Abstract
Gene therapy for cystic fibrosis (CF) is making encouraging progress into clinical trials. However, further improvements in transduction efficiency are desired. To develop a novel gene transfer vector that is improved and truly effective for CF gene therapy, a simian immunodeficiency virus (SIV) was pseudotyped with envelope proteins from Sendai virus (SeV), which is known to efficiently transduce unconditioned airway epithelial cells from the apical side. This novel vector was evaluated in mice in vivo and in vitro directed toward CF gene therapy. Here, we show that (i) we can produce relevant titers of an SIV vector pseudotyped with SeV envelope proteins for in vivo use, (ii) this vector can transduce the respiratory epithelium of the murine nose in vivo at levels that may be relevant for clinical benefit in CF, (iii) this can be achieved in a single formulation, and without the need for preconditioning, (iv) expression can last for 15 months, (v) readministration is feasible, (vi) the vector can transduce human air–liquid interface (ALI) cultures, and (vii) functional CF transmembrane conductance regulator (CFTR) chloride channels can be generated in vitro. Our data suggest that this lentiviral vector may provide a step change in airway transduction efficiency relevant to a clinical programme of gene therapy for CF.
Introduction
Cystic fibrosis (CF) is a fatal genetic disorder caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, one function of which is to act as a chloride channel in airway epithelial cells. CF is characterized by recurrent chest infections, increased airway secretions, and eventually respiratory failure.1 Although symptomatic treatments have successfully increased median survival to ~36 years, definitive novel therapeutic approaches aimed at the basic defect are clearly needed.
Given the propensity of certain viruses for infection of the respiratory tract, CFTR gene transfer using these vectors has been extensively studied. However, to date no viral vector has met the requirements for clinical use.2 Three major problems have been encountered. Gene transfer efficiency is generally poor, at least in part because the respective receptors for the viral vectors appear to be predominantly localized to the basolateral surface of the airway epithelium. Second, penetration of the augmented mucus layer typical of CF is generally poor. Finally, the ability to administer viral vectors repeatedly, mandatory for such transient expression systems in the treatment of a lifelong disease, is limited. With these limitations in mind, we describe a novel vector that is able to circumvent some of the difficulties described above.
We have previously described the use of Sendai virus (SeV) vectors for airway gene transfer.3,4 SeV is a single-stranded RNA virus, belongs to the family of Paramyxoviridae, and is able to overcome the first two of the above-noted difficulties. First, gene transfer to the airway epithelium is highly efficient, because the sialic acid and cholesterol receptors needed for transduction are present on the apical surface of airway epithelial cells. Further, SeV uses a cytoplasmic expression system, thereby removing the limitations imposed by the nuclear membrane. Second, although mucus does act as a partial barrier to SeV-mediated gene transfer, the very high expression levels generated allow this limitation to be readily tolerated. However, despite our best efforts to date, we have been unable to overcome the third impediment to clinical translation, namely repeated application of SeV.
A different solution to this problem would theoretically be provided by transduction of progenitor or stem cells, normally used to replenish the airway epithelium. If this was feasible, “single-hit” gene therapy with a viral vector might overcome this remaining hurdle. However, a significant further issue is the identity and localization of these progenitor or stem cells. Existing data suggest the presence of specialized cells, whose identity and site varies with progression along the branching of the airways.5
In addition, the ciliated epithelium may be able to renew or repair itself; the lifespan of these epithelial cells has historically been estimated to be around 3 months (ref. 6), but has been extended to 6 months in trachea and 17 months in lung in a most recent publication.7 Irrespective of the appropriate target cell, an integrating vector would clearly be needed to achieve the goal of “single-hit” gene therapy. Further, given our present lack of conclusive data regarding the identity of airway stem cells, a vector that could transduce cells irrespective of their level of turnover, would be clearly advantageous.
In contrast to retroviral vectors that can only transduce proliferating cells,8 lentiviruses are able to produce gene expression in nondividing cells, including those of the airway epithelium. However, to allow for receptor-mediated cell entry these vectors require pseudotyping to allow display of appropriate ligands for their cognate receptors. The most common pseudotyping, with glycoproteins of the vesicular stomatitis virus (VSV-G), produces efficient transduction of a broad range of cells, and the virus can be readily concentrated and purified by high-speed centrifugation.9,10 However, they are not able to transduce airway epithelial cells in vivo when delivered via the apical surface. This, in turn, relates both to the difficulties such vectors have in penetrating the overlying mucus, and the lack of apically localized receptors on the epithelial cells.11,12,13 Thus, the use of detergents such as lysophosphatidylcholine or ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetraacetic acid, help to breach these barriers, thereby allowing the vector to penetrate to the basolateral surface where the appropriate receptors reside. However, the suitability of such an approach in the bacteria-laden airways of CF patients is debatable. Thus, several groups have examined the use of a number of different pseudotyped lentiviral vectors for their airway epithelial transduction efficiency, including envelope glycoproteins of paramyxoviruses,14 filoviruses,15,16,17 and orthomyxoviruses.18 Although encouraging data have been generated, we are not aware of a vector that fulfills the three key requirements of (i) efficient transduction of airway epithelial cells without the need for chemical pretreatment, (ii) long-term transgene expression, and (iii) the ability to be produced to high vector titers suitable for clinical application.19
We describe here the development of a replication-defective lentiviral vector20 derived from the simian immunodeficiency virus (SIV) of the African green monkey.21 Using novel strategies, we have been able to pseudotype this vector with the key SeV envelope proteins, hemagglutinin-neuraminidase (HN) and fusion (F) protein.14 The HN and F proteins function, respectively, to attach to sialic acids, the receptor of SeV, and mediate cell fusion for vector entry to target cells. We have optimized vector production and transgene expression level of this F/HN-pseudotyped SIV vector by introducing the central polypurine tract (cPPT)22 and the Woodchuck hepatitis virus posttranscriptional regulatory elements (WPRE).23,24 We show that this F/HN-pseudotyped SIV vector can efficiently transduce nasal epithelial cells from the apical surface in vivo, resulting in transgene expression sustained for periods far beyond the proposed lifespan of airway epithelial cells. Importantly, we show that readministration is feasible. Finally, we demonstrate that this vector can transduce a fully differentiated human airway epithelium and that functional CFTR chloride channels can be generated after transduction with F/HN-SIV carrying the human CFTR complementary DNA in vitro. This vector may, therefore, be able to produce a step change in airway transduction efficiency relevant to a clinical programme of CF gene therapy.
Results
F/HN-pseudotyped SIV vectors can be generated and produced at high titers
To accomplish pseudotyping of SIV vector with SeV envelope proteins, we have previously described that modifications of the F and HN proteins were needed.14 Briefly, the cytoplasmic domain of the F protein was truncated to four amino acids, and the HN protein was fused with the cytoplasmic tail of the SIV transmembrane envelope protein. These modifications enabled the incorporation of F and HN-derived proteins into vector virions, generating an F/HN-pseudotyped SIV vector.
We next constructed a series of gene transfer vectors in which the cPPT and/or WPRE were inserted into the parent gene transfer vector.20 Their function was first evaluated using VSV-G pseudotyped SIV vectors encoding green fluorescent protein (GFP). As expected, insertion of cPPT and WPRE increased vector production, from 3.8 × 106 transduction units (TU)/ml (cPPT−/WPRE−) to 1.4 × 107 TU/ml (cPPT+/WPRE−) and 6.7 × 106 TU/ml (cPPT−/WPRE+), respectively. The insertion of both elements (cPPT+/WPRE+) led to the highest titer production (3.9 × 107 TU/ml). Because the number of particles produced in the supernatant (~2 × 108/ml) was similar in all cases, the introduction of these elements increased the quality of the vector by improving the TU to particle ratio from 1:100 to 1:10. Importantly, consistent with these data, insertion of these sequences into the SeV-F/HN-pseudotyped vectors encoding GFP increased virus production from 1 × 105 TU/ml (cPPT−/WPRE−) to 5 × 107 TU/ml (cPPT+/WPRE+), leading to an improvement in TU to particle ratio from 1:100 to 1:20. Thus, the cPPT/WPRE-containing vector was used in all subsequent experiments. The F/HN-pseudotyped SIV vector (Supplementary Figure S1, Supplementary Materials and Methods) could be further concentrated from 2.0 ± 0.9 × 107 to 6.1 ± 2.0 × 109 TU/ml (n = 3) through centrifugation, making high titer use in vivo feasible.
F/HN-SIV-GFP transduces the nasal epithelium of mice
We evaluated the in vivo gene transduction efficiency of the SeV-F/HN-pseudotyped SIV vector in the nasal airway epithelium of mice. This tissue was chosen because the characteristic pathophysiological abnormalities of CF are reproduced in the nasal, but not the lower airways of CF knockout mice. In addition, in contrast to the murine lung, cell composition in the murine nasal epithelium is more akin to the human lung and contains both submucosal glands, as well as mucous-secreting cells, thereby allowing the evaluation of whether the vector is able to penetrate a mucus-enriched airway surface fluid layer. As a part of this study, ~100 mice were treated (for details see individual figure legends). The overall survival over the course of the >15 months study was 100%.
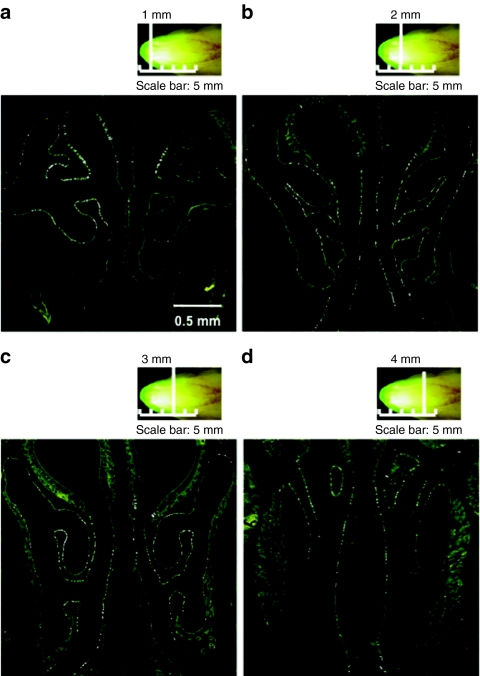
To examine transduction efficiency of respiratory epithelial cells, we administered F/HN-pseudotyped SIV vector encoding GFP to the nose of mice at a dose of 4 × 108 TU/mouse. At 30 days after transfection, GFP-positive respiratory epithelial cells were identified and quantified on histological sections collected 1–4 mm from the tip of the nasal bone (Figure 1). Transduction efficiency along the nasal septum was 4.5 ± 0.7% (mean ± SEM, n = 8). This corresponds favorably with the ~5% of cells estimated to require transduction for generating clinical benefit.
Figure 1.
Transduction of mouse nasal epithelium with F/HN-SIV-GFP. The murine nose was perfused in vivo with F/HN-SIV-GFP (4 × 108 TU/mouse) vector and gene expression analyzed 30 days after transduction (n = 8). (a,b) In situ imaging of GFP expression in the nasal cavity. (c,d) Microscopic imaging of GFP in histological sections. The sections were collected (a) 1 mm, (b) 2 mm, (c) 3 mm, and (d) 4 mm into the nasal tissue (vertical white lines). GFP-positive cells appear as small white punctuate signals.
F/HN-SIV transduces cells in both the respiratory and olfactory epithelium
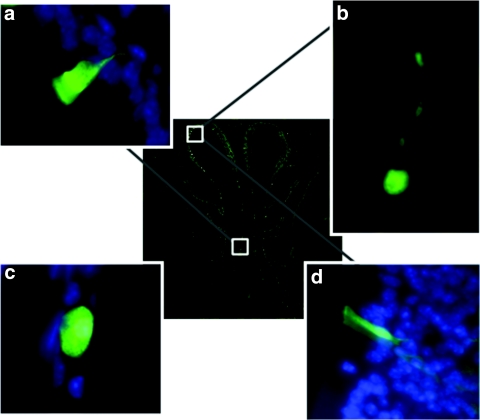
Using high-power microscopic analysis, we were able directly to detect and identify GFP fluorescent cells 30 days after transfection. The murine nasal epithelium consists of squamous cells in the anterior part of the nose, changing to respiratory epithelium in the mid-portion and neuroepithelial olfactory epithelium in the dorsoposterior regions.25 The majority (~69%) of GFP-positive cells were ciliated respiratory epithelial cells (Figure 2a) followed by neuronal cells (21%) in the olfactory epithelium (Figure 2b) and squamous cells (7%) (Figure 2c). The number of neuronal cells transduced was very few in the anterior part of the nose and increased in the posterior region as expected. The remainder (3%) were mainly non-neuronal cells in the olfactory epithelium, most likely sustentacular cells (Figure 2d), a major constituent of the olfactory epithelium. Interestingly, we did not detect GFP-expressing goblet cells, a cell type commonly transduced in VSGV-SIV perfused lysophosphatidylcholine-preconditioned nasal epithelium (data not shown).
Figure 2.
Determination of cell types transduced. The transduced GFP-positive cells were identified using fluorescent microscopy (original magnification ×63) 30 days after administration of F/HN-SIV-GFP (4 × 108 TU/mouse) vector to the mouse nose. (a) Ciliated respiratory epithelial cell, (b) neuronal cell in olfactory epithelium, (c) squamous epithelial cell, (d) non-neuronal cell in olfactory epithelium. The central image shows a cross-section through the mouse nose and white boxes indicate regions in mouse nasal epithelium where respective transduced cell types were found. Panels a, b, and d were rotated ~45°, 130°, and 180° counter clockwise, respectively, to improve clarity of the figure.
GFP expression persists for >1 year after F/HN-SIV-GFP transduction
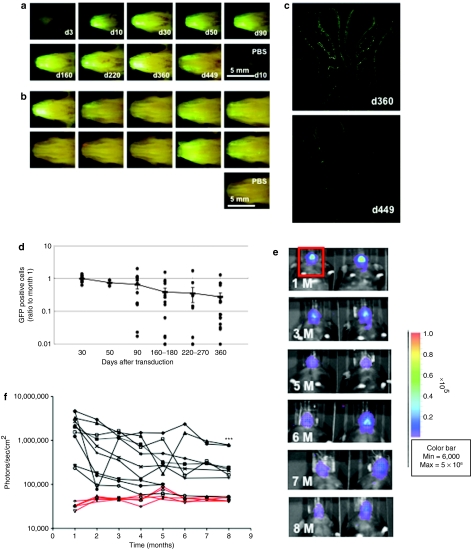
To determine the duration of gene expression, mice were perfused with F/HN-SIV-GFP (4 × 108 TU/mouse) or phosphate-buffered saline (PBS) and harvested 3–449 days after transfection (Figure 3a,b). F/HN-SIV-mediated GFP expression was visible in the nasal cavity using in situ imaging as early as 10 days post-transduction and was still detectable in 7 out of 10 and 3 out of 10 mice at 360 and 449 days after a single administration of the vector, respectively. At this time point, GFP-positive cells appeared to be randomly distributed within the tissue similar to the distribution observed 30 days after transfection (Figure 3c). GFP was undetectable in lysophosphatidylcholine-preconditoned VSV-G-SIV-GFP-transduced tissue (data not shown). Histological sections were used to quantify the number of GFP-positive cells over time. As a whole, the number of GFP-positive cells gradually declined over time by ~70% (Figure 3d). In some mice, marked decline of GFP-positive cells were observed after 90 days post-transduction; however, in the other mice the number of transduced cells were maintained well even at 360 days post-transduction to the similar level of that at 30 days post-transduction. In addition, it is important to note that GFP-positive cells were still detectable in 16 out of 17 mice 12 months after transduction.
Figure 3.
Duration of green fluorescent protein (GFP) expression after transduction with F/HN-SIV-GFP. Mouse nasal tissue was perfused with F/HN-SIV-GFP (4 × 108 TU/mouse or phosphate-buffered saline (PBS) and gene expression was analyzed at indicated time points after transduction. (a) Representative in situ images of GFP expression in the nasal cavity from mice analyzed 3–449 days after transduction. (b) In situ imaging of GFP expression in the nasal cavity from 10 mice analyzed 360 days after transduction. (c) Representative microscopic images of GFP expression in histological sections 360 and 449 days after transduction. GFP-positive cells appear as small white punctuate signals. (d) Quantification of transduced cells. GFP-positive cells were quantified on histological sections taken 2 mm into the nasal tissue of the nose. Data from 30 to 360 days after transduction are represented both by mean ± SEM and individual values (ratio to GFP cells positive on day 30). The number (n) per group are 13 (day 30), 3 (day 50), 12 (day 90), 14 (day 160–180), 10 (day 220–270), and 17 (day 360). (e) Bioluminescence in vivo imaging 1 (1 M) to 8 months (8 M) after transduction with F/HN-SIV-lux. Representative images of 2 out of 6 mice are shown. Red box indicates area chosen for quantification of photon emission. (f) Quantification of in vivo bioluminescence over time after transduction with F/HN-SIV-lux (black lines) or PBS (red lines). Each line represents photon emission over time in one animal. ***P < 0.005 compared to bioluminescence one month after gene transfer.
We also used in vivo bioluminescence imaging after nasal perfusion with F/HN-SIV-lux to assess gene expression over time in the same animal (Figure 3e).
Figure 3f shows quantification of photon emission after intraperitoneal injection of luciferin substrate from 1 to 8 months after transduction. Although persistence of gene expression far exceeds the expected lifespan of airway epithelial cells of 100 days, possibly indicating progenitor cell integration, repeated bioluminescence in vivo imaging after F/HN-SIV-lux transduction shows a gradual decline in photon emission over an 8-month period (month 1: 2.2 × 106 ± 4.9 × 105, month 8: 2.7 × 105 ± 8.8 × 104, n = 10/group, P < 0.005), but was still significantly (P < 0.01) higher than the PBS control (4.5 × 104 ± 2.0 × 103, n = 5) This result is consistent with the decline in GFP-positive cells described above. In addition, we analyzed all data using a repeat measure test to determine whether the decline in bioluminescence stabilizes during the 8-month study period. The analysis showed that gene expression significantly (P < 0.05) declined for the first 4 months but then stabilized with expression levels from 5 to 8 months not being different compared to the 4-month levels.
The SIV vector–transduced cells show clustering after induced regeneration of the epithelium
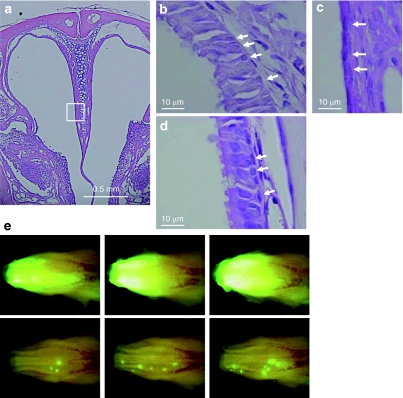
To ascertain further whether F/HN-SIV induced chromosomal integration into nasal respiratory progenitor or stem cells, we artificially induced cell division after SIV vector transduction by damaging the nasal tissue with the detergent (polidocanol),6 which has previously been shown to strip the surface epithelium within a few hours, while retaining basal cells able to regenerate the epithelium within 7 days (Figures 4a–d). At 7 and 28 days after vector transduction (4 × 108 TU/mouse, n = 3) the nasal tissue was perfused with 2% polidocanol (10 µl/mouse) and gene expression analyzed 4 weeks after the last detergent treatment. Importantly, GFP-expressing cells now showed clustering after polidocanol treatment (Figure 4e and Supplementary Figure S2), possibly indicating origination from a common progenitor.
Figure 4.
Clustering of transduced cells after the polidocanol-mediated stripping of epithelial cells followed by rapid regeneration. Mouse nasal tissue was perfused with 10 µl of 2% (vol/vol) polidocanol (n = 3). (a) Representative low-power view (original magnification ×50) of the nasal cavity 24 hours after perfusion. Respiratory epithelium, marked by a white box was further magnified (original magnification ×200). The respiratory epithelium before the treatment is shown in b. Arrow indicates basal cells. The respiratory epithelium was completely stripped 24 hours after polidocanol perfusion, whereas the basal cell layer was (c) retained and (d) regenerated 7 days after treatment. (e) This treatment was done after transduction with F/HN-SIV vector. Seven days after transduction of nasal epithelial cells with F/HN-SIV-GFP (4 × 108 transduction units/100 µl/mouse), the nasal epithelium was stripped via perfusion with 10 µl of 2% (vol/vol) polidocanol. Polidocanol treatment was repeated again 3 weeks later. Histological sections were analyzed 58 days after vector administration (30 days after the last polidocanol treatment). In situ imaging of GFP expression in the nasal cavity of untreated mice (top panel in e) or mice treated with polidocanol (bottom panel in e). Clusters of GFP-positive cells were seen in the polidocanol-treated mice. GFP, green fluorescent protein.
SIV-mediated gene transfer can be achieved after three applications of the vector
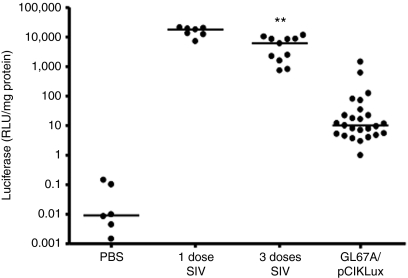
Although the above data are encouraging, gene therapy for CF will require lifelong treatment. We therefore, assessed the feasibility of readministering this vector, and compared transduction efficiency to the current optimal nonviral formulation for airway gene transfer in vivo. Figure 5 shows that following two administrations of F/HN-SIV-GFP separated by 1 month, a third administration of F/HN-SIV-lux (to prevent an immune response against the transgene) produced gene expression of ~40% of that seen following a single challenge with F/HN-SIV-lux. Further, these levels after three challenges with the SIV vector remained ~500-fold greater (P < 0.01) than seen with an optimal nonviral formulation, previously used in a CF clinical trial.
Figure 5.
Repeat administration of F/HN-SIV to nasal epithelium. Mice were transduced with F/HN-SIV-lux (1 dose) or two doses of F/HN-SIV-GFP (day 0 and day 28) followed by F/HN-SIV-lux 4 weeks later (day 56 = 3 doses). Luciferase expression was measured 30 days after F/HN-SIV-lux transduction and compared to levels achieved with the nonviral gene transfer agent GL67A complexed to a luciferase reporter gene plasmid (pCIKLux). Each dot represents one mouse. Horizontal bars indicate the median per group (**P < 0.01) compared to mice receiving GL67A/plasmid DNA. PBS, phosphate-buffered saline; RLU, relative light units.
F/HN-SIV transduces differentiated human airway epithelium
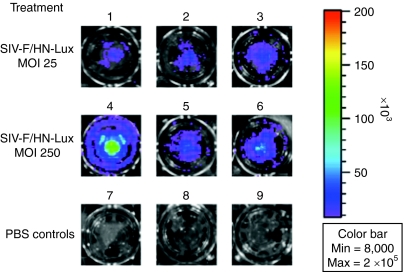
Differentiated human airway epithelium is in general difficult to transduce. In preliminary experiments, we have shown that F/HN-pseudotyped lentivirus transduced human airway cells grown as air–liquid interphase (ALI) cultures. ALIs were transfected with F/HN-SIV carrying a luciferase reporter gene at an approximate multiplicity of infection of 100 and luciferase expression was detectable 10 and 26 days after gene transfer (day 10: 53.0 ± 6.3 relative light units/mg protein, day 26: 7.9 ± 3.4 relative light units/mg protein, untransfected: 0.01 relative light units/mg protein, n = 3/group) and Figure 6. Importantly, gene transfer occurred without the need for preconditioning.
Figure 6.
Transduction of human air–liquid interface (ALIs) cultures with F/HN-SIV-lux. ALIs were transduced with F/HN-SIV-lux at an approximate multiplicity of infection of 25 (1–3) and 250 (4–6) or treated with phosphate-buffered saline (PBS) (7–9). 5 days after transduction ALIs were treated with luciferin and bioluminescent imaging performed.
Transduction with F/HN-SIV carrying the CFTR complementary DNA leads to expression of cAMP-dependent chloride channels
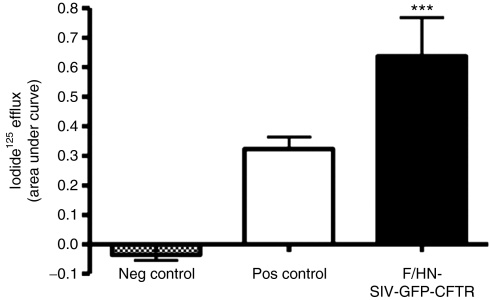
Iodide efflux is commonly used to demonstrate the presence of forskolin-activated chloride channels in vitro. In this assay, iodide is used as a surrogate for chloride due to the shorter half-life of the radioactive material. We have constructed an F/HN-SIV carrying a GFP-CFTR fusion complementary DNA construct and transduced human embryonic kidney (HEK)293T cells to assess whether functional CFTR chloride channels are generated. Fusion of GFP to the N-terminus of CFTR has previously been shown not to effect CFTR function.26,27 Figure 7 shows that cells transduced with F/HN-SIV-GFP-CFTR (multiplicity of infection 50) significantly (P < 0.001) increased cAMP-mediated efflux compared to cells treated with an F/HN-SIV-GFP control virus. We, therefore, conclude that transduction with F/HN-SIV-GFP-CFTR generates functional CFTR chloride channels in vitro.
Figure 7.
Functional confirmation of CF transmembrane conductance regulator (CFTR) production by F/HN-SIV-GFP-CFTR. HEK293T cells were transduced with F/HN-SIV-GFP-CFTR or a control virus carrying green fluorescent protein (GFP) (F/HN-SIV-GFP) at an multiplicity of infection of 500. The iodide efflux assay was performed 2 days after transduction. Cells transfected with an eukaryotic expression plasmid carrying the CFTR complementary DNA under the control of a cytomegalovirus promoter complexed to Lipofectamine 2000 were used as positive control. Data are presented as mean ± SEM. ***P < 0.001 compared to the control virus, n = 6/group. Neg, negative; pos, positive.
Discussion
Here, we show that (i) we can produce titers of a novel SIV vector pseudotyped with SeV envelope proteins appropriate for in vivo use, (ii) the vector can transduce the respiratory epithelium of the murine nose in vivo at levels that may be relevant for clinical benefit in CF, as previously suggested by in vitro mixing experiments,28 (iii) this can be achieved in a single formulation, and without the need for preconditioning, (iv) expression can last for at least half the lifespan of a mouse, (v) the vector can produce levels of gene expression ~500-fold greater than the current optimal nonviral formulation after three repeated administrations, (vi) the vector is able to transduce a fully differentiated human airway epithelium, and (vii) can produce functional cAMP-dependent CFTR chloride channels in vitro.
We inserted cPPT and WPRE sequences into the SIV vector. Both elements have previously been reported to increase gene transduction efficiency possibly due to acceleration of the movement of the preintegration complex of the vector into the nucleus,22,29,30 or other mechanisms.23,31 Interestingly, a synergistic effect of these two elements has been observed for human immunodeficiency virus–based lentiviral vectors.24 Our data are in keeping with these observations, with simultaneous insertion of cPPT and WPRE increasing productivity of both the VSV-G and SeV-F/HN-pseudotyped SIV vectors. Using these methods, we were able to reach titers of the SeV-F/HN-pseudotyped SIV vector of 5 × 107 TU/ml. Thus, this vector may be able to overcome one previously encountered important translational hurdle.
Lentiviral vectors pseudotyped with a variety of envelope proteins other than VSV-G have been described, including those from Ebola, Zaire,15,16 influenza hemagglutinin from fowl plague virus,18 and baculovirus GP64 envelopes.17 Among these, arguably it is the transduction efficiency of the baculovirus GP64-pseudotyped vectors that is most impressive, when applied in a viscoelastic gel formulation (1% methylcellulose) as a vector solvent. However, the regulatory complexities of moving two new agents into the clinic simultaneously, underline the encouraging transduction efficiency and duration we report here without the need for additions to the formulation.
The likely target for CF gene therapy are the ciliated epithelial cells, and >70% of the cells transduced by the F/HN SIV vector were of this type. This is in keeping with transduction of these cells by the “parent” SeV vector, and overcomes a second hurdle in the translation of these vectors toward the clinic. The number of cells requiring transduction for clinical benefit is a vexed, and unresolved question. In part, this may depend on which of the many functions of CFTR requires correction. Thus, if the chloride channel function predominates, in vitro data suggest that as few as 5% of cells may be sufficient.28 These values are in reach of the F/HN SIV vector described here.
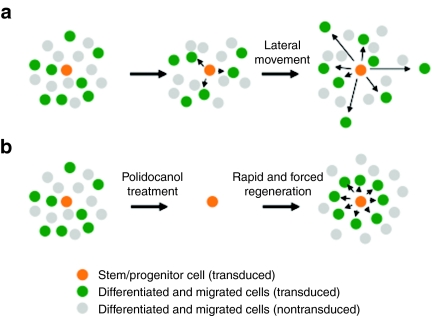
Using a human cytomegalovirus promoter, we saw expression of GFP for >360 days in 16 out of 17 mice, although gene expression gradually decreased over time when quantified as number of cells expressing the GFP reporter gene or longitudinal assessment of bioluminescent imaging. The onset of transgene expression was typically delayed, with no GFP fluorescence detected at day 3, but clearly visible by day 10. This has also been reported for an Ebola virus Z protein–pseudotyped human immunodeficiency virus vector.15 Possible explanations include the delayed movement of the preintegration complex to the nucleus, or the shutting off of promoter activity by concomitant inflammation consequent upon transduction,32,33 with subsequent expression following the resolution of inflammation. Irrespective, transgene expression was still apparent up to 449 days after transduction the longest time point assessed. Because the lifespan of terminally differentiated airway epithelial cells has been estimated at around 90 days (ref. 6), both in mice and in man, we considered whether this vector may have transduced progenitor or stem cells within the airway epithelium. We would predict that in this case we would observe clonal expansion, with clustering of transgene positive cells, and this was seen following induced regeneration of epithelial cells after polidocanol treatment. Cells derived from progenitor or stem cells thought to reside near the basement membrane have previously been shown to move laterally during differentiation.34 Thus, in the absence of epithelial damage, SIV vector–transduced progenitor or stem cell–derived cells should be observed in a scattered pattern without clustering (see schematic presentation in Figure 8a). In contrast, following epithelial stripping, a different pattern would be predicted to occur, with clusters of transduced cells becoming visible (see schematic presentation in Figure 8b). This hypothesis may explain, why we observed clusters of GFP-positive cells in damaged but not in undamaged epithelium. However, more extensive studies will be necessary to understand and more conclusively prove stem or progenitor cell transduction. Other explanations for the unexpectedly long duration of expression include (i) an alteration in the cell cycle of transduced respiratory epithelial cells, although to our knowledge this has not previously been reported, (ii) expression from -resident airway inflammatory or immune cells, for which we saw no evidence, or (iii) a >3 months half-life of respiratory epithelial cells, which has recently been suggested by Rawlins et al.7
Figure 8.
Schematic representation of epithelial cell migration in intact and damaged epithelium. (a) Scattered pattern with regeneration in normal condition. (b) Clustered formation after rapid and forced regeneration. We speculate that under normal physiological (undamaged) conditions, turnover may be comparatively slow and that newly generated epithelial cells may move laterally away from the stem or progenitor cell that they originated from a. In contrast, if rapid regeneration is forced (after tissue damage with polidocanol) stem or progenitor cells have to divide rapidly and newly generated epithelial cells may (transiently?) stay in closer proximity to the cell that they originated from b. This hypothesis may explain, why we observed clusters of GFP-positive cells in damaged, but not in undamaged epithelium.
The treatment of CF will require lifelong expression of the normal CFTR protein. Thus, despite these encouraging data showing long-lasting expression from a single administration, we assessed whether repeated application of this vector could sustain gene expression. We show that repeated mucosal administration of F/HN pseudotyped lentivirus, when given monthly over a 3-month period, is feasible and led to gene expression ~40% of that seen following a single administration. This dosing interval may be of subsequent clinical relevance. Sinn et al.35 have recently shown that seven weekly administrations of a GP64-pseudotyped FIV, given in tandem with a ciliastatic agent, are able to produce repeatable expression. Further, each study was undertaken in a different inbred mouse strain. The significant differences between the studies, yet with similar outcome, provide a growing body of evidence that such vectors can be readministered.
Differentiated human airway epithelium is in general difficult to transduce. However, we have shown here that F/HN-SIV transduced fully differentiated human airway epithelium successfully, and that reporter gene expression could be detected for at least 26 days after transduction. Importantly, gene transfer occurred without the need for preconditioning with tight junction openers or cilia static agents that are often required with other viral vectors. This provides encouraging support for its use in human trial.
In addition to demonstrating that F/HN-SIV carrying the CFTR complementary DNA was able to generate cAMP-dependent chloride channels in vitro, we also attempted to correct nasal potential difference in CF knockout mice. However, we did not detect any changes in ion transport (data not shown). Importantly, the suitability of the CF mouse nasal epithelium as a model has been put into question by two recent publications showing that the nasal bioelectrics are dominated by the olfactory rather than the respiratory epithelium.36,37 Our experience is in keeping with this observation. Transduction with SeV, which transduces respiratory and olfactory epithelium led to significant increases in chloride transport,38 whereas lentivirus and nonviral gene transfer agents,39 which predominantly transduce ciliated respiratory epithelial cells were unable to alter ion transport in the mouse nose. Until more appropriate animal models become widely available, analysis of CFTR function after gene transfer may, for certain gene transfer agents, be restricted to in vitro models.
Clearly, at least one remaining crucial hurdle is the risk–benefit ratio of these integrating vectors. The cases of leukemia in the severe combined immunodeficiency trial using a retroviral vector have been well documented, but lentiviral vectors are considered by many to be less susceptible to these problems. Further, the slowly dividing airway epithelium may represent a very different risk to the rapid turnover of bone marrow stem cells. Encouragingly, in our study of ~100 mice, over a 1-year period we saw no adverse events attributable to the vector. However, the encouraging increase in median survival of CF patients to the current ~36 years suggests that extensive toxicology studies will be needed before clinical trials can begin.
In conclusion, we suggest that the SeV-F/HN-pseudotyped SIV vector reported here may represent a further step toward translating such integrating viral vectors into clinical use. Several key hurdles have been potentially overcome, pushing these vectors into the arena as candidates for clinical trials.
Materials and Methods
Cell culture. HEK293T and 293T/17 cells (CRL-11268; ATCC, Manassas, VA) were maintained in Dulbecco's minimal Eagle's medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum and supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml).
Plasmid construction. pCAGGS-Fct4 and pCAGGS-SIVct+HN were constructed as previously described.14 The cPPT and WPRE sequences40 were inserted in the SIV-derived gene transfer plasmid.21
Production of SIV vector. Replication-defective self-inactivating SIV vector was constructed as previously described20 with minor modifications. Briefly, the SeV-F/HN-pseudotyped SIV vector was produced by transfecting 293T/17 cells (15 cm diameter culture dishes) with four plasmids complexed to Lipofectamine/Plus reagents (Invitrogen) according to the manufacturer's recommendations [Plasmid-1: 10 µg SIV-derived transfer plasmid carrying a GFP, a luciferase (lux) reporter gene, or a GFP-CFTR fusion construct,26 Plasmid-2: 3 µg packaging plasmid, Plasmid-3: 2 µg pCAGGS-Fct4, Plasmid 4: 2 µg pCAGGS-SIVct+HN]. The VSV-G pseudotyped SIV vector was produced using a similar protocol, but a pVSV-G plasmid (2 µg; Clontech, Mountain View, CA) was used instead of pCAGGS-Fct4 and pCAGGS-SIVct+HN. At 12 hours after transfection the culture medium was replaced with 30 ml serum-free Dulbecco's modified Eagle medium containing 5 mmol/l sodium butyrate. Sodium butyrate stimulates the vector production to inhibit histone deacetylase.41,42 The culture supernatant containing the SIV vector was harvested 48 hours after transfection, filtered through a 0.45 µm filter membrane, and further concentrated by high-speed centrifugation (20,000 g for 4 hours at 4 °C, Avanti JA18 rotor; Beckman Coulter, Brea, CA). The vector pellets were suspended in PBS (Invitrogen) to 100- to 200-fold concentration and stored at −80 °C.
Vector titration. The particle titer was determined using real-time reverese transcriptase-PCR. Virus RNA was purified using a QIAamp viral RNA mini-kit (QIAGEN, Strasse, Germany), and reverse transcribed using Superscript II (Invitrogen). The QuantiTect probe PCR system (QIAGEN) and primers for amplifying 131 nucleotides (bp) spanning the WPRE sequence (forward primer: 5′-ggatacgctgctttaatgcc-3′, reverse primer: 5′-acgccacgttgcctgacaac-3′) were used according to the manufacturer's protocol in an ABI PRISM 7700 Sequence Detector System (PE Applied Biosystems, Foster City, CA). SIV gene transfer plasmid DNA (3 × 104 to 2 × 106 molecules) was used as standard.
Transduction units (TU/ml) were determined by transducing 293T cells with serial dilutions of vector stock and quantification of transduced cells by GFP fluorescence (for F/HN-SIV-GFP and VSV-G-SIV-GFP) or staining with anti-luciferase antibody (for F/HN-SIV-lux). To work with a consistent virus concentration throughout the study, virus stocks were all adjusted to a final volume of 4 × 109 TU/ml. A titer of 4 × 109 TU/ml was used for all animal studies.
In vivo administration to the mouse nose. All animal studies had been approved by the DNAVEC Animal Care Committee and the Imperial College Animal Ethics Committee and were carried out according to Home Office regulations. C57BL/6N mice (female, 6–8 weeks) were used. Mice were anesthetized, placed horizontally on their backs onto a heated board, and a thin catheter (<0.5 mm outer diameter) was inserted ~2.5 mm from the tip of nose into the left nostril. Using a syringe pump (Cole-Parmer, Vernon Hills, IL), vector (100 µl) was then slowly perfused onto the nasal epithelium (1.3 µl/min) for 75 minutes. Despite perfusion of virus into the left nostril, we routinely observe transfection in both left and right nostrils, which is due to dispersion of the solutions throughout the entire nasal cavity. PBS and VSV-G-SIV transduced mice preconditioned with 1% lysophosphatidylcholine as described by Limberis et al. were used as controls.11 At indicated time points (3–360 days after transduction), mice were culled to visualize GFP expression.
In the repeat administration experiments groups of mice were transduced with either one dose of F/HN-SIV-lux (single-dose group), or two doses of F/HN-SIV-GFP (day 0, day 28), followed by F/HN-SIV-lux on day 56 (repeat-dose group). Importantly, mice receiving F/HN-SIV-lux (single-dose group) and F/HN-SIV-lux on day 56 (repeat-dose group) were of similar age and were transduced at the same time. Gene expression was analyzed 30 days after F/HN-SIV-lux administration. For comparison, mice were transfected with the cationic lipid GL67A complexed to a luciferase reporter gene as previously described43 and luciferase expression was measured 2 days after transfection.
Induced regeneration of nasal epithelial cells by polidocanol treatment. Nasal epithelial cells were stripped by polidocanol treatment according to the method described by Borthwick et al.6 with some modification. In brief, mice were anesthetized and 10 µl polidocanol (2%) were administered to the nose as a bolus by “nasal sniffing”. To confirm the stripping and regeneration of nasal epithelial cells, nasal tissue was perfused with 10 µl of 2% (vol/vol in PBS) polidocanol (nonaethylene glycol mono-dodecyl ether; SIGMA, St Louis, MO) and histological analysis undertaken 24 hours and 7 days after treatment (n = 3/group). To analyze transduction of possible progenitor or stem cells, we first administered F/HN-SIV-GFP (4 × 108 TU/mouse) vector to mouse nasal epithelium. Seven days after transduction, nasal tissue was perfused with 10 µl of 2% (vol/vol in PBS) polidocanol, and this treatment was repeated again 3 weeks later. Histological sections were analyzed 58 days after vector administration (30 days after the last polidocanol treatment).
Bioluminescent imaging. Mice were injected intraperitoneally with 150 mg/kg of D-luciferin (Xenogen, Alameda, CA) 10 minutes before imaging and were anesthetized with isoflurane. Bioluminescence (photons/s/cm2/sr) from living mice was measured using an IVIS50 system (Xenogen) at a binning of 4 for 10 minutes, using the software programme Living Image (Xenogen). For anatomical localization a pseudocolor image representing light intensity (blue: least intense, red: most intense) was generated using Living Image software and superimposed over the grayscale reference image. To quantify bioluminescence in the nose, photon emission in a defined area (red box) was measured by marking a standardized area for quantification. The size of the red box was kept constant and was placed over the heads of the animals as indicated in the figure. Importantly, the areas were marked using the grayscale reference image to avoid bias.
Tissue preparation for histological assessment of GFP expression. Mice were culled and the skin was removed. The head was cut at eye level and skin, jaw, tongue, and the soft tip of the nose were carefully removed. For in situ imaging of GFP expression in the nasal cavity, GFP fluorescence was detected using fluorescence stereoscopic microscopy (Leica, Ernst Leitz Optische Werke, Germany). Subsequently, the tissue was fixed in 4% paraformaldehyde (pH 7.4) overnight at room temperature and was then submerged in 20% EDTA (pH 7.5 for 5 days) for decalcification. The EDTA solution was changed at least every second day. After decalcification, the tissue was incubated in 15% sucrose overnight at room temperature and was then embedded in Tissue Mount (Chiba Medical, Soka, Japan). Ten micrometer sections were cut at six different positions in each mouse head (~0–6 mm from the tip of nasal bone). GFP expression was observed using a fluorescent microscope (Leica). Quantification and identification of cell types were carried out on six levels per mouse using a ×40 or ×63 objective. Prolonged image exposure was necessary to capture the structure of the nasal epithelium using fluorescent microscopy. This led to pixel saturation of GFP-positive cells and caused GFP-positive cells to appear almost white rather than the common green appearance that we, and others, observe under higher magnification.
Transduction of ALI cultures. Fully differentiated airway epithelial cells grown as ALI cultures were purchased from Epithelix (Geneva, Switzerland). ALIs were transfected with F/HN-SIV-lux at a multiplicity of infection ranging from ~25 to ~300. The virus was dissolved in 50 µl PBS and applied to the apical surface. After 6 hours, the virus was removed and ALIs were incubated for 10–26 days. The basolateral medium was changed every 48 hours during this incubation period. At specified time points, the ALIs were lysed in 100 µl reporter lysis buffer and luciferase expression was quantified using the Luciferase Assay System (Promega, Southampton, UK) according to the manufacturer's instructions. The total protein content of the cultures was quantified using the BioRad protein assay kit (BioRad, Hemel Hempstead, UK). Each sample was assayed in duplicate. Luciferase expression was then presented as relative light units/mg total protein. For bioluminescence imaging 100 µg luciferin in PBS were added to the apical membrane.
Iodide efflux assay. HEK293T cells were transfected with F/HN-SIV-GFP-CFTR or an F/HN-SIV-GFP control virus at a multiplicity of infection of 500 and cultured for 2 days. CFTR chloride channel activity was assayed by measuring the rate of 125iodide efflux as previously described.44 The 125iodide efflux rates were normalized to the time of forskolin/IBMX addition (time 0). Curves were constructed by plotting rates of 125iodide efflux against ime. To reflect the cumulative levels of 125iodide efflux following agonist-stimulation, all comparisons are based on areas under the time-125iodide efflux curves. The area under the curve was calculated by the trapezium rule. Experiments were carried out in duplicate (n = 6 wells/group/experiment).
Statistical analysis. Normal distribution was assessed for all data and parametric or nonparametric statistical analysis was performed as appropriate. Data in Figure 3f were analyzed using the Mann–Whitney U-test to compare bioluminescence at month 1 and 8, as appropriate for nonparametric data. In 2 out 10 mice, bioluminescence had returned to baseline levels before month 8 and these mice did not undergo additional bioluminescence in vivo imaging. For the final quantification of gene expression at 8 months the mean bioluminescence of the PBS control cohort was used for these two mice. In addition, data in Figure 3f were analyzed using a Friedman repeat measure test followed by Dunn's multiple comparison post hoc test, as appropriate for nonparametric data.
Data in Figure 5 were analyzed by Kruskal–Wallis followed by Dunn's Multiple Comparison test, as appropriate for nonparametric data. Data in Figure 6 were analyzed by ANOVA followed by Bonferroni's Multiple Comparison post hoc test, as appropriate for normal distributed data. The null hypothesis was rejected at P < 0.05.
SUPPLEMENTARY MATERIALFigure S1. SeV F and HN envelope proteins are incorporated into SIV vector particles and form infectious SIV pseudotyped vectors.Figure S2. GFP-positive cluster in F/HN-SIV-GFP transduced and polidocanol-treated mouse nasal epithelium.Materials and Methods.
Supplementary Material
SeV F and HN envelope proteins are incorporated into SIV vector particles and form infectious SIV pseudotyped vectors.
GFP-positive cluster in F/HN-SIV-GFP transduced and polidocanol-treated mouse nasal epithelium.
Acknowledgments
We acknowledge Hitoshi Iwasaki and Akihiro Iida for help in drafting the manuscript, and Kentaro Washizawa, and Satoshi Fujikawa for their excellent technical assistance. We thank Genzyme Corporation for supplying GL67. This work was in part funded by the Cystic Fibrosis Trust and the Dr Benjamin Angel Senior Lectureship (U.G.).
REFERENCES
- Pilewski JM., and , Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79 1 Suppl:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- Griesenbach U, Geddes DM., and , Alton EW. Gene therapy progress and prospects: cystic fibrosis. Gene Ther. 2006;13:1061–1067. doi: 10.1038/sj.gt.3302809. [DOI] [PubMed] [Google Scholar]
- Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–973. doi: 10.1038/79463. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Griesenbach U, Shiraki-Iida T, Shu T, Hironaka T, Hou X, et al. A defective nontransmissible recombinant Sendai virus mediates efficient gene transfer to airway epithelium in vivo. Gene Ther. 2004;11:1659–1664. doi: 10.1038/sj.gt.3302334. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF. Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol. 2001;24:649–652. doi: 10.1165/ajrcmb.24.6.f206. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR., and , Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Rawlins EL., and , Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR., and , Wilson JM. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992;90:2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M., and , Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JK, Miyanohara A, LaPorte P, Bouic K, Burns JC., and , Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis M, Anson DS, Fuller M., and , Parsons DW. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum Gene Ther. 2002;13:1961–1970. doi: 10.1089/10430340260355365. [DOI] [PubMed] [Google Scholar]
- Johnson LG, Vanhook MK, Coyne CB, Haykal-Coates N., and , Gavett SH. Safety and efficiency of modulating paracellular permeability to enhance airway epithelial gene transfer in vivo. Hum Gene Ther. 2003;14:729–747. doi: 10.1089/104303403765255138. [DOI] [PubMed] [Google Scholar]
- Johnson LG, Olsen JC, Naldini L., and , Boucher RC. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Iida A, Ueda Y., and , Hasegawa M. Pseudotyped lentivirus vectors derived from simian immunodeficiency virus SIVagm with envelope glycoproteins from paramyxovirus. J Virol. 2003;77:2607–2614. doi: 10.1128/JVI.77.4.2607-2614.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger GP, Weiner DJ, Yu QC., and , Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- Medina MF, Kobinger GP, Rux J, Gasmi M, Looney DJ, Bates P, et al. Lentiviral vectors pseudotyped with minimal filovirus envelopes increased gene transfer in murine lung. Mol Ther. 2003;8:777–789. doi: 10.1016/j.ymthe.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, et al. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor α. J Virol. 2003;77:5902–5910. doi: 10.1128/JVI.77.10.5902-5910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay T, Patel M, Pickles RJ, Johnson LG., and , Olsen JC. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther. 2006;13:715–724. doi: 10.1038/sj.gt.3302715. [DOI] [PubMed] [Google Scholar]
- Copreni E, Penzo M, Carrabino S., and , Conese M. Lentivirus-mediated gene transfer to the respiratory epithelium: a promising approach to gene therapy of cystic fibrosis. Gene Ther. 2004;11 Suppl 1:S67–S75. doi: 10.1038/sj.gt.3302372. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Nakamaru K, Ido E, Terao K, Hayami M., and , Hasegawa M. Development of novel simian immunodeficiency virus vectors carrying a dual gene expression system. Hum Gene Ther. 2000;11:1863–1874. doi: 10.1089/10430340050129486. [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Miura T, Hasegawa A, Morikawa S, Tsujimoto H, Miki K, et al. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988;333:457–461. doi: 10.1038/333457a0. [DOI] [PubMed] [Google Scholar]
- Manganini M, Serafini M, Bambacioni F, Casati C, Erba E, Follenzi A, et al. A human immunodeficiency virus type 1 pol gene-derived sequence (cPPT/CTS) increases the efficiency of transduction of human nondividing monocytes and T lymphocytes by lentiviral vectors. Hum Gene Ther. 2002;13:1793–1807. doi: 10.1089/104303402760372909. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D., and , Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SC, Harder B, Brzezinski M, Flint LY, Seppen J., and , Osborne WR. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum Gene Ther. 2001;12:1103–1108. doi: 10.1089/104303401750214311. [DOI] [PubMed] [Google Scholar]
- Jacob A, Faddis BT., and , Chole RA. Chronic bacterial rhinosinusitis: description of a mouse model. Arch Otolaryngol Head Neck Surg. 2001;127:657–664. doi: 10.1001/archotol.127.6.657. [DOI] [PubMed] [Google Scholar]
- Ban H, Inoue M, Griesenbach U, Munkonge F, Chan M, Iida A, et al. Expression and maturation of Sendai virus vector-derived CFTR protein: functional and biochemical evidence using a GFP-CFTR fusion protein. Gene Ther. 2007;14:1688–1694. doi: 10.1038/sj.gt.3303032. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Loffing J, Schwiebert EM, Loffing-Cueni D, Halpin PA, Karlson KH, et al. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in Madin-Darby canine kidney cells. J Biol Chem. 1998;273:21759–21768. doi: 10.1074/jbc.273.34.21759. [DOI] [PubMed] [Google Scholar]
- Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, et al. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1123–L1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- Sirven A, Pflumio F, Zennou V, Titeux M, Vainchenker W, Coulombel L, et al. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood. 2000;96:4103–4110. [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of immunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- Donello JE, Loeb JE., and , Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ., and , Bromberg JS. Promoter attenuation in gene therapy: interferon-γ and tumor necrosis factor-α inhibit transgene expression. Hum Gene Ther. 1997;8:2019–2029. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- Sung RS, Qin L., and , Bromberg JS. TNFα and IFNγ induced by innate anti-adenoviral immune responses inhibit adenovirus-mediated transgene expression. Mol Ther. 2001;3 5 Pt 1:757–767. doi: 10.1006/mthe.2001.0318. [DOI] [PubMed] [Google Scholar]
- Slack JM. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Arias AC, Brogden KA., and , McCray PB., Jr Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J Virol. 2008;82:10684–10692. doi: 10.1128/JVI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BR, Rogers TD, Boucher RC., and , Ostrowski LE. Ion transport across CF and normal murine olfactory and ciliated epithelium. Am J Physiol, Cell Physiol. 2009;296:C1301–C1309. doi: 10.1152/ajpcell.00578.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski LE, Yin W, Diggs PS, Rogers TD, O'Neal WK., and , Grubb BR. Expression of CFTR from a ciliated cell-specific promoter is ineffective at correcting nasal potential difference in CF mice. Gene Ther. 2007;14:1492–1501. doi: 10.1038/sj.gt.3302994. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Griesenbach U, Iida A, Farley R, Wright AM, Zhu J, et al. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007;14:1371–1379. doi: 10.1038/sj.gt.3302991. [DOI] [PubMed] [Google Scholar]
- Griesenbach U, Sumner-Jones SG, Holder E, Munkonge FM, Wodehouse T, Smith SN, et al. 2009Limitations of the murine nose in the development of non-viral airway gene transfer Am J Respir Cell Mol Biol(epub ahead of print). [DOI] [PubMed]
- Girones R, Cote PJ, Hornbuckle WE, Tennant BC, Gerin JL, Purcell RH, et al. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc Natl Acad Sci USA. 1989;86:1846–1849. doi: 10.1073/pnas.86.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka Y, Cannon PM, Ramsdale EE, Griffiths JC, Romano G, Kingsman SM, et al. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Glynn J, Jin MJ, Jolly DJ, Yee JK., and , Chen ST. Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol. 1999;73:1828–1834. doi: 10.1128/jvi.73.3.1828-1834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenbach U, Meng C, Farley R, Cheng SH, Scheule RK, Davies MH, et al. In vivo imaging of gene transfer to the respiratory tract. Biomaterials. 2008;29:1533–1540. doi: 10.1016/j.biomaterials.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Dérand R, Bulteau-Pignoux L., and , Becq F. Comparative pharmacology of the activity of wild-type and G551D mutated CFTR chloride channel: effect of the benzimidazolone derivative NS004. J Membr Biol. 2003;194:109–117. doi: 10.1007/s00232-003-2030-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SeV F and HN envelope proteins are incorporated into SIV vector particles and form infectious SIV pseudotyped vectors.
GFP-positive cluster in F/HN-SIV-GFP transduced and polidocanol-treated mouse nasal epithelium.