Abstract
Glycogen storage disease type Ia (GSD-Ia) patients deficient in glucose-6-phosphatase-α (G6Pase-α or G6PC) manifest disturbed glucose homeostasis. We examined the efficacy of liver G6Pase-α delivery mediated by AAV-GPE, an adeno-associated virus (AAV) serotype 8 vector expressing human G6Pase-α directed by the human G6PC promoter/enhancer (GPE), and compared it to AAV-CBA, that directed murine G6Pase-α expression using a hybrid chicken β-actin (CBA) promoter/cytomegalovirus (CMV) enhancer. The AAV-GPE directed hepatic G6Pase-α expression in the infused G6pc−/− mice declined 12-fold from age 2 to 6 weeks but stabilized at wild-type levels from age 6 to 24 weeks. In contrast, the expression directed by AAV-CBA declined 95-fold over 24 weeks, demonstrating that the GPE is more effective in directing persistent in vivo hepatic transgene expression. We further show that the rapid decline in transgene expression directed by AAV-CBA results from an inflammatory immune response elicited by the AAV-CBA vector. The AAV-GPE-treated G6pc−/− mice exhibit normal levels of blood glucose, blood metabolites, hepatic glycogen, and hepatic fat. Moreover, the mice maintained normal blood glucose levels even after 6 hours of fasting. The complete normalization of hepatic G6Pase-α deficiency by the G6PC promoter/enhancer holds promise for the future of gene therapy in human GSD-Ia patients.
Introduction
Glycogen storage disease type Ia (GSD-Ia or von Gierke disease, MIM 232200) is an autosomal recessive disorder caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC) that catalyzes the hydrolysis of glucose-6-phosphate to glucose and phosphate in the terminal step of gluconeogenesis and glycogenolysis.1 GSD-Ia patients manifest a phenotype of disturbed glucose homeostasis characterized by fasting hypoglycemia, hepatomegaly, nephromegaly, hypercholesterolemia, hypertriglyceridemia, hyperuricemia, lactic acidemia, and growth retardation.1 There is no cure for GSD-Ia, but many of the disease symptoms can be managed or improved using dietary therapies2,3 to maintain normoglycemia. Although the therapies are sufficiently successful to enable patients to attain near normal growth and pubertal development, the underlying pathological process remains uncorrected and patients continue to suffer from hyperlipidemia, hyperuricemia, hypercalciuria, hypocitraturia, and lactic acidemia.1,4,5 As a result, long-term complications including osteoporosis, gout, renal disease, pulmonary hypertension, and hepatic adenomas, which may undergo malignant transformation, still persist in GSD-Ia patients and become increasingly significant in older patients.1,4,5
To develop new therapies for GSD-Ia, we had previously generated G6Pase-α-deficient (G6pc−/−) GSD-Ia mice that manifest all of the symptoms of human GSD-Ia.6,7 If left untreated, the mice rarely survive weaning and live no more than 3 months, mimicking the lethality of the untreated human patients. Using these mice, we and others8,9,10 have investigated G6Pase-α gene transfer in vivo mediated by recombinant adeno-associated virus (AAV) pseudotypes 2/1 (AAV1)11,12 and 2/8 (AAV8)13,14 vectors. Using AAV1 expressing murine G6Pase-α directed by a hybrid chicken β-actin (CBA) promoter and a cytomegalovirus (CMV) enhancer,15 we8 showed that G6Pase-α activity in the liver of the infused G6pc−/− mice at age 24–57 weeks could be elevated to 11% of normal activity, resulting in markedly improved survival and normalized metabolic profiles of the infused mice; however, blood glucose levels after fasting were not examined. Similar extended therapeutic outcomes were observed in G6pc−/− mice treated with an AAV8 vector expressing canine G6Pase-α under the control of nucleotides −1372 to −11 of the canine G6PC 5′-flanking sequence that contain a TATA box, a CCAAT box, and putative binding sites for liver-enriched transcription factors.9 At age 7 months, the AAV-G6PC-infused mice had 29% of wild-type hepatic G6Pase-α activity but exhibited subnormal blood glucose and subnormal fasting glucose levels.9 The best AAV-G6PC-mediated gene therapy results for GSD-Ia to date were observed in G6pc−/− mice treated with an AAV8 vector expressing human G6Pase-α under the control of nucleotides −298 to +128 of the human G6PC 5′-flanking sequence,10 which contain hepatocyte nuclear factor 1 and 3 binding motifs, cyclic adenosine monophosphate (cAMP) response elements, and insulin response elements with a TATA box at nucleotides −28 to −22.16,17 In that study, there was a restoration of hepatic G6Pase-α activity to wild-type levels and the infused mice exhibited normoglycemia.10 However, at age 26 weeks, blood glucose levels in the treated mice were lower than those in the wild-type littermates. In addition, fasting blood glucose levels in the infused animals were lower than those in wild-type mice, and the infused mice had elevated hepatic glycogen content and exhibited a mild hepatomegaly.10 Because the human and murine G6Pase-α have an overall 88% amino acid identity, sharing an identical topography and active site,18 it is not surprising that the human G6Pase-α protein is capable of correcting murine GSD-Ia.10
The inability of the human G6PC promoter at nucleotides −298 to +128 to completely correct hepatic G6Pase-α deficiency raised the question whether additional enhancer elements 5′ of nucleotide −298 of the human G6PC promoter were essential for optimal in vivo hepatic G6PC expression. Sequence analysis of nucleotides −2864 to −298 region of the human G6PC 5′-flanking region predicts the presence of multiple transcription factor binding sites, which might further control in vivo expression of the G6PC gene. In this study, we constructed AAV-GPE, an AAV8 vector expressing human G6Pase-α under the control of the human G6PC promoter/enhancer at nucleotides −2864 to −1 and show that AAV-GPE corrects the metabolic abnormalities of murine GSD-Ia. We also show that AAV-GPE is more efficient in directing persistent, in vivo, hepatic transgene expression than the widely used hybrid CBA promoter/CMV enhancer. Significantly, the infused animals exhibit wild-type levels of hepatic G6Pase-α activity with blood glucose levels indistinguishable from the control mice. Importantly, the infused G6pc−/− mice no longer suffer the fasting hypoglycemia typical of human GSD-Ia patients.
Results
AAV-GPE infusion directs long-term hepatic G6Pase-α expression
To examine the in vivo impact of sequences upstream of nucleotides −298 of the human G6PC promoter element previously studied,10 we constructed AAV-GPE, an AAV8 vector expressing human G6Pase-α under the control of nucleotides −2864 to −1 of the human G6PC 5′-flanking region. Because there is no standard age at which to initiate AAV-mediated gene therapy in mice8,9,10 and there is evidence that the loss of efficiency and persistence of gene transfer is influenced by the increased rate of hepatocellular proliferation associated with liver growth,19 we chose to infuse G6pc−/− mice at three different ages, 2-day-old, 2-week-old, or 4-week-old, and examine hepatic G6Pase-α expression out to 24 weeks of age. Despite the difference in age, each group of mice was infused with the same dose of AAV-GPE (1.2 × 1011 viral particles (vg)/mouse). Metabolic profiles of the infused animals were monitored during the 24-week study and all measurements compared to those of their G6pc+/+/G6pc+/− littermates and 4- to 6-week-old untreated G6pc−/− mice. GSD-Ia is an autosomal recessive disorder and previous studies have shown that the phenotype of the G6pc+/+ and G6pc+/− littermates are indistinguishable and wild type.6
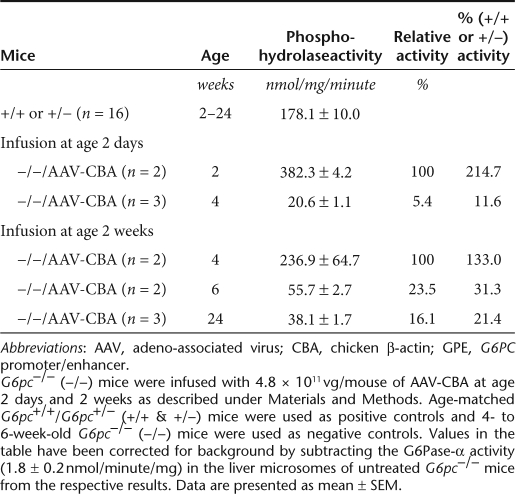
There were no premature deaths in the infused G6pc−/−animals for the duration of the 24-week study, regardless of the age of infusion. In G6pc−/− mice infused at age 2 days with AAV-GPE (1.2 × 1011 vg/mouse, equivalent to 6 × 1013 vg/kg), hepatic G6Pase-α activity was 77.6% of control activity at age 2 weeks, declining to 16.2% at age 4 weeks and 6.5% of control activity at age 6 weeks (Table 1). However beyond 6 weeks, the levels of hepatic G6Pase-α activity stabilized out to 24 weeks (Table 1). Therefore, expression dropped 11.9-fold over the entire 24-week study, with most of the drop occurring within the first 6 weeks.
Table 1.
Hepatic G6Pase activity in G6pc−/− mice infused with 1.2 × 1011 vg/mouse of AAV-GPE
In contrast, in G6pc−/− mice infused at age 2 weeks with AAV-GPE (1.2 × 1011 vg/mouse, equivalent to 1.5 × 1013 vg/kg) hepatic G6Pase-α activity 2 weeks post-infusion, at age 4 weeks, was 2.4-fold higher than the activity in their G6pc+/+/G6pc+/− littermates (Table 1), reaching 433.4 ± 11.1 nmol/mg/minute. Although hepatic G6Pase-α activity did subsequently undergo a 2.6-fold decline between ages 4 and 6 weeks, it resulted in a near normal hepatic G6Pase-α activity (174.0 ± 22.4 nmol/mg/minute) being maintained from age 6 weeks on for the duration of the 24-week study (Table 1). Similarly, in G6pc−/− mice infused with AAV8-GPE at age 4 weeks with the same dosage (1.2 × 1011 vg/mouse, equivalent to 1 × 1013 vg/kg), hepatic G6Pase-α activity at age 24 weeks was 335.6 ± 40.2 nmol/minute/mg, 1.9-fold higher than the activity in the control littermates (Table 1). These findings are consistent with the previous proposal19 that the loss of efficiency and persistence of gene transfer is influenced by the increased rate of hepatocellular proliferation associated with liver growth. Injections at later stages of development, when the rate of liver growth is lower, resulted in less gene expression loss.
The distribution of the G6Pase-α transgene expression in the liver was investigated. As expected, there was no stainable G6Pase-α activity in the liver sections of untreated G6pc−/− mice (Figure 1). In G6pc+/+/G6pc+/− mice, enzyme histochemical analysis showed G6Pase-α distributed throughout the liver but with significantly higher levels in proximity to blood vessels (Figure 1).
Figure 1.
Histochemical analysis of G6Pase-α activity in the liver of AAV-GPE and AAV-CBA infused G6pc−/− mice. Freshly sectioned liver specimens were analyzed for G6Pase-α activity using the method of lead trapping of phosphate generated by G6P hydrolysis.29 The age of the mice when infused is indicated at the far left (2 days, 2 weeks, or 4 weeks). The age of the mice at the time of sectioning is indicated by the labeling closest to the panels: 2W, 2 weeks; 4W, 4 weeks; 6W, 6 weeks; 24W, 24 weeks of age. Treatments are indicated by: (−/−) untreated G6pc−/−; (+/+) untreated G6pc+/+; (−/− GPE) G6pc−/− mice infused with 1.2 × 1011 vg/mouse of AAV-GPE; (−/− CBA) G6pc−/− mice infused with 4.8 × 1011 vg/mouse of AAV-CBA. In each pair of images, the left panel is ×50 magnification and the right panel ×200. AAV, adeno-associated virus; CBA, chicken β-actin; GPE, G6PC promoter/enhancer; G6P, glucose-6-phosphate.
In G6pc−/− mice infused at age 2 days with AAV-GPE, hepatic G6Pase-α activity was distributed throughout the liver at age 2 weeks (Figure 1). Unlike wild-type mice, the expression was uneven with foci that stained stronger than in the control livers. The stained G6Pase-α activity markedly decreased from age 2 to 4 weeks (Figure 1), consistent with a 4.8-fold decline in phosphohydrolase activity (Table 1). Again, the stained G6Pase activity decreased and stabilized at age 6 weeks and older (data not shown).
In G6pc−/− mice infused with AAV-GPE at age 2 or 4 weeks, enzyme histochemical analyses again showed that the G6Pase-α transgene was distributed throughout the liver with foci containing significantly higher levels of enzymatic activity. Again, G6Pase-α activities estimated by histochemical analyses (Figure 1) were in agreement with quantitative phosphohydrolase assays (Table 1). In G6pc−/− mice infused with AAV-GPE at age 2 weeks, there were cells in the liver that stained less intensely than cells in the wild-type livers. Therefore, a normal pattern of hepatic G6Pase-α expression was not restored in AAV-GPE-infused mice despite exhibiting wild-type G6Pase-α activity.
AAV-CBA infusion directs lower levels of hepatic G6Pase-α expression
The CBA promoter/CMV enhancer has been widely used to direct high levels of hepatic transgene expression.15 However, the CMA promoter/enhancer is known to be silenced by extensive CpG and non-CpG methylation.20,21 Previous in vivo experiments using AAV-CBA, an AAV8 vector expressing murine G6Pase-α under the control of the hybrid CBA promoter/CMV enhancer had shown poor expression,8 possibly related to CMV promoter methylation. To compare the in vivo efficacy of hepatic gene transfer between AAV-CBA and AAV-GPE, G6pc−/− mice were infused with an increased dose of AAV-CBA at 4.8 × 1011 vg/mouse in a similar manner to the AAV-GPE experiments—at ages 2 days and 2 weeks and followed to 24 weeks of age.
For G6pc−/− mice infused at 2 days of age, hepatic G6Pase-α activity at age 2 weeks was 2.8-fold higher in AAV-CBA-infused mice compared to AAV-GPE-infused mice (Tables 1 and 2), reflecting the fourfold higher dosage of AAV8-CBA infused. However, hepatic G6Pase-α activity in the neonatally AAV-CBA-infused animals declined rapidly to 20.6 ± 1.1 nmol/mg/minute at age 4 weeks (Table 2), an 18.6-fold decline in 2 weeks, compared to a 4.8-fold decline in neonatal G6pc−/− mice infused with AAV-GPE (Table 1). Because the CBA and GPE are in identical vector background, this finding suggests that the CBA promoter/CMA enhancer is less efficient in directing persistent in vivo hepatic transgene expression than the G6PC promoter/enhancer.
Table 2.
Hepatic G6Pase activity in G6pc−/− mice infused with 4.8 × 1011 vg/mouse of AAV-CBA
In G6pc−/− mice infused with 4.8 × 1011 vg/mouse of AAV-CBA at age 2 weeks, hepatic G6Pase-α activity was 236.9 ± 64.7 at age 4 weeks (Table 2), which was 1.83-fold lower than 4-week-old G6pc−/− mice infused at age 2 weeks with 1.2 × 1011 vg/mouse of AAV-GPE (Table 1). Moreover, hepatic G6Pase-α activity continued to decline with the CBA vector from 55.7 ± 2.7 nmol/mg/minute at age 6 weeks to 38.1 ± 1.7 nmol/mg/minute at age 24 weeks (Table 2). This was in contrast to the levels expressed with the GPE vector that stabilized over this time period at near wild-type levels (Table 1).
Enzyme histochemical analyses of AAV-CBA-infused animals showed that the activity staining (Figure 1) was similar to those of the GPE vector, unevenly distributed with numerous foci that stained stronger than that in the control livers (Figure 1). But consistent with the quantitative phosphohydrolase assays (Table 2), the overall stain intensities were significantly lower with increasing age of the infused mice.
AAV-GPE infusion corrects pathological manifestations of GSD-Ia
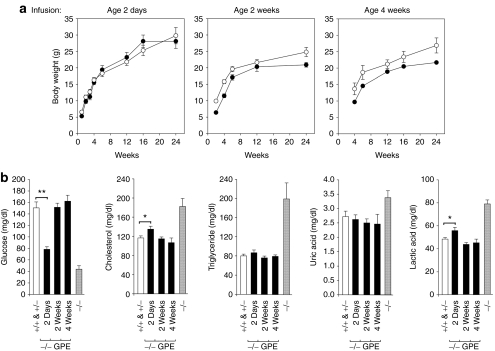
G6pc−/− mice under glucose therapy are growth retarded and by 2 weeks of age their average body weight is approximately 60% of their G6pc+/+/G6pc+/− littermates.6 Neonatal G6pc−/− mice infused with AAV-GPE had a markedly improved growth rate and the body weights of the infused animals were comparable to the control mice (Figure 2a). G6pc−/− mice infused at age 2 or 4 weeks with AAV-GPE exhibited a growth curve that paralleled their G6pc+/+/G6pc+/− littermates but at lower values, consistent with the lower starting body weights of G6pc−/− mice before commencing gene therapy (Figure 2a).
Figure 2.
Phenotype analysis of AAV-GPE-infused G6pc−/− mice. (a) Postnatal development. Data for female G6pc−/− mice infused with 1.2 × 1011 vg/mouse of AAV-GPE and their female G6pc+/+/G6pc+/− littermates are shown. The age at infusion is shown above the graph. (open circles), G6pc+/+/G6pc+/− mice; (closed circles), AAV-GPE-infused G6pc−/− mice. (b) Blood glucose, cholesterol, triglyceride, uric acid, and lactic acid levels. Because of the similarities of the respective metabolites in each group, data shown are pooled data of age 6–24 weeks. (+/+ & +/−), G6pc+/+/G6pc+/−, (−/−), G6pc−/−, or (−/− GPE), G6pc−/− mice infused with AAV-GPE at age 2 days (n = 36), 2 weeks (n = 24), or 4 weeks (n = 9). Data are presented as mean ± SEM. *P < 0.05 and **P < 0.005. AAV, adeno-associated virus; GPE, G6PC promoter/enhancer.
Under glucose therapy, the G6pc−/− mice continue to manifest hypoglycemia, hypercholesterolemia, hypertriglyceridemia, hyperuricemia, and lactic acidemia.6,7 In contrast, the AAV-GPE-infused G6pc−/− mice had normal blood glucose profiles (Figure 2b) and none of the infused animals suffered from the frequent hypoglycemic seizures typical of the untreated G6pc−/− mice6 and human GSD-Ia patients.1 Blood glucose levels in neonatally infused G6pc−/− mice were significantly lower than their control littermates (Figure 2b), suggesting that hepatic G6Pase-α activity restored to 6.5% of control levels is insufficient to maintain normal blood glucose profile. In contrast, blood glucose levels in G6pc−/− mice infused with AAV-GPE at age 2 or 4 weeks were indistinguishable from those in their control littermates (Figure 2b). AAV-GPE infusion also normalized serum cholesterol, triglyceride, uric acid, and lactic acid levels, although neonatally infused G6pc−/− mice had slightly higher levels of blood cholesterol and lactic acid (Figure 2b).
Hepatomegaly is another clinical presentation in GSD-Ia and is primarily caused by excess glycogen and lipid deposition.1 No histological abnormality was observed in the liver tissue sections of the unaffected and AAV-GPE-transduced mice at age 24 weeks (Figure 3a). Glycogen content in the liver of 24-week-old G6pc+/+/G6pc+/− mice averaged 1.89 ± 0.17 nmol glucosyl units per mg protein (Figure 3a). In neonatally AAV-GPE-infused animals, the glycogen content at 24 weeks of age was significantly higher at 4.65 ± 0.19 nmol glucosyl units per mg protein, indicative of the glycogen storage defect observed in GSD-Ia mice. In contrast, the mice receiving infusions at 2 or 4 weeks of age showed wild-type levels of glycogen at 24 weeks of age, namely, 1.61 ± 0.39 and 1.65 ± 0.19 nmol glucosyl units per mg protein, respectively (Figure 3a), indicative of the absence of the characteristic histology of GSD-Ia disease at this stage of development.
Figure 3.
Histological and liver glycogen and lipid analyses in wild-type and AAV-GPE-infused G6pc−/− mice at 24 weeks of age. Representative results are shown. (+/+), G6pc+/+/G6pc+/− mice (n = 5) and (−/− GPE) G6pc−/− (−/−) mice infused with 1.2 × 1011 vg/mouse of AAV-GPE mice at age 2 days (n = 3), 2 weeks (n = 3), or 4 weeks (n = 3). (a) H&E stained liver sections at original magnifications of ×200 and hepatic glycogen contents. Each plate represents an individual mouse, so two mice are shown for each age of infusion. For quantitation, the data for glycogen are presented as mean ± SEM. **P < 0.005. (b) Oil red O staining at original magnifications of ×400 and quantitative histochemical measurement of lipid deposition. For quantitation, the lipid imaged with Oil red O staining was converted into pixel density units using Adobe Photoshop and data are presented as mean ± SEM. AAV, adeno-associated virus; GPE, G6PC promoter/enhancer.
Oil red O staining showed that the lipid contents in AAV-GPE-infused animals were similar to that in the G6pc+/+/G6pc+/− littermates at age 24 weeks (Figure 3b). For quantitative histochemical measurement, lipid imaged with Oil red O stain was converted into pixel density units using Adobe Photoshop. Results in Figure 3b showed that the density units in the liver of AAV-GPE-treated G6pc−/− mice were lower than those in the control mice, although the difference was not statistically significant (Figure 3b). Taken together, these results indicate AAV-GPE-infused G6pc−/− mice exhibited no histological abnormality and had normal glycogen and fat contents in the liver.
AAV-GPE-infused G6pc−/− mice exhibit normal fasting glucose and glucose tolerance profile
Fasting blood glucose levels were examined in 12- and 14-week-old G6pc−/− mice infused with AAV-GPE at age 2 and 4 weeks, respectively. Blood glucose levels in G6pc+/+/G6pc+/− mice were unchanged after 6 hours of fasting (Figure 4a). Importantly, blood glucose levels in G6pc−/− mice infused with AAV-GPE at either age 2 or 4 weeks were also unchanged after 6 hours of fasting (Figure 4a), demonstrating that the infused G6pc−/− mice no longer suffer from fasting hypoglycemia, characteristics of GSD-Ia.1 Similar fasting experiments with the untreated G6pc−/− mice resulted in rapid hypoglycemia followed by hypoglycemic seizures after only a short fast.
Figure 4.
Glucose homeostasis in AAV-GPE-infused G6pc−/− mice. G6pc−/− mice were infused with 1.2 × 1011 vg/mouse of AAV-GPE at age 2 weeks or 4 weeks and fasting blood glucose or glucose tolerance analyses were conducted at 10 weeks post-infusion at age 12 weeks (n = 4) and 14 weeks (n = 4), respectively. Age matched G6pc+/+/G6pc+/− mice (n = 4) were used as controls. (a) Fasting blood glucose analysis. (b) Glucose tolerance test. G6pc+/+/G6pc+/− or AAV-GPE-infused G6pc−/− mice were fasted for 6 hours, injected subcutaneously with 0.25 ml of 10% dextrose, then sampled for blood every 30 minutes via the tail vein. Data are presented as mean ± SEM. (open circles), G6pc+/+/G6pc+/− mice; (closed circles), AAV-GPE-infused G6pc−/− mice. AAV, adeno-associated virus; GPE, G6PC promoter/enhancer.
Studies have shown that overexpression of hepatic G6Pase-α may induce diabetes.22,23,24 Because hepatic G6Pase-α activity in 24-week-old G6pc−/− mice infused at age 4 weeks with AAV-GPE was nearly twofold higher than the activity in their G6pc+/+/G6pc+/− littermates, we conducted glucose tolerance test in 14-week-old G6pc−/− mice infused at age 4 weeks with AAV-GPE. As a control, we also conducted glucose tolerance test in 12-week-old G6pc−/− mice infused at age 2 weeks with AAV-GPE exhibiting wild-type levels of hepatic G6Pase-α activity. Results in Figure 4b showed that glucose tolerance profiles in the infused G6pc−/− mice were indistinguishable from that of control littermates.
Absence of immune response against human G6Pase-α
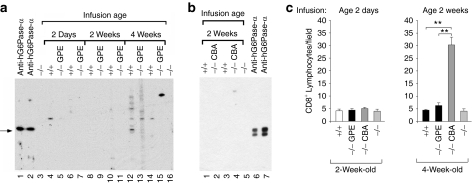
To determine whether a humoral response directed against human G6Pase-α is generated in the infused G6pc−/− mice, we performed western blot analysis using sera from mice infused with AAV-GPE (Figure 5a) or AAV-CBA (Figure 5b). As a positive control, we used a rabbit anti-human G6Pase-α antiserum that also recognizes murine G6Pase-α.25 No antibodies directed against G6Pase-α were detected in any of the AAV-GPE-infused (Figure 5a, lanes 5, 7, 9, 11, 13, and 15) or AAV-CBA-infused (Figure 5b, lanes 2 and 4) G6pc−/− mice that lived to age 24 weeks. Furthermore, there were no endogenous antibodies directed against G6Pase-α present in the sera of G6pc+/+/G6pc+/− littermates or untreated G6pc−/− mice (Figure 5).
Figure 5.
Analysis of anti-G6Pase-α antibodies and hepatic infiltration of CD8+ lymphocytes. Microsomal proteins from Ad-human G6Pase-α or Ad-mouse G6Pase-α infected COS-1 cells were electrophoresed through a single 12% polyacrylamide-SDS gel and transferred onto a PVDF membrane. Membrane strips, representing individual lanes on the gel were individually incubated with the appropriate antiserum. The antigen–antibody complex was visualized as described under Materials and Methods. (a) Antibodies against human G6Pase-α. Lanes 1 and 2: anti-human (h) G6Pase-α antiserum (1:3000 dilution); lanes 3 and 16: serum samples (1:200 dilution) from G6pc−/− mice (−/−), lanes 4, 6, 8, 10, 12, and 14: serum samples (1:200 dilution) from 24-week-old G6pc+/+/G6pc+/− (+/+) mice, or serum samples (1:200 dilution) from 24-week-old G6pc−/− mice infused with AAV-GPE (−/− GPE) at age 2 days (lanes, 5 and 7), 2 weeks (lanes 9 and 11), or age 4 weeks (lanes 13 and 15). (b) Antibodies against mouse G6Pase-α. Lanes 1 and 3: serum samples (1:200 dilution) from 24-week-old G6pc+/+/G6pc+/− mice, lanes 2 and 4: serum samples (1:200 dilution) from 24-week-old G6pc−/− mice infused with AAV-CBA (−/− CBA) at age 2 weeks, lane 5, serum sample (1:200 dilution) from G6pc−/− mice, lanes 6 and 7; anti-hG6Pase-α antiserum (1:3000 dilution) that recognizes murine G6Pase-α.25 Each lane denotes an individual mouse. The arrow denotes G6Pase-α. (c) Quantification of hepatic CD8+ lymphocyte infiltration. CD8+ lymphocytes were scored at age 2 weeks in G6pc−/− mice infused with AAV-GPE (−/− GPE) or AAV-CBA (−/− CBA) at age 2 days or at age 4 weeks in G6pc−/− mice infused with AAV-GPE or AAV-CBA at age 2 weeks; 2- and 4-week-old G6pc+/+/G6pc+/− (+/+) and G6pc−/− (−/−) mice were used as controls. Results are the mean ± SEM. Each point represents the average of three to four animals. **P < 0.005. AAV, adeno-associated virus; CBA, chicken β-actin; GPE, G6PC promoter/enhancer; PVDF, polyvinylidene fluoride; SDS, sodium dodecyl sulfate.
AAV-CBA infusion elicits increased hepatic CD8+ lymphocyte infiltration
The absence of detectable antibodies against G6Pase-α in the liver of AAV-CBA-infused G6pc−/− mice suggests that a cell-mediated immune response to the G6Pase-α transgene is not the cause for the rapid decline in transgene expression directed by this vector. Another possibility is an inflammatory immune response elicited by the AAV-CBA vector. We therefore examined hepatic CD8+ lymphocyte infiltration 2 weeks following infusion of G6pc−/− mice with AAV-CBA or AAV-GPE. In 2- and 4-week-old wild-type or G6pc−/− mice, hepatic CD8+ lymphocyte counts were low (Figure 5c). In G6pc−/−mice infused with AAV-CBA or AAV-GPE at age 2 days, hepatic CD8+ lymphocyte counts at age 2 weeks were similar for each of the vector types and comparable to the counts in untreated 2-week-old wild-type or G6pc−/− animals (Figure 5c). In G6pc−/− mice infused with AAV-GPE at age 2 weeks, hepatic CD8+ lymphocyte counts remained low at age 4 weeks. In contrast, in G6pc−/− mice infused with AAV-CBA at age 2 weeks, hepatic CD8+ lymphocyte counts were markedly increased at age 4 weeks (Figure 5c). The results suggest that an inflammatory response elicited by AAV-CBA may explain, at least in part, the rapid decline and low efficacy of hepatic G6Pase-α expression directed by the CBA promoter/CMV enhancer.
Discussion
Previous gene therapy studies using the mouse model of GSD-Ia have shown that G6Pase-α-expressing AAV vectors directed by the CBA promoter/CMV enhancer,8 the canine G6PC promoter,9 or the human G6PC promoter at nucleotides −298 to +128 of the G6PC 5′ flanking region10 deliver the G6Pase-α transgene to the liver and achieve extended correction of this disorder. However, although these studies have shown promise, none have been capable of completely correcting hepatic G6Pase-α deficiency. In this study, we explored the in vivo efficacy of hepatic G6Pase-α gene delivery in G6pc−/− mice using AAV-GPE, an AAV8 vector expressing human G6Pase-α driven by an extended region of the human G6PC 5′ flanking region at nucleotides −2864 to −1. Studies have shown that the efficiency and persistence of AAV-mediated hepatic gene transfer are lower during early development because the fast rate of hepatocellular proliferation associated with liver growth, which can dilute out the number of cells, effectively infected with AAV.19 We therefore examined the impact of infusing G6pc−/− mice at three different ages, 2-day-old, when hepatocellular proliferation is high; 2 weeks old, when hepatocellular proliferation is decreasing; and 4 weeks old, where the rate of hepatocellular proliferation has dropped dramatically.
All three infusion strategies were successful in restoring hepatic G6Pase-α activity in the G6pc−/− mice. Biochemical measurement of hepatic microsomes isolated from 24-week-old G6pc−/− mice treated at age 2 days, 2 weeks, or 4 weeks with the same dose (1.2 × 1011 vg/mouse) of AAV-GPE showed sustained restoration of 6.5, 97, and 188%, respectively, of normal liver G6Pase-α activity. This suggests that infusion of older mice, when the majority of the hepatocellular proliferation has dropped, is the most effective strategy for persistent transgene expression in the liver. All of the infused G6pc−/− mice grew normally, displayed no histological abnormalities in the liver, exhibited normalized blood metabolite profiles, and had no detectable anti-G6Pase-α antibodies. Importantly, G6pc−/− mice infused with AAV-GPE at age 2 or 4 weeks expressed hepatic G6Pase-α activity equal or higher than the activity in their G6pc+/+/G6pc+/− littermates, both by quantitative phorphohydrolase assays and by enzyme histochemical analyses. Unlike neonatally infused G6pc−/− mice that exhibited subnormal blood glucose levels and increased hepatic glycogen storage, G6pc−/− mice infused at an older age displayed normal blood glucose levels, normal hepatic storage of glycogen and lipid indistinguishable from their G6pc+/+/G6pc+/− littermates, and no longer suffered from hypoglycemia even after 6 hours of fasting. The total normalization of hepatic G6Pase-α deficiency indicates that in vivo glucose homeostasis can be adequately maintained by AAV-GPE-mediated gene therapy.
We also compared the efficacy of AAV-GPE-mediated G6Pase-α gene delivery to the liver of G6pc−/− mice with AAV-CBA, a murine G6Pase-α-expressing AAV8 vector driven by the widely used hybrid CBA promoter/CMV enhancer.15 Both vectors efficiently delivered the transgene to the liver but the persistence of transgene expression directed by the two vectors differed markedly. In AAV-GPE-infused G6pc−/− mice, hepatic G6Pase-α activity underwent a 12-fold decline from age 2 weeks to adulthood. However, in AAV-CBA-infused G6pc−/− mice, hepatic G6Pase-α activity underwent a 95-fold decline from age 2 weeks to adulthood. The primary mediators of AAV-directed gene expression in the liver are extrachromosomal forms of the AAV vectors,26 which become diluted or lost during hepatocellular proliferation.19 Although this may explain the 12-fold decline in hepatic G6Pase-α expression from age 2 to 6 weeks in AAV-GPE-mediated gene transfer, this is unlikely to explain why the AAV-CBA-mediated hepatic G6Pase-α expression declined 95-fold over a similar period. Another possibility is the generation of antibodies directed against the vector encoded G6Pase-α, but there was no evidence of this in our study. A further possibility is an inflammatory immune response against the AAV-CBA vector, which is absent with the AAV-GPE vector. In this respect, we did find elevated hepatic CD8+ lymphocyte infiltration elicited by the AAV-CBA vector which correlated with the rapid decline in transgene expression and low efficacy of this vector. Notably, there was no change in hepatic CD8+ lymphocyte infiltration in G6pc−/− mice infused with AAV-GPE. Whether this is the sole or primary mechanism of inactivation remains to be determined in more definitive future studies. The other plausible mechanism may also include methylation inactivation of the CMV enhancer in the CBA construct.20,21 Whatever the mechanisms impacting the CBA/CMV construct it is clear nucleotides −2864 to −1 of the G6PC 5′ flanking region, containing the native G6PC promoter/enhancer, is much more effective in directing persistent hepatic transgene expression and illustrates one of the advantages of seeking native control elements for gene therapy.
We have previously shown that when equivalent physical doses of AAV8 and AAV1 vectors were infused into G6pc−/− mice, the AAV8 vector directed almost twice the level of hepatic transgene expression than the AAV1 vector; however, only the AAV1 vector can direct kidney expression.8 As expected, AAV-GPE in an AAV8 vector directed a very low level of renal G6Pase-α expression and the infused G6pc−/− mice continued manifesting nephromegaly (data not shown). The different AAV serotypes display distinct tissue tropism, believed to be related to the distribution of their receptors on target cells. The primary receptors for AAV1 are alpha-2,3-linked or alpha-2,6-linked sialic acid27 while the primary receptor for AAV8 is the 37/67 kd laminin receptor.28 Therefore to correct G6Pase-α deficiency in both the liver and the kidney, infusion of both recombinant AAV8 and AAV1 vectors may be required.
Koeberl et al.10 showed that infusing 2-week-old G6pc−/− mice with 1 × 1013 vg/kg of an AAV8 vector expressing human G6Pase-α under the control nucleotides −298 to +128 of the human G6PC 5′ flanking region resulted in wild-type levels of G6Pase-α activity in the liver at age 26 weeks. However, the authors have noted that the levels of G6Pase-α expression detected by enzyme histochemical analysis were lower in AAV-treated G6pc−/−hepatocytes than that seen in the wild-type mouse liver.10 Moreover, although the infused animals exhibited normoglycemia, at age 26 weeks their blood glucose levels were lower than the wild-type mice.10 In addition, fasting blood glucose levels in the infused animals were lower than in the wild-type mice, and the infused mice had higher hepatic glycogen content and exhibited a mild hepatomegaly.10 This is in contrast to the present study where we have shown that G6pc−/− mice infused at age 2 weeks with 1.2 × 1011 vg/mouse (equivalent to 1.5 × 1013 vg/kg) of AAV-GPE harbored no metabolic abnormalities associated with hepatic G6Pase-α deficiency. In summary, the differences in the promoter as well as enhancer elements used in directing G6Pase-α expression are key factors. In this regard, additional regulatory elements contained within nucleotides −2864 to −298 of the G6PC 5′ flanking region are vital for optimal G6PC expression in vivo.
In summary, using a murine model of GSD-Ia, we have demonstrated that AAV-GPE-mediated gene therapy, when administered at 2–4 weeks of age, when hepatocellular proliferation has decreased, can effectively deliver the G6Pase-α transgene to the liver and completely normalize hepatic G6Pase deficiency in G6pc−/− mice. The therapy restores normal blood glucose and metabolite levels and normal glycogen and lipid storage in the liver, and is sufficient to prevent the development of fasting hypoglycemia. These are indeed promising developments. Future studies will address the impact of the therapy on the kidney and the longer term impact on mice beyond 24 weeks of age. Our results do suggest that G6Pase-α gene transfer may one day offer a therapeutic approach to the management of human GSD-Ia, especially for GSD-Ia patients in their teens who have not developed the severe long-term complication of hepatocellular adenomas or renal disease.
Materials and Methods
Construction of pUF11-G6PC-GPE and preparation AAV vectors. The pUF11-G6PC-GPE plasmid, containing human G6Pase-α under the control of the human G6PC promoter/enhancer was constructed by modifying pUF11-mG6Pase-α-CBA8 where the murine G6Pase-α is driven by the CBA promoter/CMV enhancer15 as follows: The Tkp-neo fragment from pUF11-mG6Pase-α-CBA was excised by XhoI/SphI digestion, the remaining vector gel purified, polished with T4 DNA polymerase, then self-ligated to yield pUF11-mG6Pase-α-CBA-[Tkp-neo] −/−. The mG6Pase-α, along with the CBA promoter/CMV enhancer, in pUF11-mG6Pase-α-CBA-[Tkp-neo] −/− was then substituted with the human G6Pase-α cDNA at 5′-SbfI and 3′-NotI sites, yielding pUF11-G6PC. Polymerase chain reaction was then used to clone nucleotides −2864 to −1 of the G6PC 5′-flanking region containing the human G6PC promoter/enhancer. The polymerase chain reaction template was a bacterial artificial chromosome containing the human G6PC gene (Invitrogen Life Technologies, Carsbad, CA) and the primer pairs were: 1S (5′-CCTTTGAGAATCCACGGTGT-3′) and 2AS (5′-CCTCATTTCCTTGGCACCTC-3′) that contain additional KpnI and XbaI sites at the 5′ and 3′ ends, respectively. The KpnI-XbaI fragment that contains the G6PC promoter/enhancer was then ligated into the KpnI-XbaI linearized pUF11-G6PC, to yield pUF11-G6PC-GPE-1. Next polymerase chain reaction was used to clone the chimeric intron from the pCI vector (Promega, Madison, WI) using a primer pair, 3S (5′-AGGTAAGTATCAAGGTTACA-3′) and 4AS (5′-ACCTGTGGAGAGAAAGGCAA-3′) that contains additional SpeI and SbfI sites at the 5′ and 3′ ends, respectively. This chimeric intron was then ligated as a SpeI-SbfI fragment into the SpeI-SbfI linearized large fragment of pUF11-G6PC-GPE-I, to yield pUF11-G6PC-GPE. All constructs were verified by DNA sequencing.
AAV-GPE and AAV-CBA were produced using pUF11-G6PC-GPE and pUF11-mG6Pase-α-CBA, respectively, and generated, purified, and tittered at the University of Florida Powell Gene Therapy Center Vector Core Laboratory as previously described.8 Vector genome quantitation was performed by the University of Florida NGVL Toxicology Core using real-time polymerase chain reaction with primers and probes directed against the G6PC or the CBA promoter.
Infusion of G6pc−/− mice with AAV vectors. All animal studies were conducted under an animal protocol approved by the NICHD Animal Care and Use Committee. A glucose therapy, which consists of intraperitoneal injection of 25–100 µl of 15% glucose, every 12 hours, was administered to the G6pc−/− mice as described previously.6 Mice that survived weaning were given unrestricted access to Mouse Chow (Zeigler Bros, Gardners, PA).
The AAV vector was infused into 2-day-old G6pc−/− mice via the temporal vein, and infused into 2- or 4-week-old G6pc−/− mice via the retro-orbital sinus. Age-matched G6pc+/+/G6pc+/− as well as 4- to 6-week-old G6pc−/− mice were used as controls. For the virus-infused mice, glucose therapy was terminated immediately after infusion.
Glucose tolerance testing of 12- or 14-week-old AAV-GPE-infused G6pc−/− mice consisted of fasting for 6 hours, prior to blood sampling, followed by the injection of 0.25 ml of 10% dextrose subcutaneously, and repeated blood sampling via the tail vein every 30 minutes for an additional 2 hours.
Phosphohydrolase assays. Microsome isolation and phosphohydrolase assays were determined essentially as described previously.6 Reaction mixtures (100 µl) contained 50 mmol/l cacodylate buffer, pH 6.5, 10 mmol/l glucose-6-phosphate, and appropriate amounts of microsomal preparations were incubated at 37 °C for 10 minutes. Disrupted microsomal membranes were prepared by incubating intact membranes in 0.2% deoxycholate for 20 minutes at 0 °C. Nonspecific phosphatase activity was estimated by pre-incubating disrupted microsomal preparations at pH 5.0 for 10 minutes at 37 °C to inactivate the acid labile G6Pase-α.
Enzyme histochemical analysis of G6Pase-α was performed by incubating 10-µm thick liver tissue sections for 10 minutes at room temperature in a solution containing 40 mmol/l Tris-maleate pH 6.5, 10 mmol/l glucose-6-phosphate, 300 mmol/l sucrose, and 3.6 mmol/l lead nitrate.29 The trapped lead phosphate was visualized following conversion to the brown colored lead sulfide.29
Phenotype analyses. Blood samples were collected from the tail vein. Blood glucose, total cholesterol, and uric acid were analyzed using kits obtained from Thermo Electron (Louisville, CO). Triglycerides were measured with a kit from Sigma Diagnostics (St. Louis, MO) and lactate measured with a kit from Trinity Biotech (St. Louis, MO).
For hematoxylin and eosin (H&E) and Oil red O staining, liver sections were preserved in 10% neutral buffered formalin, and sectioned at 4–10 µm thickness. The stained sections were visualized using the Axioskop2 plus microscope and the AxioVision 4.5 software (Carl Zeiss, Thornwood, NY). For quantitative histochemical measurement of lipid accumulation, the Oil red O stain was converted into pixel density units using Adobe Photoshop CS3 (Adobe System, San Jose, CA).
To determine the glycogen content of the liver, tissue was homogenized with HCl, boiled for 10 minutes, and neutralized with sodium acetate to a final pH of 4.5.29 The hydrolyzed tissue was then digested with amylo-α-1,4-α-1,6-glucosidase and the released glucose was measured using a kit obtained from Sigma Diagnostics. Glycogen content is reported as nmol glucosyl units per mg of hepatic protein.
Antibody assays. Antibodies against human or murine G6Pase-α were detected by western blot analysis. Microsomal proteins from Ad-human G6Pase-α or Ad-mouse G6Pase-α infected COS-1 cells25 were resolved by electrophoresis through a 12% polyacrylamide-sodium dodecyl sulfate gel and trans-blotted onto polyvinylidene fluoride membranes (Millipore, Bedford, MA). The membrane was placed in a Multiscreen Apparatus (Bio-Rad Laboratories, Hercules, CA) containing multiple channels. The membrane strip under each channel was incubated with a rabbit anti-human G6Pase-α serum25 diluted 1:3000, or serum samples from AAV-GPE-infused or AAV-CBA-infused animals diluted 1:200. Serum samples from untreated G6pc−/− and G6pc+/+/G6pc+/− littermates diluted 1:200 were used as controls. After overnight incubation, the membrane strips were then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Kirkegarrd & Perry Laboratories, Gaithersburg, MD). The immunocomplex was visualized by the chemiluminescent system using the SuperSignal West Pico Chemiluminescent substrate from Pierce (Rockford, IL).
CD8+ lymphocyte immunodetection. Mouse livers were snap frozen, embedded in O.C.T. (Sakura Finetek, Terrance, CA), and sectioned at 8 µm thickness. The sections were fixed in acetone for 10 minutes at −20 °C, dried, washed with phosphate-buffered solution (PBS), blocked with PBS containing 2% of bovine serum albumin for 30 minutes, and incubated at 4 °C overnight with a rabbit polyclonal antibody against CD8 (Abcam, Cambridge, MA) in PBS supplemented with 1% bovine serum albumin. After washes with PBS, the sections were incubated for 1 hour at 25 °C in the dark with a goat anti-rat IgG antibody conjugated with Alexa Flura 488 (Invitrogen). Following washes with PBS, the labeled cells were mounted with an anti-fade, water-based mounting medium containing DAPI (Vector Laboratories, Burlingame, CA) and visualized using the Axioskop2 plus fluorescence microscope (Carl Zeiss). The CD8+ cells were counted in ten randomly selected fields at 200-fold magnification and reported as the mean average.
Statistical analysis. The unpaired t-test was performed using the GraphPad Prism Program, version 4 (GraphPad Software, San Diego, CA). Values were considered statistically significant at P < 0.05.
Acknowledgments
This research was supported by the Intramural Research Program of the NICHD, NIH and a grant (#5P01DK058327-08) from NIDDK, NIH. We thank The Children's Fund for Glycogen Storage Disease Research for financially supporting this work.
REFERENCES
- Chou JY, Matern D, Mansfield BC., and , Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- Greene HL, Slonim AE, O'Neill JA, Jr, Burr IM. Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N Engl J Med. 1976;294:423–425. doi: 10.1056/NEJM197602192940805. [DOI] [PubMed] [Google Scholar]
- Chen YT, Cornblath M., and , Sidbury JB. Cornstarch therapy in type I glycogen-storage disease. N Engl J Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- Weinstein DA, Somers MJ., and , Wolfsdorf JI. Decreased urinary citrate excretion in type 1a glycogen storage disease. J Pediatr. 2001;138:378–382. doi: 10.1067/mpd.2001.111322. [DOI] [PubMed] [Google Scholar]
- Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K., and , Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I) Eur J Pediatr. 2002;161 Suppl 1:S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- Lei KJ, Chen H, Pan CJ, Ward JM, Mosinger B, Jr, Lee EJ, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- Kim SY, Weinstein DA, Starost MF, Mansfield BC., and , Chou JY. Necrotic foci, elevated chemokines and infiltrating neutrophils in the liver of glycogen storage disease type Ia. J Hepatol. 2008;48:479–485. doi: 10.1016/j.jhep.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Allamarvdasht M, Pan CJ, Sun MS, Mansfield BC, Byrne BJ, et al. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13:321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Sun BD, Damodaran TV, Brown T, Millington DS, Benjamin DK, Jr, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Pinto C, Sun B, Li S, Kozink DM, Benjamin DK, Jr, et al. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiler SA, Conlon TJ, Song S, Tang Q, Warrington KH, Agarwal A, et al. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Ther. 2003;10:1551–1558. doi: 10.1038/sj.gt.3302046. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z., and , Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Daly T, Gao C, Flotte TR, Song S, Byrne BJ, et al. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum Gene Ther. 2001;12:563–573. doi: 10.1089/104303401300042500. [DOI] [PubMed] [Google Scholar]
- Lin B, Morris DW., and , Chou JY. The role of HNF1alpha, HNF3gamma, and cyclic AMP in glucose-6-phosphatase gene activation. Biochemistry. 1997;36:14096–14106. doi: 10.1021/bi9703249. [DOI] [PubMed] [Google Scholar]
- Streeper RS, Svitek CA, Chapman S, Greenbaum LE, Taub R., and , O'Brien RM. A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcription by insulin. J Biol Chem. 1997;272:11698–11701. doi: 10.1074/jbc.272.18.11698. [DOI] [PubMed] [Google Scholar]
- Chou JY., and , Mansfield BC. Molecular genetics of type 1 glycogen storage diseases. Trend Endocrinol Metab. 1999;10:104–113. doi: 10.1016/s1043-2760(98)00123-4. [DOI] [PubMed] [Google Scholar]
- Cunningham SC, Dane AP, Spinoulas A, Logan GJ., and , Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Brooks AR, Harkins RN, Wang P, Qian HS, Liu P., and , Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Majumdar SS, Alam P, Gulati N., and , Brahmachari V. Epigenetic regulation of cytomegalovirus major immediate-early promoter activity in transgenic mice. Gene. 2009;428:20–24. doi: 10.1016/j.gene.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Liu Z, Barrett EJ, Dalkin AC, Zwart AD., and , Chou JY. Effect of acute diabetes on rat hepatic glucose-6-phosphatase activity and its messenger RNA level. Biochem Biophys Res Commun. 1994;205:680–686. doi: 10.1006/bbrc.1994.2719. [DOI] [PubMed] [Google Scholar]
- Antinozzi PA, Berman HK, O'Doherty RM., and , Newgard CB. Metabolic engineering with recombinant adenoviruses. Annu Rev Nutr. 1999;19:511–544. doi: 10.1146/annurev.nutr.19.1.511. [DOI] [PubMed] [Google Scholar]
- Clore JN, Stillman J., and , Sugerman H. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes. 2000;49:969–974. doi: 10.2337/diabetes.49.6.969. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shieh JJ, Pan CJ, Sun MS., and , Chou JY. The catalytic center of glucose-6-phosphatase. HIS176 is the nucleophile forming the phosphohistidine-enzyme intermediate during catalysis. J Biol Chem. 2002;277:32837–32842. doi: 10.1074/jbc.M201853200. [DOI] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L., and , Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Miller E, Agbandje-McKenna M., and , Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H., and , Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch HF. Chemomorphology of liver parenchyma. Qualitative histochemical distribution patterns and quantitative sinusoidal profiles of G6Pase, G6PDH and malic enzyme activity and of glycogen content. Prog Histochem Cytochem. 1981;14:1–92. [PubMed] [Google Scholar]