Abstract
OBJECTIVE
To compare the microcirculatory velocity distribution in type 2 diabetic patients and nondiabetic control subjects at baseline and after local heating.
RESEARCH DESIGN AND METHODS
The skin blood flow response to local heating (44°C for 20 min) was assessed in 28 diabetic patients and 29 control subjects using a new velocity-resolved quantitative laser Doppler flowmetry technique (qLDF). The qLDF estimates erythrocyte (RBC) perfusion (velocity × concentration), in a physiologically relevant unit (grams RBC per 100 g tissue × millimeters per second) in a fixed output volume, separated into three velocity regions: v <1 mm/s, v 1–10 mm/s, and v >10 mm/s.
RESULTS
The increased blood flow occurs in vessels with a velocity >1 mm/s. A significantly lower response in qLDF total perfusion was found in diabetic patients than in control subjects after heat provocation because of less high-velocity blood flow (v >10 mm/s). The RBC concentration in diabetic patients increased sevenfold for v between 1 and 10 mm/s, and 15-fold for v >10 mm/s, whereas no significant increase was found for v <1 mm/s. The mean velocity increased from 0.94 to 7.3 mm/s in diabetic patients and from 0.83 to 9.7 mm/s in control subjects.
CONCLUSIONS
The perfusion increase occurs in larger shunting vessels and not as an increase in capillary flow. Baseline diabetic patient data indicated a redistribution of flow to higher velocity regions, associated with longer duration of diabetes. A lower perfusion was associated with a higher BMI and a lower toe-to-brachial systolic blood pressure ratio.
Laser Doppler flowmetry (LDF) has frequently been used to measure blood flow abnormalities in diabetic patients (1–5). A reduced vascular response to local heating, due to loss of the active neurogenic vasodilation, has been observed in type 1 and type 2 diabetic patients using LDF (1,6) and caused by loss in endothelium nitric oxide (NO) synthesis in type 2 diabetic patients (7). Another microvascular abnormality has also been suggested—an increased flow through the arteriovenous shunts resulting in a reduced capillary flow (8–12). This is most evident during provocations such as postocclusive reactive hyperemia and in patients with severe neuropathy, but it can also be observed in diabetic patients at rest who have no complications (11).
Although differences in blood flow between diabetic patients and control subjects have been found using LDF during heating and other provocation protocols, results during baseline conditions have been more contradictory (6). Studies have reported a lower blood flow in diabetic patients compared with control subjects (3), no difference (1,4), and even tendencies toward a higher blood flow in diabetic patients (11). The conflicting results are partly explained by the different measurement positions used, for which differences have been seen especially between glabrous (e.g., palms and soles) and nonglabrous (hairy) skin (6). Although authors usually specify the measurement position in detail, unfortunately it is common that the specification of the LDF instrumentation used is omitted, in terms of operating wavelength and probe type/source-detector separation. The measurement depth is highly dependent on these two properties, especially the latter (13), and therefore some of the discrepancies in the reported results may be explained by the differences in instrumentation.
Local warming of skin causes a biphasic increase in skin blood flow as measured by LDF. The initial increase in blood flow that sometimes temporarily declines is mediated by a C-fiber axon reflex, whereas the second prolonged phase requires NO (14,15). These and other studies use LDF for elucidating NO synthase mechanisms (5,7,16,17) for quantification of abnormalities in the endothelium-dependent NO vasodilation (18) and other microvascular research issues (19,20).
Studying the microcirculation in a clinical setting using LDF may be difficult because the method provides only relative measures on skin blood flow (14). Large local spatial and temporal variations in blood flow have also been reported (21). Furthermore, measures are given for an unknown measurement volume that varies not only with instrumentation but also with the optical properties at the measurement site and with variations in the blood flow itself (13). This further obstructs the physiologic interpretation of the results.
We have developed a new LDF signal analysis based on simulations of light transport in an adaptive mathematical model of tissue. As the perfusion estimates achieved with this method are presented in physiologically relevant units (grams erythrocytes [RBC]/100 g tissue × mm/s) in a given output volume (3 mm3), the method is called quantitative LDF (qLDF). Besides the benefits of a constant output volume and physiologically relevant units, the method also provides a means of differentiating the blood flow into various flow velocity regions (22). This velocity differentiation can potentially be used to associate differences in the velocity distribution to specific vessel types because large vessels normally have a much higher blood flow velocity than smaller vessels.
The aim of the present study was to compare the velocity distribution of the microcirculatory blood flow between patients with diabetes type 2 and normal control subjects during baseline and local warming. To our knowledge, this has previously not been possible, and the differences in the velocity distribution can be used to draw conclusions about the type of vessels that are mainly involved in the pathologic disturbances of the microcirculation in diabetes type 2.
RESEARCH DESIGN AND METHODS
The patients included in this study are part of a larger study called Cardiovascular Risk Factors in Patients with Diabetes—a Prospective Study in Primary Care (CARDIPP) that was launched in 2005 with the aim of identifying markers for cardiovascular disease to facilitate earlier and individually adjusted intervention in middle aged patients with type 2 diabetes. The nondiabetic control subjects were part of a parallel control study called CAREFUL. Within the CARDIPP and CAREFUL studies, parameters such as height, weight, and blood pressure were measured. The investigation included medical history, also covering data on diabetes duration and ongoing medication. Blood specimens were drawn in the morning after a 10-h overnight fast. Aortic pulse wave velocity was measured with an applanation tonometry (Sphygmocor) over the carotid and femoral arteries as an indicator of arterial stiffness. Intima-media thickness of the carotid arteries was evaluated using a B-mode ultrasound, as a measure of subclinical atherosclerosis. The toe-brachial index (TBI) (toe systolic blood pressure–to–brachial systolic blood pressure ratio) was used as a measure of peripheral arterial disease. (For more details about these studies, see refs. 23 and 24.) The protocol was approved by the local ethics committee (D. no. M26-05).
Based on accessibility, a small number of the subjects included in CARDIPP and CAREFUL were asked to join the extended study in which microcirculatory parameters in the foot were also measured using a modified LDF instrument (Perimed, Järfälla, Sweden). Recordings were made using a custom-made probe with 1 light-emitting fiber placed in the center, 1 light-collecting fiber placed 0.25 mm (center-to-center distance) from the light-emitting fiber, and another 11 light-collecting fibers placed in a circle 1.2 ± 0.1 mm from the light-emitting fiber. All fibers were made of silica with a 0.125 mm diameter and NA 0.37, and the light source was a 780 nm laser diode. Backscattered light collected at the two source-detector distances was separately detected and sampled at 50 kHz for later analysis. The measurement probe was placed in a thermostatic probe holder (PF 450; Perimed) that was able to adjust the skin temperature and assured a minimum pressure contact between the probe tip and the examined skin. All measurements were performed on the dorsum of one foot 3–6 cm proximal of the toes between the metatarsals, avoiding visible vessels (1). The recordings lasted for 25 min. In the beginning of the recording, the probe holder was heated to 32°C, defined as the baseline, and after approximately 5 min, the temperature was increased to 44°C, which lasted throughout the rest of the measurement. All measurements were performed in a room kept at a temperature of 23–25°C and the subjects were placed in the supine position. The subjects were acclimatized in the room for at least 15 min before the measurement. The measurements were done at approximately the same time of day for the two groups, at mean 11:58 a.m. (SD 1.51 h) for control subjects and at 11:13 a.m. (1:32 h) for diabetic patients. The extended protocol was approved by the local ethics committee (D. no. M26-05 T41-08).
From the recorded signals, Doppler power spectra were calculated, calibrated, and averaged in 5-s intervals for the two different source-detector separations (22,25). Conventional LDF perfusion measures (26) were calculated for both separations from the calculated and averaged Doppler power spectra. These measures are given in arbitrary units with unknown and nonconstant sampling volumes. Besides the conventional perfusion measures, the quantitative LDF method was used to estimate the perfusion in a physiologically relevant unit (grams RBC per 100 g tissue × millimeters per second). The value is relevant for an output volume of a 3 mm3 half-sphere, that is, within a 1.13 mm radius from the emitting fiber. The perfusion measure is divided into three velocity regions: v <1 mm/s, 1 < v < 10 mm/s, and v >10 mm/s. Furthermore, the total perfusion is also presented (sum of all three regions). The method, presented in Fredriksson et al. (22), is based on a model adaption of the Doppler power spectra to the measured spectra at the two source-detector separations simultaneously.
Blood perfusion was measured in 28 diabetic patients and in 29 control subjects. Three diabetic patients and 3 control subjects were excluded because of varicose veins, 1 diabetic patient was excluded because of a skin surface temperature above 32°C, 1 control patient was excluded because of chronic obstructive pulmonary disease, and 1 control patient was excluded because of suspected poor contact between the probe and the skin. In two of the measurements, one for a diabetic patient and one for a control subject, the method was not able to find a model that matched the measured spectra (χ2 >0.5) and they were therefore excluded. The remaining diabetic patients and control subjects are described in Table 1. Among the 23 included diabetic patients, 7 were treated with diet and exercise only, 13 were also treated with oral hypoglycemic agents but no insulin, and 3 were treated with insulin. Eleven diabetic patients were taking metformin, 2 were taking sulfonylureas, 2 were taking thiazolidinediones, and 1 was treated with a dipeptidyl peptidase IV inhibitor. Three individuals did not take any antihypertensive medication, and the most frequently used antihypertensive drugs were ACE inhibitors or angiotensin receptor blockers (14 subjects), followed by 8 subjects taking β-blockers, 5 taking diuretics, and 4 taking calcium channel blockers. Twenty subjects were treated with statins. Four subjects were current smokers, and 12 were former smokers. Microalbuminuria, defined as an albumin-to-creatinine ratio >30 μg/mg, was present in 1 subject. History of cardiovascular disease was prevalent in 3 subjects. The baseline measurement was excluded for 3 diabetic patients because of a too short acclimatization period (<15 min).
TABLE 1.
Characteristics of diabetes patients and nondiabetic control subjects
| Control subjects | Diabetic patients | |
|---|---|---|
| n | 23 | 23 |
| Sex (M/F) | 8/15 | 14/9 |
| Age (years) | 62 ± 6 | 60 ± 3 |
| Diabetes duration (years) | NA | 7 ± 6 |
| Height | ||
| Men (cm) | 178 ± 7 | 176 ± 7 |
| Women (cm) | 166 ± 7 | 164 ± 5 |
| Weight | ||
| Men (kg) | 84 ± 16 | 91 ± 8 |
| Women (kg) | 67 ± 10 | 78 ± 9* |
| BMI (kg/m2) | 25 ± 3 | 29 ± 3‡ |
| Glucose (mmol/l) | 6.0 ± 0.8§ | 8.1 ± 2.1‡§ |
| Triglyceride (mmol/l) | 1.3 ± 0.8 | 1.9 ± 1.4*¶ |
| Cholesterol (mmol/l) | 5.8 ± 1.0 | 4.2 ± 0.6‡¶ |
| LDL cholesterol (mmol/l) | 3.5 ± 0.8§ | 2.4 ± 0.6‡# |
| HDL cholesterol (mmol/l) | 1.7 ± 0.6 | 1.1 ± 0.3‡¶ |
| A1C (%) | NA | 6.0 ± 0.9§ |
| Systolic blood pressure (mmHg) | 120 ± 12 | 122 ± 10 |
| Diastolic blood pressure (mmHg) | 74 ± 8 | 72 ± 7 |
| Pulse wave velocity (m/s) | 8.9 ± 1.7 | 11.2 ± 2.0‡¶ |
| Left ventricular mass | ||
| Men (g) | 106 ± 11‖ | 127 ± 34 |
| Women (g) | 106 ± 29 | 93 ± 36§ |
| Carotid intima-media thickness (mm) | 0.67 ± 0.12 | 0.75 ± 0.13* |
| TBI | 0.89 ± 0.16¶ | 0.93 ± 0.12 |
Data are means ± SD. Statistical comparisons of diabetic patients vs. nondiabetic control subjects:
*P < 0.05,
‡P < 0.001.
§One value missing;
‖two values missing;
¶three values missing;
#five values missing.
Statistical comparisons between diabetic patients and control subjects were performed using the nonparametric Mann-Whitney U test. Bivariate correlations were evaluated using Pearson product-moment analysis. Outlier data were excluded in the correlation analysis, using an upper limit of the mean + 3 SD of diabetic patient data. P < 0.05 was considered significant.
RESULTS
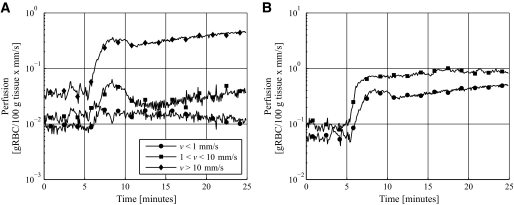
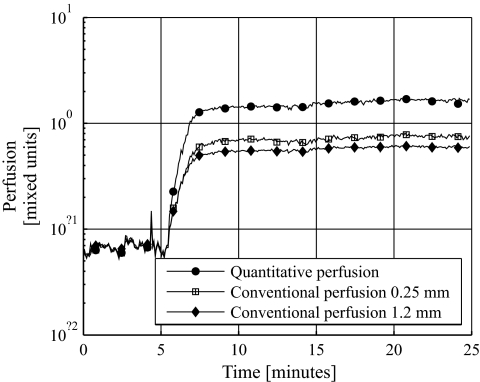
Examples of the qLDF perfusion measures during the heat provocation are shown in Fig. 1. In Fig. 1A, typical behavior of the perfusion in the three different velocity regions is shown. Only a small increase was found in the low-velocity region, whereas a larger increase occurred in the mid- and high-velocity regions. In Fig. 1B, two typical heat responses of the total perfusion (all velocities) are shown. In the first case (Fig. 1B, circles), the perfusion decreases after the initial heat-induced increase, and shortly thereafter the perfusion increases again. In the second case (squares), this biphasic response is not visible and the perfusion quickly settles on a more or less constant level after the temperature rise. Both types of responses occurred in both diabetic patients and controls and were approximately equally common. In Fig. 2, a comparison of the response of the qLDF perfusion estimate and the conventional LDF (cLDF) perfusion estimates at the two source-detector separations is shown. The cLDF perfusion estimates have been normalized so that they equal the qLDF perfusion estimate during baseline (the first 5 min). It can be seen that the heat response is much higher for the qLDF perfusion estimate than for the cLDF. On average for all 20 included diabetic patients (the 3 diabetic patients with too short acclimatization excluded), a 1,065% increase was observed in the qLDF total perfusion, whereas the increase was 672 and 502% for the cLDF perfusion estimates at 0.25- and 1.2 mm source-detector separation, respectively. Corresponding increases for the 23 control subjects were 1,755, 1,046, and 734%, respectively.
FIG. 1.
Typical response of the perfusion in the three different velocity regions (A) and two typical responses of the total perfusion (B).
FIG. 2.
Differences in response to heat stimuli between the qLDF perfusion estimate and the cLDF perfusion estimates at the two fiber separations. The cLDF estimates are normalized to equal the average baseline level of the qLDF perfusion estimate.
A summary of the cLDF and qLDF perfusion measures is given in Table 2. Values are given for baseline, that is, the median during the first 5 min when the probe holder was heated to 32°C, and for the plateau of the heat provocation response, that is, the median of the last 5 min. No significant differences were found at baseline. For the plateau, significant differences between diabetic patients and control subjects were found for cLDF using both source-detector separations (0.25 and 1.2 mm) and for qLDF total perfusion and for velocities >10 mm/s. A similar analysis of the perfusion during the initial peak in the heat response, that is, the median over 2 min starting 2 min after the heat onset, showed that both cLDF measures and qLDF total perfusion were significantly lower for diabetic patients than for control subjects (data not presented).
TABLE 2.
cLDF and qLDF perfusion estimates at baseline and at the plateau
| LDF | Time | Fiber separation/velocity | Control subjects | Diabetic patients |
|---|---|---|---|---|
| n | 23 | 23 | ||
| c | B | 0.25 mm separation | 46 ± 12 | 46 ± 15¶ |
| c | B | 1.2 mm separation | 242 ± 84 | 260 ± 55¶ |
| q | B | v <1 mm/s | 0.019 ± 0.012 | 0.016 ± 0.005¶ |
| q | B | 1 < v <10 mm/s | 0.016 ± 0.009 | 0.019 ± 0.009¶ |
| q | B | v >10 mm/s | 0.026 ± 0.012 | 0.032 ± 0.015¶ |
| q | B | All v | 0.061 ± 0.025 | 0.067 ± 0.019¶ |
| c | Pl | 0.25 mm separation | 485 ± 215 | 335 ± 113† |
| c | Pl | 1.2 mm separation | 1,842 ± 562 | 1,491 ± 396* |
| q | Pl | v <1 mm/s | 0.017 ± 0.019 | 0.021 ± 0.018 |
| q | Pl | 1 < v <10 mm/s | 0.20 ± 0.08 | 0.16 ± 0.06 |
| q | Pl | v >10 mm/s | 0.77 ± 0.42 | 0.53 ± 0.26* |
| q | Pl | All v | 0.98 ± 0.44 | 0.71 ± 0.28* |
Data are means ± SD. cLDF was measured using 0.25- and 1.2 mm fiber separations. qLDF was measured in different velocity regions. B, baseline, median over first 5 min; c, conventional, arbitrary units; q, g RBC/100 g tissue × mm/s; Pl, plateau, median over last 5 min.
¶Baseline measurements were excluded for three diabetic patients because of too short acclimatization. Statistical comparisons of diabetic patients vs. control subjects:
*P < 0.05;
†P < 0.01.
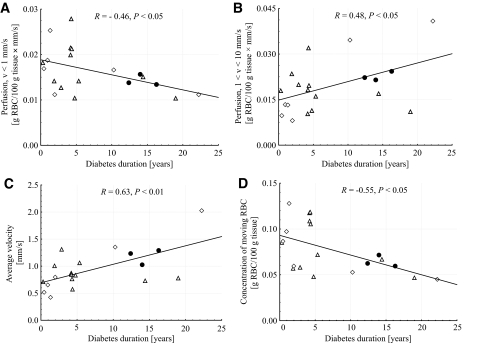
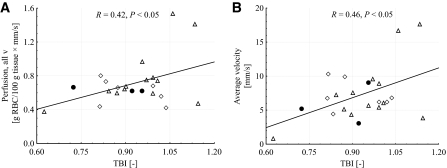
Bivariate correlation analysis for diabetic patient data were performed at baseline and at 44°C for the two conventional LDF perfusion estimates, the quantitative perfusion in the different velocity regions and the total perfusion, the concentration of moving blood cells (Table 3) and their average velocity (Table 4), versus duration of diabetes, A1C and TBI. Significant correlations were found at baseline for duration versus the quantitative perfusion in the low-velocity region (R = −0.46, P < 0.05) and in the mid-velocity region (R = 0.48, P < 0.05), the average velocity (R = 0.63, P < 0.01), and the average concentration (R = −0.55, P < 0.05). The relations between duration and qLDF data at baseline are presented in Fig. 3. No significant correlations were found for A1C versus any of the perfusion estimates. Significant correlations were found after heat for TBI versus the conventional perfusion at 1.2 mm separation (R = 0.42, P < 0.05), the quantitative total perfusion (R = 0.42, P < 0.05), and the average velocity (R = 0.46, P < 0.05). The relations between TBI and qLDF data after heat are presented in Fig. 4.
TABLE 3.
cLDF and qLDF concentration of moving blood cells at baseline and at the plateau
| LDF | Time | Fiber separation/velocity | Control subjects | Diabetic patients |
|---|---|---|---|---|
| n | 23 | 23 | ||
| c | B | 0.25 mm separation | 0.61 ± 0.18 | 0.57 ± 0.16¶ |
| c | B | 1.2 mm separation | 0.86 ± 0.14 | 0.83 ± 0.084¶ |
| q | B | v <1 mm/s | 0.085 ± 0.064 | 0.069 ± 0.027¶ |
| q | B | 1 < v < 10 mm/s | 0.0064 ± 0.0034 | 0.0075 ± 0.0034¶ |
| q | B | v >10 mm/s | 0.00,076 ± 0.00032 | 0.00,091 ± 0.00033¶ |
| q | B | All v | 0.092 ± 0.065 | 0.077 ± 0.027¶ |
| c | Pl | 0.25 mm separation | 0.78 ± 0.12 | 0.74 ± 0.14 |
| c | Pl | 1.2 mm separation | 0.96 ± 0.05 | 0.96 ± 0.046 |
| q | Pl | v <1 mm/s | 0.039 ± 0.043 | 0.061 ± 0.090 |
| q | Pl | 1 < v < 10 mm/s | 0.056 ± 0.022 | 0.048 ± 0.017 |
| q | Pl | v >10 mm/s | 0.018 ± 0.0069 | 0.013 ± 0.0052* |
| q | Pl | All v | 0.11 ± 0.051 | 0.12 ± 0.083 |
Data are means ± SD. cLDF was measured using 0.25- and 1.2 mm fiber separations. qLDF was measured in different velocity regions. B, baseline, median over first 5 min; c, conventional, arbitrary units; q, g RBC/100 g tissue; Pl, plateau, median over last 5 min.
¶The baseline measurements were excluded for three diabetic patients because of too short acclimatization. Statistical comparisons of diabetic patients vs. normal control subjects:
*P < 0.05.
TABLE 4.
cLDF and qLDF velocity estimates at baseline and at the plateau
| LDF | Time | Fiber separation/velocity | Control subjects | Diabetic patients |
|---|---|---|---|---|
| n | 23 | 23 | ||
| c | B | 0.25 mm separation | 78 ± 20 | 84 ± 26¶ |
| c | B | 1.2 mm separation | 288 ± 110 | 313 ± 66¶ |
| q | B | All v | 0.83 ± 0.40 | 0.94 ± 0.37¶ |
| c | Pl | 0.25 mm separation | 627 ± 317 | 461 ± 165* |
| c | Pl | 1.2 mm separation | 1,925 ± 629 | 1,558 ± 438* |
| q | Pl | All v | 9.7 ± 4.7 | 7.3 ± 3.9* |
Data are means ± SD. cLDF was measured using 0.25- and 1.2 mm fiber separations. qLDF was measured in different velocity regions. B, baseline, median over first 5 min; c, conventional, arbitrary units; q, mm/s; Pl, plateau, median over last 5 min.
¶The baseline measurements were excluded for three diabetic patients because of too short acclimatization. Statistical comparisons of diabetic patients vs. normal control subjects:
*P < 0.05.
FIG. 3.
Relation between diabetes duration and quantitative LDF perfusion in low-velocity (A) and mid-velocity (B) region, average velocity (C), and the concentration of moving erythrocytes (D) at baseline. Diabetes treatments were insulin (●), oral hypoglycemic agents (▵), and diet and exercise (♢), respectively.
FIG. 4.
Relation between TBI and quantitative LDF perfusion for all velocities (A) and the average velocity of moving erythrocytes (B) after heat. Diabetes treatments were insulin (●), oral hypoglycemic agents (▵), and diet and exercise (♢), respectively.
Bivariate correlation analysis for control data were performed at baseline and after heat for the two conventional LDF perfusion estimates, the quantitative perfusion in the different velocity regions and the total perfusion, the concentration of moving blood cells and their average velocity, versus TBI. No significant correlations were found.
Bivariate correlations between the cLDF perfusions at both fiber distances and the total qLDF perfusion and BMI, LDL, glucose level, and pulse wave velocity, respectively, were performed for diabetic patients and control subjects separately after heat provocation. No significant correlation was found in the control group between any LDF perfusion measure and any clinical variable. For diabetic patients, LDL was related to cLDF perfusion at 0.25 mm separation (R = −0.49, P < 0.05) and BMI was related or borderline related to three perfusion measures (cLDF perfusion at 0.25 mm fiber separation, R = −0.49, P < 0.05; cLDF perfusion at 1.2 mm fiber separation, R = −0.40, P < 0.06; qLDF total perfusion R = −0.41, P < 0.06).
DISCUSSION
In about half of the measurements, both for the diabetic patients and control subjects, a distinct biphasic heat response could be observed. The first part of this biphasic response is foremost related to the fast axon reflex, whereas the second part is related to the NO-induced response (14,15). In the measurements where this biphasic response is not visible, the two phases are overlapping. The biphasic response becomes more evident when the heat increase is slower (15). An attenuated response has previously been reported for both the axon reflex and the NO-induced response in diabetic type 2 patients (7,27) and a significant difference between diabetic patients and control subjects in the increase from baseline to both the initial peak and the late plateau was also found in the current study. The level of the increase was similar to that reported by Colberg et al. (1), who used a similar protocol.
Figure 2 shows an example of the perfusion response to heat for both the cLDF perfusion estimates at the two different source-detector separations and for the qLDF perfusion estimate. It illustrates that the relative increase from baseline to the late plateau was higher for the qLDF than for the cLDF estimates. The response for the 1.2 mm source-detector separation was weaker than for the 0.25 mm separation for the cLDF estimates. Actually, the increase for qLDF was higher than the increase in the cLDF for all measurements but one, and the increase for the long fiber separation was generally lower than the increase for the short one. This difference between the qLDF and cLDF perfusion estimates is due to the well-known nonlinearity to the blood tissue fraction in cLDF perfusion, which is not present in qLDF perfusion (22). The nonlinearity is not severe, nor does it have a great impact on the results in this study. However, when measuring on sites with higher blood tissue fraction than the dorsum of the foot, which has a relatively low blood tissue fraction, this may be of major importance. A method has previously been presented to compensate for the nonlinearity in the cLDF perfusion estimates (28). However, that method was designed and evaluated using plastic phantoms, and its usefulness for in vivo measurements has been questioned by others (29,30).
Table 2 shows that the high velocities are affected the most by the heat provocation and that they also differ the most between the diabetic patients and the control subjects. In contrast, no significant increase is found in the perfusion for the lowest velocity region, and no significant difference between the diabetic patients and the control subjects is found in the two lowest velocity regions. Knowing that the thermoregulation involves mostly the larger vessels in the microcirculation where the velocity is generally higher, it is expected that the high velocities are affected the most by the heat provocation. The unchanged low-velocity flow and the highly increased high-velocity flow indicate that the increased flow due to the heat provocation is shunted from the artery to the vein side. The lower increase of the high-velocity blood flow for diabetic patients compared with control subjects thus strongly suggests that the maximal arteriovenous shunting capacity is reduced in diabetic patients. Furthermore, a decreasing perfusion response to heat was associated with increasing BMI and decreasing TBI and, similarly, a decreasing average velocity with decreasing TBI. These relationships were observed only in diabetic patients and not in control subjects. The clinical importance of these findings remains to be answered in the future.
Relatively large SDs were found for all perfusion estimates presented in Table 2, both at baseline and during the heat provocation. This may not only reflect intraindividual differences but may also be a result of large spatial inhomogeneities in the microcirculation (21). Although Colberg et al. (1) argue that performing the measurements in a thermoneutral environment should reduce these inhomogeneities, which should be further reduced by the 32°C baseline level of the probe holder, the study would have benefited from measurements at multiple sites. For baseline measurements that must not exceed a few minutes, multiple measurement sites are recommended for future studies. Another limitation of this study is that we were not able to control for differences in antihypertensive medication between the two groups.
As stated in the Introduction, results concerning the microvascular blood flow in diabetic patients in baseline have been contradictory (1,3,4,11). In this study, no significant differences were found between the diabetic patients and the control group (Table 2), but a correlation between baseline flow and diabetes duration was found (Fig. 3). It is very interesting to observe that this correlation was the opposite for the lowest and middle velocity regions, as resolved with the quantitative LDF method. The intriguing interpretation of this would be to relate the perfusion of the low-velocity region (v <1 mm/s) to nutritive capillary flow, and the perfusion in the middle velocity region (1 < v < 10 mm/s) to flow in arterioles, venules, and arteriovenous shunts. This interpretation is supported by the findings that the arteriovenous shunt flow is increased in diabetic patients at baseline, resulting in a reduced nutritive capillary flow (8–12), although the maximal capacity of these shunting vessels is reduced.
In conclusion, by using a novel quantitative LDF method we have been able to compare the velocity distribution between diabetic patients and control subjects during baseline and after a local heat provocation. Our main findings are that 1) the heat provocation increases the blood flow for velocities over 1 mm/s, whereas blood flow at lower velocities is unchanged, 2) the reduced perfusion increase after local heating that is observed in diabetic patients compared with control subjects is found for velocities >10 mm/s, and 3) a reduced low-velocity flow (<1 mm/s) and increased mid-velocity flow (1–10 mm/s) are related to diabetes duration at baseline conditions. The physiologic interpretations of these results are that 1) the thermoregulation is a process involving primarily large, high-velocity vessels and the increased flow is shunted from the artery to the vein side, 2) the maximal arteriovenous shunting capacity is reduced in diabetic patients; and 3) a long diabetes duration is associated with a reduced nutritive capillary flow due to an increased arteriovenous shunt flow at baseline conditions. All of this has previously been known or suspected, but not proven by direct measurements on the microcirculation.
ACKNOWLEDGMENTS
The study was financed by Perimed and Linköping University through the Center for Excellence Noninvasive Medical Measurements (NIMED)-Center for Biomedical Data Processing and by the Swedish Government Agency for Innovation Systems (VINNOVA) and Perimed through the research collaboration program between companies and academia within bioscience (VINNOVA D. no. 2008-00149). The CARDIPP study was supported by grants from the Medical Research Council of Southeast Sweden, the Diabetes Research Center at the University Hospital in Linköping, the Swedish Research Council (D.no. 12661), and the medical faculty at Linköping University.
No potential conflicts of interest relevant to this article were reported.
Author contributions: I.F. wrote the manuscript, discussed study design and methods, and collected and processed data. M.L. discussed study design and methods, collected data, and discussed and reviewed the manuscript. F.H.N., T.L., and C.Ö. discussed study design and methods, provided clinical data, and discussed and reviewed the manuscript. T.S. wrote the manuscript, discussed study design and methods, collected data, and performed statistical data analysis.
Footnotes
Clinical trial reg. no. NCT01049737, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Colberg SR, Parson HK, Nunnold T, Herriott MT, Vinik AI: Effect of an 8-week resistance training program on cutaneous perfusion in type 2 diabetes. Microvasc Res 2006;71:121–127 [DOI] [PubMed] [Google Scholar]
- 2.Khan F, Elhadd TA, Greene SA, Belch JJ: Impaired skin microvascular function in children, adolescents, and young adults with type 1 diabetes. Diabetes Care 2000;23:215–220 [DOI] [PubMed] [Google Scholar]
- 3.Rendell M, Bamisedun O: Diabetic cutaneous microangiopathy. Am J Med 1992;93:611–618 [DOI] [PubMed] [Google Scholar]
- 4.Stansberry KB, Peppard HR, Babyak LM, Popp G, McNitt PM, Vinik AI: Primary nociceptive afferents mediate the blood flow dysfunction in non-glabrous (hairy) skin of type 2 diabetes: a new model for the pathogenesis of microvascular dysfunction. Diabetes Care 1999;22:1549–1554 [DOI] [PubMed] [Google Scholar]
- 5.Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R: Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998;47:457–463 [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Erbas T, Park TS, Pierce KK, Stansberry KB: Methods for evaluation of peripheral neurovascular dysfunction. Diabetes Technol Ther 2001;3:29–50 [DOI] [PubMed] [Google Scholar]
- 7.Kilo S, Berghoff M, Hilz M, Freeman R: Neural and endothelial control of the microcirculation in diabetic peripheral neuropathy. Neurology 2000;54:1246–1252 [DOI] [PubMed] [Google Scholar]
- 8.Boulton AJ, Scarpello JH, Ward JD: Venous oxygenation in the diabetic neuropathic foot: evidence of arteriovenous shunting? Diabetologia 1982;22:6–8 [DOI] [PubMed] [Google Scholar]
- 9.Edmonds ME, Roberts VC, Watkins PJ: Blood flow in the diabetic neuropathic foot. Diabetologia 1982;22:9–15 [DOI] [PubMed] [Google Scholar]
- 10.Fagrell B, Jörneskog G, Intaglietta M: Disturbed microvascular reactivity and shunting—a major cause for diabetic complications. Vasc Med 1999;4:125–127 [DOI] [PubMed] [Google Scholar]
- 11.Jörneskog G, Brismar K, Fagrell B: Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia 1995;38:474–480 [DOI] [PubMed] [Google Scholar]
- 12.Ward JD, Boulton AJ, Simms JM, Sandler DA, Knight G: Venous distension in the diabetic neuropathic foot (physical sign of arteriovenous shunting). J R Soc Med 1983;76:1011–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredriksson I, Larsson M, Strömberg T: Measurement depth and volume in laser Doppler flowmetry. Microvasc Res 2009;78:4–13 [DOI] [PubMed] [Google Scholar]
- 14.Kellogg DL, Jr, Zhao JL, Wu Y: Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 2008;295:H123–H129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minson CT, Berry LT, Joyner MJ: Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 2001;91:1619–1626 [DOI] [PubMed] [Google Scholar]
- 16.Gooding KM, Hannemann MM, Tooke JE, Clough GF, Shore AC: Maximum skin hyperaemia induced by local heating: possible mechanisms. J Vasc Res 2006;43:270–277 [DOI] [PubMed] [Google Scholar]
- 17.Lenasi H, Strucl M: The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur J Appl Physiol 2008;103:719–726 [DOI] [PubMed] [Google Scholar]
- 18.Medow MS, Glover JL, Stewart JM: Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation 2008;15:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi M, Carpi A, Galetta F, Franzoni F, Santoro G: The investigation of skin blood flowmotion: a new approach to study the microcirculatory impairment in vascular diseases? Biomed Pharmacother 2006;60:437–442 [DOI] [PubMed] [Google Scholar]
- 20.Schmidt C, Adechokan S, Mouhli J: Laser-Doppler flowmetry in peripheral arterial occlusive disease. Correlations with transcutaneous oximetry. J Mal Vasc 1996;21:294–298 [PubMed] [Google Scholar]
- 21.Tenland T, Salerud EG, Nilsson GE, Öberg PA: Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp 1983;2:81–90 [PubMed] [Google Scholar]
- 22.Fredriksson I, Larsson M, Strömberg T: Model-based quantitative laser Doppler flowmetry in skin. J Biomedical Optics In process [DOI] [PubMed] [Google Scholar]
- 23.Dahlén EM, Länne T, Engvall J, Lindström T, Grodzinsky E, Nyström FH, Östgren CJ: Carotid intima-media thickness and apolipoprotein B/apolipoprotein A-I ratio in middle-aged patients with Type 2 diabetes. Diabet Med 2009;26:384–390 [DOI] [PubMed] [Google Scholar]
- 24.Wijkman M, Länne T, Engvall J, Lindström T, Ostgren CJ, Nystrom FH: Masked nocturnal hypertension–a novel marker of risk in type 2 diabetes. Diabetologia 2009;52:1258–1264 [DOI] [PubMed] [Google Scholar]
- 25.Larsson M, Strömberg T: Toward a velocity-resolved microvascular blood flow measure by decomposition of the laser Doppler spectrum. J Biomed Opt 2006;11:014024 [DOI] [PubMed] [Google Scholar]
- 26.Nilsson GE, Salerud EG, Strömberg T, Wårdell K: Laser Doppler perfusion monitoring and imaging. In Biomedical Photonics Handbook Vo-Dinh T. Eds. Boca Raton, FL, CRC Press, 2003, p. 15-1–15-24 [Google Scholar]
- 27.Vinik AI, Erbas T, Park TS, Stansberry KB, Scanelli JA, Pittenger GL: Dermal neurovascular dysfunction in type 2 diabetes. Diabetes Care 2001;24:1468–1475 [DOI] [PubMed] [Google Scholar]
- 28.Nilsson GE: Signal processor for laser Doppler tissue flowmeters. Med Biol Eng Comput 1984;22:343–348 [DOI] [PubMed] [Google Scholar]
- 29.Barnett NJ, Dougherty G, Pettinger SJ: Comparative study of two laser Doppler blood flowmeters. J Med Eng Technol 1990;14:243–249 [DOI] [PubMed] [Google Scholar]
- 30.Petoukhova AL, Steenbergen W, Morales F, Graaff R, de Jong ED, Elstrodt JM, de Mul FF, Rakhorst G: Instrument-independent flux units for laser Doppler perfusion monitoring assessed in a multi-device study on the renal cortex. Microvasc Res 2003;66:83–90 [DOI] [PubMed] [Google Scholar]