Abstract
We evaluated the potential of lung-directed gene therapy for α1-antitrypsin (AAT) deficiency using an adeno-associated virus type 6 (AAV6) vector containing a human AAT (hAAT) complementary DNA (cDNA) delivered to the lungs of mice and dogs. The results in normal and immune-deficient mice showed that hAAT concentrations were much higher in lung fluid than in plasma, and therapeutic levels were obtained even in normal mice. However, in normal mice an immune response against the vector and/or transgene limited long-term gene expression. An AAV6 vector expressing a marker protein verified that AAV6 vectors efficiently transduced lung cells in dogs. Delivery of AAV6-hAAT resulted in low levels of hAAT in dog serum but therapeutic levels in the lung that persisted for at least 58 days to 4 months in three immunosuppressed dogs. Expression in the serum was not detectable after 45 days in one nonimmune suppressed dog. A lymphoproliferative response to AAV capsid but not to hAAT was detected even after immunosuppression. These results in mice and dogs show the feasibility of expression of therapeutic levels of AAT in the lungs after AAV vector delivery, and advocate for approaches to prevent cellular immune responses to AAV capsid proteins for persistence of gene expression in humans.
Introduction
Severe deficiency of α1-antitrypsin (AAT) increases the risk of early onset pulmonary emphysema and cirrhosis of the liver. It is estimated that 100,000 Americans have severe AAT deficiency caused by mutations in the gene coding for the 52-kd AAT glycoprotein.1 The major function of AAT is to protect tissues against neutrophil elastase, and pulmonary emphysema associated with AAT deficiency is due to the unrestrained proteolytic activity of neutrophil elastase on lung connective tissue leading to alveolar destruction. AAT is primarily synthesized by the liver and is secreted into the blood where it circulates and diffuses into the lung parenchyma. AAT is also made by lung epithelial cells and macrophages.
Many allelic variants of AAT have been identified, but the Z and S alleles are most commonly associated with severe AAT deficiency. About 4% of Northern Europeans carry the Z allele, which when homozygous is associated with circulating AAT levels that are approximately tenfold below the normal MM genotype levels of 1.9–3.5 mg/ml.2,3 The homozygous SS variant is found in 28% of Southern Europeans, and although it results in AAT levels that are 60% of normal it is not associated with pulmonary disease.2,3 Based on measurement of circulating AAT levels in SZ individuals with and without pulmonary emphysema, it is estimated that AAT serum levels of ≥570 µg/ml (11 µmol/l) or lung lining fluid levels of ≥52 µg/ml (1 µmol/l) prevent lung destruction.4,5 An increase in circulating hAAT is not expected to improve liver disease in those with ZZ-AAT polymers trapped in the liver. However, it has been reported that emphysema is the major cause of death (72%), whereas liver cirrhosis and cancer account for only 10 and 3%, respectively.6
Introduction of the normal human AAT (hAAT) by gene transfer could increase circulating AAT and prevent pulmonary destruction. Substantial production of AAT protein will be required to achieve blood levels in the therapeutic range. Several tissue targets have been studied to achieve this goal. Sustained secretion of hAAT from murine liver is possible using an adeno-associated virus type 2 (AAV2) vector in portal vein injections.7 Efforts using less invasive delivery by muscle injection have led to phase I clinical trials.8 Unfortunately, of the 12 subjects who received an AAV2 vector encoding hAAT, only one showed a low level of M-AAT (82 nmol/l) at the 1 month time point and all others were negative. It is known that other AAV types can more efficiently transduce muscle cells such as AAV1 and AAV6,9,10 and a change of AAV vector type may lead to improved results.11 Intrapleural administration of AAV5 has also resulted in persistent high lung and serum levels of AAT in mice.12 The concerns with these three routes of administration are that they are invasive, can induce tissue injury and inflammation, and allow the spread of vector via the circulation. In contrast, administration to the lung can be noninvasive and limit systemic vector spread. Direct administration to the lung would also allow for production of the therapeutic protein in the organ where destruction from elastase actually occurs.
We have tested many vector types for their ability to efficiently transduce lung cells, and vectors based on AAV6 are by far the most effective in mice.13 Therefore, we first evaluated the potential of lung-directed gene therapy for AAT deficiency using an AAV6 vector containing an hAAT complementary DNA (cDNA) in normal and immune-deficient mice, and found that therapeutic levels of hAAT could be produced in plasma and lung fluid. However, results obtained in mice may not predict clinical outcomes in humans. The random-bred dog has been a useful model for predicting human responses to bone marrow and organ transplantation regimens. Therefore, we next tested whether we could obtain therapeutic levels of hAAT in dogs and whether immune responses to an AAV6-hAAT would clear expression of hAAT following lung delivery of the vector, and if so, whether temporary immunosuppression could result in sustained high-level AAT in plasma and lung fluid of dogs.
Results
hAAT expression in mice
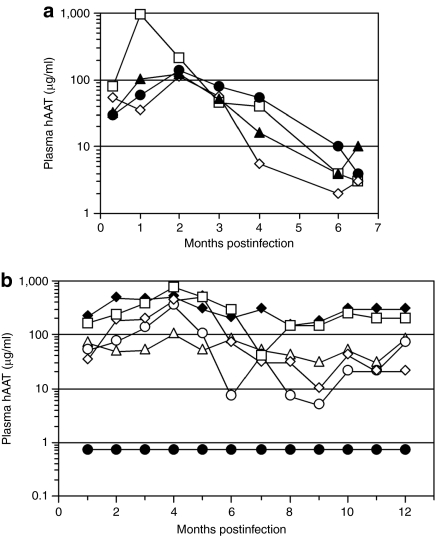
Our previous studies have shown that the combination of a vector-bearing AAV6 capsid proteins and a transgene containing a hybrid promoter consisting of a cytomegalovirus enhancer, β-actin promoter and splice donor, and a β-globin splice acceptor (CAG promoter) promotes efficient transduction of all types of lung epithelial cells in mice at rates of 90% or more.14 Given these impressive results it seemed possible that we might be able to produce therapeutic levels of hAAT in mice following AAV6 vector transduction. We constructed an AAV vector with AAV2 terminal repeats, the CAG promoter, an M-type hAAT cDNA, and a simian virus 40 polyadenylation signal, and produced this vector by transient transfection of human embryonic kidney 293 cells with the vector and AAV packaging plasmids. Vectors were administered dropwise to the nares of lightly sedated mice and hAAT was measured in plasma as a function of time (Figure 1). Vector administration to normal mice resulted in a peak of expression at 1–2 months that approached the therapeutic level (570 µg/ml) with a drop-off in expression to ≤10 µg/ml by 6 months (Figure 1a). In contrast, vector administration to immunodeficient Rag2-knockout (Rag2) mice resulted in stable hAAT expression for a year at levels close to what would be therapeutic in humans (Figure 1b). These results show the feasibility of expression of therapeutic levels of AAT in blood following AAV vector transduction of lung, and that hAAT produced in the lung can be distributed into the circulation. However, in normal mice an immune response against the vector and/or transgene limits long-term gene expression.
Figure 1.
Human AAT expression in normal and immunodeficient mice. (a) hAAT in blood plasma from C57BL/6 mice that received 5 × 1010 (closed symbols) or 1 × 1011 vg (open symbols) of the ACAGhAAT(AAV6) vector. (b) Plasma hAAT in C57BL/6 Rag2 immunodeficient mice that received 1011 (open symbols) or 2 × 1011 vg (closed diamond) of the ACAGhAAT(AAV6) vector, or 1011 vg of an AAV6 vector encoding alkaline phosphatase [ARAP4(AAV6)] (closed circles) as a negative control. AAT, α1-antitrypsin; AAV, adeno-associated virus; hAAT, human AAT.
Bronchoalveolar lavage (BAL) fluid taken from these mice at the time of sacrifice was analyzed for the level of hAAT by enzyme-linked immunosorbent assay (ELISA). The fold dilution of epithelial lining fluid (ELF) in the BAL sample was determined by a urea assay and was used to derive the concentration of hAAT in mouse ELF (Table 1). Comparison of the plasma and ELF concentrations of hAAT in normal mice given either 0.5 or 1.0 × 1011 vector genomes (vg) of ACAGhAAT(AAV6) showed that the ELF had 17–29-fold higher levels of hAAT than those of plasma. ELF hAAT levels in immunodeficient Rag2 mice were 1.2–14-fold higher than the plasma hAAT. Analysis by ELISA indicated the presence of antibodies to hAAT in normal mouse plasma (Table 1) that may act as inhibitors in serologic assays to detect hAAT concentrations. The results showed that hAAT concentrations were higher in ELF than in plasma, and therapeutic levels (≥52 µg/ml) were obtained even in normal mice.
Table 1.
hAAT and hAAT antibodies in normal C57BL6 mice at 7 months and in immunodeficient Rag2 mice at 13 months after AAV vector administration
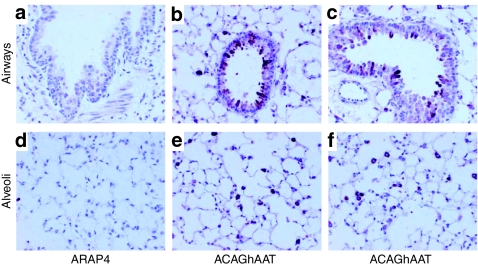
An hAAT-specific antibody revealed hAAT protein expression in epithelial cells of the small and large airways (Figure 2b,c) and in alveolar cells of the lung parenchyma (Figure 2e,f). hAAT was also less frequently found in smooth muscle cells underlying the airway and in lung vasculature (data not shown). These results show that hAAT can be expressed in many cell types of the murine lung, as has been found previously for human placental alkaline phosphatase (AP) or β-galactosidase.14,15
Figure 2.
Immunostain for hAAT expression in mouse lungs. Immunodeficient C57BL/6 Rag2 mice were given 1011 vg of ACAGhAAT(AAV6) or ARAP4(AAV6) as a negative control and lungs were harvested at 3 months and stained with an antibody specific for hAAT in formalin-fixed mouse lung. Expression (purple stain) was seen in airway epithelium (top row), alveolar cells (bottom row), and in some vascular cells (data not shown). AAV6, adeno-associated virus type 6; hAAT, human α1-antitrypsin.
AAV vector gene transfer to lungs of dogs
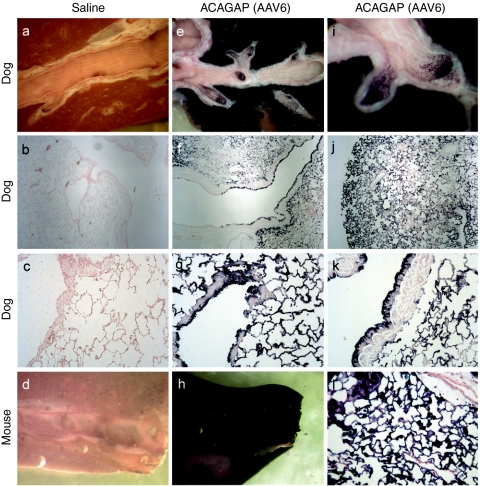
We first tested the efficiency of AAV vector-mediated gene transfer to cells in the dog lung using an AAV6 vector encoding AP, ACAGAP(AAV6). The dog was immunosuppressed with cyclosporine (CSP; 15 mg/kg daily, days −1 to 60) and mycophenolate mofetil (MMF; 7.5 mg/kg daily, days 0–60) to minimize potential immune-mediated clearance of transduced cells, and was given 2 × 1013 vg of ACAGAP(AAV6) in the right lung and saline in the left lung by using a microsprayer inserted into the airway under bronchoscopic guidance. Histochemical staining for AP was done on right and left lungs at day 60. The lungs showed efficient transduction by the AAV6 vector in distal lung airway epithelial cells and alveolar cells (Figure 3). One major difference compared to results in mice was that the mice exhibit efficient transduction of airway epithelial cells of both the large (proximal) and small (distal) airways (Figure 3h,l) whereas the dog showed a clearly reduced transduction in the large airway epithelium (Figure 3e,f,i). Poor transduction in the large airways of the dog was not due to poor vector exposure because the microsprayer used to deliver the vector was placed just in the proximal end of either the right or left main stem bronchus well upstream of the pseudostratified airway epithelium of the large proximal airways. However, the dog showed robust AP expression in alveolar regions (Figure 3g,j,k) which are the sites of destruction from neutrophil elastase and therefore the sites at which AAT is needed.
Figure 3.
AP expression in lungs of dogs and mice following administration of ACAGAP(AAV6). ACAGAP(AAV6) or saline was administered to lungs of animals and lungs were stained for AP expression (purple/black stain) 2 months later. AAV6, adeno-associated virus type 6; AP, alkaline phosphatase.
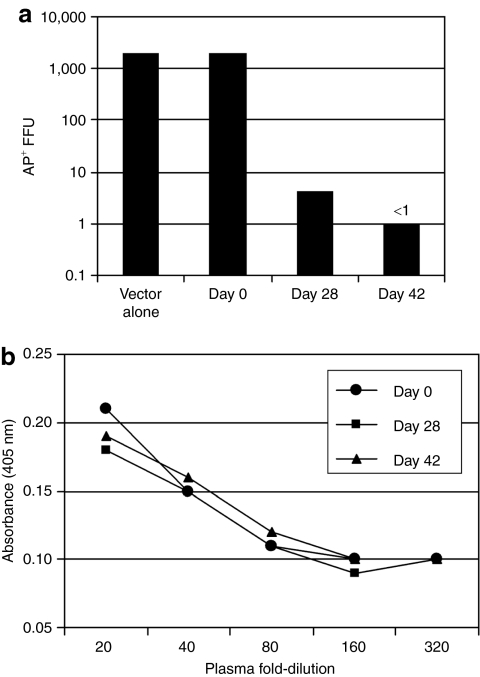
A virus neutralization assay and an ELISA assay were done to detect immune responses to AAV6 capsid and AP proteins, respectively. Plasma isolated from blood taken at day 0 did not exhibit neutralizing activity whereas a 1:100 dilution of day 28 plasma inhibited vector transduction by 500-fold, and the day 48 sample completely inhibited vector transduction (Figure 4a). In contrast, an ELISA for antibodies against AP did not show any reactivity above the day 0 plasma level (Figure 4b). The results show that the immune suppression regimen did not prevent a humoral immune response to capsid proteins.
Figure 4.
Immune response to AAV vector and AP protein. (a) Neutralization of an AAV6 vector by 1:100 dilutions of blood plasma and (b) measurement of antibodies against AP by ELISA of plasma harvested at the indicated times from a dog given ACAGAP(AAV6). AAV6, adeno-associated virus type 6; AP, alkaline phosphatase.
hAAT expression in normal and immunosuppressed dogs
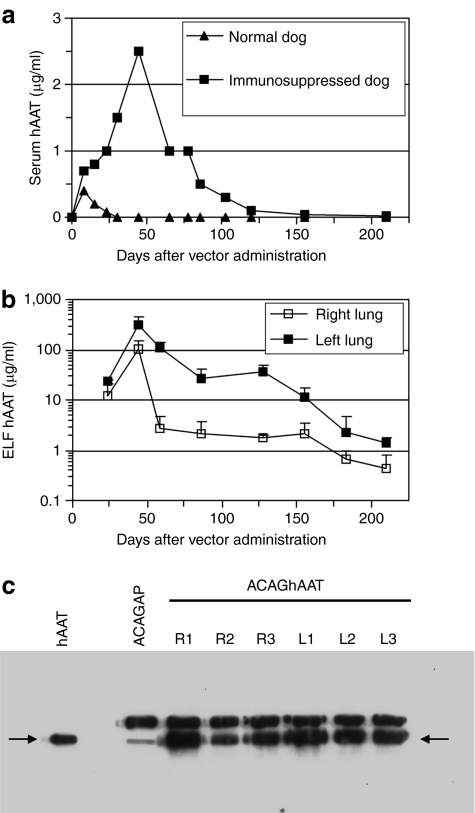
Two dogs were given 3 × 1013 vg of the ACAGhAAT(AAV6) vector split equally between the right and left lungs. This dose was estimated to give therapeutic levels in dogs that were 10–12 kg or 500 times the weight of mice used in previous experiments. One animal was immunosuppressed with CSP (30 mg/kg daily, days −1 to 90) and MMF (15 mg/kg daily, days 0–60), and the other was not. Serum values of hAAT in the immune competent dog showed an increase at day 7 but then declined to background levels by the fourth week (Figure 5a). In contrast, the immunosuppressed dog showed a higher level of serum hAAT that declined over 4 months (Figure 5a). At day 21 BAL fluids were obtained from both dogs. The immunocompetent dog did not have detectable levels of hAAT in the BAL fluid (not shown) whereas the immunosuppressed dog showed significant levels of hAAT in both right and left lungs (Figure 5b). The hAAT concentrations achieved (100 and 300 µg/ml) were higher than therapeutic levels (≥52 µg/ml) at day 44, then decreased and persisted at subtherapeutic levels for the duration of the experiment (7 months). Although turn-off of expression or turn-over of lung cells may result in some loss of expression, the differences in the amount and duration of hAAT between the immune-suppressed and normal dogs clearly show that there is a need for immune suppression to achieve any meaningful gene expression. However, the immunosuppressive regimen used was not sufficient to achieve long-term therapeutic gene expression.
Figure 5.
Expression of hAAT in normal and immunosuppressed dogs. (a) Serum samples from normal and immunosuppressed dogs taken at various times after vector administration were analyzed by ELISA for hAAT levels. (b) Expression of hAAT in ELF of the immunosuppressed dog. Three BAL samples from each lung at each time point were analyzed by ELISA to determine hAAT concentration, and ELF levels were calculated from urea assays to determine fold dilution of ELF in BAL fluid. The means ± SD are shown. (c) BAL fluids from immunosuppressed dogs given ACAGhAAT(AAV6) or ACAGAP(AAV6) were obtained at day 44 and day 60, respectively, and western analysis was done to detect expression of hAAT. Ten µl portions of three BAL fluid samples obtained sequentially from the right (R) and left (L) lungs were analyzed. Ten nanogram of an hAAT standard is shown in the left lane. The arrow marks the position of hAAT. Canine AAT is shown in the lane containing the sample from the ACAGAP(AAV6)-treated dog. The rabbit antibody is specific for human AAT in ELISA. In denaturing conditions such as that used for the western analysis, the antibody also recognizes canine AAT. AAT, α1-antitrypsin; AAV6, adeno-associated virus type 6; BAL, bronchial alveolar lavage; ELF, epithelial lining fluid; ELISA, enzyme-linked immunosorbent assay; hAAT, human AAT.
A western blot analysis was done on BAL fluids collected at day 44 from the immunosuppressed dog given the ACAGhAAT vector and a control BAL sample was from day 60 of the dog given the ACAGAP vector (Figure 5c). These BAL samples were compared to an hAAT standard. hAAT protein is marked by an arrow. The rabbit antibody recognized dog AAT in denatured conditions as seen in the ACAGAP-treated animals. There are two protein sizes with the larger one being more abundant in the control sample. hAAT is seen in each of the BAL fluid samples taken from the right and left lung of the dog given ACAGhAAT. Its size is similar to the lower molecular weight form of canine AAT.
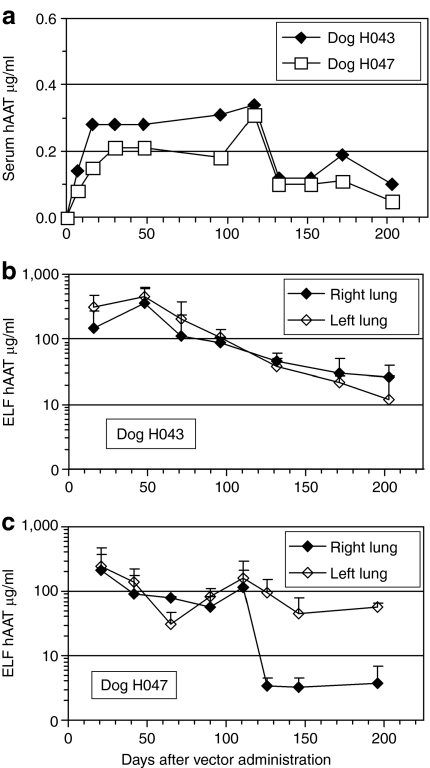
We administered the hAAT AAV vector to two more dogs and tested whether persistent therapeutic expression of hAAT could be obtained by increasing the duration of immunosuppression and decreasing the vector dose to 1 × 1013 vg split equally between both lungs. MMF (15 mg/kg daily) was given from days 0 to 120. CSP (10 mg/kg daily) was given from days −1 to 160 for dog H043. H047 given the same CSP dose exhibited digital papillomitosis on day 57 so the drug was discontinued for 3 weeks during which the lesion regressed, after which CSP dosing was resumed at the initial dose. With both immunosuppressive regimens, we saw the duration of hAAT expression increase to at least 200 days in the serum (Figure 6a), and therapeutic levels were observed in the lung for 120 days followed by subtherapeutic levels through day 200 (duration of experiment) (Figure 6b,c).
Figure 6.
Expression of hAAT in two immunosuppressed dogs. (a) Expression of hAAT in serum. (b,c) Expression of hAAT in epithelial lining fluid (ELF). hAAT, human α1-antitrypsin.
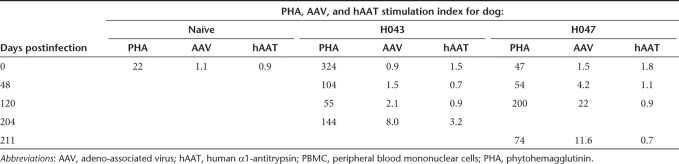
In spite of the immunosuppression regimen, a lymphoproliferation assay done on peripheral blood mononuclear cells taken at days 0, 48, and 204 or 211 showed reactivity to AAV capsid in both animals (Table 2). H047 but not H043 exhibited a proliferative response to AAV capsid at day 120. This may be indicative of some consequence of the 3 weeks respite from CSP dosing from day 57 to 78. Overall, the stimulation index against AAV capsid increased from 0.9 to 8.0 and from 1.5 to 11.0 by day 200 for H043 and H047, respectively. In contrast to the reactivity to AAV capsid, there was a mild response to hAAT in one animal (H043, stimulation index of 3.2) at day 211.
Table 2.
T-cell responses detected by lymphoproliferation assay of dog PBMC
Discussion
Current therapies for AAT deficiency include smoking and dust exposure prevention, bronchodilators, lung transplantation, and hAAT protein augmentation. Advanced cases of pulmonary emphysema resulting from AAT deficiency can be treated by lung transplantation, but unless accompanied by transplantation of a liver producing normal AAT, destruction of the transplanted lung ensues. Intravenous administration of hAAT purified from human plasma to bring the circulating level of hAAT into a range not associated with pulmonary disease (≥0.6 mg/ml) has been performed for over two decades in the United States and in some European countries.16,17 Most patients receive weekly intravenous infusions of 3–5 g AAT (60 mg/kg body weight) purified from pooled human plasma. However, this therapy is expensive and is limited by the supply of purified AAT. A major concern regarding this therapy is that conclusive documentation of its effectiveness at preventing AAT-associated lung disease is still lacking, but it is reasonable to expect that it is effective from a biochemical perspective. There are also concerns about exposure to pathogens transmitted in human plasma. In response to this, transgenic animals have been used to produce hAAT. However, glycosylation variants in nonhuman hosts caused a rapid elimination of animal-derived hAAT in patients; making a strong case for an endogenous source of hAAT. Lung epithelial cells seem to be an ideal target for gene therapy to treat AAT deficiency because the lung is where hAAT is needed most and local production of hAAT should be the most effective.
The proteinase/antiproteinase theory of the development of emphysema supports the idea that the concentration of hAAT in the lung interstitium is critical to the development of emphysema. In this study, it is most likely that the concentration of hAAT would be lower in the interstitium than that in the ELF and might be subtherapeutic. However, studies in patients with AAT deficiency have shown that the number of neutrophils in the airways is markedly increased18,19 suggesting that there is recruitment of these cells. The low concentrations of AAT in the ELF fail to inhibit elastase that then stimulates macrophages to release a leukotriene that recruits more neutrophils that releases more elastase. This cascade of events results in amplification of the neutrophil and macrophage population. The resultant migration of these cells through the interstitium is thought to be critical to the development of connective tissue damage leading to emphysema.5 The net result of producing hAAT in airway cells would be indirect protection of the interstitium. We show here that nasal delivery of an AAV6 vector encoding hAAT to mice can raise both the ELF and circulating level of hAAT to therapeutic range, and bronchial delivery of the same vector to dogs results in lung fluid levels of hAAT in the therapeutic range, indicating that a similar approach in humans may be successful.
Current data indicate that the main hurdle to using AAV vectors for in vivo gene delivery is the potential development of host immune responses to AAV proteins and/or the transgene product. These responses can destroy transgene-expressing cells and can limit the success of vector readministration to increase transgene expression. Mice are relatively tolerant of AAV vector-mediated transduction and can express many vector-encoded foreign proteins for long periods. In contrast, robust immune responses to transgene products and to AAV capsid proteins that dramatically limit transgene expression have been demonstrated in dogs20,21 and by results of the present study, predicting that cellular immunity to AAV vectors will likely be an obstacle in humans. In line with our studies, recent clinical trials have identified problems not observed in small and some large animal models, including nonhuman primates.22 Transient transaminitis developed shortly after vector administration in a trial of hepatic AAV2-mediated factor IX transfer in hemophilia B patients and was accompanied by the rise of AAV2 capsid-specific CD8+ T cells. In this trial, transgene expression was sustained for <8 weeks.23 In addition, several immunogenic epitopes of the capsid proteins of AAV2 and AAV8 have been identified in humans.23,24 These data indicate that additional studies of immunogenicity of AAV vectors are warranted so that strategies can be developed to permit long-term transgene expression.
It is unlikely that immune responses will develop to AAT produced by an AAV vector in gene therapy recipients with severe AAT deficiency because most produce mutant forms of AAT that are very similar to the normal M-type AAT, making these subjects immunotolerant of the normal M-type AAT. Furthermore, many of these subjects have been receiving AAT augmentation for years without developing antibodies against AAT.
The main components of the AAV vector virions are the vector genomes and the capsid proteins. Immune responses can arise in response to the large amount of AAV vector required for effective gene therapy. It has been reported that intracellular degradation of capsids of AAV serotype 2 takes far longer than other capsid serotypes such as 6 or 8.25 Unless gene transfer is to an immunologically privileged site, such as perhaps the retina where intact AAV particles were detected 6 years later,22 residual capsid protein can target the cells for elimination by the immune system.
We have investigated a combination of two immunosuppressive drugs; CSP, a calcineurin inhibitor, and MMF, an antimetabolite, as initial strategies for suppressing immune responses to AAV. The combination of CSP and MMF is synergistic both for enhancing engraftment and controlling graft-versus-host disease in a dog model of hematopoietic cell transplantation and is effective in preventing rejection of solid organ transplants.26,27,28 This combination has been translated successfully to the human hematopoietic cell transplantation setting.29,30 Our initial results with CSP and MMF to abrogate the immune responses to AAV have been encouraging, leading to long-term expression of hAAT in blood and lungs of dogs. However, therapeutic levels declined over time. T-cell depletion by antithymocyte globulin in addition to CSP and MMF may permit long-term therapeutic levels of hAAT as have been shown for canine microdystrophin in a dog model of Duchenne muscular dystrophy.21 In light of the side effects of the CSP, MMF, and antithymocyte globulin regimen, developing strategies for more specific immunosuppression that might be less toxic would be worthwhile.
In conclusion, we show here that bronchial delivery of an AAV6 vector encoding a marker protein (AP) to a dog resulted in robust AP expression in distal airway and alveolar epithelial cells. Delivery of an AAV6 vector encoding hAAT to mice can raise both the ELF and circulating level of hAAT into the therapeutic range, and bronchial delivery of the same vector to dogs results in lung fluid levels of hAAT in the therapeutic range, suggesting that a similar approach in humans may be successful. However, reduction of immune responses to the AAV vector will be critical for effective gene therapy using this approach.
Materials and Methods
Cell culture. Human embryonic kidney 293 cells31 and HTX cells [an approximately diploid subclone of human HT-1080 fibrosarcoma cells (ATCC CCL-121)] were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum.
AAV vector plasmids. The AAV vector ACAGAP14 contains the hybrid promoter enhancer CAG,32 the cDNA for human placental AP, and a simian virus 40 polyadenylation signal. The ACAGhAAT vector contains an hAAT cDNA in place of the AP cDNA in ACAGAP. The ARAP4 vector contains the AP cDNA expressed from a Rous sarcoma virus promoter and enhancer.33 All three vectors contain AAV2 inverted terminal repeats. The plasmid pDGM6,34 which encodes adenovirus helper, AAV2 replication, and AAV6 packaging functions, was co-transfected with the vector plasmids to generate AAV6 vectors. The AAV vector plasmids pACAGAP and pACAGhAAT were propagated in the Sure bacterial strain (Stratagene, La Jolla, CA). The packaging and helper plasmids were propagated in the DH5α strain of Escherichia coli.
AAV vector production and characterization. AAV6 vectors were generated by co-transfection of vector and helper plasmids into 293 cells. Concentration and purification of AAV vectors was done as previously described.35 Southern analysis was done to determine the number of vg in the vector preparations as described previously.36
AAV vector delivery and BAL of mouse airways. Animal studies were performed in accordance with guidelines set forth by the institutional review office of the Fred Hutchinson Cancer Research Center (Seattle, WA). Eight- to ten-week-old C57BL6 or C57BL6 Rag2-knockout mice were obtained from Taconic (Germantown, NY). Mice received vector by nasal aspiration as described.35 Mouse plasma samples were obtained by retro-orbital bleeds and were stored at −80 °C. Mice were euthanized in a CO2 chamber and the lungs exposed by dissection. Before removal of the lung from the lung cavity, an incision was made into the trachea by a 20G needle, and a polyethylene tubing (PE10) attached to a clipped 25G needle was inserted into the trachea. A string was tied around the trachea and tubing to prevent leakage of liquid. BAL was done by attaching a 1-ml syringe to the needle and instilling 1 ml of phosphate-buffered saline and liquid withdrawn by negative pressure upon pulling back of the syringe plunger to recover BAL fluid. BAL was repeated so that 2 ml of saline was given yielding 1–1.5 ml of BAL fluid. BAL fluid was centrifuged at 1,100 g for 10 minutes and at 2,200 g for another 10 minutes to remove cells and debris, and was stored at −80 °C.
AAV vector delivery and BAL of dog lungs. Canine studies were performed in accordance with guidelines set forth by the institutional review office of the Fred Hutchinson Cancer Research Center. Dogs were out-bred beagle mix, both male (three) and female (two), from 7 to 12 months of age and weighing from 7.5 to 12.5 kg at the beginning of the study. Premedication was done using acepromazine 0.025 mg/kg, glycopyrrolate 0.011 mg/kg, and butorphanol 0.2 mg/kg. Anesthesia induction and maintenance was with propofol to effect. Intubation was with an 8 mm or larger single-luminal endotracheal tube to allow insertion of the endoscope. The AAV6 vector was delivered directly to the tracheobronchial tree using a microsprayer (PennCentury, Philadelphia, PA) inserted through a 5.0-mm flexible fiberoptic bronchoscope. The bronchoscope was positioned in the proximal end of either the left or right mainstem bronchus. Under direct visualization, the microsprayer was inserted through the suction channel and advanced 1–3 mm beyond the distal tip of the scope; taking care to avoid airway trauma. The vector was sprayed into the right mainstem bronchi for the ACAGAP(AAV6) vector, and into both the right and the left mainstem bronchi for the ACAGhAAT(AAV6) vector. Three milliliter of vector or saline was instilled using a 5 ml glass high-pressure syringe. Following the procedure the dogs were kept in the special recovery cage until full recovery from anesthesia. The immune suppression regimen consisted of CSP (10–30 mg/kg/day) and MMF (15 mg/kg/day) given in two doses each day. Serum was tested to determine that the target level of CSP was obtained (200–400 ng/ml).
Diagnostic BAL was performed as described. Once the endotracheal tube was secured the dog was placed into a lateral recumbency position. The fiberoptic endoscope was passed through the endotracheal tube to the level of the segmental bronchi and gently wedged to transiently occlude the segmental bronchial orifice. BAL fluids were obtained from segmental bronchi by instilling 10-ml aliquots of sterile normal saline which were then immediately aspirated into a sterile collection trap. A total of three 10-ml aliquots were instilled and collected sequentially into a given lung. Blood samples were clotted at room temperature and serum was obtained by centrifugation as was done for mouse plasma. BAL samples were processed as they were for mice.
Staining of mouse lungs for hAAT. Immunostaining for hAAT in mouse lungs were done as previously described.37
AP stain of dog lungs. The dog treated with ACAGAP(AAV6) was euthanized and lungs were excised and perfused with 2% paraformaldehyde in phosphate-buffered saline by injection into the main bronchi of each lung segment. The lung segments were than cut into slices of 1-cm thickness and placed into fixative at 20:1 volume of lung. Fixation occurred overnight at room temperature. The next day, the lung tissues were washed 5 times with phosphate-buffered saline (1 hour per wash) and then heat-inactivated at 68 °C for 75 minutes. Histochemical staining for AP was done as described.38
Determination of hAAT concentration by ELISA. Rabbit antibody to hAAT (Sigma-Aldrich, St Louis, MO) was used as the primary antibody to coat 96-well plates. After binding and washing, serial tenfold dilutions of plasma (starting at 1:100) or BAL fluid (starting at 1:10) were added. In parallel, serial threefold dilutions of hAAT (Calbiochem, La Jolla, CA) were added to other wells to provide a standard for determining hAAT levels in samples. After overnight incubation wells were washed and incubated with secondary antibody conjugated to horse-radish peroxidase. Afterwards, peroxidase substrate was added and absorbance at 405 nm was read after 20–30 minutes.
ELISA assay for serum antibodies against AP. Human placental AP (80 µg/ml, Calbiochem) was used to coat wells of 96-well plates. Next diluted dog serum was added and binding was done at 4 °C overnight. Afterwards, rabbit anti-dog immunoglobulin G conjugated to peroxidase was added. Then peroxidase substrate was added and reaction was read as done for ELISA for hAAT. Mouse monoclonal to AP (Sigma-Aldrich) and goat anti-mouse immunoglobulin G were used in positive control wells.
Sandwich ELISA assay for mouse serum antibodies against hAAT. Rabbit anti-hAAT antibodies (1:250) were used to coat multiwell plates overnight at 4 °C. The next day, hAAT (10 µg/ml) was added and allowed to bind to the rabbit antibodies for 2–3 h, and was followed by incubation with blocking buffer for 2–3 h. Next mouse serum test samples (diluted 1:100) were added and incubated overnight at 4 °C. The wells were then incubated with goat anti-mouse immunoglobulin G conjugated to peroxidase for 1–2 h. Then peroxidase substrate was added and absorbance was read 30 minutes later at 405 nm.
Urea assay to determine fold dilution of ELF in BAL fluids. To calculate the fold dilution of ELF in BAL fluids, a urea assay (catalog #10-542-946-035; R-Biopharm AG, Darmstadt, Germany) was done on both plasma and BAL fluids obtained in parallel from mice or dogs. Determination of urea content was based on determining nitrogen content after Kjeldahl-digestion or oxidation of nicotinamide-adenine dinucleotide by glutamate dehydrogenase when ammonia and 2-oxoglutarate are present. Briefly, 100 µl of BAL or 4 µl of plasma (in 100 µl of saline) were added to 100 µl solution containing 2-oxoglutarate (3.7 mg/ml) and nicotinamide-adenine dinucleotide (4 mg/ml). Then 25 µl of urease (9 U/ml) and 75 µl of double-distilled water were added. The absorbance of mixture was read at 340 nm. Afterwards, 2 µl of glutamate dehydrogenase (833 U/ml) was added and absorbance was read 20–30 minutes later at the completion of reaction. The amount of ammonia in the sample was determined in parallel samples without addition of urease. This value was subtracted to obtain the urea content. Urea values obtained for plasma was compared to those for BAL fluids. The fold difference was used as the dilution factor of the ELF in the BAL fluid. The dilution factor was used to derive the concentration of hAAT in ELF from the level in BAL fluid.
Neutralization assay. Detection of neutralizing antibodies against AAV6 vectors was done as described.39
Western blots. hAAT was visualized using western blot analysis. The western blot was done according to standard procedures. Rabbit anti-hAAT (Sigma-Aldrich) was used as the primary antibody and was diluted 1:250 in phosphate-buffered saline.
Lymphoproliferation assay. Lymphoproliferation response to AAV and hAAT was assessed in vitro by a [3H]thymidine incorporation assay.20 Peripheral blood mononuclear cells were grown in the presence of 1011 vg of AAV or hAAT (6 µg/ml, Calbiochem). Medium and phytohemagglutinin (10 µg/ml; Sigma-Aldrich) were used as negative and positive controls, respectively.
Acknowledgments
We thank Siu-Ling Lam for excellent technical assistance, Kelly Hudkins and Charles Alpers for providing histology support as part of the Pathology Core of the Core Center for Gene Therapy at the University of Washington, and James Allen and the Chamberlain Laboratory at the University of Washington for production of the AAV vectors used in the dog studies. This work was supported by grants DK47754, CA15704 and AR056949 from the National Institutes of Health.
REFERENCES
- Stoller JK., and , Aboussouan LS. Myths and misconceptions about α1-antitrypsin deficiency. Arch Intern Med. 2009;169:546–550. doi: 10.1001/archinternmed.2009.25. [DOI] [PubMed] [Google Scholar]
- Lomas DA., and , Parfrey H. α1-antitrypsin deficiency. 4: Molecular pathophysiology. Thorax. 2004;59:529–535. doi: 10.1136/thx.2003.006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RJ., and , Zellner KM. α1-antitrypsin deficiency: incidence and implications. Dimens Crit Care Nurs. 2005;24:255–60. doi: 10.1097/00003465-200511000-00001. [DOI] [PubMed] [Google Scholar]
- Sandhaus RA. α1-Antitrypsin deficiency. 6: new and emerging treatments for α1-antitrypsin deficiency. Thorax. 2004;59:904–909. doi: 10.1136/thx.2003.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusriwil H., and , Stockley RA. -1-antitrypsin replacement therapy: current status. Curr Opin Pulm Med. 2006;12:125–131. doi: 10.1097/01.mcp.0000208452.57854.c6. [DOI] [PubMed] [Google Scholar]
- Stoller JK, Tomashefski J, Jr, Crystal RG, Arroliga A, Strange C, Killian DN, et al. Mortality in individuals with severe deficiency of α1-antitrypsin: findings from the National Heart, Lung, and Blood Institute Registry. Chest. 2005;127:1196–1204. doi: 10.1378/chest.127.4.1196. [DOI] [PubMed] [Google Scholar]
- Song S, Embury J, Laipis PJ, Berns KI, Crawford JM., and , Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299–1306. doi: 10.1038/sj.gt.3301422. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 α1-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Lu Y, Choi YK, Campbell-Thompson M, Li C, Tang Q, Crawford JM, et al. Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J Gene Med. 2006;8:730–735. doi: 10.1002/jgm.896. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Conlon TJ, Poirier A, Campbell-Thompson M., and , Byrne BJ. Preclinical characterization of a recombinant adeno-associated virus type 1-pseudotyped vector demonstrates dose-dependent injection site inflammation and dissemination of vector genomes to distant sites. Hum Gene Ther. 2007;18:245–256. doi: 10.1089/hum.2006.113. [DOI] [PubMed] [Google Scholar]
- De B, Heguy A, Leopold PL, Wasif N, Korst RJ, Hackett NR, et al. Intrapleural administration of a serotype 5 adeno-associated virus coding for α1-antitrypsin mediates persistent, high lung and serum levels of α1-antitrypsin. Mol Ther. 2004;10:1003–1010. doi: 10.1016/j.ymthe.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Allen JM., and , Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Lam SL., and , Miller AD. High-efficiency promoter-dependent transduction by adeno-associated virus type 6 vectors in mouse lung. Hum Gene Ther. 2007;18:344–354. doi: 10.1089/hum.2006.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MP, Miller AD, Zabner J., and , Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- Köhnlein T., and , Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121:3–9. doi: 10.1016/j.amjmed.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, et al. Replacement therapy for alpha1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316:1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- Hubbard RC, Fells G, Gadek J, Pacholok S, Humes J., and , Crystal RG. Neutrophil accumulation in the lung in α1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest. 1991;88:891–897. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HM, Kramps JA, Burnett D., and , Stockley RA. Lung lavage fluid from patients with alpha 1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci. 1987;72:373–381. doi: 10.1042/cs0720373. [DOI] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Stieger K, Schroeder J, Provost N, Mendes-Madeira A, Belbellaa B, Le Meur G, et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther. 2009;17:516–523. doi: 10.1038/mt.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu Q, Yang P, Hsu HC., and , Mountz JD. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z., and , Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 2003;196:176–196. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- Yu C, Seidel K, Nash RA, Deeg HJ, Sandmaier BM, Barsoukov A, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC., and , Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K., and , Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Allen JM, Halbert CL., and , Miller AD. Improved adeno-associated virus vector production with transfection of a single helper adenovirus gene, E4orf6. Mol Ther. 2000;1:88–95. doi: 10.1006/mthe.1999.0010. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL., and , Miller AD. AAV-mediated gene transfer to mouse lungs. Methods Mol Biol. 2004;246:201–212. doi: 10.1385/1-59259-650-9:201. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW., and , Miller AD. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella-Lowell I, Zusman B, Foust K, Loiler S, Conlon T, Song S, et al. Enhancing rAAV vector expression in the lung. J Gene Med. 2005;7:842–850. doi: 10.1002/jgm.759. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Standaert TA, Wilson CB., and , Miller AD. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]