Abstract
Gene therapy of solid cancers has been severely restricted by the limited distribution of vectors within tumors. However, cellular vectors have emerged as an effective migratory system for gene delivery to invasive cancers. Implanted and injected multipotent mesenchymal stromal cells (MSCs) have shown tropism for several types of primary tumors and metastases. This capacity of MSCs forms the basis for their use as a gene vector system in neoplasms. Here, we review the tumor-directed migratory potential of MSCs, mechanisms of the migration, and the choice of therapeutic transgenes, with a focus on malignant gliomas as a model system for invasive and highly vascularized tumors. We examine recent findings demonstrating that MSCs share many characteristics with pericytes and that implanted MSCs localize primarily to perivascular niches within tumors, which might have therapeutic implications. The use of MSC vectors in cancer gene therapy raises concerns, however, including a possible MSC contribution to tumor stroma and vasculature, MSC-mediated antitumor immune suppression, and the potential malignant transformation of cultured MSCs. Nonetheless, we highlight the novel prospects of MSC-based tumor therapy, which appears to be a promising approach.
Introduction
Tumor invasiveness and metastasis are the main causes of death in cancer patients and present challenging scientific and clinical problems. Glioblastoma multiforme (GBM) is an aggressive and invasive neoplasm characterized by extensive neovascularization. GBM cells grow in a highly invasive pattern along blood vessels and white matter tracts in the brain. The median survival time for GBM patients undergoing conventional treatment (i.e., surgery, radiotherapy, and chemotherapy) is only 14.6 months.1 The shortcomings of conventional GBM therapy can be attributed, at least in part, to a failure to target the invasive tumor cells. It is therefore obvious that effective treatment against GBM and other highly invasive tumors must include the killing not only of cancer cells in the main tumor mass but also of tumor cells that have dispersed deeply into surrounding normal tissue. Experimental evidence suggests that the growth of GBM is maintained by cancer stem cells (CSCs) residing within a perivascular niche.2,3 CSCs are a sub-population of cancer cells that maintain and propagate tumor growth.4 However, whether CSCs are present in GBM is still a matter of controversy.4
This article reviews evidence that multipotent mesenchymal stromal cells (MSCs) can act as a gene therapy vector system with the potential to migrate to and within invasive solid cancers. We focus on GBM as a model for invasive tumors, but we also include findings from other experimental tumor models.
MSCs
Bone marrow mononuclear cells contain a rare population of nonhematopoietic mesenchymal progenitor cells that, in in vitro culture, adhere and give rise to fibroblastoid colonies (fibroblastoid colony-forming units). Upon further culture, these cells are referred to as mesenchymal stromal cells or marrow stromal cells. Cultured MSCs have also been commonly (and incorrectly) called mesenchymal stem cells; however, cultured MSCs do not fulfill stringent stem cell criteria, in contrast to their uncultured in vivo precursors. Nevertheless, cultured MSCs possess a number of intriguing properties (such as proliferation and differentiation capacities, stroma function, and immunomodulatory properties) that make them suitable candidates for cell therapy applications.
MSCs display adipogenic, chondrogenic, osteogenic, and myogenic differentiation capacities (Figure 1a–c) and possibly others.5,6 For a review on MSC differentiation capacities, see Caplan.5 At present, no single surface marker is available that specifically identifies MSCs. Therefore, MSCs are defined by the expression of combinations of certain surface markers, including CD73+, CD90+, CD105+, CD146+, CD271+, and STRO-1+, and by the lack of expression of hematopoietic markers, such as CD34 and CD45 (refs. 7,8). Additional properties of MSCs include the capacity to form a hematopoietic microenvironment that is capable of supporting the long-term maintenance and differentiation of hematopoietic stem cells.9 MSCs play an important role in tissue regeneration and have been used to experimentally repair tissue damage in various disease conditions.10 MSCs also possess immunosuppressive properties through the modulation of cytotoxic T cells, antigen-presenting cells, natural killer cells, and B cells,11 and several ongoing promising clinical studies are investigating the potent immunomodulatory effect of MSCs (e.g., in patients who have developed severe acute graft-versus-host disease after allogeneic stem cell transplantation).12
Figure 1.
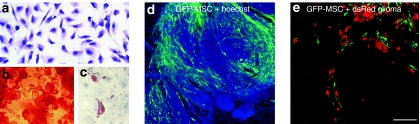
Multipotent mesenchymal stromal cells (MSCs) in vitro and within gliomas. (a) Spindle-shaped morphology of rat bone marrow–derived MSCs in vitro. MSCs possess the capacity to differentiate into (b) osteoblasts and (c) adipocytes upon induction of differentiation. (d) Implantation of enhanced green fluorescent protein (eGFP)–expressing rat MSCs (green) into orthotopic rat gliomas (Hoechst, blue). MSCs spread extensively within the glioma but largely avoid adjacent normal brain parenchyma. (e) eGFP-MSCs migrate specifically along invasive DsRed-labeled glioma cells (red). a–c, Bar = 50 µm; d, bar = 200 µm; and e, bar = 100 µm. Figure 1a–c,e are reproduced from Bexell et al.26 with permission from the publisher.
Most of the available information concerns human MSCs, especially for bone marrow–derived MSCs, because human MSCs are relatively easy to culture (in contrast to other species, such as mice). In addition to bone marrow, culture of MSCs has been reported from most other organs, including adipose tissue, skeletal muscle, pancreas, placenta, dental pulp, and umbilical cord blood.6,8 In this review, we focus on bone marrow–derived MSCs except where otherwise stated. Recent findings indicate that primary MSCs are localized perivascularly and that MSCs share many properties with pericytes. However, not all primary MSCs are found adjacent to vessels, and not all pericytes are MSCs.6,13,14
MSCs Display Tumor-Tropic Capacities
Gene therapy utilizing viral vectors to deliver antitumor substances has been successful in experimental cancer studies, but most clinical studies have had only limited success.15 The inefficient spread of vectors within the tumor and the inability to reach invasive tumor cells distant from the tumor bulk can, at least in part, explain these shortcomings. In light of this, the discovery that implanted neural stem cells (NSCs) are able to migrate throughout normal brain tissue to experimental gliomas, where NSCs can deliver a cytotoxic substance, is promising.16 Notably, the implantation of interleukin-4-producing NSCs into gliomas shows considerably better therapeutic efficiency than the retrovirus-mediated in vivo transfer of interleukin-4 (ref. 17). Subsequently, NSCs, MSCs, endothelial, hematopoietic, skin-derived, and endometrial precursor cells have been utilized as migratory cellular vectors to tumors.18,19,20,21,22,23
The first evidence of the tropism of MSCs to gliomas was demonstrated by implantation of rat MSCs into rats bearing syngeneic gliomas.20 Intracranially implanted MSCs were found to migrate to and disperse throughout the tumor mass. MSCs are also able to migrate along the corpus callosum toward established gliomas in the contralateral hemisphere.20,24 The tumor-tropic migratory capacity of MSCs is further strengthened by findings that human MSCs specifically home to human gliomas of immunocompromised mice following injections into the ipsilateral and contralateral carotid arteries.24 Another research group showed that rat bone marrow–derived multipotent adult progenitor cells (MAPCs), a population of progenitor cells distinct from MSCs, implanted directly into rat gliomas or in the vicinity spread extensively within gliomas, whereas implanted rat fibroblasts do not migrate but remain at the injection site adjacent to the tumors.25 Our laboratory has shown that intratumorally implanted rat MSCs possess the ability to migrate to invasive rat glioma extensions and distant tumor microsatellites. MSCs, however, largely avoid normal brain gray matter (Figure 1d,e).26 The attraction of MSCs to tumors is not limited to gliomas but has been reported for several experimental tumor models, including malignant melanoma,27 Kaposi's sarcoma,28 colon cancer,29 ovarian cancer,30 pancreatic cancer,31 Ewing sarcoma,32 fibrosarcoma,33 breast cancer,34 and renal cell carcinoma.35 The tumor-specific migratory pattern makes it possible to utilize intratumorally administered MSCs for delivery of toxic substances to the main tumor mass, as well as to invasive parts of the tumor, without adverse effects on normal brain tissue. Indeed, the tumor-specific migratory capacity of implanted/injected MSCs is fundamental for the development of MSCs as vehicles in cancer therapy. In this respect, MSCs show clear therapeutic advantages compared to vehicles with a more limited distribution potential, such as viruses, antibodies, nanoparticles, and liposomes.
MSCs have been delivered to a variety of different tumor models using intravenous (i.v.),27 intra-arterial,24 and intraperitoneal injections,30 and intracerebral,24 intracerebroventricular,36 and intratumoral implantation,20 as well as intratracheal administration.37 Both the route of MSC delivery and tumor localization have a decisive influence on the extent of MSC engraftment into tumors. Intratumoral implantation of rat MSCs results in a much more efficient distribution of MSCs within rat gliomas compared to i.v. administration.26 However, i.v.-injected human MSCs were shown to engraft into a mouse model of pulmonary metastasis produced by i.v. injection of human melanoma cells.27 The i.v.-injected MSCs are initially trapped within both normal lung and tumor tissue, but over time, MSCs persist within tumors, whereas their numbers gradually decrease in normal lung tissue.27 It is likely that the tumor microenvironment promotes a permissive niche for MSC survival as compared to normal lung tissue. Although easy to administer, systemically injected MSCs might cause adverse effects if they locate to normal organs. In fact, reports have shown that the majority (>90%) of i.v.-injected human MSCs are trapped within the lungs of mice and do not reach the arterial system.10,38,39 Experimental evidence thus indicates that intratumoral administration, in addition to producing a superior tumor infiltration, is a safer mode of delivery.
The important role of the tumor microenvironment for MSC survival is further strengthened by findings of absent or minimal MSC survival upon grafting into nontumoral tissue (see, e.g., Koponen et al.40). Factors that might promote MSC survival within tumors are discussed below.
Mechanisms of MSC Migration and Homing to Tumors
One early report found that epidermal growth factor, platelet-derived growth factor (PDGF)-BB, and stromal-derived factor-1, but not vascular endothelial growth factor (VEGF), are key factors mediating the tropism of human MSCs to human glioma cells in vitro.24 Mouse MSCs transduced with the epidermal growth factor receptor exhibit enhanced in vitro and in vivo migration toward mouse gliomas as compared to nontransduced MSCs.41 Other investigators have reported that glioma-produced angiogenic cytokines, such as VEGF-A, transforming growth factor-β1, interleukin-8, and neurotrophin-3, are mediators of human MSC migration to tumors in vitro.42,43 Angiogenic cytokines are involved in tumor angiogenesis, indicating that similar pathways are used for tumor angiogenesis and MSC migration within tumors.42 Furthermore, in vivo inhibition of angiogenic signaling factors using sunitinib (a broad-spectrum receptor tyrosine kinase inhibitor) is associated with decreased vessel formation and decreased MSC migration.26 The tropism of grafted MSCs to neoplastic vessels should be considered from the perspective of findings that endogenous bone marrow–derived periendothelial vascular mural cells (pericytes) contribute to tumor stroma and neovascularization.44,45,46 Thus, it seems that an inherent MSC dependence on angiogenic signaling factors confers, at least in part, the migratory specificity of grafted and endogenous MSCs. This “neoangiotropism” of grafted MSCs might preferentially direct these cells toward cancerous tissue characterized by active angiogenesis. This feature could possibly be exploited to gain maximum therapeutic benefit where it is most needed, namely, in the most rapidly growing parts of the tumor.
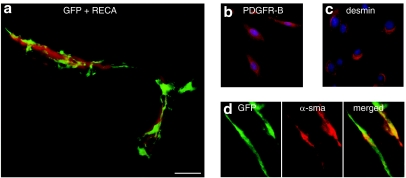
Recent data suggest that MSCs share important characteristics with pericytes.6,13 Pericytes are important regulators of microvessel blood flow and interact with and support endothelial cells. Within tumors, pericytes are thought to regulate vessel integrity, maintenance, and function47 (for a review on the role of pericytes in health and disease, see Bergers and Song48). Similar to the way that endogenous MSCs localize mainly to a perivascular niche within the bone marrow, implanted rat MSCs track and localize to a tumor perivascular niche following implantation into rat gliomas.26 Within gliomas, most implanted MSCs continue to express pericyte markers, such as neuron-glia 2, PDGF-receptor-B, and α-smooth muscle actin, but not endothelial markers (Figure 2).26
Figure 2.
MSCs share pericyte characteristics. (a) Implanted rat eGFP+ MSCs (green) are attracted to perivascular niches within gliomas. Tumor blood vessels are delineated by the endothelial cell marker rat endothelial cell antigen (RECA, red). MSC expression of the pericyte markers (b) platelet-derived growth factor receptor-B (PDGFR-B) and (c) desmin in vitro. (d) Implanted eGFP-MSCs (green) express pericyte marker α-smooth muscle actin (red) within tumors. a, Bar = 100 µm; b,c, bar = 50 µm; d, bar = 20 µm.
In addition to the “neoangiotropism” of MSCs, inflammatory mediators released from the tumor cells or tumor stroma or from surrounding peritumoral reactive cells help to attract MSCs to neoplasms; MSCs home to sites of nonspecific tissue damage and inflammation, where MSCs contribute to wound healing.49,50 Tumors and their immediate surroundings can be considered “wounds that never heal”51 and contain high numbers of inflammatory cells and cytokines that attract MSCs, e.g., via monocyte chemotactic protein-1(ref. 52). Therefore, it is possible that the extensive and tumor-specific migration capacity of implanted and injected MSCs represents a common response to injury-induced inflammation. For a detailed review of MSC migration and inflammation, see Spaeth et al.53
Owing to the lack of specific MSC sub-population markers, most studies have isolated, propagated, and characterized MSCs by their adherent growth in selected fetal calf serum (FCS)–containing medium, differentiation assays, and surface marker expression. This procedure, however, does not take into account the wide heterogeneity of MSCs in culture. Given differences in isolation and culture protocols, it might be difficult to compare studies on MSC migration mechanisms between different research groups. MSC heterogeneity is beginning to be elucidated,54 but there are still large knowledge gaps. Recently, it was shown that a sub-population of human MSCs that display high matrix metalloproteinase 1 (MMP1) expression migrate toward glioma cells to a much greater extent compared with a subset of low-MMP1-expressing MSCs.55 In addition to clarifying mechanisms of MSC migration, this study stresses the importance of identifying and characterizing sub-populations in cultured MSC preparations. Obviously, we need to gain further detailed information about the molecular mechanisms determining MSC migratory behavior before we can exploit the full potential of these cells as vectors in cancer therapy.
Effects of MSCs on Tumor Growth
The grafting of MSCs not modified to produce a therapeutic transgene but often carrying a marker gene to allow for visualization, e.g., enhanced green fluorescent protein, has produced conflicting results with respect to tumor growth. Native MSCs have been shown to suppress tumor growth in models of glioma,20 Kaposi's sarcoma,28 malignant melanoma,56,57 Lewis lung carcinoma,56 colon carcinoma,58 and other tumor models.59 Suppression of Kaposi's sarcoma growth is associated with MSC inhibition of the Akt protein.28 Otsu et al. found that implantation of mouse MSCs into established subcutaneous mouse melanomas results in lower vascular density and inhibition of tumor growth.57 In contrast, we and others have found no apparent effect on tumor growth following implantation/injection of MSCs.26,30,60 On the other hand, several studies have reported that MSCs can augment tumor growth.61,62,63,64 Promotion of tumor growth is possibly mediated by MSC production of immunosuppressive factors and by the contribution of MSCs to tumor stroma and tumor vascularization.65 Beckermann et al. reported that the injection of human MSCs into nude mice carrying xenografts of human pancreatic tumors results in an increase in tumor vessel density.66 The different effects of MSCs on tumor growth are striking and illustrate the complexity of the role of MSCs in cancer. It is possible that the heterogeneity of cultured MSCs (i.e., multiple MSC sub-populations with different properties), differences in tumor cells and models, the use of MSCs from different species and species-specific interactions between tumor cells, MSCs, and hosts account for some of the differences observed.
MSC Delivery of Prodrug-Converting Enzymes
Clinical gene therapy in malignant glioma was pioneered utilizing gene transfer of the herpes simplex virus-thymidine kinase (HSV-tk) gene in combination with the systemic administration of ganciclovir.15 HSV-tk transfer was mediated by stereotactic or intraoperative intratumoral injections of, e.g., adenoviral vectors. Within tumors, HSV-tk converts (phosphorylates) the prodrug ganciclovir into its toxic form, which inhibits DNA synthesis, leading to cell death. In addition, there is a substantial bystander effect that leads to cell death of neighboring cells. However, as discussed previously, there is a need for a more efficient and specific vector system to achieve a substantial therapeutic effect.15 The migratory capacity of transduced MAPCs/MSCs permits more efficient distribution of the HSV-tk gene within tumors as compared to injections of viral vectors. Accordingly, HSV-tk has been successfully transferred via MAPCs to experimental gliomas25 and via MSCs to pancreatic cancer67 and gliomas.36 Significant antitumor effects were demonstrated by the administration of MAPCs/MSCs expressing the HSV-tk gene in combination with systemically administered ganciclovir through bystander-mediated tumor cell killing.25,67 The migratory capacity of the transduced MSCs/MAPCs is crucial for therapeutic efficiency because administration of transduced nonmigratory fibroblasts does not lead to similar therapeutic effects.25,36
Adipose tissue–derived MSCs have been used to deliver another prodrug-converting enzyme, cytosine deaminase, followed by systemic administration of the prodrug 5-fluorocytosine, which is converted to the active toxic drug 5-fluorouracil in tumors. This therapeutic regimen has proven efficacious in the treatment of experimental colon carcinoma,60 prostate tumor growth,68 and melanoma growth.69
Cytokine Delivery by MSCs
MSCs can also be utilized to deliver immunomodulatory cytokines in order to augment the host's antitumor immune response. Tumor regression by cytokine transfer using MSCs has been demonstrated in several tumor models using a variety of immunostimulatory substances, including interleukin-2 (ref. 20), interleukin-12 (refs. 70,71), interleukin-18 (ref. 72), interferon-α,41 interferon-β,27,73 and CXC3CL1 (fractalkine).74 The therapeutic effects are often attributed to the increased tumor infiltration of antitumor immune cells, e.g., CD4+ and CD8+ T cells and natural killer cells.20,74,75 Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) has gained attention in cancer gene therapy because of its capacity to induce apoptosis specifically in tumor cells. Recent studies demonstrate the ability of umbilical cord blood–derived or bone marrow–derived TRAIL-producing MSCs to effectively inhibit the growth of gliomas.76,77,78 and breast cancer–derived lung metastases.79 Work from our group has demonstrated regression of rat gliomas as a result of combining peripheral immunization using interferon-γ-transduced autologous tumor cells with the intratumoral delivery of interleukin-7 by rat MSCs.75
MSC Delivery of Oncolytic Viruses
A major obstacle to the use of oncolytic viruses in the treatment of experimental tumors using direct viral transfer has been the high immunogenicity of the viral particles. Oncolytic viruses are often neutralized by an immunological reaction before they can exert substantial antitumor effects. Furthermore, limited vector spread within tumors makes the efficient treatment of invasive tumors difficult.80 To overcome this problem, cellular vectors for oncolytic viruses are being explored.81 By using cells as vehicles, viruses are protected from the host immune system while being delivered to the tumor site. Human MSCs loaded to deliver a conditionally replicating oncolytic adenovirus (CRAd) can migrate to gliomas and release CRAds that infect human glioma cells.82 MSC-based delivery of oncolytic adenoviruses has demonstrated therapeutic effects in mice bearing human ovarian cancer and human gliomas.30,83
Pericyte-Like MSCs as Therapeutic Vehicles
The finding that MSCs localize to tumor vasculature upon intratumoral implantation offers opportunities for therapeutic targeting, especially of vascularized tumors.26 The tumor neovasculature is critical for tumor growth,84 and implanted MSCs may be utilized as vehicles for the delivery of antiangiogenic substances to vascularized tumors. Combinatorial targeting of both tumor endothelium and tumor pericytes has been shown to synergistically affect tumor vascularization and tumor growth.85 The association of implanted MSCs with tumor vessels might thus offer an opportunity to locally and specifically target both tumor endothelial cells and tumor pericytes. In addition, implanted perivascular MSCs are strategically located to deliver substances to target putative CSCs/tumor-initiating cells known to reside within a perivascular niche.2,86
Recent findings demonstrating that MSCs can be considered members of the pericyte family and that intratumorally grafted MSCs display pericyte markers allow one to speculate that implanted MSCs could possibly function as tumor pericytes. Tumor pericytes contribute to vascular normalization, a process in which otherwise leaky and nonfunctional tumor blood vessels become “normalized,” which allows for more regular blood flow.87 This in turn can be exploited for therapeutic benefit because vascular normalization leads to an increased influx of antitumor immune effector cells.87 Accordingly, it would be interesting to address the question of whether implanted MSCs contribute to normalization of tumor vasculature and thus enhance the antitumor immune response. On the other hand, the addition of pericytes to tumors could possibly also lead to negative effects on blood flow and tumor growth, and certainly, more research is required to address these questions. Table 1 lists selected studies using native or genetically modified MSCs in various tumor models.
Table 1.
Selected examples of different effects of MSCs in cancer models
In Vivo Imaging of MSCs
Clinical application of cellular vectors for cancer therapy will require sensitive and specific methods for in vivo imaging of vector distribution and transgene expression. Imaging must also allow for sensitive and early detection of treatment effects. Noninvasive in vivo imaging in gene therapy applications has previously been reviewed in detail.88 Today, methods to investigate stem cell homing and migration to tumors include in vivo bioluminescence imaging of firefly luciferase–expressing MSCs, magnetic resonance imaging (MRI) of cells containing biocompatible superparamagnetic iron oxide nanoparticles, and positron emission tomography imaging. These imaging modalities allow for the noninvasive serial detection of implanted/injected cells.
MRI requires a costly imaging device but offers several advantages over the other techniques: no radioactive labeling is needed, more than one physiological parameter can be studied using different pulse sequences, and the spatial resolution is superior to that of other imaging modalities.88 MRI has been utilized to detect iron-labeled MSCs,28,89,90,91 Sca1+ bone marrow–derived cells,92 and NSCs93 upon local or systemic administration of cells to tumors. As few as 1,000 labeled human MSCs were detectable by MRI 1 month following co-injection with breast cancer cells.89 Furthermore, MRI allows for the detection of i.v.-injected human MSCs homing to multiple lung metastases of breast cancer.89 Moreover, iron-labeled Sca1+ bone marrow–derived cells that incorporate into newly formed vessels have been utilized to detect ongoing tumor angiogenesis using MRI.92 It is possible that pericyte-like MSCs might be used not only to deliver antiangiogenic substances to tumors but also to detect treatment effects of antiangiogenic therapy.
Bioluminescence imaging has been used to track the tropism of luciferase gene-transduced MSCs to breast and ovarian tumor models34 and lung metastases of breast cancer94 as well as to track NSC migration to experimental brain tumors.95 Although this is a very sensitive technique, it is hampered by poor spatial resolution compared to MRI and poor light penetration through tissue, essentially limiting this mode of imaging to studies in small animals.
Tumor targeting using human MSCs has also been studied using positron emission tomography,29 which is very sensitive but requires radiolabeling of cells prior to grafting. Again, spatial resolution is poor, and the short decay times of the radioligands make it difficult for sequential imaging of cell vector distribution over time.
Concerns for the Development of MSC-Based Tumor Therapy
To translate MSC-based anticancer strategies into clinical therapy, it is essential to identify and minimize treatment-associated risks. Indeed, potential hazards linked to the use of MSC gene therapy vectors have emerged.
Currently, MSCs are usually cultured in FCS-containing medium, even for clinical use. Unquestionably, this is a concern because FCS is a very complex supplement containing an undefined mixture of proteins, growth factors, hormones, amino acids, etc., and possibly also infectious agents such as prions. FCS is therefore considered a high-risk ancillary material when used for the manufacture of cell, gene, and tissue-engineered products. Thus, FCS alternatives, such as platelet lysate, have been investigated for MSC culturing.96,97,98 Moreover, several companies have developed serum-free MSC culture systems, which might be used in a clinical setting, provided that media production is performed under Good Manufacturing Practice conditions. On the other hand, the safety of allogeneic MSC therapy with regard to bacterial and viral transmission is not a major concern because MSC donors and products can be screened with an efficiency similar to that used for volunteer blood donors and stem cell donors. Moreover, using off-the-shelf third-party MSCs allows for repetitive donor testing before cells are released for transplantation.
Another possible risk for clinical application is that MSC culturing, which is necessary to obtain sufficient numbers for therapeutic use, might result in malignant transformation. It has been reported that human MSCs can be cultured safely for standard expansion periods (6–12 weeks99,100). However, conflicting data exist on the risk of the malignant transformation of murine and human MSCs following long-term ex vivo culturing.99,101 Clearly, for clinical use, well-defined in vitro procedures avoiding long-term passage of MSC cultures will be required, along with thorough investigation of possible chromosomal abnormalities.
MSCs can also contribute to the tumor neovascular network and to tumor stroma formation.65,73 Human MSCs can switch phenotypes into tumor-associated fibroblast-like cells and provide structural support that stimulates tumor growth, e.g., by the production of interleukin-6 (refs. 65,102). The role of MSCs is ambiguous with regard to tumor metastasis. It has been shown that tumor pericytes can limit tumor cell metastasis.103 The loss of mouse tumor vessel pericytes dramatically increases blood-borne tumor cell dissemination by destabilizing tumor microvessels.103 In contrast, when mixed with breast cancer cells, human MSCs were shown to increase the metastatic potential.63 This effect is dependent on MSC secretion of the chemokine CCL5, which acts in a paracrine fashion on the cancer cells and enhances their invasive capacity.63
Immunomodulatory properties of MSCs can suppress the antitumor immune response.62 MSCs exert immunosuppressive effects via several mechanisms, including suppression of T-cell proliferation and cytokine production.104 However, the effects of MSCs on immune effector cells are diverse and complex. MSCs can both suppress and promote T-cell proliferation,105 and recent results show that MSCs require a sufficiently strong, ongoing immune response to exert their immunosuppressive potential.106 In addition, the magnitude and mechanisms of MSC-mediated immune suppression vary among different species.107
Finally, bone marrow–derived cells can undergo fusion with tumor cells, which can potentially result in increased growth, drug resistance and metastatic ability of tumor cells.108 Obviously, this phenomenon needs to be clarified before MSCs can be utilized as vectors in clinical therapy.
The functional role of MSCs/pericytes in cancer growth, invasion, and metastasis is currently a subject of controversy and intense research (Table 2).109 Hopefully, the identification of MSC sub-populations and the development of standardized culture protocols will help to solve some of these controversies. However, even when using standardized MSC culture protocols and defined MSC sub-populations, it is likely that the effect of MSCs/pericytes on tumors will vary depending on the tumor type and localization. Nevertheless, the above-mentioned concerns should not halt the ongoing development of MSC-based tumor gene therapy but rather justify the need for further studies.
Table 2.
Critical issues for development of MSC-based cancer therapy
Prospects for Clinical MSC-Based Tumor Therapy
A clinical scenario in which genetically modified MSCs (or alternative cells) are implanted into inoperable tumors or into parts of partially resected invasive tumors (e.g., GBM) can be envisaged. MSCs could be harvested quickly from third-party allogeneic donors, characterized, subtyped, and then transduced with therapeutic and diagnostic transgenes prior to implantation. The operative procedure would likely include injections at multiple sites within the tumor, possibly using stereotactic technique. The MSC intratumoral distribution pattern within experimental invasive tumors suggests that human MSCs implanted into a patient's tumors will migrate and deliver their therapeutic substance to the parts of the tumor that are inaccessible to surgery. Interestingly, a recent study showed that tumor irradiation enhances the migration of injected mouse MSCs toward tumors in mice.110 This effect might be due to irradiation-induced increased inflammatory components that attract MSCs to tumors. Thus, synergy between treatment modalities might be possible, and treatment effects could possibly be followed by serial MRI of labeled MSCs.
It is clear that the successful translation of experimental findings into the clinic is challenging. Issues such as MSC biosafety, culturing protocols, choice of therapeutic transgenes, and efficient and minimally invasive delivery protocols, as well as cost-effectiveness, need to be resolved before MSC-based therapy can be brought into routine clinical use (Table 2). Still, MSCs might, by virtue of their capacity to target tumor extensions and distant metastases, constitute a powerful treatment modality in our future repertoire of therapy options for otherwise incurable invasive cancers. Exciting prospects include the potential use of pericyte-like perivascular MSCs in antiangiogenic tumor therapy and the eradication of microvascular niche-residing CSCs in highly malignant gliomas.
Acknowledgments
We apologize to those investigators whose work has not been cited because of space limitations. We thank Ariane Tormin for providing photographs, and Salina Gunnarsson and Seema Rosqvist for valuable comments on the manuscript. The Bengzon Laboratory is supported by the Swedish Cancer Society, Magnus Bergvall Foundation, the Royal Physiographic Society in Lund, and the Hans and Märit Rausing Charitable Trust. The Scheding Laboratory is supported by the Swedish Research Council, the Crafoord Foundation, the Lundgren Foundation, John and Augusta Persson's Foundation, and the Swedish Governmental Agency for Innovation Systems (Vinnova). Both laboratories receive funding from the Swedish Childhood Cancer Foundation, Gunnar Nilsson's Cancer Foundation, and ALF (Government Public Health grant).
REFERENCES
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. (2005Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma N Engl J Med 352987–996. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Stiles CD., and , Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Baksh D, Song L., and , Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF., and , Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BJ., and , McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Developmental Committee of the European Group for Blood and Marrow Transplantation. (2008Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study Lancet 3711579–1586. [DOI] [PubMed] [Google Scholar]
- Au P, Tam J, Fukumura D., and , Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Pulkkanen KJ., and , Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL., and , Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- Moore XL, Lu J, Sun L, Zhu CJ, Tan P., and , Wong MC. Endothelial progenitor cells' “homing” specificity to brain tumors. Gene Ther. 2004;11:811–818. doi: 10.1038/sj.gt.3302151. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- Pisati F, Belicchi M, Acerbi F, Marchesi C, Giussani C, Gavina M, et al. Effect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal models. Cancer Res. 2007;67:3054–3063. doi: 10.1158/0008-5472.CAN-06-1384. [DOI] [PubMed] [Google Scholar]
- Tabatabai G, Bähr O, Möhle R, Eyüpoglu IY, Boehmler AM, Wischhusen J, et al. Lessons from the bone marrow: how malignant glioma cells attract adult haematopoietic progenitor cells. Brain. 2005;128 Pt 9:2200–2211. doi: 10.1093/brain/awh563. [DOI] [PubMed] [Google Scholar]
- Han X, Meng X, Yin Z, Rogers A, Zhong J, Rillema P, et al. Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle. 2009;8:606–610. doi: 10.4161/cc.8.4.7731. [DOI] [PubMed] [Google Scholar]
- Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- Miletic H, Fischer Y, Litwak S, Giroglou T, Waerzeggers Y, Winkeler A, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17:183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ., and , Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT., and , Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- Kallifatidis G, Beckermann BM, Groth A, Schubert M, Apel A, Khamidjanov A, et al. Improved lentiviral transduction of human mesenchymal stem cells for therapeutic intervention in pancreatic cancer. Cancer Gene Ther. 2008;15:231–240. doi: 10.1038/sj.cgt.7701097. [DOI] [PubMed] [Google Scholar]
- Duan X, Guan H, Cao Y., and , Kleinerman ES. Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into Ewing sarcoma tumors. Cancer. 2009;115:13–22. doi: 10.1002/cncr.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Tang J, Song C, Yang Z, Hirst DG, Zheng QJ, et al. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009;11:516–526. doi: 10.1080/14653240902960429. [DOI] [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ding Q, Wu Z, Jiang H., and , Fang Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010;290:157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Uchibori R, Okada T, Ito T, Urabe M, Mizukami H, Kume A, et al. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009;11:373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- Xin H, Sun R, Kanehira M, Takahata T, Itoh J, Mizuguchi H, et al. Intratracheal delivery of CX3CL1-expressing mesenchymal stem cells to multiple lung tumors. Mol Med. 2009;15:321–327. doi: 10.2119/molmed.2009.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M., and , Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs (Print) 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen JK, Kekarainen T, E Heinonen S, Laitinen A, Nystedt J, Laine J, et al. Umbilical cord blood-derived progenitor cells enhance muscle regeneration in mouse hindlimb ischemia model. Mol Ther. 2007;15:2172–2177. doi: 10.1038/sj.mt.6300302. [DOI] [PubMed] [Google Scholar]
- Sato H, Kuwashima N, Sakaida T, Hatano M, Dusak JE, Fellows-Mayle WK, et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12:757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- Schichor C, Birnbaum T, Etminan N, Schnell O, Grau S, Miebach S, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Aghi M., and , Chiocca EA. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther. 2005;12:994–1005. doi: 10.1016/j.ymthe.2005.07.693. [DOI] [PubMed] [Google Scholar]
- Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL., and , Tse VC. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K., and , Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., and , Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bergers G., and , Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D., and , Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG., and , Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- Spaeth E, Klopp A, Dembinski J, Andreeff M., and , Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- Tormin A, Brune JC, Olsson E, Valcich J, Neuman U, Olofsson T, et al. Characterization of bone marrow-derived mesenchymal stromal cells (MSC) based on gene expression profiling of functionally defined MSC subsets. Cytotherapy. 2009;11:114–128. doi: 10.1080/14653240802716590. [DOI] [PubMed] [Google Scholar]
- Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P, et al. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27:1366–1375. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ, Hertens E., and , Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S., and , Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson LB, Varas L, Kjellman C, Edvardsen K., and , Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75:248–255. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245–251. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- Kucerova L, Altanerova V, Matuskova M, Tyciakova S., and , Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zischek C, Niess H, Ischenko I, Conrad C, Huss R, Jauch KW, et al. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z., and , Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18:223–231. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R, et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- Elzaouk L, Moelling K., and , Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006;15:865–874. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Hong X, Miller C, Savant-Bhonsale S., and , Kalkanis SN.2009Antitumor treatment using interleukin- 12-secreting marrow stromal cells in an invasive glioma model Neurosurgery 641139–1146.discussion 1146 [DOI] [PubMed] [Google Scholar]
- Xu G, Jiang XD, Xu Y, Zhang J, Huang FH, Chen ZZ, et al. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol Int. 2009;33:466–474. doi: 10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- Gunnarsson S, Bexell D, Svensson A, Siesjö P, Darabi A., and , Bengzon J. Intratumoral IL-7 delivery by mesenchymal stromal cells potentiates IFNgamma-transduced tumor cell immunotherapy of experimental glioma. J Neuroimmunol. 2010;218:140–144. doi: 10.1016/j.jneuroim.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- Menon LG, Kelly K, Yang HW, Kim SK, Black PM., and , Carroll RS. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27:2320–2330. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebinger MR, Eddaoudi A, Davies D., and , Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., and , Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AT., and , Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM., and , Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., and , Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E., and , Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, et al. High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–488. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- Räty JK, Liimatainen T, Unelma Kaikkonen M, Gröhn O, Airenne KJ, Jumani Airenne K, et al. Non-invasive Imaging in Gene Therapy. Mol Ther. 2007;15:1579–1586. doi: 10.1038/sj.mt.6300233. [DOI] [PubMed] [Google Scholar]
- Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009;69:8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jiang Q, Jiang F, Ding G, Zhang R, Wang L, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23:281–287. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Glod J, Arbab AS, Noel M, Ashari P, Fine HA, et al. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- Thu MS, Najbauer J, Kendall SE, Harutyunyan I, Sangalang N, Gutova M, et al. Iron labeling and pre-clinical MRI visualization of therapeutic human neural stem cells in a murine glioma model. PLoS ONE. 2009;4:e7218. doi: 10.1371/journal.pone.0007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009;27:1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X., and , Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003;14:1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- Müller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8:437–444. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- Schallmoser K, Rohde E, Reinisch A, Bartmann C, Thaler D, Drexler C, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185–196. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X, Håkansson J, Ståhlberg A, Lindblom P, Betsholtz C, Gerhardt H, et al. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and , Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11:570–583. doi: 10.1080/14653240903079377. [DOI] [PubMed] [Google Scholar]
- Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, et al. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 2009;41:2607–2611. doi: 10.1016/j.transproceed.2009.06.119. [DOI] [PubMed] [Google Scholar]
- Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- Pawelek JM., and , Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8:377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- Lazennec G., and , Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit. Stem Cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]