Abstract
CD4+ T cells contribute to the antitumor T-cell response as both effectors that promote tumor rejection and helpers that facilitate the activation of other antitumor effector cells, such as CD8+ T cells. Maximal engagement of both effector and helper CD4+ T-cell responses is a desirable attribute of cancer vaccines. We have employed the B16F10 murine melanoma model and a series of recombinant adenovirus (Ad) vaccines expressing mutant forms of the tumor antigen, dopachrome tautomerase, to investigate the relationship between antigen processing and the antitumor CD4+ T-cell response. Our results have revealed an unexpected dichotomy in the generation of helper and effector CD4+ T-cell responses where CD4+ T effector responses are dependent upon protein processing and trafficking, whereas CD4+ T helper responses are not. The results have important implications for strategies aimed at augmenting antigen immunogenicity by altering intracellular processing and localization.
Introduction
CD4+ T cells are a key component of the adaptive immune response against tumors and increasing evidence suggests that CD4+ T cells can be highly potent antitumor effectors.1,2,3,4,5 When tumor cells express major histocompatibility complex (MHC) II, as in the case of B-cell leukemias, CD4+ T cells are capable of recognizing tumor cells directly, leading to upregulation of death-inducing ligands and secretion of cytotoxic granules.6,7,8,9,10 CD4+ T cells can also mediate rejection of MHC II–negative tumors through indirect mechanisms following in situ activation by APCs that have engulfed tumor-derived antigens. Activated CD4+ T cells can promote tumor rejection indirectly through the release of tumor-suppressing cytokines11,12,13,14 and the recruitment of innate effector cells.15,16 This indirect pathway is an important facet of the tumor-specific effector CD4+ T cells as this mechanism allows CD4+ T cells to reject tumors that escape CD8+ T-cell recognition by downmodulation of MHC I.15,16,17,18 Recent work from both animal and human studies has underscored the potency of effector CD4+ T cells for cancer immunotherapy and even suggested that CD4+ T cells may be more potent than CD8+ T cells when compared on a cell-per-cell basis.19,20
Previous investigations by our group have revealed an important role for CD4+ T cells in the protective immunity produced by a prototype melanoma vaccine comprising a recombinant human adenovirus (Ad) type 5 vector expressing human dopachrome tautomerase (hDCT; vector name = AdhDCT). Immunization with AdhDCT can render immunocompetent mice completely protected against tumor challenge and can promote regression of established tumors when combined with cyclophosphamide.21,22,23 Using a combination of antibody depletion and gene-deficient mice, we have shown that CD4+ T cells play a significant role in the antitumor response produced by immunization with AdhDCT.21,22,24 Following AdhDCT immunization, DCT-specific CD4+ T cells act as helpers for the CD8+ T-cell response and effectors that are capable of effectuating tumor rejection and skin depigmentation.21 Using synthetic peptides, we identified a heteroclitic CD4+ T-cell epitope (hDCT89–101) in the hDCT that functions as a target for helper CD4+ T cells that promote CD8+ T-cell immunity.22 Immunization with the murine homologue of DCT (mDCT) produces only a weak CD8+ T-cell response. By converting Gln86 and Asn92 in mDCT to Leu and His, respectively, it is possible to engineer the mDCT protein to carry the heteroclitic epitope found in hDCT.22 The CD8+ T-cell response produced by the mutant protein was tenfold greater than the response to wild-type mDCT and comparable to the response produced by hDCT, confirming the helper function of the CD4+ T cells directed against this epitope.22 Interestingly, CD4+ T cells directed against hDCT89–101 do not effectuate tumor rejection.22 Therefore, we refer to hDCT89–101-specific CD4+ T cells as helpers. We have been unable to define a target for the CD4+ T cells that promote tumor rejection, but they can be identified functionally using tumor challenge studies in mice that lack CD8+ T cells.21,24 In the context of this article, we will refer to the latter population as effectors because they effectuate tumor rejection. Further, we have uncovered an interesting dichotomy with regard to the processes involved in CD4+ T cell–mediated antitumor immunity and autoimmunity whereby the former was dependent on IL-4/STAT6 signaling, whereas the latter required IFN-γ/STAT4 signalling.24 Our current efforts are directed at exploiting this dichotomy and maximizing CD4+ T cell–mediated tumor rejection while minimizing autoimmune sequelae.
In this article, we describe an Ad vector that expresses a mutant form of hDCT lacking the dominant CD8+ T-cell epitope, SVYDFFVWL (AdhDCTΔVYD). This vector was created to facilitate studies of CD4+ T cell–dependent antitumor immunity without mitigating effects of hDCT-specific CD8+ T cells. Surprisingly, this mutant failed to elicit protective CD4+ T-cell immunity. Characterization of the hDCTΔVYD protein and analysis of additional mutants revealed that the protective CD4+ T-cell response was dramatically affected by intracellular processing of hDCT, whereas the helper CD4+ T-cell response was not. These results have important implications for vaccine design and indicate that care must be taken when manipulating antigens in an effort to increase their immunogenicity.
Results
Immunization with a mutant hDCT that lacks the immunodominant CD8 epitope, SVYDFFVWL, results in loss of both CD8+ and CD4+ T cell–mediated tumor protection
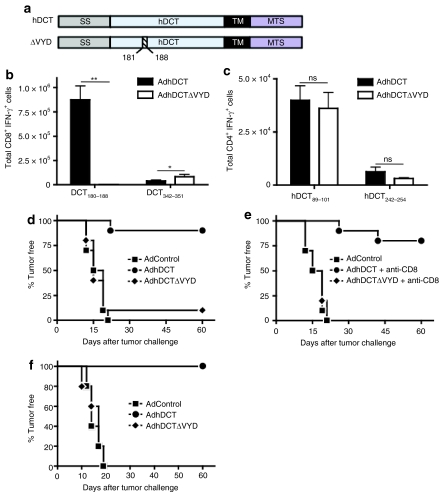
Previous reports have demonstrated that the hDCT epitope, SVYDFFVWL (DCT180–188), is alone sufficient to produce protective immunity.25,26 Therefore, we created a variant of hDCT (hDCTΔVYD, Figure 1a) that lacked this epitope in an effort to generate an antigen that would only evoke protective CD4+ T-cell immunity. The serine residue at position 180 was not removed in order to preserve a putative N-linked glycosylation motif (N-X-S) found immediately upstream of DCT180–188 (178NCS180). Mice were immunized with Ad vaccines expressing either wild-type hDCT (AdhDCT) or the mutant (AdhDCTΔVYD) to examine immunogenicity via intracellular cytokine staining (ICS) analysis (see Supplementary Figure S1 for example of the flow cytometry data). As expected, the hDCT180–188-specific response was fully abrogated following immunization with AdhDCTΔVYD, whereas CD8+ T-cell immunity to the subdominant epitope, hDCT342–351, was intact (Figure 1b). In fact, immunity to hDCT342–351 was significantly increased (P < 0.05) in mice immunized with AdhDCTΔVYD. We previously demonstrated that the magnitude of the CD8+ T-cell response to hDCT342–351 was dependent upon helper CD4+ T cells responsive to the heteroclitic epitope hDCT89 101 (ref. 22). The robust CD8+ T-cell response to hDCT342–351 suggests that the mutation in AdhDCTΔVYD did not impair the development of helper CD4+ T cells. Indeed, examination of CD4+ T-cell immunity to the dominant and subdominant CD4+ T-cell epitopes in hDCT, hDCT89–101, and hDCT242–254 revealed no differences between the wild-type and mutant vaccines (Figure 1c).
Figure 1.
Immunization with AdhDCTΔVYD abrogates CD4+ T cell–mediated tumor protection while T-cell responses to the known helper CD4 epitopes are maintained. (a) Schematic representation of the hDCTΔVYD variant (SS, endoplasmic reticulum signal sequence; TM, transmembrane domain; MTS, melanosomal transport signal). (b,c) T-cell responses to the known (b) CD8 epitopes (hDCT180–188 and hDCT342–351) and (c) CD4 epitopes (hDCT89–101 and hDCT242–254) were assessed via intracellular cytokine staining. Results are shown as mean ± SEM from 10 mice pooled from two separate experiments and represent the absolute number of antigen-specific cells per spleen (*P < 0.05 and **P < 0.0005 compared to AdhDCT; NS, not significant). (d–f) Mice (n = 10) were immunized intramuscularly with 108 plaque-forming units of AdControl, AdhDCT, or AdhDCTΔVYD. (d) Wild-type mice, (e) CD8+ T cell–depleted wild-type mice, and (f) CD8-deficient mice were challenged subcutaneously with 2 × 104 B16F10 cells 7 days after immunization. Depletion of CD8+ T cells was performed by intraperitoneal injection of purified 2.43 antibodies starting on 3 days and 1 day prior to tumor challenge and subsequently administered once a week until tumors developed. A rat IgG was used as control treatment.
Our previous work demonstrated that both CD4+ T cells and CD8+ T cells were capable of independently protecting against lethal challenge with B16F10 following immunization with AdhDCT,21,22 and it was necessary to ablate both cell populations to abrogate the protective effect.21 Indeed, we show that CD8+ T-cell depletion prior to tumor challenge does not impair protective immunity imparted by AdhDCT (compare Figure 1d,e). Therefore, we expected AdhDCTΔVYD to provide robust protection against B16F10 challenge through a CD4+ T cell–dependent mechanism. Surprisingly, the protective response produced by AdhDCTΔVYD was largely attenuated relative to AdhDCT (Figure 1d). The modest protective immunity afforded by AdhDCTΔVYD was lost following depletion of CD8+ T cells, indicating that the protection was provided by immunity elicited by subdominant CD8+ T-cell epitopes, like hDCT342–351 (Figure 1e).
We have previously determined that CD8-deficient mice can be used to study CD4+ T-cell protective immunity generated by immunization with AdhDCT.22,24 Therefore, we also evaluated protection against B16F10 following immunization with AdhDCTΔVYD in CD8-deficient mice. Similar to the scenario in CD8-depleted wild-type mice, immunization with AdhDCT provided complete protection against B16F10, whereas no protection was afforded by immunization with AdhDCTΔVYD (Figure 1f). The surprising observation that removal of a CD8+ T-cell epitope attenuated the protective CD4+ T-cell response prompted us to further investigate the mechanisms underlying the generation of helper and effector CD4+ T-cell responses following AdhDCT immunization.
Comprehensive epitope mapping of the region surrounding the deletion in AdhDCTΔVYD did not reveal any previously unidentified CD4 epitopes
It seemed paradoxical that the helper CD4+ T-cell response elicited by hDCT was unaffected by the deletion of residues 181–188, whereas the protective CD4+ T-cell response was fully attenuated. The simplest explanation for this observation is that the deletion also affects a CD4+ T-cell epitope. Using a combination of IFN-γ, IL-4, and IL-10 enzyme-linked immunosorbent spot assays, we have conducted extensive mapping of the DCT T-cell epitopes in both CD8-deficient mice (data not shown) and wild-type mice.22 We could not identify a CD4+ T-cell epitope that correlated with tumor rejection. Based on the current results, we prepared a comprehensive set of synthetic peptides that spanned the entire region deleted in hDCTΔVYD (Supplementary Table S1). ICS analysis of these peptides using splenocytes from CD8-deficient mice immunized with AdhDCT failed to reveal new reactivities (Supplementary Table S1). These results argue against the likelihood that removal of residues 181–188 disrupted a CD4+ T-cell epitope in hDCT.
Mutation of the putative glycosylation site 178NCS180 results in impaired protein trafficking and attenuated CD4+ T cell–mediated protection
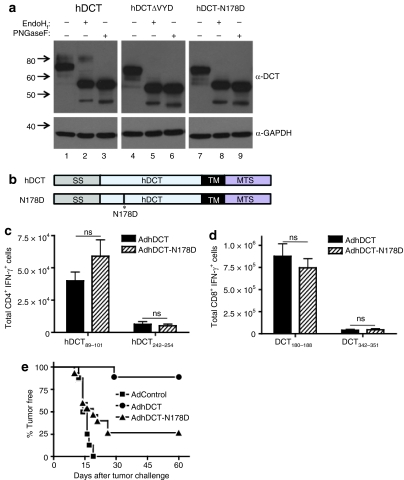
Protein conformation can significantly impact the presentation of epitopes on MHC II. As an example, Golgher et al. determined that recognition of gp90 by CD4+ T cells was dependent on the peptide conformation that was controlled by glycosylation.27 Because the deletion in hDCTΔVYD was adjacent to a putative N-linked glycosylation site, we suspected that glycosylation at this site may have been disrupted. On western blot analysis, hDCT resolves as two distinct glycoforms of 69 and 80 kd28 (Figure 2a, lane 1). When we compared the migration pattern of hDCTΔVYD to hDCT by western blot, we observed that the higher molecular weight form (80 kd) was lacking from hDCTΔVYD (Figure 2a, lane 4) and that minor, smaller molecular weight forms (<69 kd) were appearing. These results were reminiscent of a previous report where glycosylation of TRP-1 was disrupted.29 Based on the proximity of the deletion in hDCTΔVYD to a putative glycosylation site (178NCS180), we suspected that this mutation may have disrupted glycosylation at N178. We, therefore, created an hDCT mutant where N178 was replaced with D178 (hDCT-N178D, Figure 2b) to determine whether lack of glycosylation at this site was the determining factor in the impaired immunogenicity of AdhDCTΔVYD. The hDCT-N178D mutant, similarly to hDCTΔVYD, also failed to produce the higher molecular weight form (Figure 2a, lane 7), and both mutants seemed to give rise to the development of minor glycoforms that were <69 kd but >59 kd (the estimated molecular weight of the protein).
Figure 2.
Immunization with hDCT variants lacking mature N-linked glycosylation results in impaired CD4+ T cell–mediated tumor protection while T-cell responses to the known helper CD4 epitopes are maintained. (a) Western blot analysis reveals lack of mature N-linked glycosylation in hDCTΔVYD and hDCT-N178D protein variants. 293T cells were transfected for 24 hours with plasmid DNA expressing hDCT, hDCTΔVYD, or hDCT-N178D. Lysates were prepared in RIPA lysis buffer, and equal amounts of protein (~20 µg) were treated with the glycosidase enzymes EndoHf or PNGaseF for 24 hours at 37 °C. The entire reaction was diluted in 2× gel loading buffer and resolved by 10% SDS-PAGE. DCT was probed for using α-TRP-2 (C-9) and developed by ECL, followed by reprobing with α-GAPDH as a loading control. (b) Schematic representation of the hDCT-N178D variant (SS, endoplasmic reticulum signal sequence; TM, transmembrane domain; MTS, melanosomal transport signal). (c,d) T-cell responses to the known (c) CD4 epitopes (hDCT89–101 and hDCT242–254) and (d) CD8 epitopes (hDCT180–188 and hDCT342–351) were assessed via intracellular cytokine staining. Results are shown as mean ± SEM from 10 mice pooled from two separate experiments and represent the absolute number of antigen-specific cells per spleen (NS, not significant). (e) CD8-deficient mice (n = 10–15) were immunized intramuscularly with 108 plaque-forming units of AdControl, AdhDCT, or AdhDCT-N178D. Mice were challenged subcutaneously with 2 × 104 B16F10 cells 7 days after immunization.
The 69 kd glycoform of hDCT is found in the endoplasmic reticulum (ER) following translocation of the protein from the cytoplasm and addition of initial N-linked glycans; this glycoform appears to represent the majority of the hDCT when expressed from recombinant Ad (Figure 2a, lane 1). The 80 kd glycoform is found in the Golgi and represents a more mature form of the protein that harbors complex glycan structures as well as additional post-translational modifications.28 This glycoform is only a minor component of the hDCT produced by AdhDCT infection, suggesting that the majority of the virally expressed protein remains in the ER. To confirm the localization of the various glycoforms of hDCT, the proteins were subjected to digestion with glycosidases.28 EndoHf specifically cleaves N-linked glycans that are attached in the ER, whereas PNGaseF cleaves all sugar chains, including complex structures added in the Golgi. The fully deglycosylated proteins migrated at an approximate weight of 59 kd (Figure 2a, lanes 3, 6, and 9), which is consistent with the estimated molecular weight of hDCT. As predicted, EndoHf treatment caused the 69 kd band to appear as 59 kd but did not affect the 80 kd band (Figure 2a, lanes 2, 5, and 8) supporting the assertion that the 80 kd represents a more mature form of the protein that has migrated to the Golgi. Because neither hDCT-N178D nor hDCTΔVYD gave rise to the 80 kd glycoform of DCT, we conclude that these proteins do not migrate to the Golgi.
We next evaluated the immunogenicity of AdhDCT-N178D. As in the case of AdhDCTΔVYD, the CD4+ T-cell responses to hDCT89–101 and hDCT242–254 were comparable to those elicited by AdhDCT (Figure 2c). To confirm that the helper functions were unaffected, we also examined the CD8+ T-cell responses and found that CD8+ T-cell immunity was intact as well (Figure 2d). Thus, despite the protein trafficking defect, AdhDCT-N178D evokes a helper T-cell response comparable to AdhDCT. To examine the effector CD4+ T-cell response produced by AdhDCT-N178D, we immunized CD8-deficient mice as described above.22,24 Similar to AdhDCTΔVYD, protective immunity produced by AdhDCT-N178D was significantly impaired compared to AdhDCT (Figure 2e). Thus, although the helper CD4+ T-cell response produced by AdhDCT-N178D was unimpaired relative to AdhDCT, the effector CD4+ T-cell response was significantly attenuated.
Deglycosylation of hDCT putative N-linked glycosylation sites does not generate any new CD4 epitopes
Because the presence of the 80 kd molecular weight glycoform of hDCT correlated with the generation of protective CD4+ T-cell immunity, we considered a previous report involving tyrosinase where a T-cell epitope was generated by cytoplasmic deglycosylation of N-linked glycans, which resulted in the conversion of asparagine to aspartic acid.30 We hypothesized that the generation of the CD4 effector epitope(s) may be dependent upon a similar event. So, we synthesized peptides covering all putative N-linked glycosylation motifs (N-X-C/S/T) and substituted asparagine residues for aspartic acid residues (Supplementary Table S2). Potential reactivity to these peptides was evaluated via ICS analysis. Results obtained from these experiments failed to uncover any new CD4 reactivities, indicating that conversion of asparagines to aspartic acid following deglycosylation of hDCT does not give rise to measurable T-cell responses following immunization with AdhDCT (Supplementary Table S2).
Immunization with a trafficking-defective variant of hDCT results in abrogated CD4+ T cell–mediated tumor protection
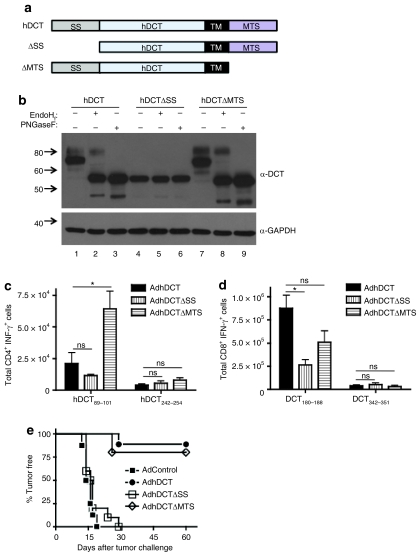
The melanosomal transport signal (MTS) present in melanoma-associated antigens (gp100, TRP-1/gp75, and TRP-2/DCT) can direct antigens to the MHC II compartment and enhance CD4+ T-cell stimulation.31,32 Therefore, the impaired effector CD4+ T-cell response observed following immunization with AdhDCTΔVYD and AdhDCT-N178D may reflect improper intracellular trafficking rather than the inability to generate a conformational or glycosylation-dependent effector CD4+ T-cell epitope. To address the possibility that targeting of hDCT is important for the generation of the effector CD4+ T-cell responses, we removed two key targeting elements from the protein: the ER signal sequence (SS, residues deleted: 2–23)33 and the putative MTS (residues deleted: 491–496) (Figure 3a). hDCTΔSS lacks the ER signal sequence and thus is expected to remain cytoplasmic. hDCTΔMTS lacks the melanosomal targeting signal, but retains the transmembrane domain and is expected to traffic through the Golgi without being redirected to melanosomes. As expected, western blot analysis shows that hDCTΔSS completely lacks N-linked glycosylation (Figure 3b, lanes 4–6). Interestingly, hDCTΔMTS actually appeared to be more stable than hDCT and gave rise to higher levels of the mature 80 kd glycoform (Figure 3b, lane 7). With regard to the generation of CD4+ T-cell immunity, both AdhDCTΔSS and AdhDCTΔMTS were able to elicit hDCT89–101-and hDCT242–254-specific CD4+ T-cell responses of a similar magnitude to AdhDCT (Figure 3c). In fact, the response to hDCT89–101 was significantly higher in mice immunized with AdhDCTΔMTS compared to AdhDCT (P < 0.05), likely due to the fact that this protein variant appears to accumulate to higher levels. hDCT180–188-specific CD8+ T-cell responses produced by AdhDCTΔSS were reduced relative to AdhDCT (P < 0.005; Figure 3d), consistent with the observation that this mutant is expressed at lower levels than the wild-type protein; however, the CD8+ T-cell response to hDCT342–351 was intact, and the response to hDCT180–188 was still markedly higher than the response to the murine form of the protein (mDCT) that cannot elicit CD4+ T-cell help22 indicating that the helper response produced by Ad hDCTΔSS was intact. Likewise, the CD8+ T-cell response produced by AdhDCTΔMTS was comparable to the wild-type vaccine indicating that the helper CD4+ T-cell response evoked by this vaccine was intact as well (Figure 3d).
Figure 3.
Immunization with AdhDCTΔSS, which is glycosylation-defective, but not AdhDCTΔMTS, abrogates CD4+ T cell–mediated tumor protection while T-cell responses to the known helper CD4 epitopes are maintained. (a) Schematic representation of the hDCTΔSS and AdhDCTΔMTS variants (SS, endoplasmic reticulum signal sequence; TM, transmembrane domain; MTS, melanosomal transport signal). (b) Western blot analysis reveals lack of mature N-linked glycosylation in hDCTΔSS variant, but not AdhDCTΔMTS variant. 293T cells were transfected with plasmid DNA expressing hDCT, hDCTΔSS, hDCTΔMTS, or GFP for 24 hours. Lysates were prepared in RIPA lysis buffer and equal amounts of protein (~20 µg) were treated with the glycosidase enzymes EndoHf or PNGaseF for 24 hours at 37 °C. The entire reaction was diluted in 2× gel loading buffer and resolved by 10% SDS-PAGE. DCT was probed for using α-TRP-2 (C-9) and developed by ECL, followed by reprobing with α-GAPDH as a loading control. (c,d) T-cell responses to the known (c) CD4 epitopes (hDCT89–101 and hDCT242–254) and (d) CD8 epitopes (hDCT180–188 and hDCT342–351) were assessed via intracellular cytokine staining. Results are shown as mean ± SEM from 10 mice pooled from two separate experiments and represent the absolute number of antigen-specific cells per spleen (*P < 0.05 compared to AdhDCT; NS, not significant). (e) CD8-deficient mice (n = 10–15) were immunized intramuscularly with 108 plaque-forming units of AdControl, AdhDCT, AdhDCTΔSS, or AdhDCTΔMTS. Mice were challenged subcutaneously with 2 × 104 B16F10 cells 7 days after immunization.
Generation of protective CD4+ T-cell immunity was next evaluated in CD8-deficient mice. As shown in Figure 3e, CD4+ T cell–mediated tumor protection was completely abrogated in mice immunized with AdhDCTΔSS, whereas mice immunized with AdhDCTΔMTS displayed tumor protection comparable to AdhDCT. We can conclude from these latter observations that the MTS is not critical for the production of either helper or effector CD4+ T-cell responses. Deletion of the ER signal sequence, similar to the ΔVYD and N178D mutations, impaired the generation of effector CD4+ T-cell immunity, whereas the helper response remained unaffected.
AdhDCTΔVYD fails to elicit protective CD4+ T-cell immunity in DCT-deficient mice
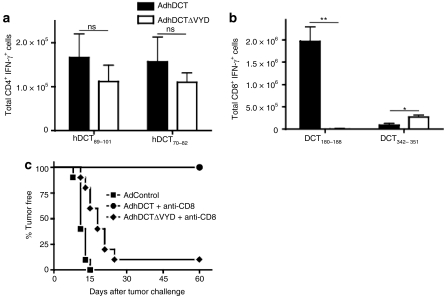
We have previously demonstrated that CD4+ T-cell immunity to DCT is more stringently regulated than the CD8+ T-cell response.22 Therefore, this dichotomy between helper and effector CD4+ T-cell responses may not be a general property of the DCT antigen, but rather simply reflects a lack of CD4+ T-cell effectors that can respond to DCT in wild-type mice. To address this possibility, we have immunized DCT-deficient mice with the mutant viruses to determine whether a similar dichotomy exists under conditions where CD4+ T-cell immunity to hDCT is less stringently regulated. Inoculation of DCT-deficient mice with AdhDCT evokes a more robust CD4+ T-cell response than wild type mice.22 Interestingly, reactivity to a dominant CD4 epitope, DCT70–82, appears only in DCT-deficient mice. This epitope is fully conserved between the murine and human forms of the protein,22 suggesting that it could be targeted for rejection by DCT-specific CD4+ T cells. We immunized DCT-deficient mice with either AdhDCT or AdhDCTΔVYD, and depleted the CD8+ T cell prior to tumor challenge to visualize CD4+ T cell–dependent tumor rejection. The immune response elicited by AdhDCTΔVYD to the dominant CD4+ T-cell epitopes and subdominant CD8+ T-cell epitope was comparable to AdhDCT (Figure 4a,b). However, only the mice immunized with AdhDCT were protected from challenge following CD8+ T-cell depletion (Figure 4c).
Figure 4.
Immunization of DCT-deficient mice with AdhDCTΔVYD abrogates CD4+ T cell–mediated tumor protection while T-cell responses to the known helper CD4 epitopes are maintained. (a,b) T-cell responses to the known (a) CD4 epitopes (hDCT89–101 and hDCT242–254) and (b) CD8 epitopes (hDCT180–188 and hDCT342–351) were assessed via intracellular cytokine staining. Results are shown as mean ± SEM from 10 mice pooled from two separate experiments and represent the absolute number of antigen-specific cells per spleen (*P < 0.05 and **P < 0.0005 compared to AdhDCT; NS, not significant). (c) DCT-deficient mice (n = 10–15) were immunized intramuscularly with 108 plaque-forming units of AdControl, AdhDCT, or AdhDCTΔVYD. Mice were challenged subcutaneously with 2 × 104 B16F10 cells 7 days after immunization. Depletion of CD8+ T cells was performed by intraperitoneal injection of purified 2.43 antibodies starting on 3 days and 1 day prior to tumor challenge and subsequently administered once a week until tumors developed. A rat IgG was used as control treatment.
Discussion
In the present report, we provide evidence that the generation of helper and effector CD4+ T-cell responses can be markedly affected by antigen structure and processing. Variants of the hDCT antigen that harbor mutations within the region of a putative glycosylation motif (178NCS180) show altered protein processing. Consistent with this observation, a previous report has suggested that the N178 glycosylation site in hDCT, which is highly conserved, is associated with protein translocation.34 As a result, it is likely that glycan maturation and other post-translational modifications on the mutant hDCT proteins, which occur in the Golgi, are impaired in cells infected with AdhDCT-N178D and AdhDCTΔVYD. Protective immunity correlated with the ability of the hDCT protein to mature to the higher molecular weight glycoform, pointing to a role for protein trafficking and subsequent post-translational modifications in the generation of protective hDCT-specific CD4+ T-cell immunity. Although the exact nature of the epitope generated from this higher molecular weight form remains unknown, it is clear that it must have a more complex structure than can be estimated from the linear protein sequence. A variety of protein modifications have been shown to influence epitope processing and presentation, including the following: isoaspartylation, phosphorylation, glycosylation, and deamination.30,35,36,37,38 Although some post-translational modifications are enzymatic in nature, others arise at physiological conditions and can be more frequent in a stressed environment resulting from disease, inflammation, and trauma, for instance.39 Our lab continues to investigate the nature of the CD4+ T-cell effector epitope, but it remains elusive. Strikingly, neither the helper CD4+ T-cell response nor the CD8+ T-cell response appeared to be influenced in the same way. Results obtained from DCT-deficient mice demonstrate that the dichotomy in the generation of helper and effector CD4+ T-cell responses following immunization with AdhDCT is not a reflection of the available CD4+ T-cell repertoire but is actually an intrinsic property of the hDCT protein.
It is unclear at this time how the lack of glycan maturation influences the generation of antitumor CD4+ T cells. A number of reports have demonstrated the importance of glycosylation in the generation of T-cell epitopes. Specific glycosylated MUC-1 epitopes are recognized by CD4+ T cells that do not crossreact with the corresponding unglycosylated peptides.38 Similarly, CD4+ T cells reactive with the envelope protein (gp90) from an endogenous murine leukemia virus were only reactive with the glycosylated form of the protein.27 In the latter case, the T cells did not appear to recognize glycosylated residues. Rather, glycosylation appeared to be important for establishing the correct orientation of the peptide for CD4+ T-cell recognition.40 Whether a similar phenomenon can explain the generation of DCT epitopes that promote tumor rejection remains to be established. It has previously been reported that impairment of glycosylation and redirection of glycosylated proteins to the cytosol can enhance CD8+ T-cell immunity;29,41 however, our results do not support these strategies as being a universal approach. We conducted extensive epitope mapping on all of the mutant proteins described in this article (data not shown) and did not uncover any novel T-cell epitopes. Therefore, according to our results, disruption of trafficking and processing of hDCT does not improve immunogenicity and, as observed for the effector CD4+ T-cell response, such disruption can actually impair protective immunity. Thusly, the impact of protein mutations must be considered on an empirical basis and cannot be broadly assumed.
MHC II loading and presentation has classically been linked to the uptake of exogenous antigens. However, endogenous proteins that traffic through the endocytic pathway, such as DCT, can be loaded directly onto MHC II.42,43 Given our results, it is possible that the different CD4+ T-cell responses may actually be influenced by the endogenous and exogenous routes for MHC II loading. Perhaps, effector epitopes can only be generated via the endogenous pathway, and, therefore, these epitopes are not produced from the mutant proteins that fail to exit the ER. In contrast, the helper epitopes may be produced by either pathway and, thus, are generated from all of the mutant proteins. In an effort to capitalize on endogenous loading of MHC II following uptake of recombinant virus vaccines by APCs, a number of strategies have been developed to selectively target tumor-associated antigens to MHC II compartments for enhanced antigen presentation to CD4+ T cells. For instance, Wu et al. have used the sorting signal of lysosomal-associated membrane protein 1 fused to an antigen to direct it toward lysosomes, which then intersect with MHC II compartments.44 A similar approach developed by another group involves coupling of the trafficking signal contained in the invariant chain Ii to a tumor antigen.45 The application of such targeting strategies has been associated with elevated CD4+ T-cell immunity and corresponding increased CD8+ T-cell immunity, presumably as a result of elevated CD4+ T-cell help. Our results failed to establish a linkage between antigen targeting and the development of CD4+ T-cell help for CD8+ T-cell immunity. Indeed, targeting antigen to the endocytic pathway is not a universal method to augment CD4+ T-cell immunity, and other studies utilizing similar strategies failed to show improved T-cell immunity.46,47,48
Overall, our studies offer novel insight into the processes required to elicit CD4+ T-cell immunity and demonstrate that distinct pathways can exist to generate helper and effector CD4+ T cells. Indeed, mature glycosylation, and likely post-translational modifications, appear to be involved in the generation of protective CD4+ T-cell antitumor immunity following immunization with hDCT, a clinically relevant tumor-associated antigen, whereas helper CD4+ T-cell responses were not influenced by these post-translational events. These observations have important implication for the design of cancer vaccination platforms aimed at eliciting CD4+ T-cell immunity.
Materials and Methods
Mice and cell culture. Six- to eight-week-old female C57BL/6 mice were purchased from Charles River Breeding Laboratory (Wilmington, MA). CD8-deficient mice49 and DCT-deficient mice50 were bred in the pathogen-free Central Animal Facility at McMaster University. All of our investigations have been approved by the McMaster Animal Research Ethics Board. C57BL/6 mouse-derived melanoma B16F10 cell line was cultured in MEM-F11 medium supplemented with 10% fetal bovine serum (Invitrogen, Burlington, Ontario, Canada), 2 mmol/l L-glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin (Life Technologies, Carlsbad, CA), 1× concentration of sodium pyruvate, nonessential amino acids, and vitamin solution (Gibco, Carlsbad, CA). RPMI medium containing fetal bovine serum, L-glutamine, penicillin, and streptomycin was used for ICS assays.
Recombinant Ads. All replication-deficient Ads used in this study contain deletions in their E1 and E3 regions.22 The expression cassettes were inserted in the E1 region under the control of the murine cytomegalovirus (CMV) promoter and the SV40 polyadenylation sequence. Ad vectors were created according to the two-plasmid rescue method using 293 cells. AdhDCT expresses the full-length human DCT gene. AdhDCTΔVYD was created using standard PCR techniques and encodes a mutated form of hDCT lacking the immunodominant CD8 epitope hDCT181–188 (VYDFFVWL). AdhDCT-N178D encodes hDCT in which a point mutation at position 178, where an asparagine (N) was mutated to an aspartic acid (D), was introduced via site-directed mutagenesis. AdhDCTΔSS and AdhDCTΔMTS were created by standard PCR techniques in which hDCT harbors deletions within the intracellular localization sequences previously identified33 [SS, ER signal sequence (hDCTΔ2–23); MTS, melanosomal targeting sequence (hDCTΔ491–496)]. AdLCMV-GP33/61 encodes the sequences corresponding to residues 33–41 and 61–80 of the lymphocytic choriomeningitis virus glycoprotein and was used as a negative control (referred to as AdControl in figures).
Immunization, immunodepletion, and tumor challenge. 108 plaque-forming units of Ad vector was prepared in 100 µl sterile PBS and injected in both rear thighs (50 µl/thigh) of each mouse. Mice were challenged subcutaneously on day 7 after immunization with 2 × 104 B16F10 cells. Tumor growth was monitored daily and measured twice a week over a period of 60 days. Immunodepletion studies were conducted using the 2.43 monoclonal antibody (anti-CD8; American Type Culture Collection, Manassas, VA), which was prepared in our laboratory. A rat IgG (Sigma, St Louis, MO) was used as control. The depletion efficiency of CD8+ T cells was >98% by flow cytometry using antibodies of a different clone (data not shown).
Peptides. hDCT180–188 (immunodominant CD8 epitope) was purchased from Dalton Chemical Laboratories (Toronto, Ontario, Canada). The following peptides were ordered from Biomer Technology (Pleasanton, CA): hDCT342–351 (subdominant CD8 epitope), hDCT89–101 (immunodominant CD4 epitope), and hDCT242–254 (subdominant CD4 epitope). 15-mer overlapping peptides surrounding the hDCT180–188 epitope (Supplementary Table S1) are part of a previously described hDCT library.22 15-mer peptides overlapping putative N-linked glycosylation sites (N-X-C/S/T) within hDCT were synthesized in which asparagine (N)-to-aspartic acid (D) mutations were incorporated (Supplementary Table S2; Pepscan Systems, Lelystad, the Netherlands). All peptides were dissolved in DMSO and stored at −20 °C.
Monoclonal antibodies. The following monoclonal antibodies were purchased from BD Pharmingen (San Diego, CA): anti-CD3ε-PE (clone 145-2C11), anti-CD8α-PE-Cy5 (clone 53-6.7), anti-CD4-PE-Cy7 (clone RM4-5), anti-IFN-γ-APC (clone XMG1.2), anti-CD16/CD32 (Fc Block; clone 2.4G2), and anti-CD28 (clone 37.51).
ICS. ICS was performed as described previously22 with the following modifications: (i) splenocytes were harvested 10 days after immunization, (ii) cells were stimulated with 1 µg/ml and 10 µg/ml peptide concentrations to detect CD8+ and CD4+ T-cell responses, respectively, and (iii) no anti-CD49d was added to the stimulations. DMSO was used as negative control. Lymphocytes were examined by flow cytometry (BD FACSCanto; BD Pharmingen) and analyzed using FlowJo software (Tree Star, Ashland, OR). Results are shown as absolute numbers of antigen-specific cells per spleen, and background values have been subtracted. T-cell responses were considered meaningful when the percentage of DCT-specific cells was 2× background values and >0.02%. Results reported as frequencies are also available in Supplementary Figure S2.
Western immunoblotting and glycosidase assays. 293T cells transfected with plasmid DNA using Lipofectamine 2000 (Invitrogen) were harvested after 24 hours in modified RIPA buffer (1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS) containing protease inhibitors [PMSF and protease inhibitor cocktail (Sigma)]. Protein concentration was determined via a Bradford assay (BioRad, Hercules, CA). For glycosidase assays, 20 µg of proteins were treated with EndoHf or PNGaseF according to the recommended conditions. The glycosidase-treated lysate was diluted 1:2 in SDS gel loading buffer (BioRad), boiled for 5 minutes, electrophoresed on 10% SDS-PAGE gels, transferred to nitrocellulose membranes and probed for DCT using α-TRP-2 (C-9) (Santa Cruz Biotechnology, Santa Cruz, CA) and polyclonal goat α-mouse IgG-HRP (Cedarlane, Burlington, Ontario, Canada). GAPDH was used as a loading control, and the membranes were probed with α-GAPDH antibodies (Sigma) following stripping of the blot (50 mmol/l Tris, pH 6.8, 2% SDS, 40 µl/ml β-mercaptoethanol for 1 hour at RT). Development was done using ECL.
Statistical analysis. A two-tailed, unpaired Student's t-test was used for the analysis. Differences between means were considered significant at P < 0.05. Results were generated using GraphPad Prism 4.0b software (GraphPad Software, LaJolla, CA).
SUPPLEMENTARY MATERIALFigure S1. Example of FACS analysis.Figure S2. Compiled intracellular cytokine staining results reported as T cell frequencies.Table S1. List of hDCT peptides surrounding the sequence deleteda in the AdhDCTΔVYD vector and peptide reactivity as measured by ICSb.Table S2. List of hDCT peptides containing an asparagine (N)-to-aspartic acid (D) mutationa of putative N-linked glycosylation sites (motif: N-X-C/S/T) and peptide reactivity as measured by ICSb.
Supplementary Material
Example of FACS analysis.
Compiled intracellular cytokine staining results reported as T cell frequencies.
List of hDCT peptides surrounding the sequence deleteda in the AdhDCTΔVYD vector and peptide reactivity as measured by ICSb.
List of hDCT peptides containing an asparagine (N)-to-aspartic acid (D) mutationa of putative N-linked glycosylation sites (motif: N-X-C/S/T) and peptide reactivity as measured by ICSb.
Acknowledgments
Funding for this work was provided by the Ontario Cancer Research Network. We thank Chuyan Ying for preparing the antibodies used in these experiments. D.B. was supported by a research studentship from the Canadian Institutes of Health Research. M.S.V. was supported by research studentships from the Natural Sciences and Engineering Research Council and the Ontario Graduate Scholarships program.
REFERENCES
- Ossendorp F, Mengedé E, Camps M, Filius R., and , Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC., and , Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–6343. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]
- Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH., and , Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002;62:6438–6441. [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG., and , Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ., and , Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Rivoltini L, Mancini M, Ng J, Hartzman RJ., and , Rosenberg SA. Melanoma-specific CD4+ T lymphocytes recognize human melanoma antigens processed and presented by Epstein-Barr virus-transformed B cells. Int J Cancer. 1994;58:69–79. doi: 10.1002/ijc.2910580113. [DOI] [PubMed] [Google Scholar]
- Perez SA, Sotiropoulou PA, Sotiriadou NN, Mamalaki A, Gritzapis AD, Echner H, et al. HER-2/neu-derived peptide 884-899 is expressed by human breast, colorectal and pancreatic adenocarcinomas and is recognized by in-vitro-induced specific CD4(+) T cell clones. Cancer Immunol Immunother. 2002;50:615–624. doi: 10.1007/s002620100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner EJ, Mascarenhas J, Bishop J, Yoo DH, Chadburn A, Crow MK, et al. CD4+ T-cell induction of Fas-mediated apoptosis in Burkitt's lymphoma B cells. Blood. 1996;88:1375–1382. [PubMed] [Google Scholar]
- Thomas WD., and , Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–2200. [PubMed] [Google Scholar]
- Echchakir H, Bagot M, Dorothée G, Martinvalet D, Le Gouvello S, Boumsell L, et al. Cutaneous T cell lymphoma reactive CD4+ cytotoxic T lymphocyte clones display a Th1 cytokine profile and use a fas-independent pathway for specific tumor cell lysis. J Invest Dermatol. 2000;115:74–80. doi: 10.1046/j.1523-1747.2000.00995.x. [DOI] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ., and , Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Qin Z., and , Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Fong T, Koch AE, Peterson JD, Waltenbaugh C, Tepper RI, et al. Inhibition of angiogenesis by interleukin 4. J Exp Med. 1998;188:1039–1046. doi: 10.1084/jem.188.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Davis ID., and , Wilks AF. The paracrine role of tumour-derived mIL-4 on tumour-associated endothelium. Int J Cancer. 1997;72:664–672. doi: 10.1002/(sici)1097-0215(19970807)72:4<664::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D., and , Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky HI, Lazenby A, Hayashi RJ., and , Pardoll DM. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med. 1994;179:1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch J, Fraser K, Lane C, Putzu K, Adema GJ, Zhang QJ, et al. CTL-dependent and -independent antitumor immunity is determined by the tumor not the vaccine. J Immunol. 2004;172:5200–5205. doi: 10.4049/jimmunol.172.9.5200. [DOI] [PubMed] [Google Scholar]
- Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C, Leitch J, Tan X, Hadjati J, Bramson JL., and , Wan Y. Vaccination-induced autoimmune vitiligo is a consequence of secondary trauma to the skin. Cancer Res. 2004;64:1509–1514. doi: 10.1158/0008-5472.can-03-3227. [DOI] [PubMed] [Google Scholar]
- Kianizad K, Marshall LA, Grinshtein N, Bernard D, Margl R, Cheng S, et al. Elevated frequencies of self-reactive CD8+ T cells following immunization with a xenoantigen are due to the presence of a heteroclitic CD4+ T-cell helper epitope. Cancer Res. 2007;67:6459–6467. doi: 10.1158/0008-5472.CAN-06-4336. [DOI] [PubMed] [Google Scholar]
- Grinshtein N, Ventresca M, Margl R, Bernard D, Yang TC, Millar JB, et al. High-dose chemotherapy augments the efficacy of recombinant adenovirus vaccines and improves the therapeutic outcome. Cancer Gene Ther. 2009;16:338–350. doi: 10.1038/cgt.2008.89. [DOI] [PubMed] [Google Scholar]
- Zhang S, Bernard D, Khan WI, Kaplan MH, Bramson JL., and , Wan Y. CD4+ T-cell-mediated anti-tumor immunity can be uncoupled from autoimmunity via the STAT4/STAT6 signaling axis. Eur J Immunol. 2009;39:1252–1259. doi: 10.1002/eji.200839152. [DOI] [PubMed] [Google Scholar]
- Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J, Brück J, Lenz J, Büchs S., and , Tüting T. Peripheral CD8+ T cell tolerance against melanocytic self-antigens in the skin is regulated in two steps by CD4+ T cells and local inflammation: implications for the pathophysiology of vitiligo. J Invest Dermatol. 2005;124:144–150. doi: 10.1111/j.0022-202X.2004.23538.x. [DOI] [PubMed] [Google Scholar]
- Golgher D, Korangy F, Gao B, Gorski K, Jaffee E, Edidin M, et al. An immunodominant MHC class II-restricted tumor antigen is conformation dependent and binds to the endoplasmic reticulum chaperone, calreticulin. J Immunol. 2001;167:147–155. doi: 10.4049/jimmunol.167.1.147. [DOI] [PubMed] [Google Scholar]
- Negroiu G, Dwek RA., and , Petrescu SM. The inhibition of early N-glycan processing targets TRP-2 to degradation in B16 melanoma cells. J Biol Chem. 2003;278:27035–27042. doi: 10.1074/jbc.M303167200. [DOI] [PubMed] [Google Scholar]
- Guevara-Patiño JA, Engelhorn ME, Turk MJ, Liu C, Duan F, Rizzuto G, et al. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bartido S, Yang G, Qin J, Moroi Y, Panageas KS, et al. A role for a melanosome transport signal in accessing the MHC class II presentation pathway and in eliciting CD4+ T cell responses. J Immunol. 1999;163:5820–5826. [PubMed] [Google Scholar]
- Lepage S., and , Lapointe R. Melanosomal targeting sequences from gp100 are essential for MHC class II-restricted endogenous epitope presentation and mobilization to endosomal compartments. Cancer Res. 2006;66:2423–2432. doi: 10.1158/0008-5472.CAN-05-2516. [DOI] [PubMed] [Google Scholar]
- Jackson IJ, Chambers DM, Tsukamoto K, Copeland NG, Gilbert DJ, Jenkins NA, et al. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992;11:527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Bartido S, Setaluri V, Qin J, Yang G., and , Houghton AN. Diverse roles of conserved asparagine-linked glycan sites on tyrosinase family glycoproteins. Exp Cell Res. 2001;267:115–125. doi: 10.1006/excr.2001.5232. [DOI] [PubMed] [Google Scholar]
- Mamula MJ, Gee RJ, Elliott JI, Sette A, Southwood S, Jones PJ, et al. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J Biol Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- Depontieu FR, Qian J, Zarling AL, McMiller TL, Salay TM, Norris A, et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc Natl Acad Sci USA. 2009;106:12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housseau F, Moorthy A, Langer DA, Robbins PF, Gonzales MI., and , Topalian SL. N-linked carbohydrates in tyrosinase are required for its recognition by human MHC class II-restricted CD4(+) T cells. Eur J Immunol. 2001;31:2690–2701. doi: 10.1002/1521-4141(200109)31:9<2690::aid-immu2690>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Vlad AM, Muller S, Cudic M, Paulsen H, Otvos L, Jr, Hanisch FG, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HA., and , Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–449. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Mimura-Kimura Y, Doores K, Golgher D, Davis BG, Dwek RA, et al. Folding of an MHC class II-restricted tumor antigen controls its antigenicity via MHC-guided processing. Proc Natl Acad Sci USA. 2007;104:5983–5988. doi: 10.1073/pnas.0701307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincheff M, Zoubak S, Altankova I, Tchakarov S, Makogonenko Y, Botev C, et al. Human dendritic cells genetically engineered to express cytosolically retained fragment of prostate-specific membrane antigen prime cytotoxic T-cell responses to multiple epitopes. Cancer Gene Ther. 2003;10:907–917. doi: 10.1038/sj.cgt.7700647. [DOI] [PubMed] [Google Scholar]
- Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS., and , Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Theos AC., and , Raposo G. Melanosomes and MHC class II antigen-processing compartments: a tinted view of intracellular trafficking and immunity. Immunol Res. 2003;27:409–426. doi: 10.1385/IR:27:2-3:409. [DOI] [PubMed] [Google Scholar]
- Wu TC, Guarnieri FG, Staveley-O'Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Wang X., and , Rosenberg SA. Identification of a novel major histocompatibility complex class II-restricted tumor antigen resulting from a chromosomal rearrangement recognized by CD4(+) T cells. J Exp Med. 1999;189:1659–1668. doi: 10.1084/jem.189.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidalin O, Tanaka E, Spengler U, Trépo C., and , Inchauspé G. Targeting of hepatitis C virus core protein for MHC I or MHC II presentation does not enhance induction of immune responses to DNA vaccination. DNA Cell Biol. 1999;18:611–621. doi: 10.1089/104454999315024. [DOI] [PubMed] [Google Scholar]
- Deng H, Kowalczyk D, O I, Blaszczyk-Thurin M, Quan Xiang Z, Giles-Davis W, et al. A modified DNA vaccine to p53 induces protective immunity to challenge with a chemically induced sarcoma cell line. Cell Immunol. 2002;215:20–31. doi: 10.1016/s0008-8749(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Dobaño C, Rogers WO, Gowda K., and , Doolan DL. Targeting antigen to MHC Class I and Class II antigen presentation pathways for malaria DNA vaccines. Immunol Lett. 2007;111:92–102. doi: 10.1016/j.imlet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Fung-Leung WP, Schilham MW, Rahemtulla A, Kündig TM, Vollenweider M, Potter J, et al. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Guyonneau L, Murisier F, Rossier A, Moulin A., and , Beermann F. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol Cell Biol. 2004;24:3396–3403. doi: 10.1128/MCB.24.8.3396-3403.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example of FACS analysis.
Compiled intracellular cytokine staining results reported as T cell frequencies.
List of hDCT peptides surrounding the sequence deleteda in the AdhDCTΔVYD vector and peptide reactivity as measured by ICSb.
List of hDCT peptides containing an asparagine (N)-to-aspartic acid (D) mutationa of putative N-linked glycosylation sites (motif: N-X-C/S/T) and peptide reactivity as measured by ICSb.