Two ongoing clinical trials utilize different adeno-associated viral (AAV) vectors for liver-directed factor IX (F.IX) gene transfer with the goal of sustained therapy in patients with severe hemophilia B. Although preclinical studies have documented immune tolerance and long-term expression of F.IX in animals, the single prior clinical trial of this approach achieved only transient therapeutic gene expression and exposed preexisting immunity to the AAV vector as a major obstacle for therapy.1 Although accumulating preclinical data continue to fuel a debate over the potential impact of immune responses on hepatic AAV gene transfer, these data do not allow us to predict whether the human immune system will reject or tolerate therapy. This commentary dissects the relevant human and animal data, some of which are contradictory or allow us to draw only limited conclusions. More clinical trial data are critically needed and should ultimately help us develop better protocols.
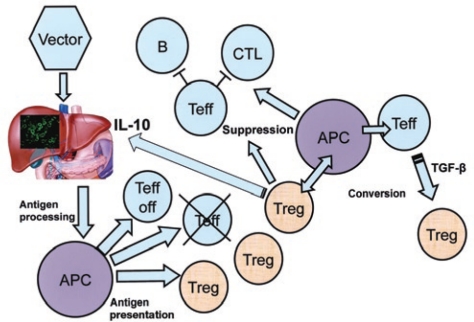
The liver performs essential functions in metabolism, detoxification, and production of plasma proteins. Therefore, it is an important target for gene therapy, not only for correction of liver disease but also for systemic delivery of therapeutic proteins. AAV vectors, based on a replication-deficient parvovirus with a small single-stranded DNA genome, have mediated long-term systemic expression of transgenes in animals, while eliciting only limited inflammatory responses in the liver. Moreover, several investigators have demonstrated induction of immune tolerance to a therapeutic gene product following gene transfer to hepatocytes (Figure 1).2
Figure 1.
Model for immune tolerance induction by liver gene transfer. Hepatocyte-restricted transgene expression leads to antigen presentation, which not only causes deletion and anergy among transgene product–specific T cells but also induces CD4+CD25+FoxP3+ Tregs. Induced regulatory T cells (Tregs) suppress B and T cells' responses (including cytotoxic T lymphocytes, CTLs) to the transgene product and convert CD4+ effector T cells (Teffs) to Tregs. In the anti-inflammatory hepatic environment, interleukin-10 (IL-10) expression by Tregs and antigen-presenting cells (APCs) further enforces suppression of CTLs. This model is based on studies from several laboratories. TGF-b, transforming growth factor-b. Adapted from ref. 2.
Approximately 30% of the total blood volume passes through a network of hepatic sinusoids every minute, delivering peripheral lymphocytes into a tolerogenic microenvironment, whose unique anatomical features promote interactions with antigen-presenting cells (APCs).3 In this hepatic environment, liver sinusoidal endothelial cells, hepatic dendritic cells, Kupffer cells, suppressive cytokines, and several types of intrahepatic lymphocytes with regulatory activity (natural killer T cells, CD4+ and CD8+ regulatory T cells, Tregs) are present, all of which have been implicated in promoting immune tolerance.2,3 A consequence of tolerogenic antigen presentation is the induction of CD4+CD25+FoxP3+ Tregs; see Figure 1).2,4 Induced Tregs suppress effector CD4+ and CD8+ T-cell responses and antibody formation to the transgene product in the liver and at extrahepatic sites, and they also help protect the liver from immune-mediated injury.
Because hepatocyte-restricted transgene expression can induce tolerance to therapeutic and autoantigens, this route of delivery is attractive both for correction of a protein deficiency and for immunomodulatory therapy.5 Hepatocyte-expressed antigen also promotes hyporesponsiveness in other organs and the systemic circulation.2,5 An obvious question is whether this concept of tolerance following gene transfer to the liver will translate to treatment of humans. Some studies caution that particular mouse models may lead to an overestimation of this ability to induce tolerance.6,7 Nevertheless, studies in nonhuman primates (NHPs) support the importance of Tregs in the development of tolerance to the transgene product,8 and other large-animal models have emphasized the importance of avoiding transgene expression in professional APCs so as to develop immune tolerance following liver gene transfer. For example, long-term F.IX expression in hemophilia B dogs with a null mutation and prolonged green fluorescent protein expression in NHPs can be achieved using hepatocyte-specific promoters.9,10
Other observations support the hypothesis that hepatic-derived antigens promote tolerance in humans. In liver transplantation, major histocompatibility complex (MHC) matching is not a prerequisite for a successful outcome, and liver allografts may enhance survival of other organs transplanted at the same time.11 Moreover, spontaneous tolerance (permitting one to discontinue immunosuppressive regimens) develops more frequently after transplantation of liver as compared with other organs.12
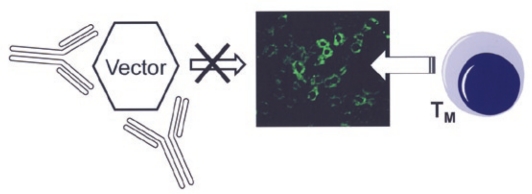
Although animal studies have shown that a therapeutic transgene product can be tolerated by the immune system, preexisting immunity to the vector capsid has emerged as an obstacle to liver-directed gene therapy to humans (Figure 2). Even very low titers of neutralizing antibodies (NABs) have been found to block AAV gene transfer to the liver. This effect has been observed in a phase I/II clinical trial of AAV2-mediated gene transfer to patients with severe hemophilia B, following AAV-mediated gene transfer to NHPs using various serotypes and following passive transfer of human or NHP sera to mice.1,10 In addition, data from the hemophilia trial have demonstrated the activation of capsid-specific CD8+ T cells.1,13 The activated cells probably represented memory T cells generated following prior natural infection with the parent virus or a related serotype. Closer examination of the results from two hemophilic subjects treated with AAV2 vectors showed a strong correlation between the time course of the CD8+ T-cell response to capsid in peripheral blood, a transient (although clinically asymptomatic) rise in systemic liver enzyme levels, and the loss of transgene expression over a period of approximately 2 months.13 Subsequent in vitro follow-up studies provided direct evidence for MHC I presentation of input capsid antigen by AAV-transduced hepatocytes, which then became targets for cytotoxic CD8+ T lymphocytes (CTLs).14 However, the development of an animal model that replicates the development of CTL activity against transduced hepatocytes—as seen in the human trial—has been elusive.15 Although it is generally accepted that some fraction of the input AAV capsid proteins is ubiquitinated and degraded by proteasomes in the target cells, it is unclear whether the level of MHC I presentation of input capsid in transduced hepatocytes in vivo will be sufficient to generate a CTL response. In addition, there are differences between immune systems and MHC presentation between species. It is also conceivable that the biology of natural infection with AAV in humans differs somewhat from other species, which may influence immune responses, and this should be studied in more detail. For example, latent genomes have been found in various human and NHP tissues.16 Finally, assays for detection of capsid-specific T cells in humans should be further refined.
Figure 2.
Obstacles to AAV liver gene transfer in humans. Preexisting immunity to adeno-associated viral vectors can potentially block gene transfer in humans through neutralizing antibodies or through targeting of transduced hepatocytes by memory CD8+ T-cell (TM) responses to capsid.
It has been proposed that transient immunosuppression or the use of alternative serotypes may help avoid activation of immune responses following AAV-mediated gene transfer to the liver. With regard to T-cell responses, one possible approach is to include a transient immune modulation regimen, similar to those used in transplantation.17 Various regimens have been reported that make use of small immunosuppressive drugs and antibodies. Interestingly, immunosuppression is now also used in enzyme replacement therapy for the lysosomal storage disorder Pompe disease, which expands our experience with immunomodulation in genetic disease. In addition to the suppression of the resulting lymphocyte response, MHC I presentation of the capsid could conceivably be directly suppressed by delivering proteasome inhibitors. On the other hand, it has been speculated that use of certain alternative AAV serotypes may circumvent the need for immunosuppression altogether. For example, AAV8 input capsid does not activate CD8+ T cells effectively in mice or in NHPs, presumably owing to a reduced capacity of such vectors to bind dendritic cells because of their reduced binding to heparin.18 Mouse studies have also shown that AAV8 is more efficient for gene transfer to hepatocytes and for induction of tolerance to the transgene product.2 However, AAV8 capsid contains epitopes that are present within other serotypes, raising the possibility that memory CD8+ T cells generated following prior natural exposure to these other serotypes might be reactivated in humans following gene transfer so as to subsequently target AAV8-transduced cells.13
In mouse liver, a vector dose of AAV8 that is 1 to 2 logs lower than AAV2 results in a similar level of transgene expression. However, such an impressive dose advantage for AAV8 has not been observed following gene transfer of clotting factors in dogs or NHPs.19 On the other hand, recent results demonstrated much more robust transduction of NHP hepatocytes with AAV8 and suggest that low titers of NABs could have partially blocked hepatic AAV8 gene transfer in previous NHP studies.10 It has been speculated that the AAV8 capsid might evade neutralization by preexisting antibodies in humans, in that it was isolated from NHPs, as opposed to AAV2, which was isolated from humans. However, published reports of the prevalence of NABs to AAV8 in humans vary considerably (from 5% to 55%), which may be due in part to difficulties with this assay.10,20
In summary, preclinical studies will continue to fuel the debate on whether the immune system will be friend or foe following liver gene transfer with AAV vectors in humans. Resolution of these issues will be facilitated by the availability of upcoming clinical trial data. A phase I/II trial on liver-directed AAV gene transfer is currently open for enrollment. This dose-escalation study of a self-complementary AAV vector for gene transfer in hemophilia B, sponsored by the St. Jude Children's Research Hospital, represents the first application of serotype 8. The vector is delivered intravenously and encodes a double-stranded genome, which leads to increased transgene expression per dose relative to a single-stranded genome. At the same time, a separate clinical trial (sponsored by The Children's Hospital of Philadelphia) of delivery of single-stranded AAV2 vector to livers of hemophilia B patients via the hepatic artery has been modified to include transient immunosuppression and is continuing to recruit patients. Both of these latter clinical trials will be conducted in adult patients. Spontaneous tolerance to a liver transplant is more often observed in pediatric patients,12 and hepatic gene transfer to neonatal animals has led to induction of tolerance to coagulation factors. Moreover, young children may also lack preexisting immunity to AAV vectors. Although inclusion of children with hemophilia may not be acceptable for these initial trials, these points should be kept in mind for future clinical studies and for other, perhaps more life-threatening diseases.
In conclusion, the liver is an important target organ for many potential gene therapies, and long-term gene engraftment has been achieved in numerous animal models. Given the potential for tolerance induction, those working on lysosomal storage disorders such as Pompe's disease hope to use hepatic AAV gene transfer as an immunomodulatory therapy to enhance enzyme replacement therapy and therapies in other organs.5 How best to accomplish these goals in humans can only be addressed by more clinical studies. Although one must approach gene transfer to a major organ with caution, the lack of additional instructive data in humans has been frustrating, and we are indebted to the investigators who are pioneering the current clinical trials in hemophilia. After all, prior experience has taught us that a “bedside to bench and back” cycle between clinical and laboratory/animal investigations is crucial to drive the field forward, and that preclinical studies alone can have substantial limitations.
REFERENCES
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- LoDuca PA, Hoffman BE., and , Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C.et al. (2007Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer Blood 1101132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Kulis MD, Young SP, Hobeika AC, Li S, Bird A.et al. (2010Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe disease Mol Ther 18353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Somanathan S., and , Wilson JM. BALB/c mice show impaired hepatic tolerogenic response following AAV gene transfer to the liver. Mol Ther. 2010;18:766–774. doi: 10.1038/mt.2009.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L.et al. (2009Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses Hum Gene Ther 20930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ., and , Sabatino DE.et al. (2007Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver Blood 1102334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J.et al. (2009Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy Blood 113797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH.et al. (2010The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques Mol Ther 18126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AH, de Creus A, Lu L., and , Thomson AW. Liver tolerance mediated by antigen presenting cells: fact or fiction. Gut. 2003;52:1075–1078. doi: 10.1136/gut.52.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesini R. Tolerance in clinical liver transplantation. Transpl Immunol. 2007;17:81–82. doi: 10.1016/j.trim.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]
- Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC.et al. (2009Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors J Clin Invest 1191688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Murphy SL, Giles-Davis W, Edmonson S, Xiang Z, Li Y.et al. (2007Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes Mol Ther 15792–800. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X.et al. (2004Clades of adeno-associated viruses are widely disseminated in human tissues J Virol 786381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R.et al. (2006Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid Nat Med 12967–971. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y.et al. (2005Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models Mol Ther 11875–888. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EGD, Gray JT, Reiss U, Nienhuis AW., and , Davidoff AM.2009Update on gene therapy in hemophilia J. Thromb Haemost. 7suppl. 2): 3418983495 [Google Scholar]