Abstract
OBJECTIVE
Various methods are used to quantify postprandial glycemia or glucose variability, but few have been compared and none are standardized. Our objective was to examine the relationship among common indexes of postprandial glycemia, overall hyperglycemia, glucose variability, and A1C using detailed glucose measures obtained during everyday life and to study which blood glucose values of the day provide the strongest prediction of A1C.
RESEARCH DESIGN AND METHODS
In the A1C-Derived Average Glucose (ADAG) study, glucose levels were monitored in 507 participants (268 type 1 diabetic, 159 type 2 diabetic, and 80 nondiabetic subjects) with continuous glucose monitoring (CGM) and frequent self-monitoring of blood glucose (SMBG) during 16 weeks. We calculated several indexes of glycemia and analyzed their intercorrelations. The association between glucose measurements at different times of the day (pre- and postprandial) and A1C was examined using multiple linear regression.
RESULTS
Indexes of glucose variability showed strong intercorrelation. Among postprandial indexes, the area under the glucose curve calculated from CGM 2 h after a meal correlated well with the 90-min SMBG postprandial measurements. Fasting blood glucose (FBG) levels were only moderately correlated with indexes of hyperglycemia and average or postprandial glucose levels. Indexes derived with SMBG strongly correlated with those from CGM. Some SMBG time points had a stronger association with A1C than others. Overall, preprandial glucose values had a stronger association with A1C than postprandial values for both diabetes types, particularly for type 2 diabetes.
CONCLUSIONS
Indexes of glucose variability and average and postprandial glycemia intercorrelate strongly within each category. Variability indexes are weakly correlated with the other categories, indicating that these measures convey different information. FBG is not a clear indicator of general glycemia. Preprandial glucose values have a larger impact on A1C levels than postprandial values.
New treatment regimens and guidelines have increasingly focused on postprandial hyperglycemia as an additional target beyond average glucose control (1). However, direct evidence for an effect of specifically controlling postprandial glucose (PPG) and glucose excursions (over and above the effect of reducing average glucose levels on long-term diabetes complications) is limited. The current debate about whether postprandial hyperglycemia and excessive glucose variability are associated with an increased risk of diabetes complications is largely based on epidemiological studies (2–6). Many of these findings (2,4–6) are based on an extrapolation of glucose levels 2 h after an oral glucose tolerance test (OGTT) as a model for the postprandial state rather than on “real-life” PPG measurements. Only a few studies (4,7,8) have also measured the effect of A1C for comparison, and these show conflicting results.
Studies examining PPG control use various methods to quantify PPG, overall hyperglycemia, and glucose variability (9), without any standardization of methods. One approach to assess the role of PPG has been to examine the extent to which it contributes to overall glucose exposure, measured as A1C (10–13). Limited evidence suggests that postprandial glycemia is the dominant contributor to overall hyperglycemia in patients with good to moderate glycemic control (A1C <8.5%), while fasting glucose levels represent the major contributor at higher A1C levels (14). These findings have been used to support the need to measure and treat PPG in order to reach clinical guideline levels of A1C (15,16). Measures of nocturnal glycemia are rarely used in the prediction of A1C. Available literature exploring the nocturnal glucose exposure is sparse and mostly focused on nocturnal hypoglycemia or assessment of glucose variability during glucose-lowering therapies.
Our aim was to examine the relationship among the most commonly used indexes of PPG, overall hyperglycemia, glucose variability, nocturnal glycemia, and A1C using glucose measures obtained during everyday activities from the A1C-Derived Average Glucose (ADAG) study. Additionally, we studied which blood glucose value(s) of the day provide the strongest prediction of mean blood glucose, as measured by A1C, especially focusing on pre- and postprandial glucose contributions to mean blood glucose levels.
RESEARCH DESIGN AND METHODS
The ADAG study was conducted at 10 centers in the U.S., Europe, and Africa from 2006 to 2008 to define the relationship between A1C and average glucose levels. A full description of the study has been published (17). A total of 268 individuals with type 1 diabetes, 159 individuals with type 2 diabetes, as well as 80 nondiabetic control subjects (aged 18–70 years) completed the study. Participants were selected based on stable glycemic control as evidenced by two A1C values within one percentage point of each other in the 5 months prior to recruitment. Individuals with a wide range of A1C levels were included. The nondiabetic control subjects had a plasma glucose level ≤5.4 mmol/l (97 mg/dl) after overnight fasting, A1C levels <6.5%, and no history of diabetes or use of antidiabetes medication. The study was approved by the human studies ethical committees at the participating institutions, and informed consent was obtained from all participants.
Measurements of glycemia.
During the study period, levels of glucose concentrations were assessed through three different methods. Continuous interstitial glucose monitoring (Medtronic Minimed, Northridge, CA) was performed four times with 4-week intervals during the 16-week study period. Monitoring periods lasted at least 48 h, during which time glucose levels were assessed every 5 min. Continuous glucose monitoring (CGM) data were accepted for analysis if there were no gaps longer than 120 min and if the mean absolute difference with the Hemocue calibration results was <18%, as recommended by the manufacturer.
For calibration purposes, and for measurement of pre- and postprandial glycemia, participants performed an eight-point self-monitoring of blood glucose (SMBG) profile (preprandial, 90 min postprandial, bedtime, and 3:00 a.m.) with the HemoCue meter (Hemocue Glucose 201 Plus; Hemocue, Ängelholm, Sweden) during the days of CGM. In addition, during the weeks when CGM was not performed, subjects performed a seven-point SMBG (the same as the eight-point profile above without the 3:00 a.m. measurement) (OneTouch Ultra; LifeScan, Milipitas, CA) for at least 3 days per week.
All blood glucose values stated are plasma equivalents.
Blood samples were analyzed for A1C levels with four different Diabetes Control and Complications Trial–aligned assays: a high-performance liquid chromatography assay, two immunoassays, and an affinity assay (all approved by the National Glycohemoglobin Study Program). The mean value at the end of the 12-week study period was used (17).
Calculated indexes of glycemia.
Indexes of glucose variability and postprandial glucose levels were calculated from the glucose monitoring data. The average blood glucose and SD were calculated based on CGM data and the seven-point SMBG (LifeScan) data. A combined average blood glucose was calculated from CGM and SMBG, weighted by the days of monitoring for each. Indexes based on CGM were calculated after exclusion of the initial 2 h of monitoring, which is considered to be an unstable calibration period.
Two indexes of intraday glucose variability were calculated based on CGM: the mean amplitude of glycemic excursions (MAGE) and the continuous overlapping net glycemic action (CONGA). MAGE is the mean of the differences between consecutive peaks and nadirs, only including changes of >1 SD of glycemic values and thus capturing only major fluctuations (18,19). For the calculation of CONGAn, the difference of the current observation and the observation n hours previously is calculated for each observation after the first n hours. The CONGAn is the SD of these differences (19). We analyzed CONGA for 1, 2, and 4 h. Both high MAGE and CONGA values indicate high intraday glucose variability.
As an indicator of overall hyperglycemia, the 24-h cumulative exposure to glucose levels above different thresholds was calculated as the area under the curve (AUC) of CGM above levels of 7.0, 11.1, and 16.7 mmol/l (or 126, 200, and 300 mg/dl, respectively). This was done for the first 24 h of each CGM period after the initial calibration period. Indexes of nocturnal blood glucose were calculated as the mean blood glucose from the CGM period 6 h prior to the fasting blood glucose (FBG) measurement. Furthermore, for each individual, a mean of all 3:00 a.m. SMBGs (HemoCue) was calculated. Also from CGM, a postprandial AUC (AUCpp) was calculated for periods of 2 or 4 h after a meal (without blood glucose thresholds), and the postprandial increment was calculated from the preprandial glucose level to the highest peak for periods of 2 or 4 h after a meal. Finally, pre- and postprandial measurements from SMBG (HemoCue) were used to calculate mean pre- and postprandial blood glucose, as well as pre- and postbreakfast, lunch, and dinner values. The prebreakfast blood glucose was used as the FBG.
Statistical analyses.
The Pearson correlation coefficient (r) was computed for each pair of glycemic indexes. This was done including only the diabetic population, as the measurements from nondiabetic participants inflate the correlations. Scatterplots of all pairs are presented with an indicator of the Pearson correlation coefficient (r).
A1C was modeled by multiple linear regression using SMBG measurements at different times of the day as explanatory variables. The association to A1C was examined in three separate analyses, including glucose before and after main meals, mean of all pre- and postmeal glucose measures, and adding nocturnal (3:00 a.m.) SMBG. These models were fitted for all individuals with diabetes and separately for type 1 and type 2 diabetes treated with and without insulin. We defined the proportion of A1C variation (SD) explained by each model as the difference between the A1C SD for each subgroup and the residual SD of the model divided by the SD.
RESULTS
Glucose monitoring in the ADAG study was completed by 507 participants. Approximately 2,700 glucose values from each participant were available for analysis. We excluded 10 nondiabetic participants from the analyses of average SMBG and SDs due to missing LifeScan measurements and 1 participant with type 1 diabetes due to erroneous, extreme HemoCue measurements of pre- and postprandial values.
Characteristics of the study population are summarized in Table 1. A1C levels were higher among those with type 1 diabetes (7.3 vs. 6.8% for type 2 diabetes, P < 0.01). Also, the degree of variability, expressed as the SD of the CGM or SMBG measurements and the calculated MAGE and CONGA, was higher among individuals with type 1 diabetes compared with those with type 2 diabetes or nondiabetic individuals (P < 0.01).
TABLE 1.
Clinical and glycemic characteristics
| All | Type 1 diabetes | Type 2 diabetes | No diabetes | |
|---|---|---|---|---|
| n | 507 | 268 | 159 | 80 |
| Age (years) | 47.6 ± 13.6 | 44.1 ± 12.9 | 56.6 ± 9.4 | 41 ± 13.8 |
| Sex (% female) | 54 | 52 | 51 | 69 |
| Ethnicity (% Caucasian) | 82 | 91 | 74 | 68 |
| BMI (kg/m2) | ||||
| Female | 28.1 ± 7 | 26.3 ± 4.7 | 32.7 ± 8.7 | 25.9 ± 5.5 |
| Male | 27.6 ± 5.1 | 26.1 ± 3.4 | 30.8 ± 6.2 | 25 ± 3.3 |
| Treatment with insulin | 65 | 100 | 38 | 0 |
| A1C (%) | 6.8 ± 1.3 | 7.3 ± 1.1 | 6.8 ± 1.1 | 5.2 ± 0.3 |
| FBG (mmol/l) | 7.8 ± 2.4 | 8.5 ± 2.5 | 7.8 ± 2.1 | 5.3 ± 0.6 |
| Average blood glucose (mmol/l) | 8.3 ± 2.2 | 9 ± 2 | 8.3 ± 2 | 5.6 ± 0.4 |
| CGM average (mmol/l) | 8.5 ± 2.2 | 9.3 ± 2 | 8.5 ± 2 | 5.8 ± 0.6 |
| SMBG average (mmol/l) | 8.2 ± 2.2 | 8.9 ± 2.1 | 8.3 ± 2 | 5.5 ± 0.5 |
| Nocturnal measures | ||||
| Mean 3:00 a.m. SMBG (mmol/l) | 8.1 ± 2.7 | 9.2 ± 2.7 | 7.6 ± 2.3 | 5.6 ± 0.7 |
| Mean nocturnal blood glucose CGM (mmol/l) | 8.0 ± 2.3 | 8.9 ± 2.3 | 7.8 ± 2.1 | 5.6 ± 0.6 |
| Prandial measures | ||||
| Preprandial SMBG (mmol/l) | 7.7 ± 2.1 | 8.4 ± 2 | 7.6 ± 1.9 | 5.4 ± 0.5 |
| Postprandial SMBG (mmol/l)* | 9 ± 2.4 | 9.7 ± 2.1 | 9.4 ± 2.2 | 6.1 ± 0.7 |
| AUCpp 2-h CGM (h/mmol/l) | 17.6 ± 4.5 | 19.1 ± 4.1 | 17.8 ± 4 | 12 ± 1.4 |
| PPG increment 2-h CGM (mmol/l)† | 2.8 ± 1.4 | 3.4 ± 1.2 | 2.7 ± 1.2 | 1.2 ± 0.5 |
| Variability measures | ||||
| CGM SD (mmol/l) | 2.7 ± 1.4 | 3.6 ± 0.9 | 2.2 ± 0.9 | 0.8 ± 0.2 |
| SMBG SD (mmol/l)§ | 3.2 ± 1.5 | 4.1 ± 1 | 2.6 ± 1 | 1.1 ± 0.4 |
| MAGE (mmol/l) | 4.8 ± 2.4 | 6.4 ± 1.8 | 3.8 ± 1.5 | 1.4 ± 0.5 |
| CONGA4 (mmol/l) | 3.7 ± 1.9 | 4.9 ± 1.3 | 2.9 ± 1.2 | 1 ± 0.3 |
Data are means ± SD or percent. Glucose values are expressed as plasma equivalent mmol/l.
*Defined as mean of 90-min postprandial self-monitored glucose levels.
†Defined as the increment from the preprandial blood glucose to highest peak 2-h postprandially.
§Defined as the SDs of all self-monitored blood glucose.
As an indicator of overall hyperglycemia, the 24-h cumulative exposure to glucose levels above selected glucose thresholds was calculated as the AUC of glucose (AUC [in hours × mmol/l]) by subgroups defined by type of diabetes and, for type 2 diabetes, insulin therapy. In each subgroup, a different proportion of participants reached each respective threshold at least at some point during the CGM period. While >80% of those with type 1 diabetes and 63% of those with type 2 diabetes on insulin treatment reached a level of 16.7 mmol/l (300 mg/dl), only 31% of those with type 2 diabetes without insulin treatment did so. One individual without diabetes reached this level briefly. (These results can be seen in the online appendix Table, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1774/DC1).
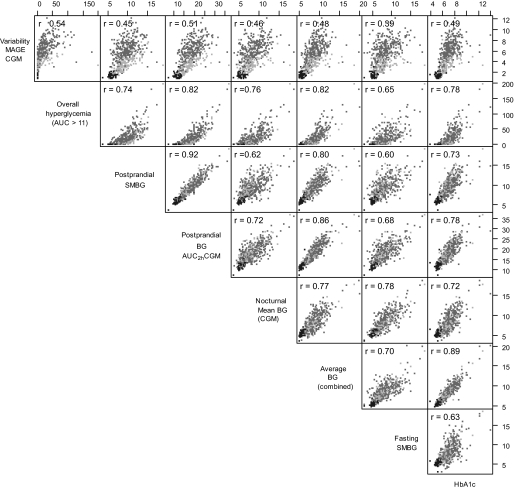
The relationship between the different indexes of glycemia is illustrated in pairwise scatterplots in Fig. 1 (and in an extended online appendix Fig. 2). Although the different indexes were strongly correlated within each category, some indexes do capture somewhat different information. All glucose variability indexes calculated from CGM were closely correlated. CONGA analyses for 1, 2, and 6 h correlated to CONGA4h with correlation coefficients of 0.94, 0.98, and 0.99, respectively (data not shown). CONGA4 and CGM SD (not shown) were both strongly correlated with MAGE (r = 0.95). The SMBG variability index (SD) correlated with the CGM indexes MAGE, the CGM SD, and the CONGA4, with coefficients of 0.83, 0.86, and 0.83, respectively. The variability measures did not correlate well with the postprandial measurements or indexes of fasting or average glycemia (illustrated in Supplemental Fig. 2 in the online appendix). The postprandial indexes calculated from CGM 2 or 4 h after meal, AUCpp2 and AUCpp4, were strongly correlated (r = 0.97) and correlated well with the SMBG postprandial measurements (r = 0.92 and 0.89, respectively) (only AUCpp2 is shown). The postprandial increment, from preprandial blood glucose level to highest blood glucose peak within a 2- or 4-h postprandial window, did not correlate well with any other measure. The correlation coefficients with SMBG postprandial measurements were as low as 0.51. The nocturnal blood glucose mean from CGM and the self-monitored 3:00 a.m. “random” night blood glucose correlated by a correlation coefficient of 0.83.
FIG. 1.
Pairwise scatter diagrams illustrating selected correlations of glycemic variables with Pearson correlation coefficients (r) for each pair of indexes (not including data for no diabetes), highlighting the different participant subgroups with different shades (type 1 diabetes, dark gray; type 2 diabetes, light gray; and no diabetes, black).
Table 2 shows the effects of specific SMBG of the day on A1C levels. Among participants with type 1 diabetes, prebreakfast, prelunch, and postlunch blood glucose measurements had the largest effect on A1C. Among participants with type 2 diabetes, prebreakfast, postlunch, and predinner values had the largest effects on A1C, regardless of insulin treatment. In general, the mean of all preprandial values predicted A1C better than the mean of all postprandial values among those with either type of diabetes both before and after adding nocturnal blood glucose to the model (statistical significant difference [P < 0.05] for the total diabetic group but not for diabetic subgroups or the nondiabetic group). The proportion of A1C variation (SD) explained by the glucose features of each model can be seen in Table 2 and can be compared with variation explained by average blood glucose. Adding a nocturnal glucose index (3:00 a.m. SMBG) only minimally increased the proportion of variation explained.
TABLE 2.
Effects of specific glucose measurements and A1C in three multiple linear regression models
| All diabetes (n = 427) | Type 1 diabetes (n = 268) | Type 2 diabetes (n = 99) |
Type 2 diabetes (n = 60) |
|
|---|---|---|---|---|
| Non–insulin-treated | Insulin-treated | |||
| A | ||||
| Prebreakfast | 0.122 (0.086–0.157)* | 0.107 (0.065–0.149)* | 0.116 (0.031–0.201)* | 0.179 (0.088–0.270)* |
| Postbreakfast | 0.020 (−0.011 to 0.050) | 0.000 (−0.37 to 0.038) | 0.029 (−0.035 to 0.093) | 0.078 (0.008–0.148)* |
| Prelunch | 0.097 (0.059–0.134)* | 0.130 (0.086–0.175)* | 0.055 (−0.053 to 0.164) | 0.002 (−0.088 to 0.091) |
| Postlunch | 0.108 (0.071–0.145)* | 0.120 (0.077–0.164)* | 0.092 (−0.009 to 0.192) | 0.103 (0.015–0.191)* |
| Predinner | 0.093 (0.058–0.128)* | 0.053 (0.012–0.095)* | 0.085 (−0.034 to 0.204) | 0.140 (0.052–0.229)* |
| Postdinner | 0.052 (0.018–0.085)* | 0.077 (0.037–0.117)* | 0.066 (−0.021 to 0.153) | −0.012 (−0.109 to 0.085) |
| A1C variation explained† | 41% | 39% | 49% | 43% |
| B | ||||
| All preprandial | 0.315 (0.267–0.362)* | 0.288 (0.227–0.349)* | 0.259 (0.164–0.354)* | 0.340 (0.207–0.473)* |
| All postprandial | 0.167 (0.123–0.211)* | 0.186 (0.129–0.242)* | 0.177 (0.094–0.259)* | 0.134 (0.024–0.245)* |
| Difference pre-/postprandial‡ | P < 0.01 | P = 0.07 | P = 0.34 | P = 0.08 |
| A1C variation explained† | 40% | 37% | 48% | 38% |
| C | ||||
| All preprandial | 0.257 (0.204–0.310)* | 0.244 (0.178–0.310)* | 0.136 (0.004–0.268)* | 0.312 (0.176–0.447)* |
| All postprandial | 0.163 (0.120–0.206)* | 0.183 (0.128–0.239)* | 0.182 (0.102–0.262)* | 0.106 (−0.008 to 0.220) |
| Nocturnal SMBG | 0.071 (0.040–0.102)* | 0.060 (0.022–0.098)* | 0.117 (0.027–0.207)* | 0.069 (−0.019 to 0.156) |
| Difference pre-/postprandial1 | P = 0.04 | P = 0.28 | P = 0.64 | P = 0.07 |
| A1C variation explained† | 41% | 38% | 50% | 39% |
| A1C variation explained by average blood glucose§ | 53% | 51% | 56% | 53% |
Data are β coefficients (in % A1C per mmol/l) from multiple linear regression models (95% CI), unless otherwise indicated. A: model including mealtime measurements. B: model including mean of all pre- and postprandial values. C: model with both prandial and nocturnal blood glucose.
*P value for estimates <0.05.
†The proportion of A1C variation (SD) explaned by the glucose features of each model.
‡P values from test of difference between pre- and postprandial estimates.
§For comparison, the A1C variation explained by average blood glucose is illustrated.
DISCUSSION
Based on frequent glucose monitoring during usual daily activities, we found, in a large set of individuals with type 1 diabetes, type 2 diabetes, or those without diabetes, that many of the commonly used indexes of glycemic variability, average glycemia, and postprandial glycemia were strongly correlated within each category. Indexes of glucose variability (CONGA, SD of CGM or SMBG, and MAGE) were especially highly correlated. These findings indicate that the different methods of characterizing glucose variability tend to convey similar information.
The putative roles of glucose variability and PPG as risk factors for diabetes complications are based on 1) studies reporting an association between excessive PPG levels and factors that may lead to development of diabetes complications (20–23), 2) epidemiological studies associating 2-h post-OGTT values with increased mortality and cardiovascular disease (2–5), and 3) a few clinical trials in very specific subgroups (e.g., pregnant women [24] and individuals with impaired glucose tolerance [25] or type 2 diabetes post-AMI [26]), which have addressed the issue with different methods and have had conflicting results. The roles of PPG and glucose variability as risk markers need further exploration, and an understanding of the differences and similarities among the different measures of PPG, overall hyperglycemia, and glucose variability is critical.
MAGE (18) has previously been described as the gold standard with which to measure variability (19,27). Our findings show that CONGA or the “simple” SD captures variability to a very similar degree as MAGE. Regarding the methods to assess PPG, we found that the postprandial AUC from CGM 2 h after a meal correlates well with SMBG postprandial measurements, with a correlation coefficient of 0.92. This suggests that a routine 90-min postprandial SMBG measurement contains much of the information about the glucose curve in the hours after a meal. The ADAG study also showed that seven-point profile SMBG levels, measured on average 3 days per week, and CGM, measured on 2 days per month, both over a 3-month period, predict average glucose and A1C similarly (17).
The postprandial increment in glucose levels (the difference from preprandial to highest postprandial value in a 2-h window) showed generally weak correlations with postprandial blood glucose levels (r = 0.45–0.51) and with indexes of average glycemia (r = 0.26–0.27). Postprandial increments have been used to assess glucose variability and PPG in other studies (14,28). The difference between the calculated increments (from CGM) and the difference between the pre- and postprandial measurements from SMBG (Table 1) might be because the latter is measured by the participant ∼90 min after eating (not necessarily capturing the highest postprandial value). One might expect large postprandial increments to reflect high glucose variability; however, the correlation with the variability measures was also only moderate (r = from 0.41 [SMBG SD] to 0.54 [CONGA4]).
As expected, A1C correlated well with average blood glucose from CGM, SMBG, and the two combined. When exploring the contribution of glucose levels from SMBG at different times of the day to average glycemia (Table 3), the preprandial glucose levels had a larger effect on A1C than postprandial glucose levels, presumably because they resemble the 24-h glucose levels (and thus the long-term exposure to glucose) more closely. This result was the same before and after including the nocturnal blood glucose index to the regression model, which, surprisingly, only lead to a small increase in the proportion of A1C variation explained.
The frequently cited article by Monnier et al. (14) concludes that postprandial glucose levels are the dominant contributor to A1C levels in patients with A1C <8.5%, while fasting glucose levels were the major contributor for patients with A1C >8.5%. The calculations underpinning this conclusion were based on AUCs derived from meal-period measurements only, thus disregarding the contribution of glucose exposure outside meal periods to A1C.
Monnier et al. (14) define postprandial glycemia as the AUC above each individual's fasting value, while preprandial glycemia is defined as the AUC between 6.1 mmol/l (110 mg/dl) and measured FBG for each individual. This approach introduces a bias when comparing the association between these two indexes and A1C. Individuals with A1C levels >8.5% will strongly tend to also have high FBG. Their postprandial AUC values will therefore be small by artifact, as only excursions above these high individual FBG values are considered postprandial glucose exposure. Simultaneously, Monnier et al.'s definition yields larger preprandial AUCs in this same group, thus introducing the reported effect. This methodological problem might explain why Monnier et al.'s results differ from our findings and those of others (12,29).
Our study shows that no single blood glucose measurement during the day accurately predicts A1C. This is in accordance with previous studies (29–31) showing that single or limited numbers of blood glucose measurements daily do not accurately reflect A1C levels. However, any insights into the specific timed glucose measurement or combination of measurements that have the largest effect on A1C can help patients and clinicians to plan optimal glucose-monitoring regimens.
Levels of FBG alone were not clear indicators of overall hyperglycemia. The correlation coefficients to indexes of hyperglycemia and average or postprandial blood glucose levels are between 0.60 and 0.70 in the present study. This adds to previous findings (30,31) showing that A1C and postchallenge blood glucose are difficult to predict from FBG values alone. Bouma et al. (30) found a correlation coefficient of 0.77 between A1C and FBG in 1,020 individuals with type 2 diabetes.
This study is based on frequent glucose monitoring during real-life activities in a large, heterogeneous population of people with diabetes and those without diabetes and provides the opportunity to assess reliably the different features of glycemia. The limitations of the study are that the ADAG study population was selected to exclude patients with severe renal/liver disease, pregnancy, and anemia. Therefore, the results may not be extrapolated to all patients with diabetes. However, patients were chosen to span a large range of A1C levels. Moreover, the ADAG study recruited a broad multicenter population, so we feel it is justifiable to draw general conclusions from our results.
The fact that participants had stable A1C (<1% A1C change 6 months prior to study) could have lead to underestimation of glucose variability. However, high levels of glucose variability were seen among our subjects despite stable A1C levels. Even though type 1 diabetes and type 2 diabetes have different glucose patterns due to different disease mechanisms, the mechanism of hemoglobin glycation is likely to be the same. The correlation of the glycemic indexes were therefore calculated for the combined group. Limitations of the MiniMed CGM system include the inability to measure glucose values <2.2 mmol/l (40 mg/dl) or >22.2 mmol/l (400 mg/dl) (measurements outside this range were treated as 2.2 or 22.2 mmol/l, respectively, for the analyses). The mean FBG is derived from prebreakfast measurements and thus can contain blood glucose not preceded by 8 h of fasting.
In summary, the role of glucose excursions and postprandial glycemia in day-to-day diabetes control and risk management is still debated. We found relatively weak correlations between variability indexes and indexes of fasting, postprandial, and mean glycemia, indicating that the variability indexes convey different information. Fasting glucose values had only a moderate correlation with other indexes, confirming that it is not a clear indicator of general glycemia. The mean of all preprandial glucose levels had a larger impact on A1C levels than postprandial glucose levels in type 1 and type 2 diabetic patients.
Supplementary Material
ACKNOWLEDGMENTS
The ADAG study is supported by research grants from the American Diabetes Association and European Association for the Study of Diabetes. Financial support was provided by Abbott Diabetes Care, Bayer Healthcare, GlaxoSmithKline, Sanofi-Aventis Netherlands, Merck & Company, Lifescan, and Medtronic Minimed. Supplies and equipment were provided by Medtronic Minimed, Lifescan, and Hemocue.
The Steno substudy was supported by research grants from the Sehested Hansen Foundation, the Clinical Development Foundation at Steno Diabetes Center, and the Danish Diabetes Association.
R.J.H. is employed by and owns stocks of Eli Lilly and Company. K.B.-J. is head of the Steno Diabetes Center, a hospital integrated in the Danish National Healthcare Service but owned by Novo Nordisk. K.B.-J. holds shares in Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Results in this article were published in abstract form and were presented as two poster presentations at the Annual Meeting of the European Association for the Study of Diabetes in 2008 and 2009.
R.B. researched data, contributed to discussion, and wrote the manuscript. J.C.K. and H.Z. researched data and reviewed/edited the manuscript. B.C. and D.M.N. researched data, contributed to discussion, and reviewed/edited the manuscript. R.J.H., J.N., and K.B.J. contributed to discussion and reviewed/edited manuscript. D.R.W. contributed to discussion and wrote the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ceriello A, Colagiuri S, Gerich J, Tuomilehto Jthe Guideline Development Group Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis 2008;18:S17–S33 [DOI] [PubMed] [Google Scholar]
- 2.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617–621 [PubMed] [Google Scholar]
- 3.Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Ziegelasch HJ, Lindner J: Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia 1996;39:1577–1583 [DOI] [PubMed] [Google Scholar]
- 4.de-Vegt F, Dekker JM, Ruhé HG, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ: Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999;42:926–931 9 [DOI] [PubMed] [Google Scholar]
- 5.Balkau B, Shipley M, Jarrett RJ, Pyörälä K, Pyörälä M, Forhan A, Eschwége E: High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998;21:360–367 [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Ferrara A: Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men: the Rancho Bernardo Study. Diabetes Care 1998;21:1236–1239 [DOI] [PubMed] [Google Scholar]
- 7.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M: Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000;23:1830–1834 [DOI] [PubMed] [Google Scholar]
- 8.Kuusisto J, Mykkänen L, Pyörälä K, Laakso M: NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes 1994;43:960–967 [DOI] [PubMed] [Google Scholar]
- 9.Rodbard D: Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther 2009;11(Suppl. 1):S55–S67 [DOI] [PubMed] [Google Scholar]
- 10.Monnier L, Colette C, Lapinski H: Global assessment for quality and safety of control in type 2 diabetic patients. Eur J Clin Invest 2004;34:37–42 [DOI] [PubMed] [Google Scholar]
- 11.Avignon A, Radauceanu A, Monnier L: Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 1997;20:1822–1826 [DOI] [PubMed] [Google Scholar]
- 12.Hillman N, Herranz L, Grande C, Vaquero PM, Pallardo LF: What is the relative contribution of blood glucose levels at different time points of the day to HbA1c in type 1 diabetes? Diabet Med 2004;21:468–470 [DOI] [PubMed] [Google Scholar]
- 13.Woerle HJ, Neumann C, Zschau S, Tenner S, Irsigler A, Schirra J, Gerich JE, Goke B: Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007;77:280–285 [DOI] [PubMed] [Google Scholar]
- 14.Monnier L, Lapinski H, Colette C: Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 15.Bloomgarden ZT: Glycemic treatment in type 1 and type 2 diabetes. Diabetes Care 2006;29:2549–2555 [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A: Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005;54:1–7 [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, Fort A: Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF: Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–655 [DOI] [PubMed] [Google Scholar]
- 19.Mcdonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ: A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther 2005;7:253–263 [DOI] [PubMed] [Google Scholar]
- 20.Ceriello A: The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev 2000;16:125–132 [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre P, Scheen AJ: The postprandial state and risk of cardiovascular disease. Diabet Med 15:S63–S68, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Heine RJ, Dekker JM: Beyond postprandial hyperglycaemia: metabolic factors associated with cardiovascular disease. Diabetologia 2002;45:461–475 [DOI] [PubMed] [Google Scholar]
- 23.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C: Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. J Am Med Assoc 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 24.de-Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, Evans AT: Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995;333:1237–1241 [DOI] [PubMed] [Google Scholar]
- 25.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M: Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003;290:486–494 [DOI] [PubMed] [Google Scholar]
- 26.Raz I, Wilson PWF, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ: Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitelaw B: What is the best way to analyse glycaemic variability using continuous glucose monitoring data? Diabetologia 2008;51:S436 [Google Scholar]
- 28.Diabetes Research in Children Network (DirecNet) Study Group Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab 2005;90:3387–3391 [DOI] [PubMed] [Google Scholar]
- 29.Bonora E, Calcaterra F, Lombardi S, Bonfante N, Formentini G, Bonadonna RC, Muggeo M: Plasma glucose levels throughout the day and HbA(1c) interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care 2001;24:2023–2029 [DOI] [PubMed] [Google Scholar]
- 30.Bouma M, Dekker JH, de-Sonnaville JJ, van-der D, de-Vries H, Kriegsman DM, Kostense PJ, Heine RJ, van-Eijk JT: How valid is fasting plasma glucose as a parameter of glycemic control in non-insulin-using patients with type 2 diabetes? Diabetes Care 1999;22:904–907 [DOI] [PubMed] [Google Scholar]
- 31.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE: Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275–278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.