Abstract
Mucopolysaccharidosis IVA (MPS IVA, Morquio A disease) is an inherited lysosomal storage disorder that features skeletal chondrodysplasia caused by deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS). Human GALNS was bioengineered with the N-terminus extended by the hexaglutamate sequence (E6) to improve targeting to bone (E6-GALNS). We initially assessed blood clearance and tissue distribution. Next, to assess the effectiveness of storage clearance and reversal of pathological phenotype, a dose of 250 U/g of enzyme was given weekly to Morquio A mice (adults: 12 or 24 weeks, newborn: 8 weeks). Sulfatase modifier factor 1 (SUMF1) was co-transfected to activate the enzyme fully. The E6-GALNS tagged enzyme had markedly prolonged clearance from circulation, giving over 20 times exposure time in blood, compared to untagged enzyme. The tagged enzyme was retained longer in bone, with residual enzyme activity demonstrable at 48 hours after infusion. The pathological findings in adult mice treated with tagged enzyme showed substantial clearance of the storage materials in bone, bone marrow, and heart valves, especially after 24 weekly infusions. Mice treated from the newborn period showed marked reduction of storage materials in tissues investigated. These findings indicate the feasibility of using tagged enzyme to enhance delivery and pathological effectiveness in Morquio A mice.
Introduction
Enzyme-replacement therapy (ERT) is an established strategy of treating lysosomal storage diseases including mucopolysaccharidoses (MPS). Tremendous progress in research toward development of ERT has been made in the last three decades. In Gaucher disease, delivery of enzyme to affected cells was achieved by modifying the N-linked carbohydrate on the enzyme to expose core mannose residues,1,2 enabling the enzyme to bind to the mannose receptor, which is highly abundant on cells of the reticuloendothelial system.3,4 These findings led to achievement of clinical management of Gaucher disease by ERT with dramatic clinical results.5
ERT is clinically available for several lysosomal storage diseases6 including MPS I,7 MPS II,8 and MPS VI.9 Those ERTs have resulted in marked improvement in the visceral organs but little or no improvement in bone or brain because the enzymes are not delivered to these tissues effectively. It is still a challenge to achieve clinical efficacy for the skeleton, especially for diseases with multi-bone involvement, like MPS I, II, IVA, IVB, VI, and VII. Most of the enzyme-based drugs are delivered to major visceral organs like liver and spleen and only a small amount of enzyme is delivered to bone. Many lysosomal enzymes have a short half-life because of rapid clearance in liver by carbohydrate-recognizing receptors, particularly the mannose receptor that is highly abundant on Kupffer cells3 or the mannose-6-phosphate receptor (M6PR). Although a fraction of enzyme reaches bone marrow, a slight amount reaches bone, especially the cartilage cells in the avascular growth plate. Thus, improvement of bone lesions in Gaucher patients by ERT is quite limited even after a long-term treatment.5,6 Similarly, very little improvement in bone was seen in clinical trials on MPS I.7 Thus, the current oligosaccharide-based ERT targeting both mannose receptor and M6PR does not work efficiently on bone and cartilage lesions. Another approach is to utilize alternative uptake mechanisms that rely on protein-based, rather than oligosaccharide-based, targeting determinants. Some studies have focused on the use of small basic peptides, like human immunodeficiency virus transactivator of transcription, to enhance the delivery to specific tissues.9,10 Others have demonstrated the feasibility of this approach using insulin-like growth factor-2, a peptide ligand for the M6PR or the low-density lipoprotein receptor.11,12,13 Such peptide-based targeting system has enhanced ERT in some tissues but not directly in bone and cartilage.
Bone formation occurs by three coordinated processes: production of osteoid matrix, its maturation, and subsequent mineralization of the matrix. To initiate mineralization in woven bone, or in growth plate cartilage, high-local concentrations of Ca2+ and PO43− ions must be reached in order to induce their precipitation into amorphous calcium phosphate, leading to hydroxyapatite crystal formation: Ca10(PO4)6(OH)2. This is achieved by membrane-bound matrix vesicles, which originate by budding from the cytoplasmic processes of the chondrocyte or the osteoblast and are deposited within the matrix during its formation. In the matrix, these vesicles are the first structure wherein hydroxyapatite crystals are observed. Hydroxyapatite is a positively charged, major inorganic component in a hard tissue (bone) and does not exist in soft tissues. Bone is continuously remodeled throughout life by resorption and formation. A drug attached to hydroxyapatite may be released in bone resorption process and targeting a drug on hydroxyapatite could be a potential strategy for a selective drug delivery to bone and cartilage cells.14,15 Some bone matrix proteins (osteopontin, bone sialoprotein, etc.) that bind to hydroxyapatite have been found to have a repetitive sequence of negatively charged acidic amino acids (Asp or Glu), a possible hydroxyapatite-binding site.16,17 In osteoblastic cell culture, osteopontin and bone sialoprotein rapidly bind to hydroxyapatite after the osteoblast secretes these proteins.18 Kasugai et al. showed that they could enhance estradiol uptake by bone and prevent osteoporosis by using Glu6 (E6) targeted hormone.19,20 We and another group have recently applied this new bone-targeting system to a large molecule, an enzyme (tissue-nonspecific alkaline phosphatase), showing that the tagged enzymes were delivered more efficiently to bone21 and that the clinical and pathological improvement in systemic bone disease, hypophosphatasia, was observed.22
We hypothesized that this new bone-targeting system could be also applied to MPS IVA (Morquio A), which involves a deficiency of the lysosomal enzyme, N-acetylgalactosamine-6-sulfate sulfatase (GALNS; EC 3.1.6.4). This enzyme is required to degrade keratan sulfate (KS) and chondroitin-6-sulfate, which are synthesized mainly in bone and cartilage. To study the long-term effectiveness of ERT without confounding immune responses, we have established Morquio A mice immunotolerant to multiple injections of human enzymes.23,24,25 The mouse model has the significant pathological phenotypes in bone and cartilage. With the availability of the tolerant Morquio A mouse model, we have applied a newly designed bone-targeting drug delivery system. We have produced and purified the mature enzymes tagged with the acidic oligopeptides for further characterization and ERT.
Sulfatases catalyze the hydrolysis of sulfate esters such as glycosaminoglycans (GAGs), sulfolipids, and steroid sulfates. Deficiency of lysosomal sulfatases leads to human diseases characterized by the accumulation of either GAGs or sulfolipids. The post-translational modification of the highly conserved Cys residue is required for catalytically active sulfatases. The Cα-formylglycine (F-Gly), conversion of Cys activates the eukaryotic sulfatases. The sulfatase modifying factor 1 (SUMF1) is the gene responsible for the oxidation of Cys to F-Gly. SUMF1 showed an enhancing effect on sulfatase activity when coexpressed with sulfatase genes in COS-7 cells.26,27 SUMF1 coexpression was used for ERT on MPS IVA mice, but did not have any effect on the biodistribution profile.28
In this study, we tested our hypothesis that the bone-targeting drug delivery system will improve the efficacy of ERT to bone and cartilage. We studied the pharmacokinetics, biodistribution, and therapeutic effect on Morquio A mouse models by E6-tagged GALNS. The results of preclinical trials on Morquio A mouse model can provide “proof of concept” and helpful information required to design future human trials of Morquio A.
Results
Characterization of human recombinant E6-GALNS
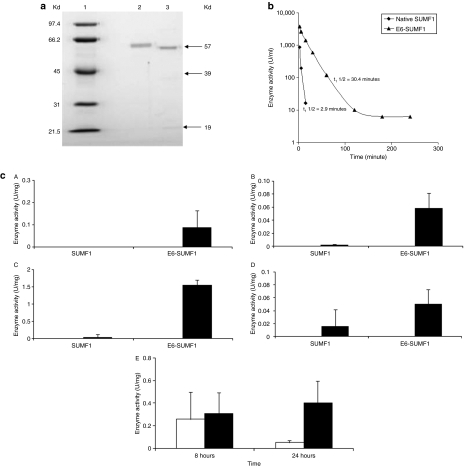
Untagged native SUMF1-GALNS previously described,29 tagged E6-GALNS, and E6-SUMF1 GALNS were isolated from Chinese hamster ovary (CHO) cells. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) analysis of the enzyme preparations showed a higher Mr of E6-SUMF1 GALNS (60 kd), compared with native SUMF1 GALNS (57, 39, and 19 kd) (Figure 1a) (there was no (SDS/PAGE) change of Mr without SUMF1, data not shown). These two untagged and tagged GALNSs differed in the degree of uptake by MPS IVA human fibroblasts, showing that native SUMF1 GALNS has a little higher uptake although no statistical difference was observed (data not shown).
Figure 1.
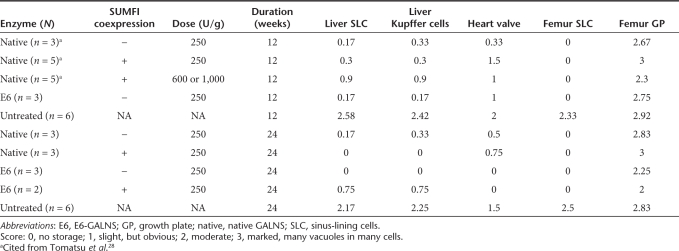
Characterization, pharmacokinetics, and biodistribution of purified GALNSs. (a) SDS-PAGE and silver staining of purified GALNSs. SDS-PAGE; the GALNSs from the gel filtration step were subjected to SDS-PAGE under reducing conditions (0.1 mmol/l DTT), followed by silver staining. Lane 1 is molecular-mass standard. Lane 2 (100 ng) is E6-SUMF1 GALNS. Lane 3 (100 ng) is native SUMF1 GALNS. Both E6-SUMF1 GALNS and native SUMF1 GALNS were purified from CHO secretions. The molecular sizes were indicated with arrows and were calculated by comparison with SDS-PAGE standards (Bio-Rad Laboratories, Hercules, CA). (b) Pharmacokinetics of GALNSs (clearance from the blood circulation). The 250 U/g of native SUMF1 and E6-SUMF1 GALNSs was administered to 3-month-old Morquio A mice (3 mice/group) in the tail vein. The blood sample was taken at 2-, 5-, 15-, 30-, 60-, 120-, 180-, 240-, 1,440, and 2,880-minutes time points. Plasma enzyme activity was assayed. The results graphed are the semilog plots of the average levels of GALNS activity (U/ml) at each time point. (c) Biodistribution of native SUMF1 and E6-SUMF1 GALNSs in femur bone, bone marrow, spine bone, and heart (clearance from the tissues). We infused 250 U/g body weight of the indicated enzymes into three Morquio A mice for each treatment. After 48 hours, the mice treated with 250 U/g were killed, and tissue samples were processed for biochemistry. Levels of enzyme observed in femur bone, bone marrow, spine bone, and heart are shown. (A) Femur bone (adult); (B) spine bone (adult); (C) bone marrow (adult); (D) heart (adult), (E) femur bone (treated from newborn). Open bar: native SUMF1 GALNS, filled bar: E6-SUMF1 GALNS. CHO, Chinese hamster ovary; DTT, dithiothreitol; GALNS, N-acetylgalactosamine-6-sulfate sulfatase; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SUMF1, sulfatase modifier factor 1.
The theoretical isoelectric point of untagged native GALNS was 6.25 whereas the pI of E6-GALNS was 5.68. The optimal pH of GALNS activity for all enzymes was also determined using 0.02 mol/l sodium acetate buffer. It was between pH 4.8 and pH 5.3. At a higher pH or lower pH, the activity decreased markedly (data not shown).
Pharmacokinetics and biodistribution
After a single intravenous dose of 250 U/g, native SUMF1 GALNS was cleared very rapidly within 15 minutes from the blood circulation of Morquio A mice with a plasma half-life of 2.9 minutes between 2 minutes and 15 minutes. E6-SUMF1 GALNS clearance was biphasic, with two half-life times—the first one with a plasma half-life of 31.7 minutes between 2 minutes and 120 minutes (Figure 1b). These findings indicated that the untagged native enzyme was undetectable after 15 minutes in blood circulation whereas the tagged GALNS had a markedly prolonged circulation. If we compare the area under the curve (AUC) of both enzymes, the AUC for E6-SUMF1 GALNS (60,967 U·min/ml) was 22 times larger compared to native SUMF1 GALNS (2,699 U min/ml). The tendency was the same when we provided a single shot of 1,000 U/g (data not shown).
The biodistribution of tagged and untagged enzymes was determined after a single injection of 250 U/g (Figure 1c). At 48 hours postinjection into adult Morquio A tolerant mice, the enzyme activities in femur bone, spine bone, and bone marrow were almost undetectable for untagged native SUMF1 GALNS whereas 0.09 ± 0.075 U/mg, 0.06 ± 0.024 U/mg, and 1.55 ± 0.14 U/mg, respectively, were detected in each tissue from mice treated with tagged E6-SUMF1 GALNS. The enzyme activities in heart were 0.016 ± 0.026 U/mg for native SUMF1 GALNS and 0.05 ± 0.023 U/mg for E6-SUMF1 GALNS (Figure 1c; A–D). The enzyme activities in liver were 0.023 ± 0.008 U/mg for native SUMF1 GALNS and 0.051 ± 0.32 U/mg for E6-SUMF1 GALNS. Thus, in most tissues examined, E6-tagged SUMF1 GALNS retained more enzyme activity than native SUMF1 GALNS. The tendency was the same when we provided a single dose of 1,000 U/g (data not shown). Delivery of each enzyme to femur bone of newborn Morquio A mice showed no difference between the two enzymes at 8 hours postinfusion whereas E6-SUMF1 GALNS was retained in femur bone more than native SUMF1 GALNS at 24 hours postinfusion (Figure 1c; E). We also made a construct of D6-tagged GALNS and obtained D6-GALNS purified enzyme. The resultant tissue distribution showed that E6-GALNS was delivered to bone and bone marrow 40–60% more than D6-GALNS (data not shown).
On the basis of the above findings, we treated adult and newborn Morquio A mice with E6- and E6-SUMF1 GALNSs and compared results with the previously published data of ERT with untagged native GALNSs.28
E6- or E6-SUMF1-GALNSs in clearance of storage in Morquio A mice
To detect quantitative differences in the effectiveness of clearance of lysosomal storage by the tagged enzyme, we developed an initial protocol in which 250 U/g of E6-GALNS enzyme was given in 12 weekly infusions to adult Morquio A tolerant mice (group 1: Figure 2). Next, to evaluate whether addition of SUMF1 and a longer term treatment will contribute to a greater treatment effect, we treated adult Morquio A tolerant mice with E6-GALNS or E6-SUMF1 GALNS in 24 weekly infusions (group 2: Figure 3). We also treated newborn Morquio A tolerant mice for 8 weeks with E6-GALNS (group 3: Figure 4) to assess early treatment. Tissues were collected and examined for depletion of storage 1 week after the last intravenous administrations of GALNS (Table 1).
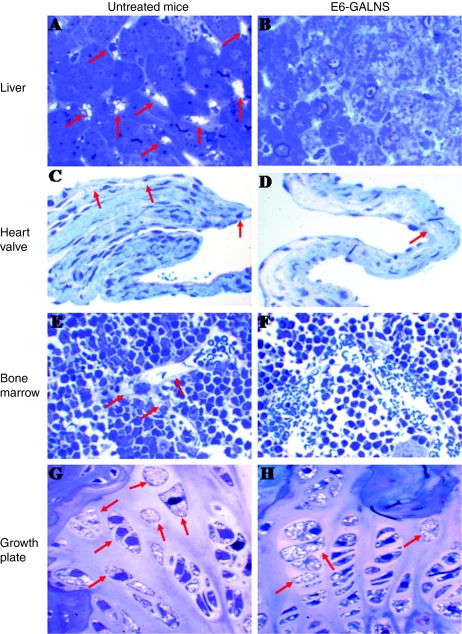
Figure 2.
ERT on Morquio A tolerant mice treated for 12 weeks with 250 U/g of E6-GALNS. Light microscopy of liver, heart valve, bone marrow, and cartilage cell layer from (A,C,E,G) untreated and (B,D,F,H) E6 GALNS-treated. The arrows show vacuoles. The (B) liver Kupffer cells and (F) bone marrow had essentially complete clearance of storage by the treatment group. (D) Heart valve showed reduction of vacuoles in a treated mouse and the (H) cartilage cells showed a slight response after 12 weeks of treatment. ERT, enzyme-replacement therapy; GALNS, N-acetylgalactosamine-6-sulfate sulfatase.
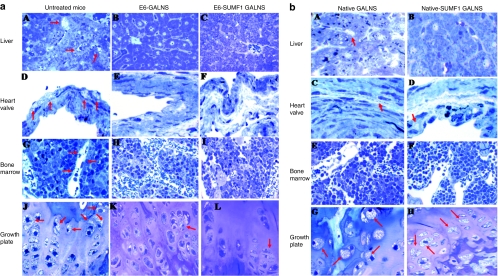
Figure 3.
ERT on Morquio A tolerant mice treated for 24 weeks with 250 U/g of (a) E6-GALNS or E6-SUMF1 GALNS and (b) native GALNS or native-SUMF1 GALNS. (a) Light microscopy of liver, heart valve, bone marrow, and cartilage cell layer from (A,D,G,J) untreated, (B,E,H,K) E6 GALNS-treated, and (C,F,I,L) E6-SUMF1 GALNS-treated mice for 24 weeks with 250 U/g. The arrows show vacuoles. (A–C) Liver. The (B,C) liver Kupffer cells have essentially complete clearance of storage by both the treatment groups whereas an (A) untreated Morquio A mouse has extensive storage in sinus-lining cells and Kupffer cells. (D–F) Heart valve. The heart valves in an untreated Morquio A mouse have abundant cytoplasmic vacuolization in the valve (arrow). A reduction of storage is seen in the valve in mice treated with (E) E6-GALNS and (F) E6-SUMF1 GALNS. (G–I) Bone marrow. The sinus-lining cells in bone marrow had essentially complete clearance of storage by both (H,I) treatment groups. The sinus-lining cells in (G) bone marrow of untreated mouse (arrow) contain storage material. (J–L) Growth plate. An untreated Morquio A mouse shows marked storage material in (J) chondrocytes. Vacuolated chondrocytes with lysosomal distension are obvious. The cartilage cells have substantial reduction of storage material in both (K,L) treated mice. (b) Light microscopy of liver, heart valve, bone marrow, and cartilage cell layer from (A,C,E,G) native GALNS and (B,D,F,H) native-SUMF1 GALNS-treated mice for 24 weeks with 250 U/g. The arrows show vacuoles. (A,B) Liver. The liver Kupffer cells had essentially complete clearance of storage by both the treatment groups. (C,D) Heart valve. A reduction of storage is seen in the valve in mice treated with (C) native GALNS and (D) native-SUMF1 GALNS. (E,F) Bone marrow. The sinus-lining cells in bone marrow had essentially complete clearance of storage by (E,F) both treatment groups. (G,H) Vacuolated chondrocytes with lysosomal distension remained still obvious. The cartilage cells had substantial storage material in both (G,H) treated mice. ERT, enzyme-replacement therapy; GALNS, N-acetylgalactosamine-6-sulfate sulfatase; SUMF1, sulfatase modifier factor 1.
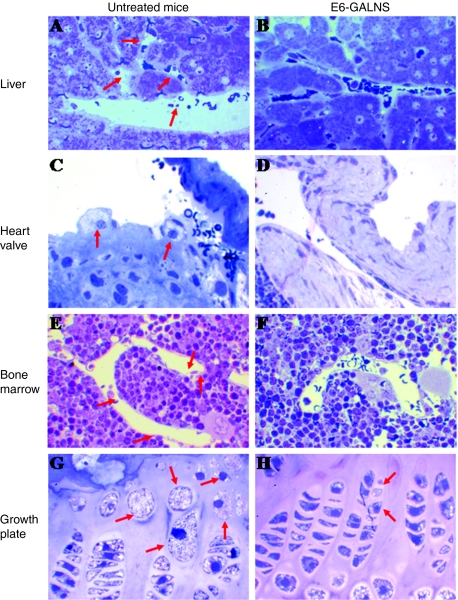
Figure 4.
ERT on newborn Morquio A tolerant mice (8 weeks with 250 U/g of E6-GALNS). Untreated mice showed numerous vacuoles in (A) liver, (C) heart valves, (E) bone marrow, and (G) chondrocytes in femur growth plates. After 8-weekly intravenous injection of E6-GALNS, reduction of storage materials was observed in (B) liver, (D) heart valves, (F) bone marrow, and (H) cartilage cell layer. ERT, enzyme-replacement therapy; GALNS, N-acetylgalactosamine-6-sulfate sulfatase.
Table 1.
Summary of pathology
All regimes of GALNS completely or almost cleared the accumulated GAGs in sinus-lining cells and Kupffer cells in liver and sinus-lining cells in bone marrow, regardless of age at the beginning of treatment (Figures 2–4).
The growth plate region in treated adult Morquio A tolerant mice showed limited improvement in reduction of storage materials after 12 weekly infusions of E6-GALNS. However, mice treated with 24 weekly infusions with E6- or E6-SUMF1 GALNS showed more substantial reduction (Figure 3a). Mice treated from birth had marked reduction of storage materials in growth plate (Figure 4), although we did not achieve complete clearance.
Ligaments and connective tissues surrounding the articular cartilage in any group of treated mice had less storage, although there was individual variation. Overall, the tendency was the same with the growth plate region. In treated adult Morquio A mice, 24 weekly infusions with E6- or E6-SUMF1 GALNS showed more improvement than 12 weekly infusions. Early treatment groups (from the newborn period) also showed more reduction although we could still see a few vacuoles. In addition, the overall organization of the growth plate region was improved in the group treated from the newborn period.
There was substantial reduction among the treated groups in the extent of clearance of storage in heart valves although some variation was observed in individual mice. More clearance of storage material was observed in mice treated for a longer term or from birth (Figures 2–4).
We previously reported the results of ERT on Morquio A mice treated with untagged native SUMF1 GALNS for 12 weeks.28 Ligaments and connective tissues surrounding the articular cartilage in treated mice had less storage with a higher dose (600 or 1,000 U/g). However, the growth plate region did not show much improvement with 250 U/kg, and the storage material was not cleared by even a higher dose.28 We also confirmed the above finding in the earlier study of less treatment effect in cartilage cells and heart valves in Morquio A mice receiving native SUMF1 GALNS for 24 weeks (Figure 3b). The current results suggest that E6-tagged enzymes showed more improvement in pathology of cartilage cell and heart valves than untagged enzymes.
It is noteworthy that murine antibodies to the heterologous human enzyme were not detected during these multiple-dose long-term studies using the Morquio A tolerant mice we described previously (data not shown here).25
Thus, the pathological lesions in sinus-lining cells and Kupffer cells in liver and sinus-lining cells in bone marrow were very responsive to any treatment. The lesions in heart valves were refractory to untagged enzymes, but responsive to tagged enzymes, especially for a longer term treatment. The lesions in cartilage cells were the most refractory and required a longer term treatment with a tagged enzyme or treatment from birth to achieve substantial improvement.
Concentration of serum KS in Morquio A mouse
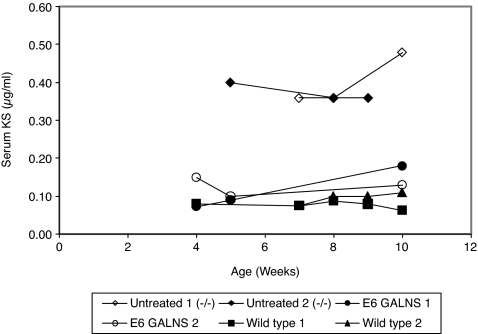
Serum samples from Morquio A mouse treated with 250 U/g of E6-GALNS, untreated Morquio A mice, or wild-type control mice were analyzed for KS (Figure 5). The baseline serum KS in wild-type controls showed an average of 0.11 ± 0.03 µg/ml and did not change with age. The baseline serum KS in untreated Morquio A tolerant mice was elevated to a level between 0.30 and 0.50 µg/ml after the mice were 4 weeks old.
Figure 5.
Level of serum KS in Morquio A mouse. Untreated Morquio A mice (n = 2), E6-GALNS treated (n = 2), and control wild type (n = 2). GALNS, N-acetylgalactosamine-6-sulfate sulfatase; KS, keratan sulfate.
Serum KS levels were greatly reduced during the 8 weeks of treatment to the baseline levels of untreated control mice (i.e., 0.09 and 0.15 µg/ml). The final serum KS in E6-GALNS treated mice was similar to that in normal control group.
Discussion
Storage of KS leads to severe skeletal deformity in MPS IVA in humans, for which there has been no effective medical treatment to date. Our previous ERT study on adult Morquio A mice treated with native SUMF1 GALNS showed only a limited pathological improvement in bone and cartilage by 12 weekly infusions independent of dose escalation.28 Such limited effect could be explained by relatively brief periods of treatment, insufficient steady-state circulating concentrations of GALNS, inability of the administered soluble GALNS to reach and to be retained at sites of bone and cartilage, or necessity of early treatment. We hypothesized that a bone-targeted form of GALNS, especially if administered for a longer term or from birth would improve the skeletal phenotype of Morquio A more. We document here that this approach for ERT using E6-GALNS prevents their characteristic pathological features, namely storage vacuoles.
This study had two main purposes: (i) to compare biochemical properties of E6 (or E6-SUMF1) tagged GALNS enzyme with native (or native SUMF1) GALNS enzyme previously described28 and (ii) to assess the response of Morquio A mice to therapy with E6- and E6-SUMF1 GALNS enzymes with varying duration and time of initiation of treatment. Coexpression of SUMF1 together with E6-GALNS complementary DNA (cDNA) resulted in an increase of specific activity (~200,000 units/mg versus 100,00 units/mg). Most other biochemical properties—molecular weight, optimal pH, and blood clearance were not affected by coexpression of SUMF1. In vitro studies of the effect of inhibitors on endocytosis of E6 (or E6-SUMF1) GALNS enzyme by Morquio A fibroblasts showed clearly that the tagged GALNS could be taken up by M6P-mediated mechanisms, which target the M6PR (data not shown).
Cosma et al.26 and Dierks et al.27 identified the C-α-formylglycine-generating-enzyme-1 (FGE) and its gene, SUMF1, which is responsible for multiple sulfatase deficiency. FGE is the post-translational modifying enzyme essential for the activation of multiple forms of sulfatases including GALNS. Normally, a sufficient amount of FGE required to modify sulfatases is constitutively expressed in the cell. However, when a specific recombinant sulfatase is excessively expressed, the relative amount of FGE is anticipated to become limiting, making it necessary to coexpress FGE. The utility of FGE for overexpression of functional sulfatases was tested in previous studies. In vitro coexpression of SUMF1 with many sulfatase cDNAs in COS cells results in a synergistic increase in enzymatic activity, indicating that SUMF1 is both an essential and a limiting factor for most sulfatases.26,27 Enhanced production by coexpressed SUMF1 was obtained in vivo in the arylsulfatase deficient mouse.29 FGE coexpression did not affect arylsulfatase mRNA or protein levels, suggesting that activation occurs at the post-translational level and that active and inactive arylsulfatase enzymes are colocalized in the cells.30 It was demonstrated that part of the recombinant overexpressed sulfatases produced for ERT without coexpressed FGE is inactive due to insufficient post-translational modification.
In this study, the pathological improvement on Morquio A mice did not show any substantial difference between two tagged enzymes (produced with or without coexpressed SUMF1) at the current dose (in units). However, it appears to be beneficial to coexpress SUMF1 in the GALNS overexpressing cell line to double the yield of recombinant active enzyme.
The recombinant E6-GALNS enzyme had marked difference from native GALNS in pharmacokinetics. The blood clearance study in Morquio A mice with the untagged enzyme showed a rapid clearance (first half-life: 2.9 minutes) compatible with those of other phosphorylated lysosomal enzymes: α-L-iduronidase, 0.9 minutes,31 α-galactosidase A, 2–5 minutes32) glycosylasparaginase, 4 minutes,33 β-glucuronidase, 5.2 minutes,34 and with nonphosphorylated, recombinant human α-N-acetylglucosaminidase (2 minutes).35 However, E6-GALNS enzyme showed marked prolonged blood clearance (half-life over 30 minutes). Thus, E6-enzyme showed much larger AUC, resulting in the longer exposure time in blood. There are two potential explanations of such prolonged blood circulation. First, by adding the acidic amino acid, the glycosylation pattern has been changed leading to substantial delay of clearance. In a recent study, treatment of human β-glucuronidase with sodium metaperiodate followed by sodium borohydride reduction (cleavage of mannose and phosphate at glycosylation site) eliminated uptake by M6PR and mannose receptor, and dramatically slowed its plasma clearance from a half-life time of <10 minutes to 18 hours, leading to almost complete reversal of storage in neocortical and hippocampal neurons by ERT on MPS VII mouse.36 Prolonged blood clearance of E6-tagged GALNS is similar to this finding with modified β-glucuronidase. Second, tagging the acidic amino acid changes the folding structure of GALNS, resulting in increase of stabilization of the enzyme in blood. This hypothesis could explain why tagged enzyme showed a 60 kd single band whereas untagged enzyme gave three smaller bands, suggesting a structural change had prevented protein processing. Further analyses of glycosylation site and three dimensional structure of E6-GALNS enzyme will answer this question.
The tissue distribution of infused enzymes in adult and newborn Morquio A mice indicated that the biodistribution of the tagged enzyme was also markedly different in bone and bone marrow compared with the untagged enzyme. The finding that tissue distribution to bone and bone marrow was greater with the tagged GALNS suggests more efficient delivery of enzyme to the targeted tissues in which the pathological improvement in model mice is desired. Such a specific bone-targeting system provides a positive effect in delivering the enzyme to bone as previously described.17,19,21 Substantial improvement of the pathological and clinical phenotypes by bone-targeted enzyme was shown previously in osteoporosis and hypophosphatasia mouse models.20,22 The present study on Morquio A has two limitations. (i) The metabolism of KS was found to be dissimilar in humans and rodents. Morquio A mouse model used here did not display a dysostosis phenotype, although histological signs of storage were evident.28 Therefore, it is difficult from the current pathological improvement in Morquio A mice to extrapolate this to a successful treatment in human Morquio A patients. (ii) The second potential limitation: a hexaglutamate sequence may cause antibody production. However, the present and previous studies on acidic oligopeptide-tagged proteins did not detect immunological responses.20,22,36 Further investigations on these two issues are needed.
Infusion of the tagged enzyme also led to more activity in heart and had a more positive effect on pathology (clearance of storage vesicles). One speculation is that a longer circulation in blood could allow enzyme to reach heart (generally hard to reach) as well as bone and bone marrow. Recent works on MPS VII mice by using a longer circulating enzyme indicated the substantial improvement in brain pathology.36,37 Brain pathology is not a dominant feature of Morquio disease.
The limited pathological response in growth plate region to 12 weekly infusions in adult Morquio A mice demonstrated the difficulty of access to the cartilage cell layers in adult mice in a short-term treatment, since the region is avascular. We did observe greater clearance of storage materials in bone, ligaments, connective tissues, and heart valves by a longer term treatment (24 weekly infusions) and by treatment from birth to age 8 weeks, though clearance was not complete.
The KS assay was not performed for all groups of mice. However, serum KS was normalized by ERT from birth. This normalization correlates with greater improvement of pathological findings in ligaments and connective tissues, when ERT is given from birth.
The drug delivery system strategy has described two aspects. One is that the membrane-bound matrix vesicles originating from the cytoplasmic processes of the chondrocyte or the osteoblast (primary target cells) contain hydroxyapatite and the enzyme with negatively charged short acidic oligopeptide is easily bound to positively charged hydroxyapatite at the targeted site. This permits efficient delivery to the site of pathology. The other aspect is that the prolonged blood clearance provides a greater chance to deliver the enzyme to bone, which are known to be hard to reach. Both factors contribute to enhancement of drug delivery to targeted tissues, leading to greater pathological improvement.
The complete remission of bone pathological lesions, especially in avascular cartilage cell layer, is still an unmet challenge. The availability of recombinant human tagged GALNS and the generation of GALNS–deficient mice25,38 have permitted the evaluation of tagged GALNS enzymes in the mouse model of Morquio A. The resulting findings provide proof-of-concept in vivo that tagged GALNS reaches heart, bone, and bone marrow more efficiently than untagged GALNS and substantially reduces the accumulated GAGs in the lysosomes of affected tissues. At the same time, it reduces the corresponding elevation in serum KS. The treatment effect is better when we treat mice for a longer term or from birth. Further studies are needed to clarify the optimal dose of ERT with tagged enzyme and to evaluate the optimal number of acidic amino acids tagged to the enzyme to maximize efficacy of drug delivery and pathological improvement without affecting enzyme activity and leading to immunological problems. These and follow-up experiments will provide important preclinical data for the design of enhanced GALNS enzyme-replacement trials in patients with Morquio A syndrome.
Materials and Methods
Murine model. Morquio A mouse tolerant to human GALNS protein (Galnstm(hC79S•mC76S)slu) was used in this study.25 Tolerant mice contained both a transgene (cDNA)-expressing inactive human GALNS in intron 1 and an active site mutation (C76S) in adjacent exon 2. We developed the Morquio A mouse tolerant to multiple infusions of human enzyme to study the long-term effectiveness and side effects of therapies in the absence of immune responses. Both mice were produced by homologous recombination in 129/Sv embryonic stem cells, by a replacement-type vector containing both the neomycin-resistance gene, for positive selection, and the thymidine kinase gene, for negative selection. After backcrossing onto C57/B6 strain, both affected mice showed progressive accumulation in the lysosomes of most tissues (liver, heart valves, brain, bone marrow, periosteum, ligaments, chondrocytes, connective tissues around the bone). All experiments were conducted with the highest standards of humane animal care approved by the local committee at Saint Louis University (St Louis, MO).
Production, purification, and characterization of the recombinant human GALNS. Three human GALNSs were produced and purified, in order to compare their pharmacokinetics, biodistribution, and pathological effects on Morquio A mice. Three GALNSs, designated “native SUMF1 GALNS,” “E6-GALNS,” and “E6-SUMF1 GALNS,” were produced by stably transfected CHO-K1 cells expressing respective human GALNS cDNA. To produce native SUMF1 GALNS and E6-SUMF1 GALNS, Chinese hamster ovary cells over expressing GALNS were stably transfected with human SUMF1 cDNA.26,27
Enzyme produced by this modified cell line provided a higher yield with a fully active form. The molecular weights of untagged native GALNS and tagged E6-GALNS were estimated by SDS/PAGE.
The theoretical isoelectric point of GALNS was determined by using Expose tool at the web site: http://www.expasy.ch/cgi-bin/pi_tool.
Both tagged GALNS enzymes were purified from the medium using a three-column procedure. Briefly, batches of medium containing the enzyme were filtered through 0.2-µm capsule filters (Pall Gellman, East Hills, NY). Filtered medium was concentrated 15-fold and then dialyzed against 20 mmol/l Tris–HCl and 50 mmol/l NaCl (pH 7.8). The concentrated and dialyzed medium containing GALNS enzyme was loaded onto a DEAE sepharose (Sigma-Aldrich, St Louis, MO) column and was eluted with a linear gradient of 0–0.5 mol/l NaCl (pH 7.8). The fractions with GALNS activity were pooled and concentrated. The concentrated fractions were applied to equilibrated Q-Sepharose (Sigma-Aldrich) column. The column was eluted with a linear gradient of 0–0.5 mol/l NaCl (pH 5.5). The fractions with GALNS activity were pooled and concentrated. The concentrated fractions were applied to Sephacryl S-100 HR (Amersham Biosciences, Piscataway, NJ) gel filtration column equilibrated. The column was eluted with equilibration buffer. Fractions with high GALNS activity were pooled, concentrated, and analyzed under denaturing conditions by SDS/PAGE gel. Aliquots of the purified and concentrated GALNS were assayed for enzyme activity using 4-methylumbelliferyl-β-D-galactopyranoside-6-sulphate as a substrate (Moscerdam Substrate, Rotterdam, the Netherlands) as described.39
One unit is defined as the enzyme catalyzing release of 1 nmol of substrate per hour. Total protein was determined by micro-Lowry protein assay40 with bovine serum albumin used as a standard. The enzyme was stored in sterile vials at −80 °C until used for infusions.
Optimal pH. Optimum pH of the enzymes was determined by incubating with the GALNS substrate in 0.1 mol/l NaCl and 0.1 mol/l sodium acetate at pH 3.0, 3.5, 4.0, 4.5, 5.0, and 5.5 for 15 minutes at 37 °C. GALNS enzyme activity was measured for each enzyme at different pH values in triplicate. The enzyme activity was expressed in U, and the percent inhibition was calculated as follows: (U without inhibitor − U with inhibitor)/(U without inhibitor) × 100.
Injections, specimen collection, and enzyme activity. To compare the pharmacokinetics of the tagged GALNS enzymes with previously described native GALNS,29 3-month-old homozygous mutant Morquio A tolerant mice were used. The tagged GALNSs were diluted in phosphate-buffered saline (PBS) and were injected intravenously through the lateral tail vein. Mock-treated mice received PBS.
To determine the clearance of the enzyme from the blood circulation, a dose of 250 U/g of native SUMF1-GALNS, E6-GALNS, and E6-SUMF1 GALNS was injected into the tail vein, and blood samples were collected at intervals after the infusion (2-, 5-, 15-, 30-, 60-, 120-, 180-, 240-, 1,440-, and 2,880-minutes time points). To establish the tissue distribution of the enzyme, tolerant mice were treated with 250 or 1,000 U/g of native SUMF1 GALNS, E6-GALNS, and E6-SUMF1 GALNS and were euthanized 48 hours later. Liver, heart, femur bone, and bone marrow were obtained. To observe enzyme delivery to the bone in newborn Morquio A mice, three Morquio A mice (day 2) treated with 250 U/g of native SUMF1 GALNS and E6-SUMF1 GALNS were sacrificed 8 hours and 24 hours later and femur bone was obtained. Mice were anesthetized by intraperitoneal injection of ketamine/ml (Sigma-Aldrich) and were perfused with 50 ml of 0.9% saline, to remove heme, which interfered with the fluorometric GALNS enzymatic assay. Tissues were homogenized immediately in 1 ml of homogenization buffer consisting of 25 mmol/l Tris–HCl, pH 7.2, and 1 mmol/l phenylmethylsulfonyl fluoride. GALNS assays on dilutions of tissue extracts were incubated for 24 hours, and the activity was expressed as U/mg protein, as determined by micro-Lowry assay.
Treatment protocols. We designed three treatment protocols. In the group 1, we treated Morquio A tolerant mice with E6-GALNS enzyme. Then to evaluate impact of SUMF1 and a longer term treatment, we conducted ERT with E6-GALNS and E6-SUMF1 GALNS in group 2. We compared with native GALNS enzyme with or without SUMF1. To study effect of early treatment, we also treated mice from birth (group 3). Group 1: Three 3-month-old Morquio A tolerant mice in each group received 250 U/g of body weight (E6-GALNS) through tail vein for 12 weeks. Group 2: Three 3-month-old tolerant mice received 250 U/g of body weight (E6-GALNS, E6-SUMF1 GALNS, native GALNS, or native SUMF1 GALNS) through tail vein for 24 weekly infusions. Group 3: Three tolerant mice received weekly injections from birth until they reached 8 week of age (250 U/g of body weight: E6-GALNS). Mice treated from birth had an initial injection into the superficial temporal vein. The second to fourth injection was intraperitoneal. The remaining injections from fifth to eighth were made into the tail vein. One mouse died during the procedure.
Mice were weighed before enzyme injection; dose was calculated according to body weight and was diluted in PBS (pH 7.2) to give a concentration of 250 U of GALNS per gram of mouse weight. All mice were sacrificed 1 week after the last injection, and the organs were removed for histopathology analysis with light microscopy. Three untreated age-matched Morquio A mice for each experiment served as controls.
Pathology: response to ERT. We examined liver, spleen, brain, heart, femur, and bone marrow from 4.5-month-old (i) Morquio A tolerant mice treated for 12 weeks with 250 U/g of E6-GALNS (n = 3) (Figure 2), (ii) 7.5-month-old Morquio A tolerant mice treated for 24 weeks with 250 U/g of E6-GALNS or E6-SUMF1 GALNS (n = 3) (Figure 3a), native or native SUMF1 GALNS (n = 3) (Figure 3b), (iii) 2.5-month-old Morquio A tolerant mice treated for 8 weeks with 250 U/g of E6-GALNS (n = 3) (Figure 4), or age-matched Morquio A mice treated with PBS buffer (n = 6 for each group). Then, all tissues were immersion-fixed in 4% paraformaldehyde/2% glutaraldehyde in PBS, postfixed in osmium tetroxide, and embedded in Spurr's resin. For evaluation of lysosomal storage by light microscopy, toluidine blue-stained 0.5-µm thick sections were examined. Tissues from the treated and untreated mice were evaluated for reduction in storage without knowledge of their treatment.
KS assay. Tandem mass spectrometry method was used for the analysis of the disaccharides produced from KS.41 API-4000 mass spectrometer equipped with a turbo ionspray was used (Applied Biosystems, Foster City, CA). KS in mouse serum was digested to disaccharide by keratanase II (Seikagaku, Tokyo, Japan). Analysis of disaccharides was performed by liquid chromatography tandem mass spectrometry using multiple reactions monitoring in negative ion mode. Separation of liquid chromatography was performed on a Hypercarb (2.0 mm i.d. ×150 mm, 5 µm) with a gradient elution of acetonitrile-0.01 mol/l ammonium bicarbonate (pH 10). Flow rate of mobile phase was 0.2 ml/minute. This method was applied to the determination of KS only in serum of mouse samples treated from birth (group 3) (the complete specimens were not available for other groups).
Acknowledgments
This work was supported by grants from NIH GM34182 Vivendy Therapeutics, Ariana's Cure Fund for Morquio, the Austrian Research Society for Mucopolysaccharidoses and Related Diseases, Bennett Foundation, Care For Carly Foundation, Care for Sota Morquio Foundation, German MPS Society, International Morquio Organization (Carol Ann Foundation), Italian MPS Society, Jacob Randall Foundation, National MPS Society, Muconetwork, and Spanish MPS Society. The content of the article has not been influenced by the sponsors.
REFERENCES
- Murray GJ. Lectin-specific targeting of lysosomal enzymes to reticuloendothelial cells. Meth Enzymol. 1987;149:25–42. doi: 10.1016/0076-6879(87)49041-1. [DOI] [PubMed] [Google Scholar]
- Furbish FS, Steer CJ, Krett NL., and , Barranger JA. Uptake and distribution of placental glucocerebrosidase in rat hepatic cells and effects of sequential deglycosylation. Biochim Biophys Acta. 1981;673:425–434. doi: 10.1016/0304-4165(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Achord DT, Brot FE, Bell CE., and , Sly WS. Human β-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978;15:269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Stahl PD, Rodman JS, Miller MJ., and , Schlesinger PH. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci USA. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Lim-Melia ER., and , Kronn DF. Current enzyme replacement therapy for the treatment of lysosomal storage diseases. Pediatr Ann. 2009;38:448–455. doi: 10.3928/00904481-20090723-09. [DOI] [PubMed] [Google Scholar]
- Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- Xia H, Mao Q., and , Davidson BL. The HIV Tat protein transduction domain improves the biodistribution of β-glucuronidase expressed from recombinant viral vectors. Nat Biotechnol. 2001;19:640–644. doi: 10.1038/90242. [DOI] [PubMed] [Google Scholar]
- Green I, Christison R, Voyce CJ, Bundell KR., and , Lindsay MA. Protein transduction domains: are they delivering. Trends Pharmacol Sci. 2003;24:213–215. doi: 10.1016/S0165-6147(03)00076-2. [DOI] [PubMed] [Google Scholar]
- LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C., and , Sly WS. Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc Natl Acad Sci USA. 2004;101:3083–3088. doi: 10.1073/pnas.0308728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince WS, McCormick LM, Wendt DJ, Fitzpatrick PA, Schwartz KL, Aguilera AI, et al. Lipoprotein receptor binding, cellular uptake, and lysosomal delivery of fusions between the receptor-associated protein (RAP) and α-L-iduronidase or acid α-glucosidase. J Biol Chem. 2004;279:35037–35046. doi: 10.1074/jbc.M402630200. [DOI] [PubMed] [Google Scholar]
- Orii KO, Grubb JH, Vogler C, Levy B, Tan Y, Markova K, et al. Defining the pathway for Tat-mediated delivery of β-glucuronidase in cultured cells and MPS VII mice. Mol Ther. 2005;12:345–352. doi: 10.1016/j.ymthe.2005.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Fujisaki J, Tokunaga Y, Takahashi T, Shimojo F, Kimura S., and , Hata T. Osteotropic drug delivery system (ODDS) based on bisphosphonic prodrug. I.v. effects of osteotropic estradiol on bone mineral density and uterine weight in ovariectomized rats. J Drug Target. 1998;5:129–138. doi: 10.3109/10611869808995866. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Franzén A., and , Heinegård D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988;263:19430–19432. [PubMed] [Google Scholar]
- Kasugai S, Fujisawa R, Waki Y, Miyamoto K., and , Ohya K. Selective drug delivery system to bone: small peptide (Asp)6 conjugation. J Bone Miner Res. 2000;15:936–943. doi: 10.1359/jbmr.2000.15.5.936. [DOI] [PubMed] [Google Scholar]
- Nagata T, Bellows CG, Kasugai S, Butler WT., and , Sodek J.1991Biosynthesis of bone proteins [SPP-1 (secreted phosphoprotein-1, osteopontin), BSP (bone sialoprotein) and SPARC (osteonectin)] in association with mineralized-tissue formation by fetal-rat calvarial cells in culture Biochem J 274(Pt 2): 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido T, Sakura N, Higashi Y, Miya K, Nitta Y, Nomura M, et al. Novel drug delivery system to bone using acidic oligopeptide: pharmacokinetic characteristics and pharmacological potential. J Drug Target. 2001;9:111–121. doi: 10.3109/10611860108997922. [DOI] [PubMed] [Google Scholar]
- Yokogawa K, Miya K, Sekido T, Higashi Y, Nomura M, Fujisawa R, et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–1233. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Tomatsu S, Gutierrez MA, Miyamoto K, Trandafirescu GG, Lopez PL, et al. Enhancement of drug delivery to bone: characterization of human tissue-nonspecific alkaline phosphatase tagged with an acidic oligopeptide. Mol Genet Metab. 2006;88:244–255. doi: 10.1016/j.ymgme.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán JL, Narisawa S, Lemire I, Loisel TP, Boileau G, Leonard P, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly WS, Vogler C, Grubb JH, Zhou M, Jiang J, Zhou XY, et al. Active site mutant transgene confers tolerance to human β-glucuronidase without affecting the phenotype of MPS VII mice. Proc Natl Acad Sci USA. 2001;98:2205–2210. doi: 10.1073/pnas.051623698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Orii KO, Vogler C, Grubb JH, Snella EM, Gutierrez M, et al. Production of MPS VII mouse (Gus(tm(hE540A × mE536A)Sly)) doubly tolerant to human and mouse β-glucuronidase. Hum Mol Genet. 2003;12:961–973. doi: 10.1093/hmg/ddg119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Gutierrez M, Nishioka T, Yamada M, Yamada M, Tosaka Y, et al. Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum Mol Genet. 2005;14:3321–3335. doi: 10.1093/hmg/ddi364. [DOI] [PubMed] [Google Scholar]
- Cosma MP, Pepe S, Annunziata I, Newbold RF, Grompe M, Parenti G, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113:445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- Dierks T, Schmidt B, Borissenko LV, Peng J, Preusser A, Mariappan M, et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(α)-formylglycine generating enzyme. Cell. 2003;113:435–444. doi: 10.1016/s0092-8674(03)00347-7. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Gutierrez M, Grubb JH, Oikawa H, Dung VC, et al. Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase. Mol Genet Metab. 2007;91:69–78. doi: 10.1016/j.ymgme.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Takakusaki Y, Hisayasu S, Hirai Y., and , Shimada T. Coexpression of formylglycine-generating enzyme is essential for synthesis and secretion of functional arylsulfatase A in a mouse model of metachromatic leukodystrophy. Hum Gene Ther. 2005;16:929–936. doi: 10.1089/hum.2005.16.929. [DOI] [PubMed] [Google Scholar]
- Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ., and , Neufeld EF. Enzyme replacement in a canine model of Hurler syndrome. Proc Natl Acad Sci USA. 1994;91:12937–12941. doi: 10.1073/pnas.91.26.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou YA, Zeidner KM, Gordon RE., and , Desnick RJ. Fabry disease: preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunder U, Kaartinen V, Valtonen P, Väänänen E, Kosma VM, Heisterkamp N, et al. Enzyme replacement therapy in a mouse model of aspartylglycosaminuria. FASEB J. 2000;14:361–367. doi: 10.1096/fasebj.14.2.361. [DOI] [PubMed] [Google Scholar]
- Sands MS, Vogler CA, Ohlemiller KK, Roberts MS, Grubb JH, Levy B, et al. Biodistribution, kinetics, and efficacy of highly phosphorylated and non-phosphorylated β-glucuronidase in the murine model of mucopolysaccharidosis VII. J Biol Chem. 2001;276:43160–43165. doi: 10.1074/jbc.M107778200. [DOI] [PubMed] [Google Scholar]
- Yu WH, Zhao KW, Ryazantsev S, Rozengurt N., and , Neufeld EF. Short-term enzyme replacement in the murine model of Sanfilippo syndrome type B. Mol Genet Metab. 2000;71:573–580. doi: 10.1006/mgme.2000.3095. [DOI] [PubMed] [Google Scholar]
- Montaño AM, Oikawa H, Tomatsu S, Nishioka T, Vogler C, Gutierrez MA, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol Genet Metab. 2008;94:178–189. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Levy B, Galvin N, Tan Y., and , Sly WS. Chemically modified β-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Orii KO, Vogler C, Nakayama J, Levy B, Grubb JH, et al. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns-/-) produced by targeted disruption of the gene defective in Morquio A disease. Hum Mol Genet. 2003;12:3349–3358. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- van Diggelen OP, Zhao H, Kleijer WJ, Janse HC, Poorthuis BJ, van Pelt J, et al. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin Chim Acta. 1990;187:131–139. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- Peterson GL. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979;100:201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Oguma T, Tomatsu S., and , Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]