Abstract
In previous studies, we demonstrated that recombinant adeno-associated virus (rAAV)-mediated gene transfer of the doxycycline (Dox)-regulatable system allows for the regulation of erythropoietin (EPO) expression in the retina of nonhuman primates after intravenous or oral administration of Dox. In addition, it was shown that administrating different amounts of Dox resulted in a dose–response dynamic of transgene expression. Adeno-associated viral gene therapy has raised hope for the treatment of patients with Leber congenital amaurosis, caused by mutations in the retinal pigment epithelium (RPE)–specific gene RPE65. The preliminary results of three clinical trials suggest some improvement in visual function. However, further improvements might be necessary to optimize vision recovery and this means developing vectors able to generate transgene expression at physiological levels. The purpose of this study was to investigate the ability of the Dox-regulatable system to regulate retinal function in RPE65−/− Briard dogs. rAAV vectors expressing RPE65 under the control of either the TetOff and TetOn Dox-regulated promoters or the cytomegalovirus (CMV) constitutive promoter were generated and administered subretinally to seven RPE65-deficient dogs. We demonstrate that the induction and deinduction of retinal function, as assessed by electroretinography (ERG), can be achieved using a Dox-regulatable system, but do not lead to any recovery of vision.
Introduction
Several ligand-dependent transcription regulatory systems have been developed.1,2,3,4 Among these, the doxycycline (Dox)-regulatable system is by far the most frequently used and the most advanced in terms of its potential application in gene therapy trials. Dox-regulatable AAV vectors have been tested to regulate transgene expression in vivo, mainly in the muscle,5,6,7,8,9 the brain,10,11 and in the retina12,13 (for review, see ref. 14).
Our group has developed a regulatory system based on the TetOn technique (rtTA.M2) that controls the expression of erythropoietin (EPO) in the eye of nonhuman primates.15,16 Three different vectors, AAV2/5CMV.TetOn.epo, AAV2/4CMV.TetOn.epo, and AAV2/4pRPE65.TetOn.epo, were injected into a total of seven primates. An induction of EPO expression was performed initially by a 3-day Dox pulse given intravenously (10 mg/kg/day). After 2–4 days after Dox administration, EPO concentrations reached a peak and declined to background levels within 10–14 days of withdrawal of the inducer drug. Maximum EPO concentrations in the anterior chamber fluid were 1,000 mU/ml, 100 mU/ml, and 10 mU/ml for animals injected with AAV2/5CMV.TetOn.epo, AAV2/4CMV.TetOn.epo, and AAV2/4pRPE65.TetOn.epo vectors, respectively. This shows a range of two orders of magnitude of possible concentrations for any given therapeutic protein, depending on the serotype and the amount of vector injected, and the promoter driving the expression of the rtTA.M2 protein. Regulated transgene expression was observed for >5 years following administration of the AAVTetOn vector.14 No immune response was observed either to transgene products, rtTA and EPO, or to the AAV capsids.
Because the possibility of oral administration of Dox is paramount when one considers advancing to clinical trials, we also evaluated the influence of oral administration upon the capacity to control transgene expression.16 In this study, we not only demonstrated the equally efficient and tight control of induction and deinduction of EPO expression following oral administration of Dox, but we were also able to show that the system is capable of continuously inducing transgene expression for a period of 6 months, without eliciting any negative side effects.
Based on these extensive preclinical experiments in the nonhuman primate model, we consider this regulatory system to be a potential useful tool for the development of gene therapy strategies in the retina.
A landmark study for retinal gene therapy was provided in a dog model by the treatment of Leber congenital amaurosis caused by mutations in the RPE65 gene.17,18,19 RPE65 is involved in the conversion of all-trans-retinal to 11-cis-retinal and in the RPE65-deficient dog, the photoreceptor function is absent despite the photoreceptor cells being initially healthy. The subretinal administration of AAV2/2 or AAV2/4 vectors expressing RPE65 restores the photoreceptor function and consequently, improves visual mobility in dim light.17,19 Initial results from three separate clinical trials of gene therapy for RPE65 deficiency have recently been reported. Each study involved the treatment of three young adults by subretinal injection of an AAV2/2 vector expressing RPE65, using either the tissue-specific RPE65 promoter20 or a chicken β-actin ubiquitous promoter.21,22 Although all three studies detected improvement in retinal sensitivity, no improvement in photoreceptor function could be detected by electroretinography (ERG) in any of the patients. A recent study in younger subjects with much less advanced disease showed the greatest improvement in vision.23 Nonetheless, certain improvements might be required for an optimal recovery of vision, including the development of vectors to generate transgene expression at physiological levels.
In our previous study in RPE65−/− Briard dogs, we had observed structural alterations of the retina specific to the subretinal injection of AAV2/2RPE65.rpe65 or AAV2/4RPE65.rpe65 or vehicle alone.19 Similar retinal complications also occurred in patients who had vector injections into areas that included the fovea with retinotomy sites within 1–1.5 mm of the foveal region.21,22 These observations suggest that subretinal injection of the therapeutic vector in humans should be preferentially performed outside but adjacent to the fovea. Although RPE65 is present only in transduced cells, the 11-cis retinal substrate is able to diffuse through cell membranes,24 and should therefore be able to diffuse to the foveal area.25 Therefore, the level of RPE65 expressed in the targeted retinal pigment epithelium (RPE) cells within the vicinity of cone photoreceptors of the fovea could constitute a key factor in the vision recovery of humans. One approach to evaluate this issue is to study regulatable RPE65 vectors. In addition, regulated vectors would be needed in the event that individual patients might respond to different doses of the gene product. The use of regulatable vectors should allow for clinical dose–response studies by varying the amount of the regulating compound.
The purpose of this study was to investigate the ability of the Dox-regulatable system (TetOff and TetOn) to regulate retinal function and to evaluate the correlation between the amount of Dox administered and the recovered vision in RPE65-deficient dogs.
Results
Vector design and subretinal administration
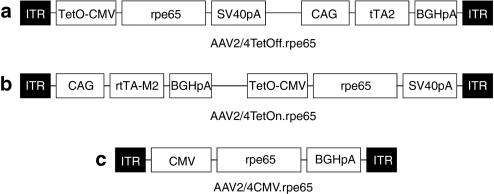
We generated the regulatable rAAV2/4TetOff.rpe65 and rAAV2/4TetOn.rpe65 vectors containing the human RPE65 cDNA under the Dox-inducible TetO-CMV promoter and the chimeric transactivator (tTA2 for the TetOff vector and rtTA.M2 for the TetOn vector) driven by the CAG promoter (CMV enhancer/chicken β-actin promoter). Given that the Dox-regulatable promoters, TetOff and TetOn, are based on a cytomegalovirus (CMV) promoter, we also generated a constitutive rAAV2/4CMV.rpe65 vector containing the human RPE65 cDNA under the control of the CMV promoter (Figure 1).
Figure 1.
Structure of rAAV vectors. (a) The AAV2/4TetOff.rpe65 vector encodes the human RPE65 cDNA under the control of the Dox-inducible TetO-CMV promoter, and the tTA chimeric transactivator (tTA2) under the control of the CAG promoter (CMV enhancer/chicken β-actin promoter). (b) The AAV2/4TetOn.rpe65 vector encodes the human RPE65 cDNA under the control of the Dox-inducible TetO-CMV promoter, and the rtTA chimeric transactivator (rtTA-M2) under the control of the CAG promoter. (c) The AAV2/4CMV.rpe65 vector encodes the human RPE65 cDNA under the control of the CMV promoter.
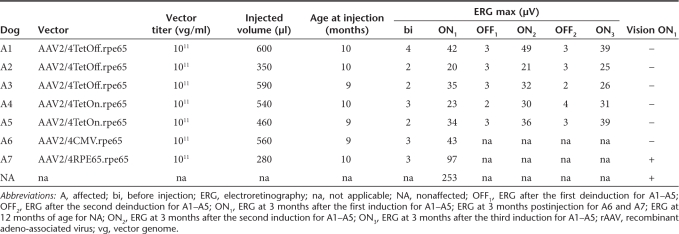
A total of seven dogs were treated: A1 through A3 were injected with AAV2/4TetOff.rpe65, A4 and A5 were injected with AAV2/4TetOn.rpe65, and A6 and A7 were treated with vectors constitutively expressing RPE65 under the control of the ubiquitous CMV promoter or the specific RPE65 promoter, AAV2/4CMV.rpe65 and AAV2/4RPE65.rpe65, respectively (Table 1). All dogs received recombinant adeno-associated virus (rAAV) unilaterally in the right retina at the age of 9–10 months. A1, A2, and A4 were treated first, followed by A3 and A5, which received injections 4 months later. Expression of the transgene RPE65 in the five dogs treated with the regulatable vectors was turned OFF and ON simultaneously in all animals.
Table 1.
List of treated dogs and rAAV doses injected
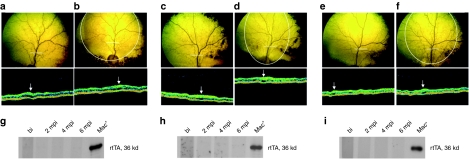
All dogs consistently received ophthalmic examination including funduscopy and optical coherence tomography to monitor retinal morphology and eventual chorioretinal alterations owing to rAAV2/4.TetOff and rAAV2/4.TetOn vectors. The fundus were normal, and the long-term optical coherence tomography monitoring documented preservation of the retinal thickness in both treated and untreated retinas, in all animals (Figure 2). Serum was also evaluated for the presence of antibodies against the chimeric transactivator protein. As assessed by western blot, none of the animals developed antibodies against tTA2 (TetOff) or rtTA.M2 (TetOn) (Figure 2).
Figure 2.
Assessment of retinal morphology and evaluation of humoral immune response against the tTA2 and rtTA.M2 proteins. (a,b) Fundus photography and OCT image of the AAV2/4TetOff.rpe65 treated eye of A2 before (a) and 6 months postinjection (b). (c,d) Fundus photography and OCT image of the AAV2/4TetOn.rpe65 treated eye of A4 before (c) and 6 months postinjection (d). (e,f) Fundus photography and OCT image of the AAV2/4CMV.rpe65 treated eye of A6 before (e) and 6 months postinjection (f). The white circle on the fundus photography indicates the zone of the bleb. The white line on the fundus photography indicates the OCT scanning path. The white arrow on the OCT image indicates the retinal vessel. (g–i) Evaluation of antibody generation against tTA2 and rtTA.M2 before and at 2, 4, and 6 months postinjection in A2 (g), A4 (h), and A6 (i). bi, before injection; mpi, months postinjection; OCT, optical coherence tomography.
Long-term regulation of retinal function in RPE65-deficient dogs following subretinal injection of the AAV2/4TetOff.rpe65 vector
Retinal function was tested using simultaneous bilateral full-field flash ERG prior to surgery and at different time points postinjection in ON and OFF states, in order to assess the kinetics of functional recovery. The same investigator (L.L.) recorded the ERGs in a standardized manner.
Cycles of Dox induction in the AAV2/4TetOff.rpe65 vector–treated dogs started at 7 months postinjection (mpi) for A1 and A2, and 3 mpi for A3.
Follow-up with ERG was 19 mpi for A1 and A2, and 15 months for A3. The ERG profiles and the kinetics of functional recovery are described in Figures 3a and 4.
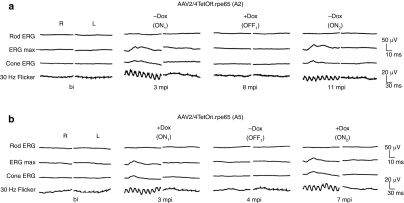
Figure 3.
Bilateral full-field electroretinographic (ERG) recordings of AAV2/4TetOff.rpe65 and AAV2/4TetOn.rpe65 treated dogs in the ON and OFF states. (a) Affected dog A2 treated with the AAV2/4TetOff.rpe65 vector before and at different time points postinjection, in the ON1, OFF1, and ON2 states. (b) Affected dog A5 treated with the AAV2/4TetOn.rpe65 vector before and at different time points postinjection, in the ON1, OFF1, and ON2 states. The top two recordings are low- and high-intensity scotopic responses, whereas the bottom two recordings show photopic responses (responses to light-adapted single flash and 30 Hz flicker stimuli, respectively). bi, before injection; mpi, months postinjection; R, right eye; L, left eye.
Figure 4.
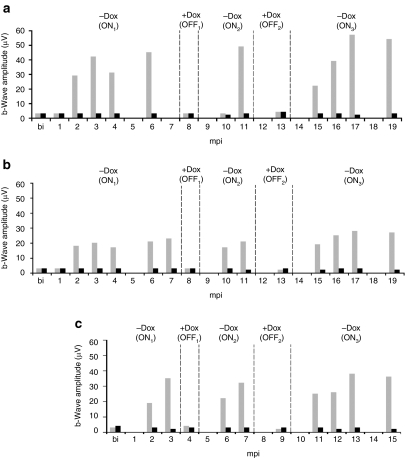
Long-term regulation of retinal function in RPE65-deficient dogs following subretinal injection of the AAV2/4TetOff.rpe65 vector. Affected dogs (a) A1, (b) A2, and (c) A3 before injection and at different time points postinjection, in the ON and OFF states. The b-wave amplitudes of the ERG max. (scotopic) of the treated and untreated eyes were measured and reported in the graphs. Gray, treated eye; black, untreated contralateral eye. The ON state was obtained in the absence of Dox, whereas the OFF state was obtained upon oral administration of Dox. bi, before injection; ERG, electroretinography; mpi, months postinjection.
All AAV2/4TetOff.rpe65-treated retinas showed tightly regulated retinal function and displayed rod and cone function recovery in the ON states (in the absence of Dox). The maximum recovered amplitude of the dark-adapted b-wave (ERG max) 3 months postinduction in the ON states was 40–50 µV for A1, 20–25 µV for A2, and 30–35 µV for A3 (Table 1 and Figure 4).
In the absence of Dox (ON1), the restoration of rod and cone photoreceptor function started at 2 mpi, reached a maximum at 3 mpi, and remained stable thereafter (Figure 4).
Upon Dox administration, the rod and cone function returned to the baseline level within 1 month (OFF1).
Two months following Dox removal (ON2, 10 mpi for A1 and A2, and 6 mpi for A3), A2 and A3 displayed a recovery of retinal function, but not A1. Three months following Dox removal (ON2, 11 mpi for A1 and A2, and 7 mpi for A3), all dogs displayed rod and cone function recovery to the same extent as that observed in the ON1 state (Figures 3a and 4).
Similar variations in retinal function were observed for the OFF2 and ON3 states. In the ON3 state, all three dogs started to recover rod and cone function 2 months after Dox removal and achieved the initial rod and cone function recovery 3 months after Dox removal (ON3, 16 mpi for A1 and A2, and 12 mpi for A3) and remained stable thereafter with some individual variations.
Long-term regulation of retinal function in RPE65-deficient dogs following subretinal injection of the AAV2/4TetOn.rpe65 vector
Cycles of Dox induction in the AAV2/4TetOn.rpe65 vector–treated dogs started 7 mpi for A4 and 3 mpi for A5.
Follow-up with ERG was 19 mpi for A4 and 15 months for A5. The ERG profiles and the kinetics of functional recovery are described in Figures 3b and 5.
Figure 5.
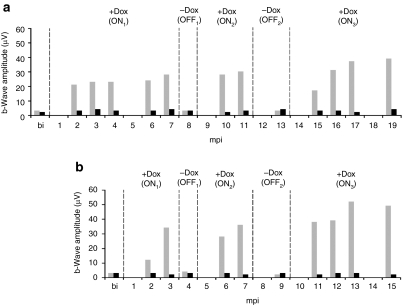
Long-term regulation of retinal function in RPE65-deficient dogs following subretinal injection of the AAV2/4TetOn.rpe65 vector. Affected dogs (a) A4 and (b) A5 before injection and at different time points postinjection, in the ON and OFF states. The b-wave amplitudes of the ERG max. (scotopic) of the treated and untreated eyes were measured and reported in the graphs. Gray, treated eye; black, untreated contralateral eye. The ON state was obtained upon oral administration of Dox, whereas the OFF state was obtained in the absence of Dox. bi, before injection; ERG, electroretinography; mpi, months postinjection.
All AAV2/4TetOn.rpe65-treated retinas showed a tightly regulated retinal function and displayed rod and cone function recovery in the ON states, in the presence of Dox. The ERG max levels 3 months postinduction in the ON states were 23–30 µV for A4, and 35–40 µV for A5 (Table 1 and Figure 5).
For these two dogs, Dox administration started the day after the subretinal injection of the vector. Restoration of rod and cone photoreceptor function started at 2 mpi and reached a maximum at 3 mpi, and remained stable thereafter in A4 (ON1, Figure 5a).
Upon Dox removal, the rod and cone function returned to the baseline level within 1 month (OFF1).
Upon the second Dox administration, dogs started to recover rod and cone function at 2 months (ON2, 10 mpi for A4 and 6 mpi for A5), and reached the level of retina function observed in the ON1 state, 3 months post-Dox administration. Similar variations in retinal function were observed for the OFF2 and ON3 states. In the ON3 state, the two dogs started to recover rod and cone function 2 months after Dox administration and reached the initial rod and cone function recovery 3 months after Dox administration (ON3, 16 mpi for A4, and 12 mpi for A5), with a slight increase in the ERG max. over time.
Both AAV2/4TetOff.rpe65 and AAV2/4TetOn.rpe65 dogs showed a tightly regulated retinal function with similar profiles. In all dogs, the maximum recovered b-wave amplitude in the first ON state (ON1) consistently occurred 3 months postinduction (in the absence of Dox for the TetOff and in the presence of Dox for the TetOn) and returned to the baseline level within 1 month following deinduction (upon administration of Dox for the TetOff, and withdrawal of Dox for the TetOn). Although the general trend of ERG profiles was similar in all Briards between the ON and the OFF states, individual variations were observed. For all animals, a slight increase in the ERG max. was observed in the ON3 state over time. In all AAV2/4TetOff.rpe65- and AAV2/4TetOn.rpe65-treated Briards, the maximum recovered amplitude of the dark-adapted b-wave was consistently between 30 and 60 µV.
Comparison between dogs treated with Dox-regulatable vectors and the control dogs that were not affected or injected with constitutive vectors
In parallel to the five dogs treated with the Dox-regulatable vectors, three additional dogs were used as controls. A6 and A7 were treated with the constitutive AAV2/4CMV.rpe65 and AAV2/4RPE65.rpe65 vectors,19 respectively. NA is a nonaffected dog (Table 1). Follow-up with ERG was 9 mpi for A6 and A7 and 9 months (9–18 months of age) for NA. The ERG profiles and the kinetics of the functional recovery are described in Figure 6.
Figure 6.
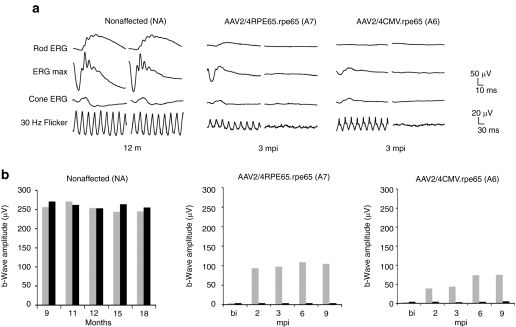
Retinal function in control dogs. (a) Bilateral full-field electroretinographic recordings of control dogs. Nonaffected dog NA at 12 months of age, affected dog A7 treated with the AAV2/4RPE65.rpe65 vector at 3 months postinjection, and affected dog A6 treated with the AAV2/4CMV.rpe65 vector at 3 months postinjection. (b) Kinetics of retinal function in control dogs. Nonaffected dog NA at different time points after birth, affected dog A7 treated in the right eye with the AAV2/4RPE65.rpe65 vector, and affected dog A6 treated in the right eye with the AAV2/4CMV.rpe65 vector at different time points postinjection. The b-wave amplitudes of the ERG max. (scotopic) of both eyes were measured at different times and reported in the graphs. Gray, right eye; black, left eye. bi, before injection; ERG, electroretinography; mpi, months postinjection.
The amplitude of the dark-adapted b-wave was ~250 µV in the nonaffected dog. In the AAV2/RPE65.rpe65-treated dog, the recovered amplitude was ~100 µV at 3 mpi and remained stable thereafter. This result was in accordance with our previous study in which all affected Briards treated between the age of 8 and 11 months (five dogs) with this same vector displayed a maximum b-wave amplitude between 86 and 131 µV.19 In spite of the larger volume of vector injected into the AAV2/4CMV.rpe65-treated dog, the amplitude was around 40 µV at 2 and 3 mpi and increased up to 74 µV between 3 and 6 months.
Assessment of vision recovery
We evaluated vision in all seven treated Briards using behavioral testing at different time points postinjection, as previously described.19 Three months postinduction for the regulatable vectors and, postinjection for the constitutive vectors, only the AAV2/4RPE65.rpe65-treated dog (A7) avoided obstacles (Table 1). The AAV2/4TetOff.rpe65- and the AAV2/4TetOn.rpe65-treated dogs never displayed recovery of vision at any time. As an example, at 11 mpi, at the peak of retinal function in the ON2 for A1 (Figure 4a, ERG max. = 49 µV), the time to negotiate the obstacle course did not change between the two configurations, either when the untreated eye was covered (20 seconds) or when the treated eye was covered (17 seconds) (Supplementary Video S1). Although retinal function slightly increased in the ON3 state (ERG max. = 57 µV) for A1, no improvement of vision was observed up to 8 months after Dox removal in the ON3 state (data not shown). Interestingly, the AAV2/4CMV.rpe65-treated dog (A6) that did not show any detectable vision improvement 3 months postvector administration (ERG max. = 43 µV) started to show signs of recovered vision from 6 months onward (ERG max. = 74 µV) (Supplementary Video S2). The time to negotiate the obstacle course was shorter when the untreated eye was covered (7 seconds), compared to the configuration in which the treated eye was covered (41 seconds).
Considered together, these results indicate that an ERG max. below 60 µV does not result in the recovery of an effective vision in RPE65-deficient dogs, as assessed with our ERG and behavioral test setup.
Discussion
In this study, we demonstrated that a single subretinal injection into RPE65-deficient dogs of an AAV2/4TetOff.rpe65 or AAV2/4TetOn.rpe65 vector containing the RPE65 transgene under a Dox-dependent expression system results in an effective regulation of retinal function for at least 19 months.
All treated dogs displayed tightly regulated retinal rod and cone function with similar induction and deinduction kinetics. The maximum amplitude of the dark-adapted b-wave consistently occurred 3 months after induction (upon withdrawal of Dox for the TetOff and upon administration of Dox for the TetOn), returning to the baseline level within 1 month of deinduction (upon administration of Dox for the TetOff and withdrawal of Dox for the TetOn).
In all AAV2/4TetOff.rpe65- and AAV2/4TetOn.rpe65-treated Briards, the maximum recovered amplitude of the dark-adapted b-wave was consistently below 60 µV. None of these treated dogs displayed vision recovery at any time.
Our group has previously shown long-term Dox-regulated transgene expression (reporter gene EPO) in the retina of nonhuman primates following subretinal injection of rAAV vectors.15,16 The results in this new study demonstrate for the first time that it is possible to use a Dox-regulatable system to regulate rod and cone function in RPE65-deficient dogs, by controlling the expression of the RPE65 therapeutic transgene.
In our previous study in nonhuman primates, we validated the TetOn system, in which the ligand (Dox) activates transcription. In the case of RPE65 gene replacement therapy, gene expression is to be maintained to a switch-on state over a long period of time, which makes the TetOff system more attractive. In this case, the activation of transgene expression depends mainly on the clearance kinetics of Dox.
Surprisingly, the induction and deinduction kinetics of rod and cone function were similar between both TetOff and TetOn systems. Similarly to our previous study in nonhuman primates, no immune response against the chimeric transactivator protein could be detected.
Unfortunately, in the ON state, all AAV2/4TetOff.rpe65- and AAV2/4TetOn.rpe65-treated dogs displayed only low levels of retinal function recovery. First, no ERG improvement was detected at 1 month postvector administration. This is in contrast to our previous study in RPE65-deficient dogs treated with the AAV2/4RPE65.rpe65 constitutive vector where restoration of rod and cone photoreceptor function started from 15 days postinjection.19 Second, using the regulatable vectors, the maximum recovered amplitude of the dark-adapted b-wave at 3 months postvector administration ranged from 20 to 42 µV. In our previous study of five AAV2/4RPE65.rpe65-treated dogs, and in the control dog A7, the maximum recovered amplitude of the dark-adapted b-wave 3 months postvector administration ranged from 94 to 110 µV.19 Moreover, given that the same administration procedure, the same vector titer, and the same ERG setup was used for all animals (in the previous study Le Meur et al.19 and in this new study), the lower level of retinal function restoration observed in the dogs treated with the regulatable vectors (AAV2/4TetOff.rpe65 and AAV2/4TetOn.rpe65) is likely to be both real and significant when compared to the dogs treated with the constitutive AAV2/4RPE65.rpe65 vector.
These small improvements in ERG were confirmed by the behavioral testing, as none of the regulatable AAV vectors made it possible for treated dogs to recover their vision. In contrast, all of the dogs treated with the AAV2/4RPE65.rpe65 vector recovered their vision (five dogs in our previous study Le Meur et al.19 and A7 in this study). Using our ERG and behavioral test setup, it appeared that a dark-adapted b-wave amplitude below 60 µV did not restore vision.
This interesting observation is in contrast to what is observed in treated human patients who displayed improvement in visual mobility but ERG recordings below detection level.20,21,23
Our previous results using regulatable AAVTetOn vectors for gene transfer in the retina of nonhuman primates14,15,16 showed that the maximum transgene expression was detected as early as 48 hours after oral Dox administration and that the regulation of transgene expression persisted for up to 5 years postsubretinal injection with no detection of deleterious side effects. This indicates that the delay in the onset and the lower levels of retinal function recovery in RPE65-deficient dogs using the TetOff or the TetOn vectors is not due to a delay in transgene expression, to the pharmacokinetics of Dox after oral administration, or to the toxicity of the Tet system.
Our results lean more toward the hypothesis suggesting that the CMV-based TetOff and TetOn promoters are not as strong as the RPE65 promoter for transgene expression in the RPE cells in vivo, and therefore lead to a later onset and a lower recovery of retinal function in treated RPE65-deficient dogs. Interestingly, although only one control animal was treated with the constitutive vector AAV2/4CMV.rpe65, it displayed an ERG improvement similar to that obtained in the AAV2/4TetOff.rpe65- and AAV2/4TetOn.rpe65-treated animals. This observation lends support to our hypothesis suggesting that the strength of the CMV and CMV-based promoters is lower than the strength of the RPE65 promoter in vivo. Additional experiments would, however, be necessary in dogs to confirm such a hypothesis.
Unfortunately, the low level of retinal function recovery using the Dox-regulated system prevented any dose–response study.
Different regulatory elements controlling expression of the RPE65 cDNA were used in the three clinical trials. Bainbridge et al. employed the RPE65 promoter used in our previous study19,20 and in A7. The other two teams employed the chicken β-actin promoter.21,22,23 Moreover, Maguire et al. inserted a modified Kozac translational initiation sequence before the RPE65 cDNA.21,26 In this condition, it is difficult to know the relative strength of expression for each of the three vectors. This could only be addressed by running parallel experiments in RPE65-deficient dogs with a similar vector containing these three different promoters.
In conclusion, we demonstrate for the first time that it is possible to use the TetOff and TetOn system to regulate retinal function in RPE65-deficient dogs. Such a regulatable system will therefore be valuable to assess the level of RPE65 expression necessary to obtain an optimal rescue of retinal function and vision. Unfortunately, the CMV-based TetOff and TetOn promoters seem much less efficient than the specific RPE65 promoter and lead to a lower level of retinal function recovery, which prevents any dose–response study or a more accurate evaluation of the promoter strength necessary for an optimized rescue of vision. Future efforts should focus on designing a strong regulatable promoter. Our results suggest that the nature of the promoter may be critical for the recovery of retinal function and vision in RPE65-deficient dogs and the need to develop AAV vectors that generate optimal RPE65 expression for the treatment of patients.
Materials and Methods
Recombinant AAV2/4TetOn.rpe65, AAV2/4TetOff.rpe65, and AAV2/4CMV.rpe65 vectors
rAAV2/4TetOff.rpe65 vector: Generation of expression cassettes encoding d2GFP (TetO.CMVmin-d2GFP-SV40pA) and the transactivator tTA2 under the control of the CAG promoter (CAG-tTA2-BGHpA) was previously described.13 The expression cassette TetO.CMVmin-hrpe65-SV40pA was produced by replacing the 951 base-pair (bp) d2GFP cDNA fragment with the 1,630-bp human RPE65 transgene fragment (kindly provided by Robin Ali, London, UK).
rAAV2/4TetOn.rpe65 vector: Generation of expression cassettes encoding EPO (TetO.CMVmin-mEPO-WPRE-SV40pA) and the transactivator rtTA.M2 under the control of the CAG promoter (CAG-rtTA.M2-BGHpA) was previously described.15 The expression cassette TetO.CMVmin-hrpe65-SV40pA was produced by replacing the 1,237-bp mEPO-WPRE fragment with the 1,630-bp human RPE65 transgene fragment.
rAAV2/4CMV.rpe65 vector: Generation of expression cassette encoding human RPE65 cDNA under the control of the human RPE65-specific promoter (RPE65-hrpe65-BGHpA) was previously described.19 The expression cassette CMV-hrpe65-BGHpA was produced by replacing the 806-bp human RPE65-specific promoter fragment with the 586-bp CMV promoter fragment.
The expression cassettes, flanked by two AAV2 ITRs, were encapsidated into AAV4 shells. Vectors were produced as described previously by Rabinowitz et al. in the Vector Core at the University Hospital of Nantes (http://www.vectors.nantes.inserm.fr). The rAAV titers were determined by dot blot and expressed as vector genomes (vg) per ml.
Subretinal injection of rAAV vectors. A colony of Briard dogs carrying the RPE65 null mutation was developed at the Boisbonne Center (Veterinary School of Nantes, Nantes, France). All animals were cared for in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. The subretinal injections were performed on 9- to 10-month-old dogs using a transvitreal approach and following vitrectomy. Procedures were conducted on dogs under isoflurane gas anesthesia as previously described.27 The protocol was approved by the Institutional Animal Care and Use Committee of the University of Nantes. All subretinal injections were performed unilaterally in the right eye. Volumes injected were between 280 and 600 µl and vector titers were 1011 vg/ml.
Fundus photography. Pupils of the animals were dilated 20 minutes before anesthesia using tropicamide (Ciba Vision Faure; Novartis, Annonay, France) and phenylephrine hydrochloride (10% Neo-Synephrine; Novartis). Dogs were anesthetized with a combination of medetomidine (Domitor; Pfizer, Paris, France) with ketamine (Imalgène; Rhone Merieux, Lyon, France).
Fundi were imaged using a Canon UVI retinal camera connected to a digital imaging system (Lhedioph Win Software; Lheritier, Saint-Ouen-l'Aumône, France).
Optical coherence tomography. Retinal morphology was assessed by optical coherence tomography (Stratus 3000; Zeiss, Jena, Germany). Dilatation of the pupils and intravenous anesthesia of the animals were performed as described above. Examination was performed at different time points, by doing a 3 mm horizontal line scan in the area located above the optical nerve head, within the treated area of the retina.
Humoral immune response against tTA2 and rtTA.M2. Detection of anti-tTA2 and anti-rtTA.M2 antibodies was performed using western blot analysis as recently described.15 Briefly, recombinant transactivator protein (200 ng) (Proteogenix, Oberhausbergen, France) was subjected to SDS-PAGE and transferred to a Hybond ECL nitrocellulose membrane (Amersham, Saclay, France) for subsequent immunoblotting. Serum (1/100) from our experimental dogs was tested for the presence of antibodies. A serum (1/100) from a macaque that have previously been shown to contain antibodies against rtTA.M2 was used as positive control (Mac+).8,9 Incubation with sera was performed overnight at 4 °C. Peroxidase-conjugated rabbit anti-dog IgG (Jackson ImmunoResearch, Baltimore, PA) was used as secondary antibody for the dog samples (1/10,000, 45 minutes at room temperature).
Peroxidase-conjugated goat anti-rhesus monkey IgG (Southern Biotechnology Associates, Birmingham, AL) was used as secondary antibody for the positive control (1/2,000, 45 minutes at room temperature).
Bilateral full-field ERG. Dogs were dark-adapted for 20 minutes, and ERGs were performed under general anesthesia. Rod and cone function was tested using simultaneous bilateral flash photopic and scotopic ERG. ERGs were recorded in a standardized fashion, according to the International Society for Clinical Electrophysiology of Vision (ISCEV),28 using a computer-based system (Neuropack µ MEB-9102K; Nihon-Kohden, Tokyo, Japan) and contact lens electrodes (ERG-jet; Microcomponents, Grenchen, Switzerland).
In fully dark-adapted dogs, attenuated white flash (0.028 cd·s/m2) was used to elicit a rod ERG b-wave. Four flashes, presented at a rate of 0.5 Hz, were used to average the rod system response. To elicit a maximal mixed rod and cone response, we used a single white light flash (2.8 cd·s/m2). The maximum mixed rod and cone responses were averaged by using three flashes, presented at a rate of 0.1 Hz. Following a 10-min light adaptation, cone function was tested with 1 Hz white light flashes and the cone flicker was tested with 30 Hz white light flashes, both in addition to a continuous rod desensitizing white background light. Ten to twenty responses were averaged for light-adapted recordings.
The b-wave amplitude of the ERG max of the treated and the untreated eyes was measured from the a-wave peak to the b-wave peak at different times postinjection and reported in the graphs. The uninjected left eye served as an intraindividual control. The method of measurement was in accordance with the recommendations in the ISCEV standard for clinical ERG.
Dox administration. Oral administration of Dox consisted of the daily uptake of 10 mg/kg Dox (Doxy 100 Gé Elerte, Aubervilliers, France). In A1, A2, and A3 injected with the AAV2/4TetOff.rpe65 vector, the ON state was obtained in the absence of Dox, whereas the OFF state was obtained upon oral administration of Dox. In A4 and A5 treated with the AAV2/4TetOn.rpe65 vector, the ON state was obtained upon administration of Dox, whereas the OFF state was obtained upon withdrawal of Dox.
Behavioral studies. Ambulation of treated and untreated RPE65−/− dogs through an obstacle course in dim light was recorded using a camcorder. An opaque lens, specifically designed for dogs, was used to alternatively cover the treated or the untreated eye. The time to negotiate the obstacle course was compared between the course for which the untreated eye was covered and the course in which the treated eye was covered.
SUPPLEMENTARY MATERIALVideo S1. Visual function of A1 was evaluated 11 months postinjection by behavioral testing. The time to negotiate the obstacle course does not change between the two configurations, either when the untreated eye is covered with an opaque contact lens (20 seconds) or when the treated eye is covered (17 seconds).Video S2. Visual function of A6 was evaluated 6 months postinjection by behavioral testing. The time to negotiate the obstacle course is shorter when the untreated eye is covered (7 seconds), compared to the configuration in which the treated eye is covered (41 seconds).
Supplementary Material
Visual function of A1 was evaluated 11 months postinjection by behavioral testing. The time to negotiate the obstacle course does not change between the two configurations, either when the untreated eye is covered with an opaque contact lens (20 seconds) or when the treated eye is covered (17 seconds).
Visual function of A6 was evaluated 6 months postinjection by behavioral testing. The time to negotiate the obstacle course is shorter when the untreated eye is covered (7 seconds), compared to the configuration in which the treated eye is covered (41 seconds).
Acknowledgments
We thank Knut Stieger (Department of Ophthalmology, Justus-Liebig-University of Giessen, Giessen, Germany) for critical reading. We also thank the Vector Core (http://www.vectors.nantes.inserm.fr) at the University Hospital of Nantes. This work was supported by the Association Française contre les Myopathies (AFM), the INSERM, and the Fondation pour la Thérapie Génique en Pays de la Loire.
REFERENCES
- Gossen M., and , Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera VM, Clackson T, Natesan S, Pollock R, Amara JF, Keenan T, et al. A humanized system for pharmacologic control of gene expression. Nat Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP., and , O'Malley BW. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Palli SR, Kapitskaya MZ, Kumar MB., and , Cress DE. Improved ecdysone receptor-based inducible gene regulation system. Eur J Biochem. 2003;270:1308–1315. doi: 10.1046/j.1432-1033.2003.03501.x. [DOI] [PubMed] [Google Scholar]
- Rendahl KG, Quiroz D, Ladner M, Coyne M, Seltzer J, Manning WC, et al. Tightly regulated long-term erythropoietin expression in vivo using tet-inducible recombinant adeno-associated viral vectors. Hum Gene Ther. 2002;13:335–342. doi: 10.1089/10430340252769842. [DOI] [PubMed] [Google Scholar]
- Agha-Mohammadi S, O'Malley M, Etemad A, Wang Z, Xiao X., and , Lotze MT. Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J Gene Med. 2004;6:817–828. doi: 10.1002/jgm.566. [DOI] [PubMed] [Google Scholar]
- Bohl D, Salvetti A, Moullier P., and , Heard JM. Control of erythropoietin delivery by doxycycline in mice after intramuscular injection of adeno-associated vector. Blood. 1998;92:1512–1517. [PubMed] [Google Scholar]
- Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Joussemet B, Bujard H, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Jiang L, Rampalli S, George D, Press C, Bremer EG, O'Gorman MR, et al. Tight regulation from a single tet-off rAAV vector as demonstrated by flow cytometry and quantitative, real-time PCR. Gene Ther. 2004;11:1057–1067. doi: 10.1038/sj.gt.3302245. [DOI] [PubMed] [Google Scholar]
- Chen Q, Xiong X, Lee TH, Liu Y, Sun QA, Wetsel W, et al. Adeno-associated virus-mediated ILK gene silencing in the rat NAc core. J Neurosci Methods. 2008;173:208–214. doi: 10.1016/j.jneumeth.2008.06.004. [DOI] [PubMed] [Google Scholar]
- McGee Sanftner LH, Rendahl KG, Quiroz D, Coyne M, Ladner M, Manning WC, et al. Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina. Mol Ther. 2001;3 5 Pt 1:688–696. doi: 10.1006/mthe.2001.0308. [DOI] [PubMed] [Google Scholar]
- Folliot S, Briot D, Conrath H, Provost N, Cherel Y, Moullier P, et al. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. J Gene Med. 2003;5:493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- Stieger K, Belbellaa B, Le Guiner C, Moullier P., and , Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Stieger K, Mendes-Madeira A, Meur GL, Weber M, Deschamps JY, Nivard D, et al. Oral administration of doxycycline allows tight control of transgene expression: a key step towards gene therapy of retinal diseases. Gene Ther. 2007;14:1668–1673. doi: 10.1038/sj.gt.3303034. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Narfström K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Phase I trial of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results. Hum Gene Ther. 2008;7:7. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE. An analysis of retinal gene therapy clinical trials. Curr Opin Mol Ther. 2009;11:540–546. [PMC free article] [PubMed] [Google Scholar]
- Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL, et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Narfström K, Ekesten B, Rosolen SG, Spiess BM, Percicot CL., and , Ofri R, Committee for a Harmonized ERG Protocol, European College of Veterinary Ophthalmology Guidelines for clinical electroretinography in the dog. Doc Ophthalmol. 2002;105:83–92. doi: 10.1023/a:1020524305726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual function of A1 was evaluated 11 months postinjection by behavioral testing. The time to negotiate the obstacle course does not change between the two configurations, either when the untreated eye is covered with an opaque contact lens (20 seconds) or when the treated eye is covered (17 seconds).
Visual function of A6 was evaluated 6 months postinjection by behavioral testing. The time to negotiate the obstacle course is shorter when the untreated eye is covered (7 seconds), compared to the configuration in which the treated eye is covered (41 seconds).