and promote in vivo bone growth into nanofibrous scaffolds in subcutaneous and bone defect models. The mechanical properties of nanofibrous structures also make them an attractive scaffold for use in cartilage tissue engineering where chondrocytic function and differentiation has been demonstrated in nanofibrous scaffolds. Polymer nanofibers are a suitable material choice for vascular grafts because of their tensile strength and directional guidance properties. Polymer nanofiber vascular grafts have been tested in vitro under static and pulsatile conditions and in vivo. In skeletal muscle, tendon and ligament tissue engineering, the tensile strength and directional alignment of polymer nanofibers are also attractive properties. Nerve tissue engineering benefits from the directional guidance offered by aligned nanofibers as well. Polymer nanofiber scaffolds can improve the viability, growth, alignment, and differentiation of neural cells in vitro and they have been applied to injuries in the peripheral and central nervous system to promote regeneration in vivo. Many in vivo studies have been conducted using nanofiber scaffolds in wound healing applications, including clinical trials [151]. Nanofiber scaffolds can provide structure for the ingrowth of cells around a wound site and provide a barrier to outside infection.
6. Cell incorporation into nanofibrous electrospun scaffolds
The success of a tissue engineering scaffold is determined by its ability to incorporate desired cell types and to promote the desired functionality of the incorporated cells. Despite great promise and widespread investigation there have been few clinically relevant successes for nanofibers as tissue engineering scaffolds. One of the main reasons for this limited success is the difficulty of incorporating cells into electrospun nanofibrous scaffolds. Electrospinning is the most versatile and widely studied method of nanofibrous scaffold fabrication, but it still remains a major challenge to fabricate electrospun scaffolds with antiquate cell incorporation or permeability. Cells can be easily incorporated into self assembling peptide nanofiber scaffolds, which is one of the major reasons for the success of this technique. There are however some major limitations to the self assembling peptide method such as, complex procedures, and limited control over fiber material, size, and arrangement. In contrast, the electrospinning technique is a very simple and versatile process that allows the fabrication of nanofibers from many types of materials and offers control over fiber size and arrangement. A feasible and practical method of quickly advancing the field of nanofibrous tissue engineering scaffolds is through the development of better methods to incorporate cells into electrospun scaffolds.
Several creative approaches have been explored to address this major challenge in polymer nanofiber scaffold fabrication. Cell incorporation into nanofibrous scaffolds may be achieved in two ways: (1) Cell incorporation inside of the scaffold during fabrication (2) Migration of cells into the scaffold in vitro or by host tissue cells after in vivo implantation. Some of the strategies for improving cell incorporation into electrospun nanofiber scaffolds include cell incorporation during fabrication, physically assisted seeding methods and the design of cell permeable scaffolds using composite techniques, layer by layer assembly, and incorporation of sacrificial materials.
6.1. Cell incorporation during fabrication
Several techniques for direct incorporation of cells into electrospun nanofiber scaffolds have been developed. Cell nanofiber constructs were fabricated by directly pipetting cell suspension into an electrospun nanofiber scaffold during collection. Nanofibers were electrospun onto a charged ring on the surface of culture media and intermittent pipetting of cells during the fabrication process resulted in nanofibrous constructs with uniform cell incorporation [101]. No significant toxicity was caused by residual solvent left on the electrspun nanofibers. A similar approach incorporated cells into an electrospun nanofiber scaffold by electrospraying a cell containing solution onto a grounded target while nanofibers were simultaneously electrospun onto the same target [209]. Cells integrated into the nanofiber constructs and no significant decrease in cell viability from the fabrication process was apparent. Fibroblast and adipose-derived adult stem cells were directly added into an aqueous PVA solution and electrospun to form nanofibers that encapsulated the cells [210]. Fibroblasts incorporated into PVA nanofibers retained viability, proliferation, and function.
6.2. Cell population by migration
When cells are not directly incorporated into a nanofiber scaffold, the scaffold may be populated by cells that migrate into the interior of the scaffold from the outer surface. The extent of cell migration into nanofiber scaffolds can be affected by both the architecture of the scaffolds and the biological or chemical cues incorporated into the scaffold.
6.2.1. Perfusion
Cells infiltration into nanofibrous scaffolds can be enhanced by physical forces, such vacuum, flow perfusion, or centrifuge [172,211]. These types of techniques can be utilized to increase the speed at which cells infiltrate nanofibrous scaffolds during in vitro seeding. Flow perfusion speeds up cell infiltration by moving cells with hydrodynamic forces and increases nutrient transport improving cell viability deeper inside the scaffolds [211].
6.2.2. Scaffold permeability
In order for cell migration into a nanofibrous scaffold to occur there must be adequate void space for cells to occupy and move around. In the case of randomly aligned electrospun mats, pore size is generally related to fiber diameter, where scaffolds fabricated from larger fibers have larger pores and thus greater permeability to cell infiltration [212]. Pham et al. [211] fabricated electrospun microfiber scaffolds with fiber diameters ranging from 2 to 10 µm. The pore size of these scaffolds increased with increasing fiber diameter from a value of around 10µm for 2µm diameter fibers, to around 40 µm for 10 µm diameter fibers. In contrast, overall porosity ranged from around 85–89% with the greatest porosity in smaller diameter fiber scaffolds. Cell infiltration into 5 µm diameter nanofiber scaffolds was severely limited by the inclusion of a thin layer of electrospun 600 nm fiber on its surface under both static and flow perfusion culture conditions [211]. Migration of human venous myofibroblasts into nanofiber scaffolds with diameters ranging from 3.4 to 12.1 µm increased with fiber diameter during 3 days of culture [213]. Even the relatively large 3.4 µm diameter fiber significantly impeded cell migration when compared to the 12.1 µm fiber scaffolds. Optimal pore size may be dependent on the specific cell type or application, but the minimum pore size necessary for infiltration must not be significantly smaller than that of the migrating cells. Small diameter nanofibers can be desirable because of the effects that fiber diameter has on cell behaviors as discussed in section 4, but pore size requirements limit the use of small diameter nanofibers in tissue engineering scaffolds. The trade off between scaffold porosity and fiber diameter in electrospun scaffolds is one of the current limiting factors of this technology in tissue engineering.
6.2.3. Strategies to increase cell permeability
Several strategies have been developed to fabricate nanofiber scaffolds that combine both small diameter nanofibers and adequate permeability for cell infiltration. Several groups have fabricated composite fiber scaffolds that combine microfibers for large pore size and structural stability and nanofibers for improved cell interactions and increased area for attachment. A starch based scaffold made by fiber bonding with 160 µm microfibers was electrospun with 400 nm nanofibers that allowed endothelial cells to span the spaces between microfibers, migrate, and organize into capillary-like structures while maintaining structural integrity and a porosity of 70% [214,215]. A composite scaffold made from direct polymer melt deposition of microfibers that included electrospun PCL/collagen nanofibers showed improved cell adhesion and proliferation [216]. Multilayered micro/nanofiber strucutures have also been fabricated by modifying electrospinning conditions to include layers of 5 µm and 600 nm fibers [211]. Nanofibers with diameters of 400–500 nm were even directly electrospun onto 30 µm microfibers, which were subsequently formed into a highly porous scaffold containing nanofibers [217].
Another method to produce nanofiber scaffolds with improved cell infiltration properties is by addition of large pores within a nanofibrous structure. One hundred micron holes etched into 500 nm fiber scaffolds by UV lithography allowed the migration of smooth muscle cells into the scaffolds [218]. Porogen leaching has also been used as a method of fabricating structures with large interconnected pores within a nanofibrous network. Silk fibroin electrospun nanofiber scaffolds were soaked in a salt containing solution and lyophilized to create a porous structure. This process formed 600–900 µm pores in a scaffolds with fiber diameters of 400 nm in contrast to an initial pore size of 1–2 µm [219]. Salt particles have also been incorporated into nanofiber meshes by mechanical addition of solid salt particles directly to an electrospun jet during fabrication [164]. Salt particles were deposited in intervals during electrospinning to form a layered structure containing regions of salt particles. Cell infiltration of depths up to 4 mm was reported in these scaffolds after 3 weeks of in vitro culture.
Similar techniques have utilized other types of sacrificial materials to create an evenly distributed enhanced porous structure within electrospun nanofibrous scaffolds. Ice crystals were introduced to nanofiber scaffolds during fabrication by cooling a collecting mandrel to −30°C while electrospinning at room temperature [220]. Ice crystals simultaneously deposited on the mandrel during electrospinning and were distributed throughout the mesh. Freeze drying resulted in a nanofiber mesh with pores around 100 times larger than in control meshes and pore size changed with the level of humidity. Larger pore size resulted in nanofiber meshes that allowed cell penetration up to 50 µm, while control meshes allowed only surface cell growth. A similar technique involves simultaneously electrospinning water soluble sacrificial fibers into a nanofibrous scaffolds to create void space after sacrificial fiber dissolution. In randomly oriented PCL nanofiber (diameter ~1 µm) scaffolds, the depth of cell penetration after 7 days was increased from 48 to 114 µm with the addition of soluble gelatin nanofibers (diameter ~1 µm) [7]. Cell penetration into PCL scaffolds containing water soluble PEO fibers increased with increasing percentage of PEO fibers [144]. In contrast, it has also been reported that nanofiber meshes with sacrificial PEO and gelatin fibers offered very limited improvement versus control meshes in an infiltration experiment due to the collapse of the structure after sacrificial fiber leaching [221].
Cell permeable materials can be added as a filler material within a nanofibrous scaffold to add space for cell penetration. Ekaputra et al. fabricated a hybrid fibrous PCL/Col mesh with regions of hydrogel matrix by simultaneously electrospinning nanofibers and electrospraying hydrogel onto a collection mandrel [221]. The depth of cell infiltration into nanofiber meshes containing electrosprayed hydrogel was increased from around 60 to 225 µm when compared to control after 10 days of culture. Composite films containing a protein matrix and nanofibers were fabricated by adding fixed nanofiber arrays to an aqueous gelatin solution [12]. After gelatination the gelatin matrix provided structural support to fragile aligned nanofiber arrays with significant void space between individual fibers.
Porous 3D nanofibrous networks have been fabricated by suspending electropun nanofibers in liquids. A unique method of dispersing hydrophobic nanofibers in aqueous solutions was developed that utilized the attachment of lipase onto the surface of electrospun polystyrene nanofibers [222]. Alcohol pretreatment of the surface modified fibers caused the tightly aggregated nanofibers to be dispersed into a loosely entangled structure with greatly increased volume, and this dispersed geometry remained when the fibers were immersed DI water as long as the fibers remained hydrated throughout the washing step. Silk fibroin nanofibers collected directly in a methanol bath formed a three dimensional structure that was retained after hydration and freeze drying [223]. Cells were able to penetrate into the porous structure of these scaffolds, while control scaffolds collected on a rotating mandrel did not allow cell penetration. Three dimensional networks of PCL nanofiber yarns were fabricated as suspensions in water by electrospinning into a novel dynamic flow collecting system [224]. These nanofiber yarn networks also retained their porous microstructure after freeze drying. It is hypothesized that these loosely dispersed nanofibers would allow improved cell penetration.
6.2.4. Layer by layer assembly ofpre-seeded nanofiber sheets
Three-dimensional nanofibrous constructs with uniform cell distributions have been fabricated with layer-by-layer stacking of thin cell containing nanofibrous sheets. A cylinder with an 8 mm diameter and a height of 3 mm was constructed by stacking thirty 100µm thick cell seeded nanofiber disks, and nourished with a perfusion bioreactor [225]. Thin sheets of nanofibers with a thickness of 10µm were collected on a wire ring with a diameter of about 15 mm and seeded with cardiomyocytes [226]. Individual layers adhered immediately when these thin cell-nanofiber sheets were stacked, and constructs of up to 5 layers were formed without incidence of core ischemia. Multilayer cell-nanofiber structures have also been formed by stacking additional layers of cell containing fiber sheets every few days during cell culture [184].
7. Concluding remarks
Many damaged or degenerated tissues cannot be treated by conventional methods. Tissue engineering presents a promising alternative to regenerate damaged and degenerated tissues by guiding the formation of new healthy tissue. An ideal matrix for promoting the formation of such tissue requires a supporting structure for cell attachment within three dimensions as well as adequate void space for cell infiltration and vascularization. Polymer nanofibers are an ideal material for assembling structures that address both of these requirements. In addition polymer nanofibers posses many other advantages as tissue engineering scaffolds, such as versatility for biofunctionalization, and the promotion of specific desired cell behaviors that are elicited by the nanofibrous architecture. Despite a variety of techniques employed to fabricate polymer nanofibers and to assemble them into structures, no optimal method has emerged that combines the potential advantages of polymer nanofibers and a truly 3-dimensional structure. The future clinical success of polymer nanofiber tissue engineering scaffolds will depend on whether new techniques are developed that allow for the fabrication of polymer nanofiber scaffolds with control over structural arrangement, material composition, and biofunctionalization, while maintaining reasonable cost and yield.
Acknowledgments
This work was made possible by the NIH/NINDS USA (R01 NS050243) and American Heart Association (09PRE2060154).
References
- 1.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 2.Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol. 1957;35:681–690. [PubMed] [Google Scholar]
- 3.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 4.Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, Hoffman MP. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev Biol. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- 7.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis-Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci U S A. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venugopal J, Low S, Choon AT, Ramakrishna S. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;84:34–48. doi: 10.1002/jbm.b.30841. [DOI] [PubMed] [Google Scholar]
- 10.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 11.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomed. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beachley V, Wen X. Effect of electrospinning parameter on the nanofiber diameter and length. Mater Sci Eng C. 2009;29:663–668. doi: 10.1016/j.msec.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berndt P, Fields G, Tirrell M. Synthetic lipidation of peptides and amino acids: monolayer structure and properties. J Am Chem Soc. 1995;117:9515–9522. [Google Scholar]
- 14.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 15.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 17.Hong Y, Legge RL, Zhang S, Chen P. Effect of amino acid sequence and pH on nanofiber formation of self-assembling peptides EAK16-II and EAK16-IV. Biomacromolecules. 2003;4:1433–1442. doi: 10.1021/bm0341374. [DOI] [PubMed] [Google Scholar]
- 18.Yokoi H, Kinoshita T, Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci U S A. 2005;102:8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G. Nanofibers. Adv Mater. 1997;9:437–439. [Google Scholar]
- 20.Liu D, De Feyter S, Cotlet M, Wiesler U-M, Weil T, Herrmann A, et al. Flourescent self-assembled polyphenylene dendrimer nanofibers. Macromolecules. 2003;36:8489–8498. [Google Scholar]
- 21.Jun HW, Yuwono V, Paramonov SE, Hartgerink JD. Enzyme-mediated degradation of peptide-amphiphile nanofiber network. Adv Mater. 2005;17:2612–2617. [Google Scholar]
- 22.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishna S, Fujuhara K, Teo WE, Lim TC, Ma Z. An introduction to electrospinning and nanofibers. Singapore: World Scientific Publishing Company; 2005. p. 396. [Google Scholar]
- 24.Czaja WK, Young DJ, Kawecki M, Brown RM., Jr The future prospects of microbial cellulose in biomedical applications. Biomacromolecules. 2007;8:1–12. doi: 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- 25.Brown EE, Laborie MP. Bioengineering bacterial cellulose/poly(ethylene oxide) nanocomposites. Biomacromolecules. 2007;8:3074–3081. doi: 10.1021/bm700448x. [DOI] [PubMed] [Google Scholar]
- 26.Ciechanska D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Textiles Eastern Europe. 2004;12:69–72. [Google Scholar]
- 27.Grimm S, Giesa R, Sklarek K, Langner A, Gosele U, Schmidt HW, Steinhart M. Nondestructive replication of self-ordered nanoporous alumina membranes via cross-linked polyacrylate nanofiber arrays. Nano Lett. 2008;8:1954–1959. doi: 10.1021/nl080842c. [DOI] [PubMed] [Google Scholar]
- 28.Porter JR, Henson A, Popat KC. Biodegradable poly(epsilon-caprolactone) nanowires for bone tissue engineering applications. Biomaterials. 2009;30:780–788. doi: 10.1016/j.biomaterials.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Tao SL, Desai TA. Aligned arrays of biodegradable poly(epsilon-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007;7:1463–1468. doi: 10.1021/nl0700346. [DOI] [PubMed] [Google Scholar]
- 30.Jeong HE, Lee SH, Kim P, Suh KY. Stretched polymer nanohairs by nanodrawing. Nano Lett. 2006;6:1508–1513. doi: 10.1021/nl061045m. [DOI] [PubMed] [Google Scholar]
- 31.Xing X, Wang Y, Li B. Nanofibers drawing and nanodevices assembly in poly(trimethylene terephthalate) Opt Express. 2008;16:10815–10822. doi: 10.1364/oe.16.010815. [DOI] [PubMed] [Google Scholar]
- 32.Nain A, Wong J, Amon C, Sitti M. Drawing suspended polymer micro-/nanofibers using glass micropipettes. Appl Phys Lett. 2006;89:1831051–1831053. [Google Scholar]
- 33.Alemdar A, Sain M. Isolation and characterization of nanofibers from agricultural residues: wheat straw and soy hulls. Bioresour Technol. 2008;99:1664–1671. doi: 10.1016/j.biortech.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Fischer TH, Valeri CR, Smith CJ, Scull CM, Merricks EP, Nichols TC, et al. Non-classical processes in surface hemostasis: mechanisms for the poly-N-acetyl glucosamine-induced alteration of red blood cell morphology and surface prothrombogenicity. Biomed Mater. 2008;3 doi: 10.1088/1748-6041/3/1/015009. 015009/1–9. [DOI] [PubMed] [Google Scholar]
- 35.Fan Y, Saito T, Isogai A. Preparation of chitin nanofibers from squid pen beta-chitin by simple mechanical treatment under acid conditions. Biomacromolecules. 2008;9:1919–1923. doi: 10.1021/bm800178b. [DOI] [PubMed] [Google Scholar]
- 36.Aden Brook Farms Website Large 3×3 bales of bright wheat straw. 2009 http://adenbrookfarms.com/order/index.php?main_page=index&cPath=2.
- 37.Rollings DA, Tsoi S, Sit JC, Veinot JG. Formation and aqueous surface wettability of polysiloxane nanofibers prepared via surface initiated, vapor-phase polymerization of organotrichlorosilanes. Langmuir. 2007;23:5275–5278. doi: 10.1021/la063604a. [DOI] [PubMed] [Google Scholar]
- 38.Mankidy P, Rajagopalan R, Foley H. Facile catalytic growth of cyanoacrylate nanofibers. Chem Commun. 2006:1139–1141. doi: 10.1039/b514600c. [DOI] [PubMed] [Google Scholar]
- 39.Jang J, Chang M, Yoon H. Chemical sensors based on highly conductive Poly(3,4-ethylene-dioxythiophene) Nanorods. Adv Mater. 2005;17:1616–1620. [Google Scholar]
- 40.Kong H, Jang J. One-step fabrication of silver nanoparticle embedded polymer nanofibers by radical-mediated dispersion polymerization. Chem Commun. 2006:3010–3012. doi: 10.1039/b605286j. [DOI] [PubMed] [Google Scholar]
- 41.Jing X, Wang Y, Wu D, Qiang J. Sonochemical synthesis of polyaniline nanofibers. Ultrason Sonochem. 2007;14:75–80. doi: 10.1016/j.ultsonch.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Li D, Huang J, Kaner RB. Polyaniline nanofibers: a unique polymer nanostructure for versatile applications. Acc Chem Res. 2009;42:135–145. doi: 10.1021/ar800080n. [DOI] [PubMed] [Google Scholar]
- 43.Chen F, Li X, Mo X, He C, Wang H, Ikada Y. Electrospun chitosan-P(LLA-CL) nanofibers for biomimetic extracellular matrix. J Biomater Sci Polym Ed. 2008;19:677–691. doi: 10.1163/156856208784089661. [DOI] [PubMed] [Google Scholar]
- 44.Jeong SI, Lee AY, Lee YM, Shin H. Electrospun gelatin/poly(L-lactide-co-epsilon-caprolactone) nanofibers for mechanically functional tissue-engineering scaffolds. J Biomater Sci Polym Ed. 2008;19:339–357. doi: 10.1163/156856208783721029. [DOI] [PubMed] [Google Scholar]
- 45.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 47.Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Chen ZC, Ekaputra AK, Gauthaman K, Adaikan PG, Yu H, Hutmacher DW. In vitro and in vivo analysis of co-electrospun scaffolds made of medical grade poly(epsilon-caprolactone) and porcine collagen. J Biomater Sci Polym Ed. 2008;19:693–707. doi: 10.1163/156856208784089580. [DOI] [PubMed] [Google Scholar]
- 50.Kwon IK, Matsuda T. Co-electrospun nanofiber fabrics of poly(L-lactide-co-epsilon-caprolactone) with type I collagen or heparin. Biomacromolecules. 2005;6:2096–2105. doi: 10.1021/bm050086u. [DOI] [PubMed] [Google Scholar]
- 51.Han I, Shim KJ, Kim JY, Im SU, Sung YK, Kim M, et al. Effect of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber matrices cocultured with hair follicular epithelial and dermal cells for biological wound dressing. Artif Organs. 2007;31:801–808. doi: 10.1111/j.1525-1594.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 52.Meng W, Kim SY, Yuan J, Kim JC, Kwon OH, Kawazoe N, et al. Electrospun PHBV/collagen composite nanofibrous scaffolds for tissue engineering. J Biomater Sci Polym Ed. 2007;18:81–94. doi: 10.1163/156856207779146114. [DOI] [PubMed] [Google Scholar]
- 53.Zeugolis DI, Khew ST, Yew ES, Ekaputra AK, Tong YW, Yung LY, et al. Electro-spinning of pure collagen nano-fibres–just an expensive way to make gelatin? Biomaterials. 2008;29:2293–2305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Gauthaman K, Venugopal JR, Yee FC, Peh GS, Ramakrishna S, Bongso A. Nanofibrous substrates support colony formation and maintain stemness of human embryonic stem cells. J Cell Mol Med. doi: 10.1111/j.1582-4934.2009.00699.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casper CL, Yang W, Farach-Carson MC, Rabolt JF. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules. 2007;8:1116–1123. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- 56.Song JH, Kim HE, Kim HW. Production of electrospun gelatin nanofiber by water-based co-solvent approach. J Mater Sci Mater Med. 2008;19:95–102. doi: 10.1007/s10856-007-3169-4. [DOI] [PubMed] [Google Scholar]
- 57.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Kim HW, Yu HS, Lee HH. Nanofibrous matrices of poly(lactic acid) and gelatin polymeric blends for the improvement of cellular responses. J Biomed Mater Res A. 2008;87:25–32. doi: 10.1002/jbm.a.31677. [DOI] [PubMed] [Google Scholar]
- 59.Meng W, Xing ZC, Jung KH, Kim SY, Yuan J, Kang IK, et al. Synthesis of gelatin-containing PHBV nanofiber mats for biomedical application. J Mater Sci Mater Med. 2008;19:2799–2807. doi: 10.1007/s10856-007-3356-3. [DOI] [PubMed] [Google Scholar]
- 60.Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 61.Geng X, Kwon OH, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26:5427–5432. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 62.Ohkawa K, Cha D, Kim H, Nishida A, Yamamoto H. Electrospinning of chitosan. Macromol Rapid Commun. 2004;25:1600–1605. [Google Scholar]
- 63.Ohkawa K, Minato K, Kumagai G, Hayashi S, Yamamoto H. Chitosan nanofiber. Biomacromolecules. 2006;7:3291–3294. doi: 10.1021/bm0604395. [DOI] [PubMed] [Google Scholar]
- 64.Chu XH, Shi XL, Feng ZQ, Gu ZZ, Ding YT. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol Lett. 2009;31:347–352. doi: 10.1007/s10529-008-9892-1. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Fang D, Hsiao BS, Chu B, Chen W. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules. 2004;5:326–333. doi: 10.1021/bm034345w. [DOI] [PubMed] [Google Scholar]
- 66.Pan H, Jiang H, Chen W. Interaction of dermal fibroblasts with electrospun composite polymer scaffolds prepared from dextran and poly lactide-co-glycolide. Biomaterials. 2006;27:3209–3220. doi: 10.1016/j.biomaterials.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 67.Wnek G, Carr M, Simpson D, Bowlin G. Electrospinning of nanofiber fibrinogen structrures. Nano Lett. 2003;3:213–216. [Google Scholar]
- 68.McManus MC, Boland ED, Simpson DG, Barnes CP, Bowlin GL. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. J Biomed Mater Res A. 2007;81:299–309. doi: 10.1002/jbm.a.30989. [DOI] [PubMed] [Google Scholar]
- 69.McManus M, Boland E, Sell S, Bowen W, Koo H, Simpson D, Bowlin G. Electrospun nanofibre fibrinogen for urinary tract tissue reconstruction. Biomed Mater. 2007;2:257–262. doi: 10.1088/1748-6041/2/4/008. [DOI] [PubMed] [Google Scholar]
- 70.Neal RA, McClugage SG, Link MC, Sefcik LS, Ogle RC, Botchwey EA. Laminin nanofiber meshes that mimic morphological properties and bioacivity of basement membranes. Tissue Eng Part C Methods. 2009;15:11–21. doi: 10.1089/ten.tec.2007.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Um IC, Fang D, Hsiao BS, Okamoto A, Chu B. Electrospinning and electro-blowing of hyaluronic acid. Biomacromolecules. 2004;5:1428–1436. doi: 10.1021/bm034539b. [DOI] [PubMed] [Google Scholar]
- 72.Ji Y, Ghosh K, Shu XZ, Li B, Sokolov JC, Prestwich GD, Clark RA, Rafailovich MH. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials. 2006;27:3782–3792. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 73.Ji Y, Ghosh K, Li B, Sokolov JC, Clark RA, Rafailovich MH. Dual-syringe reactive electrospinning of cross-linked hyaluronic acid hydrogel nanofibers for tissue engineering applications. Macromol Biosci. 2006;6:811–817. doi: 10.1002/mabi.200600132. [DOI] [PubMed] [Google Scholar]
- 74.Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell Orientation. Tissue Eng. 2005;11:1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 75.Duan Y, Wang Z, Yan W, Wang S, Zhang S, Jia J. Preparation of collagen-coated electrospun nanofibers by remote plasma treatment and their biological properties. J Biomater Sci Polym Ed. 2007;18:1153–1164. doi: 10.1163/156856207781554019. [DOI] [PubMed] [Google Scholar]
- 76.Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29:3574–3582. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Chu B, Hsiao BS. Mineralization of hydroxyapatite in electrospun nanofibrous poly(L-lactic acid) scaffolds. J Biomed Mater Res A. 2006;79:307–317. doi: 10.1002/jbm.a.30799. [DOI] [PubMed] [Google Scholar]
- 78.Yu HS, Jang JH, Kim TI, Lee HH, Kim HW. Apatite-mineralized polycaprolactone nanofibrous web as a bone tissue regeneration substrate. J Biomed Mater Res A. 2009;88:747–754. doi: 10.1002/jbm.a.31709. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Y, Leong MF, Ong WF, Chan-Park MB, Chian KS. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials. 2007;28:861–868. doi: 10.1016/j.biomaterials.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 80.Nisbet DR, Yu LM, Zahir T, Forsythe JS, Shoichet MS. Characterization of neural stem cells on electrospun poly(epsilon-caprolactone) submicron scaffolds: evaluating their potential in neural tissue engineering. J Biomater Sci Polym Ed. 2008;19:623–634. doi: 10.1163/156856208784089652. [DOI] [PubMed] [Google Scholar]
- 81.Park K, Ju YM, Son JS, Ahn KD, Han DK. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J Biomater Sci Polym Ed. 2007;18:369–382. doi: 10.1163/156856207780424997. [DOI] [PubMed] [Google Scholar]
- 82.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 83.Chua KN, Chai C, Lee PC, Ramakrishna S, Leong KW, Mao HQ. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2007;35:771–781. doi: 10.1016/j.exphem.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakowsky U, Schumacher G, Gege C, Schmidt RR, Rothe U, Bendas G. Cooperation between lateral ligand mobility and accessibility for receptor recognition in selectin-induced cell rolling. Biochemistry. 2002;41:4704–4712. doi: 10.1021/bi0117596. [DOI] [PubMed] [Google Scholar]
- 85.Houseman BT, Mrksich M. The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials. 2001;22:943–955. doi: 10.1016/s0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- 86.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29:587–596. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Li W, Guo Y, Wang H, Shi D, Liang C, Ye Z, Qing F, Gong J. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J Mater Sci Mater Med. 2008;19:847–854. doi: 10.1007/s10856-007-3087-5. [DOI] [PubMed] [Google Scholar]
- 88.Kim TG, Park TG. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006;12:221–233. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- 89.Zhang D, Chang J, Zeng Y. Fabrication of fibrous poly(butylene succi-nate)/wollastonite/apatite composite scaffolds by electrospinning and biomimetic process. J Mater Sci Mater Med. 2008;19:443–449. doi: 10.1007/s10856-006-0043-8. [DOI] [PubMed] [Google Scholar]
- 90.He W, Ma Z, Yong T, Teo WE, Ramakrishna S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26:7606–7615. doi: 10.1016/j.biomaterials.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 91.Ma K, Chan CK, Liao S, Hwang WY, Feng Q, Ramakrishna S. Electrospun nanofiber scaffolds for rapid and rich capture of bone marrow-derived hematopoietic stem cells. Biomaterials. 2008;29:2096–2103. doi: 10.1016/j.biomaterials.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 92.Chew SY, Hufnagel TC, Lim CT, Leong KW. Mechanical properties of single electrospun drug-encapsulated nanofibres. Nanotechnology. 2006;17:3880–3891. doi: 10.1088/0957-4484/17/15/045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J Control Release. 2003;89:341–353. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 95.Chew SY, Wen J, Yim EK, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–2024. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 96.Maretschek S, Greiner A, Kissel T. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J Control Release. 2008;127:180–187. doi: 10.1016/j.jconrel.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Sanders E, Kloefkorn R, Bowlin G, Simpson D, Wnek G. Two-phase electrospinning from a single electrified jet: microencapsulation of aqueous reservoirs in poly(ethylene-co-vinylacetate) fibers. Macromolecules. 2003;36:3803–3805. [Google Scholar]
- 98.Luong-Van E, Grondahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. Controlled release of heparin from poly(epsilon-caprolactone) electrospun fibers. Biomaterials. 2006;27:2042–2050. doi: 10.1016/j.biomaterials.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 99.Qi H, Hu P, Xu J, Wang A. Encapsulation of drug reservoirs in fibers by emulsion electrospinning: morphology characterization and preliminary release assessment. Biomacromolecules. 2006;7:2327–2330. doi: 10.1021/bm060264z. [DOI] [PubMed] [Google Scholar]
- 100.Li X, Su Y, He C, Wang H, Fong H, Mo X. Sorbitan monooleate and poly(L-lactide-co-epsilon-caprolactone) electrospun nanofibers for endothelial cell interactions. J Biomed Mater Res A. 2009;91:878–885. doi: 10.1002/jbm.a.32286. [DOI] [PubMed] [Google Scholar]
- 101.Yang Y, Li X, Qi M, Zhou S, Weng J. Release pattern and structural integrity of lysozyme encapsulated in core-sheath structured poly(DL-lactide) ultrafine fibers prepared by emulsion electrospinning. Eur J Pharm Biopharm. 2008;69:106–116. doi: 10.1016/j.ejpb.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 102.Taepaiboon P, Rungsardthong U, Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur J Pharm Biopharm. 2007;67:387–397. doi: 10.1016/j.ejpb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 103.Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and beta-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065–4073. doi: 10.1016/j.biomaterials.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 104.Sun Z, Zussman E, Yarin A, Wendorff J, Greiner A. Compound core-shell polymer nanofibers by co-electrospinning. Adv Mater. 2003;15:1929–1932. [Google Scholar]
- 105.Loscertales IG, Barrero A, Marquez M, Spretz R, Velarde-Ortiz R, Larsen G. Electrically forced coaxial nanojets for one-step hollow nanofiber design. J Am Chem Soc. 2004;126:5376–5377. doi: 10.1021/ja049443j. [DOI] [PubMed] [Google Scholar]
- 106.Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(epsilon-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006;7:1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- 107.Yi F, LaVan DA. Poly(glycerol sebacate) nanofiber scaffolds by core/shell electrospinning. Macromol Biosci. 2008;8:803–806. doi: 10.1002/mabi.200800041. [DOI] [PubMed] [Google Scholar]
- 108.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling Peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS ONE. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 110.Gelain F, Bottai D, Vescovi A, Zhang S. Designer self-assembling Peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mater Res A. 2006;78:157–167. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 112.Matsuzaka K, Walboomers XF, de Ruijter JE, Jansen JA. The effect of poly-L-lactic acid with parallel surface micro groove on osteoblast-like cells in vitro. Biomaterials. 1999;20:1293–1301. doi: 10.1016/s0142-9612(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 113.Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis YF. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22:1241–1251. doi: 10.1016/s0142-9612(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 114.Curtis A, Wilkinson C. Nanotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19:97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 115.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 116.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 117.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Ji Y, Ghosh K, Clark RA, Huang L, Rafailovich MH. Effects of fiber orientation and diameter on the behavior of human dermal fibroblasts on electrospun PMMA scaffolds. J Biomed Mater Res A. 2009;90:1092–1106. doi: 10.1002/jbm.a.32165. [DOI] [PubMed] [Google Scholar]
- 119.Rubenstein D, Han D, Goldgraben S, El-Gendi H, Gouma PI, Frame MD. Bioassay chamber for angiogenesis with perfused explanted arteries and electrospun scaffolding. Microcirculation. 2007;14:723–737. doi: 10.1080/10739680701410173. [DOI] [PubMed] [Google Scholar]
- 120.Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16:1151–1170. [Google Scholar]
- 121.Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Sci Technol. 2003;63:2223–2253. [Google Scholar]
- 122.Rockwood DN, Akins RE, Jr, Parrag IC, Woodhouse KA, Rabolt JF. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials. 2008;29:4783–4791. doi: 10.1016/j.biomaterials.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 124.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 125.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 126.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, et al. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 2007;83:636–645. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 127.Chua KN, Lim WS, Zhang P, Lu H, Wen J, Ramakrishna S, et al. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–2547. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 128.Meng J, Kong H, Han Z, Wang C, Zhu G, Xie S, et al. Enhancement of nanofibrous scaffold of multiwalled carbon nanotubes/polyurethane composite to the fibroblasts growth and biosynthesis. J Biomed Mater Res A. 2009;88:105–116. doi: 10.1002/jbm.a.31862. [DOI] [PubMed] [Google Scholar]
- 129.Chua KN, Chai C, Lee PC, Tang YN, Ramakrishna S, Leong KW, et al. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials. 2006;27:6043–6051. doi: 10.1016/j.biomaterials.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 130.Finne-Wistrand A, Albertsson AC, Kwon OH, Kawazoe N, Chen G, Kang IK, et al. Resorbable scaffolds from three different techniques: electrospun fabrics, salt-leaching porous films, and smooth flat surfaces. Macromol Biosci. 2008;8:951–959. doi: 10.1002/mabi.200700328. [DOI] [PubMed] [Google Scholar]
- 131.Chen M, Patra PK, Warner SB, Bhowmick S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007;13:579–587. doi: 10.1089/ten.2006.0205. [DOI] [PubMed] [Google Scholar]
- 132.McKenzie JL, Waid MC, Shi R, Webster TJ. Decreased functions of astrocytes on carbon nanofiber materials. Biomaterials. 2004;25:1309–1317. doi: 10.1016/j.biomaterials.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 133.Yuan Y, Cong C, Zhang J, Wei L, Li S, Chen Y, et al. Self-assembling peptide nanofiber as potential substrates in islet transplantation. Transplant Proc. 2008;40:2571–2574. doi: 10.1016/j.transproceed.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 134.Sangsanoh P, Waleetorncheepsawat S, Suwantong O, Wutticharoenmongkol P, Weeranantanapan O, Chuenjitbuntaworn B, et al. In vitro biocompatibility of schwann cells on surfaces of biocompatible polymeric electrospun fibrous and solution-cast film scaffolds. Biomacromolecules. 2007;8:1587–1594. doi: 10.1021/bm061152a. [DOI] [PubMed] [Google Scholar]
- 135.Elias KL, Price RL, Webster TJ. Enhanced functions of osteoblasts on nanometerdiametercarbon fibers. Biomaterials. 2002;23:3279–3287. doi: 10.1016/s0142-9612(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 136.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 137.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15:115–126. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garreta E, Genove E, Borros S, Semino CE. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215–2227. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 140.Shih YR, Chen CN, Tsai SW, Wang YJ, Lee OK. Growth of mesenchymal stem cells on electrospuntype I collagen nanofibers. Stem Cells. 2006;24:2391–2397. doi: 10.1634/stemcells.2006-0253. [DOI] [PubMed] [Google Scholar]
- 141.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 142.Bashur CA, Shaffer RD, Dahlgren LA, Guelcher SA, Goldstein AS. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:2435–2445. doi: 10.1089/ten.tea.2008.0295. [DOI] [PubMed] [Google Scholar]
- 143.Leong MF, Chian KS, Mhaisalkar PS, Ong WF, Ratner BD. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res A. 2009;89:1040–1048. doi: 10.1002/jbm.a.32061. [DOI] [PubMed] [Google Scholar]
- 144.Baker SC, Southgate J. Towards control of smooth muscle cell differentiation in synthetic 3D scaffolds. Biomaterials. 2008;29:3357–3366. doi: 10.1016/j.biomaterials.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 145.Li X, Gao H, Uo M, Sato Y, Akasaka T, Feng Q, et al. Effect of carbon nanotubes on cellular functions in vitro. J Biomed Mater Res A. 2009;91:132–139. doi: 10.1002/jbm.a.32203. [DOI] [PubMed] [Google Scholar]
- 146.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 148.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 149.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A. 2002;99:1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McBride SH, Knothe Tate ML. Modulation of stem cell shape and fate A: the role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng Part A. 2008;14:1561–1572. doi: 10.1089/ten.tea.2008.0112. [DOI] [PubMed] [Google Scholar]
- 151.Czaja W, Krystynowicz A, Bielecki S, Brown RM., Jr Microbial cellulose—the natural power to heal wounds. Biomaterials. 2006;27:145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 152.Araujo JV, Martins A, Leonor IB, Pinho ED, Reis RL, Neves NM. Surface controlled biomimetic coating of polycaprolactone nanofiber meshes to be used as bone extracellular matrix analogues. J Bio-mater Sci Polym Ed. 2008;19:1261–1278. doi: 10.1163/156856208786052335. [DOI] [PubMed] [Google Scholar]
- 153.Ito Y, Hasuda H, Kamitakahara M, Ohtsuki C, Tanihara M, Kang IK, et al. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J Biosci Bioeng. 2005;100:43–49. doi: 10.1263/jbb.100.43. [DOI] [PubMed] [Google Scholar]
- 154.Deng XL, Sui G, Zhao ML, Chen GQ, Yang XP. Poly(L-lactic acid)/hydroxyapatite hybrid nanofibrous scaffolds prepared by electrospinning. J Biomater Sci Polym Ed. 2007;18:117–130. doi: 10.1163/156856207779146123. [DOI] [PubMed] [Google Scholar]
- 155.Jeong SI, Ko EK, Yum J, Jung CH, Lee YM, Shin H. Nanofibrous poly(lacticacid)/hydroxyapatite composite scaffolds forguided tissue regeneration. Macromol Biosci. 2008;8:328–338. doi: 10.1002/mabi.200700107. [DOI] [PubMed] [Google Scholar]
- 156.Venugopal JR, Low S, Choon AT, Kumar AB, Ramakrishna S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artif Organs. 2008;32:388–397. doi: 10.1111/j.1525-1594.2008.00557.x. [DOI] [PubMed] [Google Scholar]
- 157.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 158.Catledge SA, Clem WC, Shrikishen N, Chowdhury S, Stanishevsky AV, Koopman M, et al. An electrospun triphasic nanofibrous scaffold for bone tissue engineering. Biomed Mater. 2007;2:142–150. doi: 10.1088/1748-6041/2/2/013. [DOI] [PubMed] [Google Scholar]
- 159.Kim HW, Lee HH, Chun GS. Bioactivity and osteoblast responses of novel biomedical nanocomposites of bioactive glass nanofiber filled poly(lactic acid) J Biomed Mater Res A. 2008;85:651–663. doi: 10.1002/jbm.a.31339. [DOI] [PubMed] [Google Scholar]
- 160.Kim HW, Song JH, Kim HE. Bioactive glass nanofiber-collagen nanocomposite as a novel bone regeneration matrix. J Biomed Mater Res A. 2006;79:698–705. doi: 10.1002/jbm.a.30848. [DOI] [PubMed] [Google Scholar]
- 161.Sargeant TD, Oppenheimer SM, Dunand DC, Stupp SI. Titanium foam-bioactive nanofiber hybrids for bone regeneration. J Tissue Eng Regen Med. 2008;2:455–462. doi: 10.1002/term.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28:316–325. doi: 10.1016/j.biomaterials.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 164.Cho WJ, Kim JH, Oh SH, Nam HH, Kim JM, Lee JH. Hydrophilized polycaprolactone nanofiber mesh-embedded poly(glycolic-co-lactic acid) membrane for effective guided bone regeneration. J Biomed Mater Res A. 2009;91:400–407. doi: 10.1002/jbm.a.32264. [DOI] [PubMed] [Google Scholar]
- 165.Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Bone regeneration on a collagen sponge self-assembled peptide-amphiphile nanofiber hybrid scaffold. Tissue Eng. 2007;13:11–19. doi: 10.1089/ten.2006.0120. [DOI] [PubMed] [Google Scholar]
- 166.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nanofibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shin SY, Park HN, Kim KH, Lee MH, Choi YS, Park YJ, et al. Biological evaluation of chitosan nanofiber membrane for guided bone regeneration. J Periodontol. 2005;76:1778–1784. doi: 10.1902/jop.2005.76.10.1778. [DOI] [PubMed] [Google Scholar]
- 168.Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, et al. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol. 2005;120:327–339. doi: 10.1016/j.jbiotec.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 169.Ko EK, Jeong SI, Rim NG, Lee YM, Shin H, Lee BK. In vitro osteogenic differentiation of human mesenchymal stem cells and in vivo bone formation in composite nanofiber meshes. Tissue Eng Part A. 2008;14:2105–2119. doi: 10.1089/ten.tea.2008.0057. [DOI] [PubMed] [Google Scholar]
- 170.Sargeant TD, Guler MO, Oppenheimer SM, Mata A, Satcher RL, Dunand DC, et al. Hybrid bone implants: self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials. 2008;29:161–171. doi: 10.1016/j.biomaterials.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 171.da Silva MA, Crawford A, Mundy J, Martins A, Araujo JV, Hatton PV, et al. Evaluation of extracellular matrix formation in polycaprolactone and starch-compounded polycaprolactone nanofiber meshes when seeded with bovine articular chondrocytes. Tissue Eng Part A. 2009;15:377–385. doi: 10.1089/ten.tea.2007.0327. [DOI] [PubMed] [Google Scholar]
- 172.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng Part A. 2008;14:639–648. doi: 10.1089/tea.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 174.Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res. 2008;149:47–56. doi: 10.1016/j.jss.2007.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shin HJ, Lee CH, Cho IH, Kim YJ, Lee YJ, Kim IA, et al. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed. 2006;17:103–119. doi: 10.1163/156856206774879126. [DOI] [PubMed] [Google Scholar]
- 176.Lee SJ, Yoo JJ, Lim GJ, Atala A, Stitzel J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J Biomed Mater Res A. 2007;83:999–1008. doi: 10.1002/jbm.a.31287. [DOI] [PubMed] [Google Scholar]
- 177.He W, Yong T, Teo WE, Ma Z, Ramakrishna S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005;11:1574–1588. doi: 10.1089/ten.2005.11.1574. [DOI] [PubMed] [Google Scholar]
- 178.Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10:1160–1168. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 179.Jeong SI, Kim SY, Cho SK, Chong MS, Kim KS, Kim H, et al. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials. 2007;28:1115–1122. doi: 10.1016/j.biomaterials.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 180.Inoguchi H, Kwon IK, Inoue E, Takamizawa K, Maehara Y, Matsuda T. Mechanical responses of a compliant electrospun poly(L-lactide-co-epsilon-caprolactone) small-diameter vascular graft. Biomaterials. 2006;27:1470–1478. doi: 10.1016/j.biomaterials.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 181.Matsuda T, Ihara M, Inoguchi H, Kwon IK, Takamizawa K, Kidoaki S. Mechano-active scaffold design of small-diameter artificial graft made of electrospun segmented polyurethane fabrics. J Biomed Mater Res A. 2005;73:125–131. doi: 10.1002/jbm.a.30260. [DOI] [PubMed] [Google Scholar]
- 182.He W, Ma Z, Teo WE, Dong YX, Robless PA, Lim TC, et al. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A. 2009;90:205–216. doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 183.Nottelet B, Pektok E, Mandracchia D, Tille JC, Walpoth B, Gurny R, et al. Factorial design optimization and in vivo feasibility of poly(epsilon-caprolactone)-micro- and nanofiber-based small diameter vascular grafts. J Biomed Mater Res A. 2009;89:865–875. doi: 10.1002/jbm.a.32023. [DOI] [PubMed] [Google Scholar]
- 184.Inanc B, Arslan YE, Seker S, Elcin AE, Elcin YM. Periodontal ligament cellular structures engineered with electrospun poly(DL-lactide-co-glycolide) nanofibrous membrane scaffolds. J Biomed Mater Res A. 2009;90:186–195. doi: 10.1002/jbm.a.32066. [DOI] [PubMed] [Google Scholar]
- 185.Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 186.Wu Y, Zheng Q, Du J, Song Y, Wu B, Guo X. Self-assembled IKVAV peptide nanofibers promote adherence of PC12 cells. J Huazhong Univ Sci Technol Med Sci. 2006;26:594–596. doi: 10.1007/s11596-006-0530-7. [DOI] [PubMed] [Google Scholar]
- 187.Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 188.Corey JM, Gertz CC, Wang BS, Birrell LK, Johnson SL, Martin DC, et al. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 2008;4:863–875. doi: 10.1016/j.actbio.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Valmikinathan CM, Tian J, Wang J, Yu X. Novel nanofibrous spiral scaffolds for neural tissue engineering. J Neural Eng. 2008;5:422–432. doi: 10.1088/1741-2560/5/4/007. [DOI] [PubMed] [Google Scholar]
- 190.Guo J, Su H, Zeng Y, Liang YX, Wong WM, Ellis-Behnke RG, et al. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine. 2007;3:311–321. doi: 10.1016/j.nano.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 191.Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, et al. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008;8:39. doi: 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang W, Itoh S, Matsuda A, Ichinose S, Shinomiya K, Hata Y, et al. Influences of mechanical properties and permeability on chitosan nano/microfiber mesh tubes as a scaffold for nerve regeneration. J Biomed Mater Res A. 2008;84:557–566. doi: 10.1002/jbm.a.31536. [DOI] [PubMed] [Google Scholar]
- 193.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 2004;70:286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 194.Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3:321–330. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 195.Venugopal J, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11:847–854. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- 196.Powell HM, Boyce ST. Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal-epidermal skin substitutes. J Biomed Mater Res A. 2008;84:1078–1086. doi: 10.1002/jbm.a.31498. [DOI] [PubMed] [Google Scholar]
- 197.Sun T, Mai S, Norton D, Haycock JW, Ryan AJ, MacNeil S. Self-organization of skin cells in three-dimensional electrospun polystyrene scaffolds. Tissue Eng. 2005;11:1023–1033. doi: 10.1089/ten.2005.11.1023. [DOI] [PubMed] [Google Scholar]
- 198.Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS ONE. 2008;3:e1410. doi: 10.1371/journal.pone.0001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pietramaggiori G, Yang HJ, Scherer SS, Kaipainen A, Chan RK, Alper-ovich M, et al. Effects of poly-N-acetyl glucosamine (pGlcNAc) patch on wound healing in db/db mouse. J Trauma. 2008;64:803–808. doi: 10.1097/01.ta.0000244382.13937.a8. [DOI] [PubMed] [Google Scholar]
- 200.Powell HM, Supp DM, Boyce ST. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials. 2008;29:834–843. doi: 10.1016/j.biomaterials.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 201.Meng H, Chen L, Ye Z, Wang S, Zhao X. The effect of a self-assembling peptide nanofiber scaffold (peptide) when used as a wound dressing for the treatment of deep second degree burns in rats. J Biomed Mater Res B Appl Biomater. 2009;89B:379–391. doi: 10.1002/jbm.b.31226. [DOI] [PubMed] [Google Scholar]
- 202.Blackwood KA, McKean R, Canton I, Freeman CO, Franklin KL, Cole D, et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials. 2008;29:3091–3104. doi: 10.1016/j.biomaterials.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 203.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 204.Nesti LJ, Li WJ, Shanti RM, Jiang YJ, Jackson W, Freedman BA, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A. 2008;14:1527–1537. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Part A. 2008;14:227–236. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 206.Navarro-Alvarez N, Soto-Gutierrez A, Rivas-Carrillo JD, Chen Y, Yamamoto T, Yuasa T, et al. Self-assembling peptide nanofiber as a novel culture system for isolated porcine hepatocytes. Cell Transplant. 2006;15:921–927. doi: 10.3727/000000006783981387. [DOI] [PubMed] [Google Scholar]
- 207.Hashemi SM, Soleimani M, Zargarian SS, Haddadi-Asl V, Ahmad-beigi N, Soudi S, et al. In vitro differentiation of human cord blood-derived unrestricted somatic stem cells into hepatocyte-like cells on poly(epsilon-caprolactone) nanofiber scaffolds. Cells Tissues Organs. 2009;190:135–149. doi: 10.1159/000187716. [DOI] [PubMed] [Google Scholar]
- 208.Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, et al. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant. 2008;17:111–119. doi: 10.3727/000000008783907125. [DOI] [PubMed] [Google Scholar]
- 209.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 210.van Aalst JA, Reed CR, Han L, Andrady T, Hromadka M, Bernacki S, et al. Cellular incorporation into electrospun nanofibers: retained viability, proliferation, and function in fibroblasts. Ann Plast Surg. 2008;60:577–583. doi: 10.1097/SAP.0b013e318168db3e. [DOI] [PubMed] [Google Scholar]
- 211.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 212.Eichhorn SJ, Sampson WW. Statistical geometry of pores and statistics of porous nanofibrous assemblies. J R Soc Interface. 2005;2:309–318. doi: 10.1098/rsif.2005.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Balguid A, Mol A, van Marion MH, Bank RA, Bouten CV, Baaijens FP. Tailoring fiber diameter in electrospun poly(epsilon-caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng Part A. 2009;15:437–444. doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 214.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med. 2005;16:1099–1104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 215.Santos MI, Tuzlakoglu K, Fuchs S, Gomes ME, Peters K, Unger RE, et al. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials. 2008;29:4306–4313. doi: 10.1016/j.biomaterials.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 216.Park SH, Kim TG, Kim HC, Yang DY, Park TG. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008;4:1198–1207. doi: 10.1016/j.actbio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 217.Thorvaldsson A, Stenhamre H, Gatenholm P, Walkenstrom P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules. 2008;9:1044–1049. doi: 10.1021/bm701225a. [DOI] [PubMed] [Google Scholar]
- 218.Yixiang D, Yong T, Liao S, Chan CK, Ramakrishna S. Degradation of electrospun nanofiber scaffold by short wave length ultraviolet radiation treatment and its potential applications in tissue engineering. Tissue Eng Part A. 2008;14:1321–1329. doi: 10.1089/ten.tea.2007.0395. [DOI] [PubMed] [Google Scholar]
- 219.Ki CS, Park SY, Kim HJ, Jung HM, Woo KM, Lee JW, et al. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: implications for bone regeneration. Biotechnol Lett. 2008;30:405–410. doi: 10.1007/s10529-007-9581-5. [DOI] [PubMed] [Google Scholar]
- 220.Leong MF, Rasheed MZ, Lim TC, Chian KS. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J Biomed Mater Res A. 2009;91:231–240. doi: 10.1002/jbm.a.32208. [DOI] [PubMed] [Google Scholar]
- 221.Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules. 2008;9:2097–2103. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- 222.Nair S, Kim J, Crawford B, Kim SH. Improving biocatalytic activity of enzyme-loaded nanofibers by dispersing entangled nanofiber structure. Biomacromolecules. 2007;8:1266–1270. doi: 10.1021/bm061004k. [DOI] [PubMed] [Google Scholar]
- 223.Ki CS, Kim JW, Hyun JH, Lee KH, Hattori M, Rah DK, et al. Electrospun three-dimensional silk fibroin nanofibrous scaffold. J Appl Polym Sci. 2007;106:3922–3928. [Google Scholar]
- 224.Teo WE, Liao S, Chan CK, Ramakrishna S. Remodeling of three-dimensional hierarchically organized nanofibrous assemblies. Curr Nanosci. 2008;4:361–369. [Google Scholar]
- 225.Srouji S, Kizhner T, Suss-Tobi E, Livne E, Zussman E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J Mater Sci Mater Med. 2008;19:1249–1255. doi: 10.1007/s10856-007-3218-z. [DOI] [PubMed] [Google Scholar]
- 226.Ishii O, Shin M, Sueda T, Vacanti JP. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J Thorac Cardiovasc Surg. 2005;130:1358–1363. doi: 10.1016/j.jtcvs.2005.05.048. [DOI] [PubMed] [Google Scholar]