Abstract
A cochlear implant may be used to electrically stimulate spiral ganglion neurons (SGNs) in people with severe sensorineural hearing loss (SNHL). However, these neurons progressively degenerate after SNHL due to loss of neurotrophins normally supplied by sensory hair cells (HCs). Experimentally, exogenous neurotrophin administration prevents SGN degeneration but can also result in abnormal resprouting of their peripheral fibers. This study aimed to create a target-derived neurotrophin source to increase neuron survival and redirect fiber resprouting following SNHL. Adenoviral (Ad) vectors expressing green fluorescent protein (GFP) alone or in combination with brain-derived neurotrophic factor (BDNF) or neurotrophin-3 (NT3) were injected into the cochlear scala tympani or scala media of guinea-pigs (GPs) deafened via aminoglycosides for 1 week. After 3 weeks, cochleae were examined for gene expression, neuron survival, and the projection of peripheral fibers in response to gene expression. Injection of vectors into the scala media resulted in more localized gene expression than scala tympani injection with gene expression consistently observed within the partially degenerated organ of Corti. There was also greater neuron survival and evidence of localized fiber responses to neurotrophin-expressing cells within the organ of Corti from scala media injections (P < 0.05), a first step in promoting organized resprouting of auditory peripheral fibers via gene therapy.
Introduction
Over 278 million people have a disabling hearing impairment, with this number predicted to increase in an aging population (World Health Organization, 2005). The most common form of deafness is the result of damage to or loss of sensory hair cells (HCs) in the organ of Corti [sensorineural hearing loss (SNHL)]. In severe cases of SNHL, a cochlear implant is the only therapeutic option available, bypassing lost HCs and directly stimulating spiral ganglion neurons (SGNs). However, loss of HCs leads to the secondary degeneration of SGNs due to the removal of trophic support normally provided by the HCs.1 Degeneration of SGNs is progressive over time resulting in a reduced population of target neurons available for electrical stimulation. Human temporal bone studies show greater loss of SGNs with longer durations of deafness,2,3 although the rate of loss is slower than that observed in animal deafness models.4,5
Several neurotrophins, particularly neurotrophin-3 (NT3) and brain-derived neurotrophic factor (BDNF), have been shown to play an important role in the development and maintenance of SGNs. SGNs express receptors for NT3 and BDNF, and these two neurotrophins are known to be released by the cochlear sensory epithelium, especially HCs in the organ of Corti.6,7,8,9 After SNHL and loss of this endogenous source of neurotrophins, intracochlear administration of exogenous BDNF and/or NT3 following aminoglycoside-induced SNHL in guinea-pigs (GPs) was shown to promote survival of SGNs and resprouting of their peripheral fibers.10,11,12,13 However, the peripheral fibers grew abnormally. Rather than projecting radially in a spatially precise orientation toward the organ of Corti, the resprouting fibers grew in a disorganized manner, for example, doubling back toward the inner spiral sulcus or projecting laterally along the length of the organ of Corti.12,14 As the pitch coding of the cochlear implant relies on stimulation of spatially distinct populations of SGNs, extensive and disorganized fiber resprouting may reduce the spatial precision with which electrical stimulation can be delivered. Although the interelectrode spacing of current cochlear implants is relatively large with respect to the degree of lateral deviation of resprouting fibers, even a small amount of “neural overlap” may effectively increase the spread of activation and thus lead to a deterioration in implant performance. Furthermore, such overlap would limit the benefits of future advancements to cochlear implant electrode design and stimulation strategies aimed at achieving more precise and spatially restricted stimulation. Therefore, strategies that not only provide SGN protection but also control fiber resprouting may lead to an improved electrode-neural interface.
Control over the direction of resprouting SGN peripheral fibers has been achieved with limited success in adult normal-hearing GPs via gene therapy with the Math1/Atoh1 transcription factor gene. Introduction of this gene to the scala media induced supporting cells of the organ of Corti to differentiate into cells resembling HCs. SGN peripheral fibers grew toward and made connections with the new ectopic HCs15 providing evidence that targeted resprouting of SGN fibers can be achieved with gene therapy. However, new HCs could not be generated after longer-term SNHL (1 week) when the degeneration of the organ of Corti was more severe.16 The effectiveness of HC replacement appears to be restricted to situations in which there is minimal disruption to the normal architecture of the organ of Corti, limiting the practicality of clinical translation of this technique. Although the Math1/Atoh1 gene requires healthy organ of Corti supporting cells to promote transdifferentiation into HCs, the expression of other genes, such as the neurotrophin genes, in a more degenerated organ of Corti may be possible. Several studies have detailed broad expression of reporter genes in multiple cochlear cell types of normal-hearing GPs and rats,17,18,19,20 but neither reporter gene expression in the deafened cochlea nor neurotrophin gene expression within the scala media have been examined in detail.
Expression of the BDNF gene via injection of viral vectors into the scala tympani led to protection of SGNs,21,22,23 but gene expression was extensive and would provide little directional guidance to resprouting peripheral fibers. The potential exists to localize gene expression to the organ of Corti via injection into the scala media24,25 in order to achieve SGN protection and controlled fiber regrowth. Because the developmental expression of BDNF and NT3 in HCs is critical for fiber guidance during HC innervation,26 the introduction of these neurotrophins to the scala media via viral transduction may also have an important role in the guidance of resprouting fibers after damage.
The aim of this study was to use viral transduction to produce a localized source of neurotrophins that was effective in promoting SGN survival and redirecting the resprouting of their peripheral fibers. Three adenovirus (Ad) vectors containing green fluorescent protein (GFP) with or without the genes for BDNF or NT3 were used. Ototoxically deafened GPs were unilaterally injected with Ad-GFP, or combined Ad-GFP-NT3 and Ad-GFP-BDNF (hereafter referred to as Ad-GFP-NT). The vectors were injected into the scala tympani or scala media. Cochleae were examined 3 weeks after viral injection. A cohort of normal-hearing GPs was included in the study to specifically determine the extent of hearing loss associated with the delivery of these vectors into the scala tympani and scala media, and to examine in detail gene transduction in normal organ of Corti (Table 1). The study examined the hypotheses that (i) injection of viral vectors into the scala media would result in more localized gene expression compared to scala tympani injections; and (ii) localized neurotrophin expression in the organ of Corti would protect SGNs from degeneration and limit the disorganized peripheral fiber resprouting after hearing loss.
Table 1.
GP groups for study
Results
Production of neurotrophins by Ad vectors
Expression and release of neurotrophins were tested in HT1080 cells transduced with Ad-GFP-NT3 and Ad-GFP-BDNF Ad vectors prior to injection in GPs. The amount of NT3 detected in Ad-GFP-NT3-transduced HT1080 conditioned medium and cell lysate was 5.2 and 5.6 ng/ml, respectively, and the amount of BDNF in Ad-GFP-BDNF-transduced HT1080 conditioned medium and cell lysate was 2.4 and 2.7 ng/ml, respectively.
GFP expression in deafened cochleae
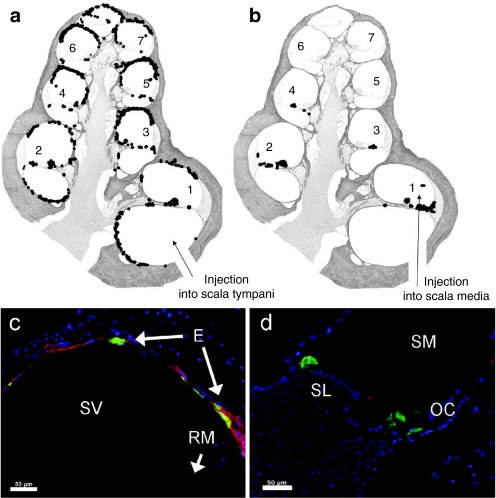
To test the hypothesis that injection of viral vectors into the scala media would result in more localized gene expression compared to scala tympani injections, the GFP reporter gene present in all viral vectors was used to study gene expression patterns in mid-modiolar sections and cochlear half-turns from deafened GPs (groups 1–4; Table 1). No difference in GFP distribution was observed between Ad-GFP- and Ad-GFP-NT-injected cochleae. Therefore, gene expression data were combined to form composite images of GFP distribution of all cochleae injected into the scala tympani (n = 11) or scala media (n = 8) surgical approaches (Figure 1a,b).
Figure 1.
Composite diagrams and photomicrographs of GFP expression from scala tympani and scala media adenoviral vector injection in 1-week deaf cochleae. (a) Following injection of Ad-GFP or Ad-GFP-NT into the scala tympani, GFP expression (represented by black dots on diagram; not drawn to scale) was detected in all turns of the cochlea, predominantly in endosteal cells lining the perilymphatic spaces (n = 11). (b) Injection of Ad-GFP or Ad-GFP-NT into the scala media resulted in GFP expression in the organ of Corti and the spiral limbus, mostly in the basal turn (n = 8). Turns are numbered for identification in Table 2. (c) In this example, injection into the scala tympani resulted in GFP expression (green) in endosteal cells (E) in the scala vestibuli (SV) (RM, Reissner's membrane). (d) Injection into the scala media (SM) resulted in GFP expression in the organ of Corti (OC) and interdental cells of the spiral limbus (SL). In both examples, sections are colabeled with anti-NF-200 (red) and DAPI (blue). Bar = 50 µm. GFP, green fluorescent protein.
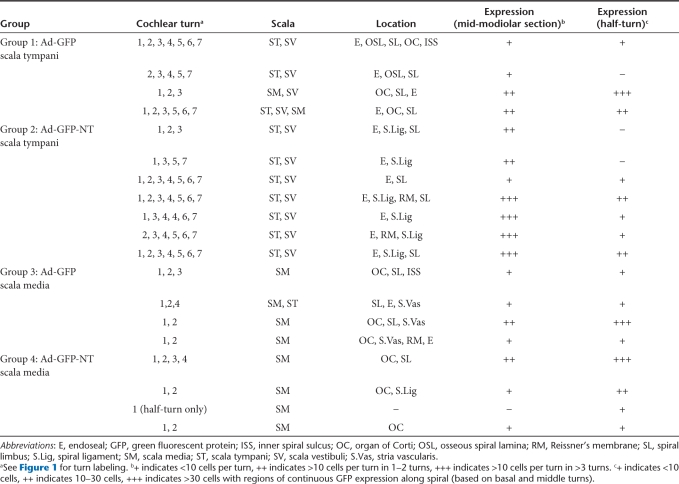
Using the scala tympani approach, GFP expression was widespread throughout all cochlear turns. The majority of GFP expression was located in cells proximal to the scala vestibuli and scala tympani and, in two GPs, also in cells within the scala media (Figure 1a). Expression was most commonly observed in the endosteal cells lining the scala vestibuli and/or scala tympani. Less frequently, GFP expression was also detected in the spiral ligament, osseous spiral lamina, spiral limbus, and very rarely in the organ of Corti, partially degenerated from the aminoglycoside deafening (Table 2, Figure 1c). Following injection into the scala media, expression was detected in the basal turn only (n = 5/8), or in the basal and middle turns (n = 3/8), and was most commonly observed within the degenerating organ of Corti and in the interdental cells of the spiral limbus, particularly those near the intersection with Reissner's membrane (Table 2, Figure 1b,d). Only rarely was GFP expression observed in other structures such as Reissner's membrane, osseous spiral lamina, and the stria vascularis (Table 2).
Table 2.
Summary of GFP expression in injected cochlea of each GP in deafened groups
Identification of transduced cells in the normal organ of Corti
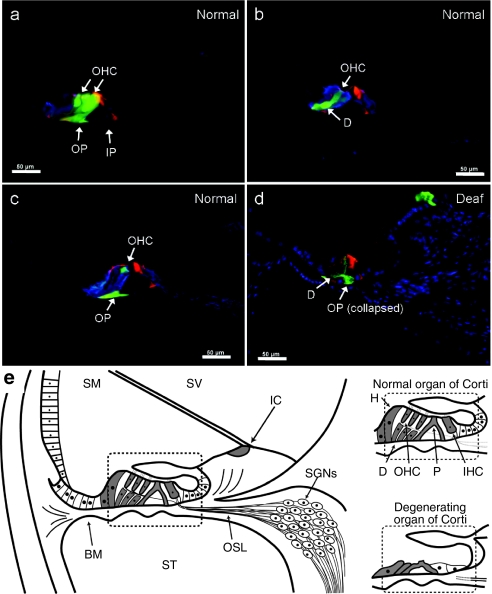
The degeneration of the organ of Corti induced by aminoglycoside treatment meant that it was not possible to precisely identify cells that expressed GFP by morphology or immunohistochemical markers. Therefore, normal-hearing GPs were used to identify the types of cells that could be transduced by Ad vectors in the intact organ of Corti after scala media injection of Ad-GFP or Ad-GFP-NT (groups 7–8; Table 1). Sections were stained with anti-calretinin (expressed in inner and outer HCs, and Deiters' cells) and phalloidin (stains pillar cells, HC stereocilia, and apical and basal planes of supporting cells). GFP expression was detected in all cells of the organ of Corti including inner and outer HCs, inner and outer pillar cells, Deiters' cells, and Hensen's cells (Figure 2). Consistent with GFP expression profiles in the deafened GPs, GFP expression was observed in interdental cells of the spiral limbus following injection into the scala media of normal-hearing GPs. A comparison of GFP expression in the normal-hearing organ of Corti and in the degenerating 1-week deafened organ of Corti is summarized in Figure 2e.
Figure 2.
Organ of Corti gene expression in normal-hearing and deafened GP groups. (a–c) Cochlear sections from normal-hearing scala media Ad-injected GPs were stained with anti-calretinin (blue) and phalloidin (red) to identify cells in the organ of Corti that could be transduced with adenoviral vectors. In these examples, GFP expression (green) was detected in inner and outer pillar cells (IP; OP), Deiters' cells (D), and outer hair cells (OHC). (d) For comparison, a phalloidin (red) and DAPI (blue)-stained cochlear section from a deafened GP injected into the scala media with Ad-GFP is also shown, highlighting that expression of GFP still occurs in supporting cells of the organ of Corti despite the partial collapse of the organ of Corti in these animals. Bar = 50 µm. (e) Summaries of GFP expression (gray shaded cells) in the normal-hearing (main picture and inset) and degenerating organ of Corti (inset) following adenoviral vector injection into the scala media is shown. BM, basilar membrane; GP, guinea-pig; H, Hensen's cell; IC, interdental cell; IHC, inner hair cell; P, pillar cell; SGNs, spiral ganglion neurons; SM, scala media; ST, scala tympani; SV, scala vestibuli.
Neurotrophin expression in deafened cochleae
To confirm that GFP expression corresponded to neurotrophin gene expression in Ad-GFP-NT-injected GPs, sections were stained with anti-BDNF and anti-NT3 antibodies. Anti-BDNF staining colocalized with 43.7% GFP+ cells of Ad-GFP-NT-injected GPs (Figure 3) and was not detected in Ad-GFP-injected GPs. However, NT3 expression was not reliably detected using the anti-NT3 antibodies on sections from Ad-GFP-NT-injected GPs despite the confirmation of NT3 production by enzyme-linked immunosorbent assay prior to injection.
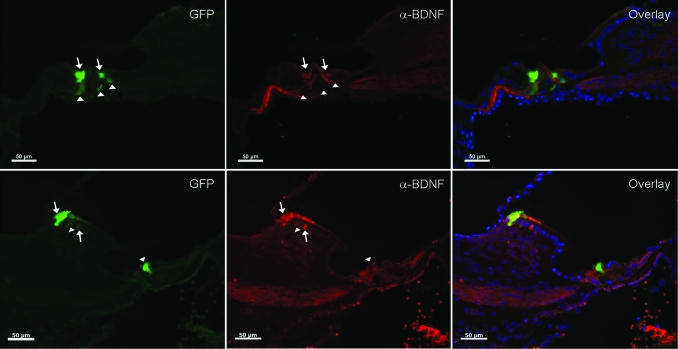
Figure 3.
BDNF expression in deafened GPs injected into the scala media with Ad-GFP-NT. Two examples of anti-BDNF antibody staining (red) colocalized with regions of GFP expression (green) in some (arrows), but not all cases (arrowheads). Because GPs were injected with combined BDNF and NT3 vectors, not every GFP+ cell would be expected to colocalize with BDNF staining. Bar = 50 µm. GFP, green fluorescent protein; GP, guinea-pig.
SGN survival in deafened cochleae
Degeneration of the organ of Corti induced by SNHL leads to secondary degeneration of SGNs. To test the hypothesis that localized neurotrophin expression in the organ of Corti would protect SGNs from degeneration after hearing loss, SGN density was examined in the basal, middle, and apical turns after Ad injections in deafened GP groups (groups 1–4; Table 1).
Basal turn. Following viral injections into the scala tympani of deafened GPs, there was no statistical difference in the densities of SGNs between injected and noninjected cochleae in the basal turn for Ad-GFP vectors (1553 ± 97 versus 1411 ± 109 SGNs/mm2, respectively) or for Ad-GFP-NT vectors (1680 ± 92 versus 1744 ± 99 SGNs/mm2, respectively). Following injections into the scala media, SGN densities were significantly lower in cochleae injected with Ad-GFP compared to noninjected cochleae (1529 ± 51 versus 1797 ± 51 SGNs/mm2, respectively) (P < 0.001, t-test). Conversely, SGN densities were significantly higher in cochleae injected with Ad-GFP-NT into the scala media (1833 ± 89 SGNs/mm2) compared to the noninjected cochleae (1177 ± 67 SGNs/mm2) (P < 0.001, t-test). Despite the average SGN density in noninjected cochleae of the Ad-GFP-NT group being lower than the average SGN densities observed in noninjected cochleae of the other groups, analysis of the raw data indicated that this difference was largely driven by the results from one GP in the Ad-GFP-NT group that exhibited more severe SGN degeneration (in all turns) in comparison to the other GPs. On a paired basis (injected versus noninjected cochlea), all GPs in the Ad-GFP-NT group had greater SGN survival in the basal turn of the injected cochlea compared to the noninjected cochlea.
Statistical comparisons of the differences in SGN density between injected and noninjected cochleae were made across experimental groups. Significantly higher SGN survival (i.e., positive SGN density differences) was observed in cochleae injected with Ad-GFP-NT into the scala media compared to all other groups [analysis of variance (ANOVA) P < 0.05 Holm–Sidak]. Loss of SGNs (i.e., negative density differences) was also significant in cochleae injected with Ad-GFP into the scala media compared to cochleae injected with Ad-GFP into the scala tympani (ANOVA P < 0.05 Holm–Sidak) (Figure 4).
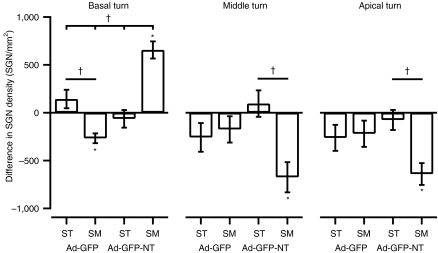
Figure 4.
Average differences in SGN densities between injected and noninjected cochleae for the basal, middle, and apical turns of 1-week deafened GPs. SGN survival with respect to the contralateral cochlea (positive difference) or loss (negative difference) was determined for each deafened experimental group (groups 1–4). In the basal turn, there was significantly greater SGN survival in cochleae injected with Ad-GFP-NT in the scala media compared to all other groups. There was also significant loss of SGNs in cochleae injected with Ad-GFP in the scala media compared to the scala tympani. In the middle and apical turns, there were significant losses of SGN in cochleae injected with Ad-GFP-NT into the scala media compared to the scala tympani (†P < 0.05 for one-way analysis of variance group comparisons; *P < 0.05 for injected versus noninjected cochlea t-test comparisons). Error bars indicate the standard error of the mean (n = 4–7 GPs per point). GFP, green fluorescent protein; GP, guinea-pig; SGN, spiral ganglion neuron; SM, scala media; ST, scala tympani.
Middle and apical turns. SGN densities were significantly lower in cochleae injected with Ad-GFP-NT into the scala media compared to the contralateral cochlea for the middle turn (1545 ± 113 versus 2186 ± 151 SGNs/mm2) (P < 0.005, t-test), and for the apical turn (1366 ± 158 versus 2041 ± 117 SGNs/mm2) (P < 0.005, t-test). Statistical comparisons across groups indicated that there was a significant difference in the extent of SGN loss in both middle and apical turns in the Ad-GFP-NT scala media group compared to the Ad-GFP-NT scala tympani group (P < 0.05, one-way ANOVAs) (Figure 4).
Peripheral fiber responses to gene expression in deafened cochleae
To test the hypothesis that localized neurotrophin expression in the organ of Corti would limit the disorganized resprouting of peripheral fibers after hearing loss, peripheral fiber trajectories and densities were examined in cochlear half-turns from deafened GPs (groups 1–4; Table 1). There were fewer peripheral fibers projecting onto the basilar membrane in the deafened groups compared to normal-hearing GPs (normal-hearing data not shown). Resprouting fibers were distinguishable from normal fibers by their branching, indirect and ectopic growth. In all deafened groups, sporadic regions of resprouting fibers were apparent; as the fibers exited the habenulae perforata, the fibers either projected toward the degenerating organ of Corti or doubled back and grew between cells of the inner spiral sulcus toward the vestibular lip of the spiral limbus.
The trajectory of fiber regrowth in the vicinity of transduced cells in the organ of Corti was examined. In Ad-GFP-NT groups, fibers tended to project toward transduced cells in the organ of Corti compared to the trajectory of fibers in Ad-GFP groups that were more random and wandering (Figure 5). Morphologically, peripheral fibers did not appear to synapse with neurotrophin-expressing cells, although synaptic markers were not used in the present study. Peripheral fiber resprouting in the region of the inner spiral sulcus was unaffected by either GFP or neurotrophin gene expression in the organ of Corti.
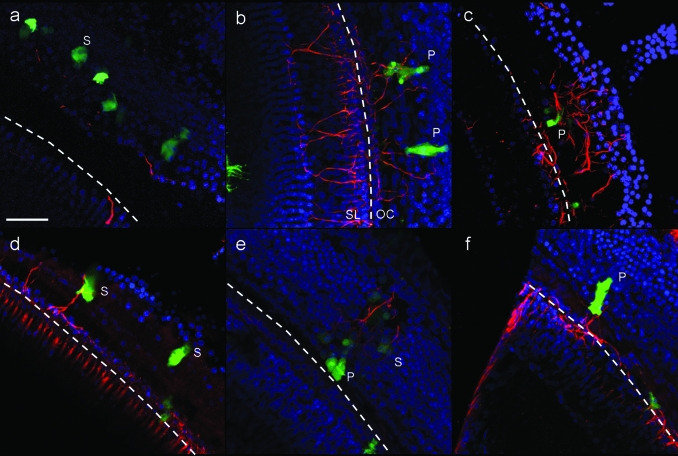
Figure 5.
Projected confocal images of peripheral fibers growing proximal to transduced cells in 1-week deafened GPs. Cochlear half-turns were stained with anti-NF-200 (red) and DAPI (blue) to visualize peripheral fiber trajectories onto the organ of Corti. Three-dimensional projections of serial Z-sections from confocal microscopy are shown here. (a) Peripheral fiber response to GFP-expressing cells (green) in the organ of Corti of 1-week deafened GP injected with Ad-GFP into the scala media. (b–f) Peripheral fiber response to neurotrophin-expressing cells in the organ of Corti of 1-week deafened GPs injected with Ad-GFP-NT into the scala media. Dotted lines indicate the position of the habenulae perforata. OC, organ of Corti; P, pillar cell; S, supporting cell (e.g., Deiters' cell or Hensen's cell); SL, spiral limbus. Bar = 50 µm and applies to all images. GFP, green fluorescent protein; GP, guinea-pig.
To quantify the peripheral fiber response to GFP or neurotrophin expression in Ad-GFP or Ad-GFP-NT groups, peripheral fiber density was examined within 10 µm regions surrounding transduced cells located in the organ of Corti in the basal turn. Based on their morphology and location, the GFP+ cells used in analysis were identified as pillar cells or other supporting cells (Deiters' or Hensen's cells). The number of NF-200 positive pixels surrounding transduced cells from Ad-GFP-NT-injected GPs (171.3 ± 27.1, n = 13 cells) was significantly higher compared to transduced cells from Ad-GFP-injected GPs (71.8 ± 24.9, n = 7 cells) (P < 0.05, t-test).
Peripheral fiber resprouting was also observed in regions where there was no gene transfer in Ad-GFP-NT groups (spontaneous resprouting). There were also a few examples of neurotrophin-expressing cells without evidence of proximal peripheral fibers (e.g., Figure 5d) and examples of transduced cells from Ad-GFP groups with proximal resprouting fibers, although these fibers were fewer in number than neurotrophin-expressing cells, and these fibers did not appear to grow as directly toward GFP-expressing cells compared to neurotrophin-expressing cells (Figure 5).
Viral injections and hearing thresholds in normal-hearing animals
Hearing thresholds were measured prior to and 21 days following injection of the viral vectors in normal-hearing GPs (groups 5–8). There was a significant shift in frequency-specific auditory brainstem response (ABR) thresholds over the treatment period (pre- to postinjection period) indicating a significant decrease in ABR sensitivity for all of the four experimental groups (two-way repeated measures ANOVA, P < 0.05). Post hoc analysis (Holm–Sidak method) indicated that threshold shifts were primarily observed in the high-frequency basal turn region (8–32 kHz) with no changes in ABR thresholds observed in the low-frequency middle turn region (1–2 kHz) (Figure 6). There was no change in ABR thresholds for the contralateral untreated cochleae over the treatment period.
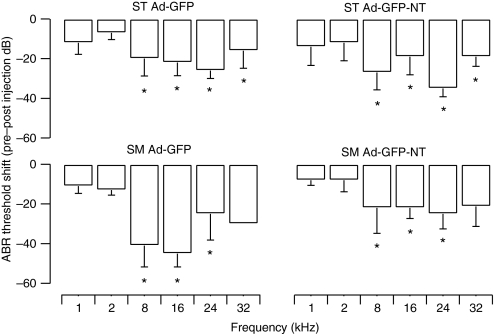
Figure 6.
Shifts in ABR threshold 1-week preinjection to 3-week postinjection in normal-hearing GPs. Shifts in ABR thresholds were observed in normal-hearing GPs following viral vector injections. Significant threshold shifts were primarily observed for high frequencies (8–32 kHz) for both scala tympani and scala media injections using either Ad-GFP-NT or Ad-GFP vectors. Threshold shifts were greater when Ad-GFP was injected into the scala media compared to the scala tympani, whereas there was no difference in threshold shifts for Ad-GFP-NT injections between the scala media and scala tympani approaches. *P < 0.05 two-way RM analysis of variance. Error bars represent standard error of the mean (n = 5 GPs per point). ABR, auditory brainstem response; GFP, green fluorescent protein; GP, guinea-pig; SM, scala media; ST, scala tympani.
There was no difference in the ABR threshold shifts between the scala media surgical approach and the scala tympani approach when Ad-GFP-NT vectors were injected (two-way ANOVA, P = 0.475). Following the injection of the Ad-GFP vector, significantly greater threshold shifts were observed with the scala media approach than with the scala tympani approach (two-way ANOVA, P < 0.05). However, the difference between threshold shifts following scala media Ad-GFP injections and scala media Ad-GFP-NT injections just failed to reach significance (main effect two-way ANOVA, P = 0.066) (Figure 6). Taken together, these findings indicated that hearing loss associated with scala media injections were not more severe than hearing loss associated with scala tympani injections.
Discussion
This study has demonstrated that neurotrophin gene expression can be localized to the organ of Corti by injecting into the scala media of deafened or normal-hearing GPs. Furthermore, localized neurotrophin gene expression in a partially degenerated organ of Corti of deafened GPs resulted in significant SGN survival in the basal turn and promoted local peripheral fiber regrowth toward cells expressing neurotrophins.
GFP expression
The basal to apical gene expression profile of the transduced cells was dependent on the route of administration of the viral injections. GFP expression following injections into the scala media was restricted to basal and middle turns. In contrast, scala tympani injection of the vectors resulted in GFP expression throughout all turns of the cochlea. High GFP expression in the organ of Corti throughout all cochlear turns has previously been achieved from a scala media surgical approach using a perfusion technique in which an Ad vector was injected into the endolymph through a cochleostomy in the basal turn and allowed to flow through the endolymph via a second cochleostomy in the apex.27 Cochlea-wide gene expression would be preferential for repairing genetic defects. However, for applications such as promoting SGN survival and peripheral fiber resprouting for improving cochlear implant performance, gene expression localized to the basal and middle turns (i.e., proximal to the electrode array) would be advantageous.
The cellular expression profile was also dependent on the route of administration of the viral vectors as well as the surface receptors expressed by each cell type. Ad infection requires a cell to express both αv-integrins and coxsackie Ad receptors. In the cochlea, integrins and coxsackie Ad receptors are reported to be coexpressed in inner and outer HCs, supporting cells (pillar cells, Deiters' cells, and Hensen's cells), endosteal cells lining the scala tympani and scala vestibule, and cells in the stria vascularis.28 In the present study, GFP expression was detected in all of these cell types (depending on the route of administration), although expression in stria vascularis was rare for all groups. GFP expression was never detected in SGNs or in any other cell type within Rosenthal's canal after injections into either the scala tympani or scala media, consistent with previous reports of the absence of coxsackie Ad receptor expression in SGNs.28
In normal-hearing GPs that received scala media injection of viral vectors, gene expression was consistently observed in all cell types of the organ of Corti and interdental cells of the spiral limbus, particularly those near the junction with Reissner's membrane. There is not a particularly prominent expression of coxsackie Ad receptors or αv-integrins in interdental cells,28 so the reason for this expression pattern is unclear. A similar expression pattern of the Math1 gene was reported following scala media injection of the Ad-Math1 vector.15 Following aminoglycoside-induced SNHL in GPs, loss of HCs occurs rapidly within 7 days,13,29 whereas loss of supporting cells in the organ of Corti occurs more gradually giving a wider window of opportunity for neurotrophin gene expression within supporting cells. Although the partial degeneration of the organ of Corti made it more difficult to identify GFP-expressing cells in the organ of Corti of deafened GP groups, in some deafened GPs, it was possible to identify pillar cells and Deiters' cells expressing GFP. To exclusively localize gene expression to supporting cells of the organ of Corti, promoters specific to these supporting cells could be used. For example, glial cell fibrillary acidic protein promoter is one such promoter that directs gene expression predominantly to supporting cells of the organ of Corti, at least in early development.30
Given that GFP expression in scala tympani–injected GPs was predominantly observed in the endosteal cells lining the scala tympani or the scala vestibuli, gene expression was rarely located in close proximity to SGNs or their peripheral fibers. Therefore, neurotrophin release from these cells would first enter the perilymph before it could affect SGNs. Even when expression was proximal to the basilar membrane, the cellular architecture of the basilar membrane and the existence of tight junctions along the apical surface of the normal organ of Corti are likely to restrict the movement of neurotrophins from the scala tympani to the scala media and limit the amount of neurotrophin to which peripheral fibers were exposed. Viral injections into the scala media meant that route of entry into the transduced cells was likely to be from the endolymphatic surface of the organ of Corti. However, the route of neurotrophins released by the Ad-GFP-NT transduced cells is not known. Some neurotrophin may be released back into the endolymph, but resprouting fibers are not directly exposed to the endolymphatic fluids (A.K. Wise, R. Pujol, J.B. Fallon and R.K. Shepherd unpublished results). Rather, fibers typically project between and under the overlying epithelial cells on the basilar membrane or the cells lining the inner sulcus. Neurotrophins may also be released through basolateral membranes of transduced cells. The observation that the density of resprouting fibers was greater around Ad-GFP-NT-transduced cells suggests that the released neurotrophin was localized to the intercellular spaces around the transduced cells and not widely dispersed.
SGN survival in deafened cochleae
An increase in SGN survival compared to the paired contralateral cochlea was limited to the basal turn of the Ad-GFP-NT scala media–injected GPs is consistent with the basal turn gene expression profile. Although there were differences in the severity of SGN degeneration between GPs, the effects of aminoglycoside deafening on SGN survival are consistent between the two cochleae of the same animal.5 Therefore, results were analyzed on a paired basis (injected side versus noninjected side). The data show a consistent finding across all GPs: that is, all injected cochleae in the Ad-GFP-NT scala media group have higher SGN densities in the basal turn than noninjected cochleae, and all injected cochleae in this group have lower SGN densities in the middle and apical turns than noninjected cochleae.
An effect on SGN survival was not observed following injections of Ad-GFP-NT into the scala tympani. Previous studies have demonstrated improved SGN survival using the scala tympani approach following continuous infusion of high concentrations of neurotrophins10,11,12 or neurotrophin gene therapy using high viral titers.21,22,23,31 Of the gene therapy studies, one group injected Ad-BDNF vectors of similar viral titers to this study into the scala tympani (1010 Ad particles/ml),23 whereas another used Ad-BDNF viral titers of 1012 Ad particles/ml (along with concurrent electrical stimulation from a cochlear implant).31 Increased SGN survival after hearing loss has also been reported with adeno-associated virus–mediated expression of BDNF using much lower viral titers (106 viral particles/ml) but delivered over many days,22 as well as with herpes simplex virus–mediated BDNF expression (using 105 infectious particles/ml).21 SGN cell bodies were specifically transduced in both of these studies, suggesting that an autocrine mechanism may account for increased SGN survival. The differences in the effects on SGN survival using the scala tympani approach between this and other studies are likely to be due to differences in the level and location of gene expression. Injection of neurotrophin gene transfer vectors into the scala media may concentrate the neurotrophins released by transduced cells as a result of the localized expression pattern or improve the accessibility of SGNs or their peripheral fibers to neurotrophins when they are expressed in the organ of Corti. Furthermore, an additional factor that is a potential contributor to SGN survival may arise indirectly from the initiation of cellular processes involved in fiber regrowth. The fiber resprouting mechanisms may potentiate or provide novel pro-survival signals to the SGNs and thus lead to an overall increase in SGN survival. However, as spontaneous resprouting was also observed in the deafened, untreated cochleae, these mechanisms themselves are unlikely to provide long-term SGN survival.
A small but significant loss of SGNs in the basal turn was observed when Ad-GFP was injected into the scala media, which may reflect the greater surgical trauma associated with accessing the scala media compared with the scala tympani. In support of this, greater hearing loss was recorded in GPs injected with Ad-GFP into the scala media in frequencies corresponding to the upper basal turn (8–16 kHz) compared to GPs injected with Ad-GFP into the scala tympani. It cannot be determined whether this significant loss of SGN density was also associated with injection of Ad-GFP-NT into the scala media as the SGN survival benefit of neurotrophin expression overrode any loss that may have occurred. There was no observable difference in hearing loss between the scala media and scala tympani approaches when using the Ad-GFP-NT vector. A significant loss of SGNs was observed in middle and apical turns of cochleae injected with Ad-GFP-NT into the scala media. This cannot be due to the surgical technique alone as no loss was observed in the Ad-GFP scala media group. Infection, inflammation, and unusual morphology were not present in these cochleae, so the reason for this finding remains obscure.
Peripheral fiber resprouting
A previous study has shown that in deafened GPs, peripheral fibers retract to the inner spiral bundle, but some fibers can be observed resprouting spontaneously onto the organ of Corti or reversing direction and growing within the inner spiral sulcus.12 In the present study, both of these patterns of fiber resprouting were observed in all deafened groups. However, when neurotrophins were expressed in supporting cells of the organ of Corti, significantly more fibers were present near cells expressing neurotrophins as indicated by NF-200 pixel analysis. Fiber resprouting in response to neurotrophin gene expression was less direct and exhibited more branching than fiber resprouting reported in response to Math1/Atoh1 gene therapy by other studies.15,32 In the latter studies, synapse formation was also confirmed in some cases.
Resprouting of peripheral fibers may lead to clinical improvements in cochlear implant functioning. Regrowth of fibers toward a cochlear implant electrode array may enable a closer interface between the electrodes and the neural population, leading to a decrease in excitation thresholds and thus a decrease in power consumption. Furthermore, controlled resprouting may also enable the development of new electrode arrays and stimulation strategies to achieve more spatially restricted neural activation on independent intracochlear electrodes, providing a means to deliver fine spatiotemporal information from the acoustic environment.
ABR thresholds
An increasing number of cochlear implant users have some residual hearing at the time of implantation. Just as it is important to protect residual hearing during electrode insertion for optimal speech perception outcomes, any adjunctive therapy for enhancing SGN survival or peripheral fiber resprouting used with cochlear implantation should also have minimal impact on residual hearing. The effects of viral vector delivery to the scala tympani and scala media on ABR thresholds were examined in normal-hearing GPs. The site of the basal turn injection corresponds approximately to the 24–32 kHz region of the cochlea. Hearing loss was greatest in the 8–32 kHz region (basal turn) for all groups and could be caused by the noise or vibrations of the surgical drill, opening of the cochlea, injection, gene expression, or a combination of these factors. It has been reported that hearing loss as a result of drilling and opening of the cochlea occurs in the 24–32 kHz region.33,34
When Ad-GFP was injected into the scala media, there was significantly more hearing loss in comparison to the Ad-GFP scala tympani group. This was not the case for the Ad-GFP-NT scala media and scala tympani groups in which no difference was found. Furthermore, there was no significant difference in ABR threshold shifts between injection of Ad-GFP into the scala media and injection of Ad-GFP-NT into the scala media. Larger group sizes may be required to elucidate whether injection into the scala media using the surgical approach described here results in greater hearing loss than injection into the scala tympani. Threshold shifts of 30–40 dB resulting from scala media injection of adeno-associated virus, Ad and Sendai virus have been reported previously, compared to 10–15 dB threshold shifts recorded for scala tympani injections.35,36,37 Furthermore, these studies indicated that there was no difference in threshold shifts between injections of the viral samples compared to carrier alone, suggesting that the hearing loss was not the result of viral transduction of cells in the organ of Corti or reporter gene expression. In all cases, the scala media was approached via a cochleostomy in the lateral wall that may cause greater or more consistent hearing losses than injection through the basilar membrane as performed in this study. With the basilar membrane approach, any physical damage to the organ of Corti caused by piercing the basilar membrane would be localized.
It is important to ensure that Ad-mediated gene expression in the cochlea does not result in damage to residual HCs. Studies using early-generation E1/E3 deleted Ad in vitro showed evidence of Ad-related damage to HC stereocilia and loss of hearing.38,39 More extensively deleted Ad vectors lacking either the E4 region or viral polymerase (such as the vectors used in this study40) appear to be less toxic to the inner ear.25,41,42 This suggests that residual Ad gene expression may be directly or indirectly (through an immune response) responsible for toxicity or hearing loss observed in earlier studies. Furthermore, protective effects of neurotrophins on HCs have been reported.43,44 The difference in ABR threshold shifts between the Ad-GFP and Ad-GFP-NT scala media groups was not significant, but was close to being significant. More data would be required to fully examine the possibility of HC protection in relation to gene transfer of NT3 or BDNF.
Neurotrophin expression
Neurotrophins were detected by enzyme-linked immunosorbent assay both in cell lysate and in cell culture media following transduction of HT1080 cells with Ad-GFP-NT3 or Ad-GFP-BDNF. After transduction of cells in GP cochleae with a combination of these vectors, BDNF expression was detected via anti-BDNF antibodies, but unfortunately, NT3 expression could not be confirmed by antibodies in vivo. Possible explanations for this are that NT3 expression may be shorter-lived than BDNF expression, NT3 expression was too low to detect by immunohistochemistry, or that Ad-GFP-NT3 did not enter cochlear cells.
Clinical considerations
The viability and safety of viral therapy as a potential clinical treatment remain to be established. There are a number of issues requiring further research. First, in a profoundly deaf cochlea, degeneration of the sensory epithelium may be so severe that very few cells remain on the basilar membrane for viral transduction. In this situation, it may not be possible to transduce suitable cellular targets meaning that viral therapy to attract and support remaining peripheral fibers might be limited. Furthermore, in cases where a successful transduction has been achieved, the longer-term fate of the transduced cells needs to be determined: do the transduced cells continue to produce neurotrophins and are the transduced cells protected from degeneration over long durations? The overall safety of viral gene therapy and neurotrophin gene transfer in humans needs to be elucidated. There are numerous clinical trials underway into the safety and toxicity of viral gene transfer vectors as well as trials that involve gene transfer of BDNF, for example, for the treatment of amyotrophic lateral sclerosis. Finally, the viability of the surgical access also needs to be established. During cochlear implant surgery in humans, the endolymphatic space can potentially be accessed by passing a micropipette apically through the round window, or alternatively by creating a cochleostomy anterior to the round window. Neither approach is trivial. The round window approach is constrained by the posterior tympanostomy, namely the access from the mastoid to the middle ear between the facial nerve and the chorda tympani. A hooked electrode would be needed to access the scala media via the posterior tympanostomy. Once the endolymphatic space has been accessed, the injection procedure would be quick and potentially provide long-term neurotrophin expression without implantation of slow-release devices.
Conclusions
This study examined gene therapy in the cochlea and demonstrated that localized sources of neurotrophins protected SGNs from degeneration after hearing loss and could redirect the regrowth of their peripheral fibers. Injection of viral vectors into the scala media resulted in more localized gene expression in the organ of Corti compared to scala tympani injections. This work provides a strong basis for establishing the effectiveness of neurotrophin gene transfer to the deafened cochlea and improving our understanding of how cochlear cells respond to neurotrophin treatment. Our study suggests that gene therapies that target cells of the organ of Corti utilizing the scala media approach are likely to be more effective in providing protective or regenerative factors to the cochlear nerves. The next step toward translating this research into the clinic is to determine the overall safety and long-term effectiveness of the technique in achieving SGN survival and targeted fiber regrowth. Furthermore, additional studies need to establish whether by controlling SGN fiber regrowth we can actually improve the nerve-electrode interface, thus enabling the implementation of new strategies to improve outcomes with cochlear implantation. Although there is a long way to go before we can achieve targeted fiber regrowth, this study presents a first step in a new approach toward improving outcomes after hearing loss.
Materials and Methods
Ad production. E1/E3/polymerase/terminal protein-deleted Ad type 5 vectors containing GFP under the control of a cytomegalovirus promoter with or without mouse NT3 or BDNF expressed via an IRES sequence (Ad-GFP, Ad-GFP-NT3, and Ad-GFP-BDNF) were generated using the AdEasy system (Stratagene, La Jolla, CA). Appropriately recombined vectors were transfected into C7-HEK 293 cells that stably expressed Ad polymerase and preterminal protein.40 Crude virus was amplified through several rounds to produce a high titer, purified using commercially available ion exchange chromatography membranes (Vivascience, Littleton, MA), and stored in Ad storage buffer (25% glycerol, 10 mmol/l Tris pH 8.0, and 2 mmol/l MgCl2) at −80 °C. The purified virus was titered on HT1080 cells and by spectrophotometry [>1011 optical particle units (OPU)/ml by OD260].45 All viral stocks were verified to be free of replication-competent Ad using a semiquantitative PCR assay for E1a and E1b.46,47 Prior to injection, Ad vectors were diluted 1:5 in artificial perilymph (125 mmol/l NaCl, 3.5 mmol/l KCl, 1.3 mmol/l CaCl2, 25 mmol/l NaHCO3, 1.2 mmol/l MgCl2, 0.75 mmol/l NaH2PO4, 5 mmol/l glucose, pH 7.4) to final concentrations of 1.32 × 1011 OPU/ml (Ad-GFP), 7.48 × 1010 OPU/ml (Ad-GFP-NT3), and 5.28 × 1010 OPU/ml (Ad-GFP-BDNF).
Ad samples were tested to ensure production of neurotrophins by infected cells. HT1080 cells seeded at 1 × 105 cells per well in a 6-well plate were infected after 24 hours with 3.74 × 108 OPU Ad-GFP-NT3 or 1.32 × 108 OPU Ad-GFP-BDNF. The conditioned media and cell lysate were harvested after 72 hours, and the amounts of NT3 and BDNF were analyzed by enzyme-linked immunosorbent assay (Promega, Sydney, Australia).
Animals. Male or female adult pigmented Dunkin–Hartley GPs were used (average weight 336 g). National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals were observed (NIH publication no. 85-23 Rev. 1985). The Animal Research Ethics Committee of the Royal Victorian Eye and Ear Hospital approved the care and use of the animals in this study (ethics #06/126AB and #09/180AB). GPs were randomly assigned to groups (Table 1).
ABR recordings
Deafened groups: The hearing status of each GP in deafened groups (groups 1–4 from Table 1) was assessed prior to deafening using computer-generated click stimuli and ABR recordings.48,49 For inclusion in the study, GPs were required to have normal hearing prior to deafening (defined as having an ABR threshold <43 dB peak-equivalent sound pressure level). Postdeafening ABRs prior to the viral injections could not be performed due to the short timeline between deafening and injection, and the detrimental effects of additional anesthesia on the health of the GPs. However, previous experience indicates that this deafening technique produces reliable threshold shifts of >50 dB.12,49 Threshold shifts of >50 dB result in loss of 80–100% HCs within 7 days. Any remaining HCs are most commonly in the apical turns of the cochlea.13,29
Normal-hearing groups: ABR thresholds from normal-hearing GPs (groups 5–8 from Table 1) were assessed to determine the effects of viral injection on hearing thresholds. Tone-pip ABR thresholds (at frequencies of 1, 2, 8, 16, 24, and 32 kHz) were determined 1 week prior to injection and 3 week postinjection.33
GP deafening. Normal-hearing GPs were deafened under anesthesia via an intravenous 100 mg/kg frusemide (Troy Laboratories, Smithfield, Australia) and subcutaneous 400 mg/kg kanamycin sulfate (Sigma-Aldrich, Castle Hill, Australia) injections.49
Cochlear injection of viral samples. Viral vectors were unilaterally injected into cochleae using aseptic techniques 1 week after deafening. The head of the GP was secured using a nontraumatic head holder under anesthesia. A retroauricular incision was made to expose the bulla. After drilling through the bulla, the cochlea was located and a small cochleostomy made into the otic capsule of the basal turn using a 2-mm diamond drill bit, gaining access to the scala tympani. Perilymph was removed using gentle suction until the basilar membrane could be visualized. A glass recording micropipette (World Precision Instruments, Sarasota, FL) with a 5–10 µm tip diameter was inserted and 5 µl of the viral preparation was injected into the scala tympani over 2 minutes. For scala media injections, the same approach was used, but after accessing the scala tympani, the recording micropipette was advanced via a stepper motor through the basilar membrane until an endocochlear potential was recorded (average 83.2 ± 3.4 mV; n = 8), indicating that the scala media had been accessed.50 Two microliters of the viral preparation was injected into the scala media (due to the smaller volume of this compartment) over 5 minutes and the micropipette was retracted 2 minutes later. The cochleostomy was sealed with connective tissue, the bulla sealed with dental cement, and the wound closed with sutures. Viral samples were diluted in artificial perilymph for both scala tympani and scala media injections in this study. Similar studies have been performed with viral samples diluted in artificial endolymph for scala media injections with no difference observed with regard to the endocochlear potential recordings, distribution of GFP expression, or effects on SGNs (data not shown).
Perfusion, cochlea processing, sectioning, and immunohistochemistry. Three weeks postinjection, GPs were euthanized with 1.5 ml pentobarbitone and intracardially perfused with 0.9% (wt/vol) saline containing 0.1% (vol/vol) heparin sodium and 0.025% (wt/vol) sodium nitrite, followed by 10% (vol/vol) neutral buffered formalin. The bullae were removed and the cochleae dissected out. Cochleae were placed in 10% (vol/vol) neutral buffered formalin for a further 24 hours and then decalcified over 14 days in 10% (wt/vol) EDTA in 0.1 mol/l phosphate buffer. Cochleae were embedded in OCT (Tissue-Tek, Torrance, CA) and sectioned on a cryostat at 12 µm through pre-modiolar and mid-modiolar planes and mounted onto SuperFrost Plus slides (Menzel-Gläser, Braunschweig, Germany), leaving the final half of the cochleae intact. This remaining half-cochlea that contained the viral injection site was cut into half-turn surface preparations.12 The Reissner's and tectorial membranes were removed for better visualization of the organ of Corti. Standard immunofluorescent protocols were followed using antibodies to neurofilament-200 (NF-200; Chemicon International, Boronia, Australia) for staining SGNs and peripheral fibers, anti-calretinin (Chemicon International) and phalloidin (Molecular Probes, Eugene, OR) for cells in the organ of Corti, antibodies to NT3 and BDNF (Santa Cruz Biotechnology, Santa Cruz, CA), and AlexaFluor secondary antibodies (Molecular Probes). Sections were examined on a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Jena, Germany). Cochlear half-turn surface preparations were viewed on a Zeiss confocal microscope.
Data analysis. All results are presented as the mean ± s.e.m.
GFP expression: The GFP reporter gene was used to assess gene expression in mid-modiolar sections and cochlear half-turns. The location of GFP expression from nine nonconsecutive mid-modiolar sections (over 324 µm) was positioned on a representative mid-modiolar image of a GP cochlea to form a composite image.
SGN survival: SGN density was analyzed from nine nonconsecutive mid-modiolar sections from each cochlea in a blinded manner. Density was determined by counting NF-200-positive SGN cell bodies within Rosenthal's canal and by measuring the area formed by the perimeter around the outermost SGNs using a Zeiss Axioplan II microscope and AxioVision software. Lower basal and upper basal SGN densities were averaged to represent SGN density in the basal turn. The same was done for middle and apical turns. Injected and noninjected (contralateral) cochleae were compared using t-tests. The difference in SGN density between the injected and noninjected cochleae of each GP was calculated (termed SGN survival when positive or SGN loss when negative) thus allowing normalized comparisons between groups, analyzed by one-way ANOVA with Holm–Sidak post hoc analysis.
Peripheral fiber response: SGN peripheral fibers were analyzed from confocal images that contained GFP+ cells. Only cells in the basilar membrane that were distal to the inner spiral bundle were used, to avoid counting fibers that normally occur within the inner spiral bundle. A subset of GFP-expressing cells were selected for detailed analysis: only GFP-expressing cells within the organ of Corti region were selected as colocalization of GFP-expressing cells and resprouting fibers was not observed elsewhere. Furthermore, due to the expression pattern summarized in Figure 1 and Table 2, analysis was restricted to GPs in scala media groups and one GP from a scala tympani group with organ of Corti gene expression (n = 2 GPs for Ad-GFP and n = 3 GPs for Ad-GFP-NT). Pixel density occupied by NF-200 labeling was measured within a boundary 10 µm from the edge of GFP+ cells. Four consecutive Z-planes with the highest pixel density were averaged. Measurements from Ad-GFP-injected GPs and Ad-GFP-NT-injected GPs were compared using a t-test.
ABR thresholds: Statistical analysis of the tone-pip ABR threshold data within each normal-hearing group (comparison of pre- and postinjection thresholds) was performed using two-way repeated measures ANOVA. To compare between experimental groups' threshold shifts, pre- and postinjection threshold differences were calculated, and a two-way ANOVA was used to evaluate statistical significance with Holm–Sidak post hoc comparisons.
Acknowledgments
We are grateful for funding support from the Royal National Institute for Deaf People, the Garnett Passe and Rodney Williams Memorial Foundation, NIDCD HHS-N-263-2007-00053-c, NIDCD DC-006437, NIDCD P30 DC-04661, NICHHD P30 HD-02774, and the Hearing Regeneration Initiative and Veterans' Hospital Administration. We appreciate the suggestions of the reviewers that made significant improvements to the manuscript. This work was performed in East Melbourne, Australia. The Bionic Ear Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program.
REFERENCES
- Lefebvre PP, Martin D, Staecker H, Weber T, Moonen G., and , Van de Water TR. TGF beta 1 expression is initiated in adult auditory neurons by sectioning of the auditory nerve. Neuroreport. 1992;3:295–298. doi: 10.1097/00001756-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Otte J, Schunknecht HF., and , Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88 8 Pt 1:1231–1246. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Young YS., and , Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Leake PA., and , Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Hardie NA., and , Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP., and , Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumäe U., and , Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumäe U., and , Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM., and , Fariñas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM., and , Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykkö I, Olivius NP, Kaksonen R, et al. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G., and , O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW., and , Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, et al. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE., and , Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL., and , Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, et al. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998;5:277–281. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- Li Duan M, Bordet T, Mezzina M, Kahn A., and , Ulfendahl M. Adenoviral and adeno-associated viral vector mediated gene transfer in the guinea pig cochlea. Neuroreport. 2002;13:1295–1299. doi: 10.1097/00001756-200207190-00016. [DOI] [PubMed] [Google Scholar]
- Praetorius M, Knipper M, Schick B, Tan J, Limberger A, Carnicero E, et al. A novel vestibular approach for gene transfer into the inner ear. Audiol Neurootol. 2002;7:324–334. doi: 10.1159/000066157. [DOI] [PubMed] [Google Scholar]
- Wareing M, Mhatre AN, Pettis R, Han JJ, Haut T, Pfister MH, et al. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61–69. doi: 10.1016/s0378-5955(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Federoff H., and , Van De Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Han JJ, Castelein CM, Carvalho GJ., and , Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112 8 Pt 1:1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R., and , Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S., and , Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Wenzel GI, Xia A, Funk E, Evans MB, Palmer DJ, Ng P, et al. Helper-dependent adenovirus-mediated gene transfer into the adult mouse cochlea. Otol Neurotol. 2007;28:1100–1108. doi: 10.1097/MAO.0b013e318158973f. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E., and , Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Ballana E, Wang J, Venail F, Estivill X, Puel JL, Arbonès ML, et al. Efficient and specific transduction of cochlear supporting cells by adeno-associated virus serotype 5. Neurosci Lett. 2008;442:134–139. doi: 10.1016/j.neulet.2008.06.060. [DOI] [PubMed] [Google Scholar]
- Venail F, Wang J, Ruel J, Ballana E, Rebillard G, Eybalin M, et al. Coxsackie adenovirus receptor and alpha nu beta3/alpha nu beta5 integrins in adenovirus gene transfer of rat cochlea. Gene Ther. 2007;14:30–37. doi: 10.1038/sj.gt.3302826. [DOI] [PubMed] [Google Scholar]
- Versnel H, Agterberg MJ, de Groot JC, Smoorenburg GF., and , Klis SF. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res. 2007;231:1–12. doi: 10.1016/j.heares.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Stone IM, Lurie DI, Kelley MW., and , Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 2005;11:843–848. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Di Polo A, Raphael Y., and , Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ., and , Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DP, Eastwood H, Richardson RT., and , O'Leary SJ. Effects of round window dexamethasone on residual hearing in a guinea pig model of cochlear implantation. Audiol Neurootol. 2008;13:86–96. doi: 10.1159/000111780. [DOI] [PubMed] [Google Scholar]
- Chang A, Eastwood H, Sly D, James D, Richardson R., and , O'Leary S. Factors influencing the efficacy of round window dexamethasone protection of residual hearing post-cochlear implant surgery. Hear Res. 2009;255:67–72. doi: 10.1016/j.heares.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Kanzaki S, Mochizuki H, Inoshita A, Narui Y, Furukawa M, et al. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum Gene Ther. 2008;19:384–390. doi: 10.1089/hum.2007.167. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Di Pasquale G, Cortez SR, Chiorini JA., and , Raphael Y. Gene transfer using bovine adeno-associated virus in the guinea pig cochlea. Gene Ther. 2009;16:990–997. doi: 10.1038/gt.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S, Shiotani A, Inoue M, Hasegawa M., and , Ogawa K. Sendai virus vector-mediated transgene expression in the cochlea in vivo. Audiol Neurootol. 2007;12:119–126. doi: 10.1159/000097798. [DOI] [PubMed] [Google Scholar]
- Holt JR, Johns DC, Wang S, Chen ZY, Dunn RJ, Marban E, et al. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol. 1999;81:1881–1888. doi: 10.1152/jn.1999.81.4.1881. [DOI] [PubMed] [Google Scholar]
- Luebke AE, Foster PK, Muller CD., and , Peel AL. Cochlear function and transgene expression in the guinea pig cochlea, using adenovirus- and adeno-associated virus-directed gene transfer. Hum Gene Ther. 2001;12:773–781. doi: 10.1089/104303401750148702. [DOI] [PubMed] [Google Scholar]
- Amalfitano A., and , Chamberlain JS. Isolation and characterization of packaging cell lines that coexpress the adenovirus E1, DNA polymerase, and preterminal proteins: implications for gene therapy. Gene Ther. 1997;4:258–263. doi: 10.1038/sj.gt.3300378. [DOI] [PubMed] [Google Scholar]
- Luebke AE, Steiger JD, Hodges BL., and , Amalfitano A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001;8:789–794. doi: 10.1038/sj.gt.3301445. [DOI] [PubMed] [Google Scholar]
- Holt JR. Viral-mediated gene transfer to study the molecular physiology of the Mammalian inner ear. Audiol Neurootol. 2002;7:157–160. doi: 10.1159/000058302. [DOI] [PubMed] [Google Scholar]
- Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA., and , Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–142. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Ruan RS, Leong SK, Mark I., and , Yeoh KH. Effects of BDNF and NT-3 on hair cell survival in guinea pig cochlea damaged by kanamycin treatment. Neuroreport. 1999;10:2067–2071. doi: 10.1097/00001756-199907130-00014. [DOI] [PubMed] [Google Scholar]
- Mittereder N, March KL., and , Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WW, Koch PE., and , Roth JA. Detection of wild-type contamination in a recombinant adenoviral preparation by PCR. BioTechniques. 1995;18:444–447. [PubMed] [Google Scholar]
- Suzuki E, Murata T, Watanabe S, Kujime Y, Hirose M, Pan J, et al. A simple method for the simultaneous detection of E1A and E1B in adenovirus stocks. Oncol Rep. 2004;11:173–178. [PubMed] [Google Scholar]
- Shepherd RK., and , Clark GM. Progressive ototoxicity of neomycin monitored using derived brainstem response audiometry. Hear Res. 1985;18:105–110. doi: 10.1016/0378-5955(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30:2614–2624. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick P, Layton MG, Rodger J., and , Robertson D. A method for introducing non-silencing siRNA into the guinea pig cochlea in vivo. J Neurosci Methods. 2008;167:237–245. doi: 10.1016/j.jneumeth.2007.08.026. [DOI] [PubMed] [Google Scholar]