Abstract
OBJECTIVE
The unraveling of the elaborate brain networks that control glucose metabolism presents one of the current challenges in diabetes research. Within the central nervous system, the hypothalamus is regarded as the key brain area to regulate energy homeostasis. The aim of the present study was to investigate the hypothalamic mechanism involved in the hyperglycemic effects of the neuropeptide pituitary adenylyl cyclase-activating polypeptide (PACAP).
RESEARCH DESIGN AND METHODS
Endogenous glucose production (EGP) was determined during intracerebroventricular infusions of PACAP-38, vasoactive intestinal peptide (VIP), or their receptor agonists. The specificity of their receptors was examined by coinfusions of receptor antagonists. The possible neuronal pathway involved was investigated by 1) local injections in hypothalamic nuclei, 2) retrograde neuronal tracing from the thoracic spinal cord to hypothalamic preautonomic neurons together with Fos immunoreactivity, and 3) specific hepatic sympathetic or parasympathetic denervation to block the autonomic neuronal input to liver.
RESULTS
Intracerebroventricular infusion of PACAP-38 increased EGP to a similar extent as a VIP/PACAP-2 (VPAC2) receptor agonist, and intracerebroventricular administration of VIP had significantly less influence on EGP. The PACAP-38 induced increase of EGP was significantly suppressed by preinfusion of a VPAC2 but not a PAC1 receptor antagonist, as well as by hepatic sympathetic but not parasympathetic denervation. In the hypothalamus, Fos immunoreactivity induced by PACAP-38 was colocalized within autonomic neurons in paraventricular nuclei projecting to preganglionic sympathetic neurons in the spinal cord. Local infusion of PACAP-38 directly into the PVN induced a significant increase of EGP.
CONCLUSIONS
This study demonstrates that PACAP-38 signaling via sympathetic preautonomic neurons located in the paraventricular nucleus is an important component in the hypothalamic control of hepatic glucose production.
To maintain glucose homeostasis, a complex glucose sensing and regulatory system has developed within the central nervous system (CNS), involving hypothalamic and hindbrain areas (1,2). Also a recent study in humans evidenced the sensitivity of the hypothalamus to small changes in blood glucose levels (3). Despite evidence from animal data for roles in glucose homeostasis of several hypothalamic nuclei such as the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), and paraventricular nucleus (PVN), the major part of the neurochemical makeup of this hypothalamic network is largely unknown.
Pituitary adenylyl cyclase-activating polypeptide (PACAP) is a highly conserved peptide from the secretin-glucagon superfamily (4), which exerts a plethora of peripheral and central effects. PACAP also affects food intake and glucose and lipid metabolism (5,6). PACAP-immunoreactive neurons and their receptors (5) are distributed widely, including the CNS and peripheral tissues such as adipose tissue and pancreas. The highest concentrations in the CNS are found in the hypothalamus (7), where PACAP-38 is produced abundantly in VMH neurons and high release rates are reported for the PVN (7–9). PACAP from retinal ganglion cells is released primarily in the hypothalamic suprachiasmatic nuclei (SCN) (10). However, because tissue-specific knockout animals are not available, it is unclear whether the effects of PACAP on glucose metabolism are mediated via central or peripheral pathways.
PACAP is structurally related to vasoactive intestinal peptide (VIP). PACAP and VIP receptors have been classified as three different subtypes: PACAP-1 receptors (PAC1R) (11), VIP/PACAP-1 receptors (VPAC1R), and VIP/PACAP-2 receptors (VPAC2R) (12,13). VIP and PACAP have a comparable affinity for VPAC1 and VPAC2 (14), whereas PACAP also has a high affinity for the PACAP-specific PAC1 receptor (15). Both PAC1R and VPAC2R are broadly expressed in the brain, including the hypothalamus, whereas VPAC1R is mainly expressed in the cerebral cortex and the hippocampus (16). The demonstration that central administration of PACAP-38 not only induces Fos immunoreactivity in several brain areas, including the PVN (17), but also increases plasma glucose levels (18) suggests that central PACAP-38 may play an important role in (glucose) metabolism.
To determine the importance of central PACAP signaling in regulating glucose metabolism, we combined intracerebroventricular administration of PACAP-38, VIP, and/or their receptor-specific agonists and antagonists, with measurements of plasma glucose concentration, endogenous glucose production (EGP), metabolic clearance rate (MCR), gluconeogenesis (GNG), and glycogenolysis. To delineate further the hypothalamic output pathway, we combined the intracerebroventricular administration of PACAP-38 with Fos immunohistochemistry and retrograde tracing from the intermediolateral column (IML) of the thoracic spinal cord, measured plasma glucose concentrations and EGP after intranuclear PVN administration of PACAP-38, and performed intracerebroventricular infusions of PACAP-38 in rats after a specific hepatic sympathetic or parasympathetic denervation.
RESEARCH DESIGN AND METHODS
Experiments were conducted with approval of the animal care committee of the Royal Netherlands Academy of Arts and Sciences. Male Wistar rats weighing 300–350 g (Harlan, Netherlands) were housed four per cage at room temperature (21 ± 1°C) with a 12/12-h light-dark schedule (lights on at 0700 h). Food and water were available ad libitum, unless stated otherwise.
Surgery preparation.
Animals underwent surgery under anesthesia with 0.8 ml/kg i.m. Hypnorm (Janssen, High Wycombe, Buckinghamshire, U.K.) and 0.4 ml/kg s.c. Dormicum (Roche, Almere, Netherlands).
Silicon catheters were inserted into the right jugular vein and left carotid artery for intravenous infusions and blood sampling, respectively. With a standard David Kopf stereotaxic apparatus, intracerebroventricular guiding cannulas were placed into the lateral cerebral ventricle; double intracerebroventricular cannulations were applied for intracerebroventricular preinfusion studies (see below). Intranuclear infusion guiding cannulas were placed into the PVN. All coordinates were adapted from our previous study (supplementary Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1398/DC1). Fixation, anticlotting treatment, and connection of catheters and probes were applied as described before (19) as well as the hepatic sympathetic, parasympathetic, and sham denervation (20).
For visualizing the PVN preautonomic neurons, 0.05–0.1 μl retrograde neuronal tracer cholera toxin subunit B (conjugated with Alexa Fluor 555, CTB-AF555; Molecular Probes, Eugene, OR) was injected into the thoracic IML unilaterally between T6 and T7 (where the liver-projecting sympathetic motor neurons are found) (21).
Experiments were performed after at least 10 days of recovery and with presurgery body weights regained. Food was restricted overnight at 20 g before the experimental day, and experimental blood sampling was started 5 h after light on.
Stable isotope tracer dilution, intracerebroventricular infusions, and blood sampling schedule.
To compare the effects of the different PACAP-38/VIP receptor (ant)agonists on EGP, the isotope dilution method was applied, using [6.6-2H2]glucose [primed (8.0 μmol/5 min) − continuous (16.6 μmol/h)] infusion (>99% enriched); Cambridge Isotopes, Andover, MA). To dissect the separate contributions of GNG and glycogenolysis to EGP, in an additional vehicle and PACAP-38 intracerebroventricular infusion group, the mass isotopomer distribution analysis method was applied using [U-13C6]glucose and [2-13C1]glycerol (22,23) (3.53 mg/ml [U-13C6]glucose, 22.8 mg/ml [2-13C1]glycerol, 2,910 μl/h bolus for 5 min, and continuous infusion). Blood samples (0.3 ml/sample) were taken at t = −5 min for background tracer enrichment and at t = 90, 95, and 100 min for determining basal plasma parameters and isotope enrichment after having reached isotope equilibrium (data are presented by averaging these three time points). After t = 100 min, single intracerebroventricular infusions of different drugs (and vehicle at 5 μl/h) were started immediately and lasted 120 min; direct infusion of PACAP-38 into PVN was performed with a fivefold lower concentration of PACAP-38 than the intracerebroventricular infusions and a 2 μl/h infusion rate. From t = 120–220 min, six blood samples were taken with 20-min intervals for determining plasma parameters. After the last blood sample, liver tissue was collected under deep anesthesia for quantitative real-time PCR (RT-PCR) studies, and subsequently animals were perfusion fixed (supplementary data 2, available in an online appendix) for Fos immunoreactivity (Fos-ir) and localizing cholera toxin subunit B (CTB)-AF555 tracer. Single Fos or double Fos/CTB and Fos/arginine-vasopressin (AVP) immunohistochemical analysis was performed. To investigate the effect of PACAP-38 on plasma epinephrine concentrations, an additional experiment with intracerebroventricular infusions of PACAP-38 and vehicle was performed. Blood was sampled (2.0 ml/sample) only at t = −5 and 90 min.
All drugs used for intracerebroventricular infusions were dissolved in a fivefold stock solution in purified water containing 30% glycerol and diluted to working solution by purified water, except for the VPAC2R antagonist, which was dissolved in 0.5% acetic acid neutralized by NaHCO3 (this vehicle did not differ from the common vehicle with respect to its effects on plasma glucose concentration [P = 0.29], EGP [P = 0.30], and MCR [P = 0.10]). PACAP-38 for the microinfusions was dissolved in 0.9% saline.
For experiments that needed preinfusion and coinfusion of receptor antagonists, a preinfusion of the receptor antagonist was started immediately after t = 100 min through the left intracerebroventricular cannula; 10 min later, the PACAP-38 was started via the right intracerebroventricular cannula.
Analytical methods.
Plasma samples were stored at −20°C for analysis. By using radioimmunoassay kits, plasma insulin (t = 100, 140, 180, and 220 min), glucagon (t = 90, 120, 160, and 200 min) (LINCO Research; St. Charles, MO), and corticosterone concentrations (all time points) (ICN Biomedicals, Costa Mesa, CA) were measured. Plasma isotope enrichments were measured using gas chromatography–mass spectrometry, and GNG was calculated by mass isotopomer distribution analysis (23–25). Plasma epinephrine and liver noradrenalin were measured by high-performance liquid chromatography with fluorescence detection after derivatization of the catecholamines with diphenylethylene diamine. Glycogen content was measured by spectrophotometry. Liver expression of phosphoenolpyruvate carboxykinase (Pepck) and glucose-6-phosphatase (G6Pase) mRNA were examined by RT-PCR (supplementary data 3, available in an online appendix) (19). Fos-ir–positive cells in the PVN from vehicle, PACAP-38, VIP (5 nmol/h), VPAC1R, VPAC2R agonist intracerebroventricular infusion, and direct injection of PACAP-38 into the PVN were quantified (supplementary data 4, available in as online appendix) (26).
Calculation and statistics.
Data from all experiments are presented as means ± SEM. EGP was calculated from isotope enrichment using adapted Steele equations (27). Glucose concentration and EGP were analyzed using a repeated-measures ANOVA to test for the effects of peptide infusions and time. Plasma epinephrine, corticosterone, glucagon, and insulin, as well as liver noradrenalin, glycogen content, and mRNA expression, were analyzed using one-way ANOVA, to compare the average among experimental groups.
RESULTS
Intracerebroventricular PACAP-38 induces hyperglycemia by stimulating endogenous glucose production.
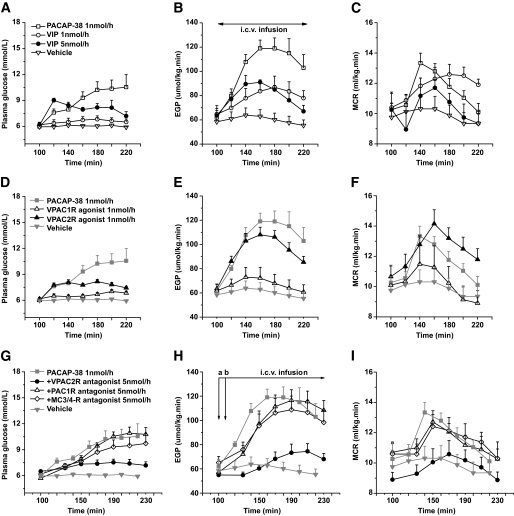
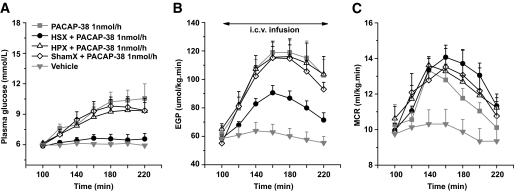
To investigate the possible contribution of the hypothalamic PACAP/VIP systems to peripheral glucose metabolism, we administered PACAP-38 and VIP, as well as a specific VPAC1-R agonist (K15,R16,L27VIP/GRF) (28) and VPAC2-R agonist, Hexa-His′ VIP(2–27) (29), by intracerebroventricular infusion into the lateral cerebral ventricle. Upon intracerebroventricular infusion of PACAP-38 for 120 min (1 nmol/h, n = 6), both plasma glucose concentration and EGP were increased in comparison with the basal state at t = 100 min (∼70 and ∼100%, respectively). ANOVA detected a significant effect of time (difference between time points is expressed by time effects Pt; Pt < 0.001 for both parameters). The PACAP-38 induced increase was also significant compared with the vehicle control group (n = 6) (difference between groups is expressed by group effects Pg; Pg = 0.001 and Pg < 0.001 for plasma glucose and EGP, respectively) (Fig. 1A and B). Intracerebroventricular infusion of VIP at the same concentration (1 nmol/h, n = 4) did not significantly change plasma glucose concentrations (Pt = 0.15) but did cause a significant increase of EGP (Pt = 0.004). This increase was slightly higher than vehicle control (Pg = 0.049) but significantly lower than PACAP-38 (Pg = 0.04). When a fivefold higher concentration of VIP (5 nmol/h, n = 5) was administered, a clear hyperglycemia was induced (∼50%, Pt < 0.001), which was significantly higher than that in the 1 nmol/h (Pg = 0.03) and vehicle (Pg < 0.001) groups and not significantly different from PACAP-38. EGP also increased significantly (Pt < 0.001) in the 5 nmol/h group; it was significantly higher than that of the vehicle control group (Pg = 0.02) but did not differ from 1 nmol/h VIP and was still significantly lower than the PACAP-38 group (Pg = 0.03). MCR increased significantly in both PACAP38-treated (Pt = 0.007) and VIP (1 nmol/h)-treated (Pt = 0.002) animals and showed a significant decrease from t = 160 min to t = 220 min in the VIP (5 nmol/h) group (Pt = 0.004) (Fig. 1C). The vehicle group showed no effects of time for the MCR. No group effect was found, although MCR was higher in PACAP-38 than in vehicle.
FIG. 1.
The effects of central administration of PACAP-38, VIP, and/or their receptor agonists and antagonists on plasma glucose concentration, EGP, and MCR. A–C: Intracerebroventricular (i.c.v.) infusion of PACAP-38 increases both plasma glucose concentrations and EGP. Similar responses could not be obtained with equimolar or five times higher concentrations of VIP. MCR during PACAP-38 and VIP (1 nmol/h) increased and during VIP (5 nmol/h) decreased along the intracerebroventricular infusion. D–F: Intracerebroventricular infusion of the VPAC2R agonist resulted in significantly higher plasma glucose levels, EGP, and MCR responses than the VPAC1R agonist. The almost similar responses induced by the VPAC2R agonist and PACAP-38 indicate that the plasma glucose, EGP, and MCR changes induced by PACAP-38 are predominantly mediated via the VPAC2R. G–I: Coinfusion of PACAP-38 with the PAC1R or VPAC2R antagonist shows that antagonizing VPAC2R, but not PAC1R, blocks a large part of the plasma glucose (G), EGP (H), and MCR (I) responses evoked by PACAP-38, thus confirming that PACAP-38 mainly acts on central VPAC2Rs to regulate plasma glucose concentrations, EGP, and MCR. Coinfusion of PACAP-38 with the MC3R/MC4R antagonist SHU9119 still induces changes in plasma glucose, EGP, and MCR. Thus, the melanocortin signaling pathway is not likely to be involved in the central PACAP-38 effects on glucose production. Antagonist infusions were started at time point “a” through the left intracerebroventricular cannula, and the PACAP-38 infusion was started 10 min later at time point “b” through the right intracerebroventricular cannula. The starting and end time points for the intracerebroventricular infusion of the different drugs in all groups are illustrated in B and H, respectively, that is, immediately after t = 100 min or t = 110 min, and end at t = 220 min or t = 230 min. The gray symbols and lines in parts (D–I) are a repeat of the PACAP-38 and vehicle data in A–C. Data are means ± SEM.
PACAP-38 induces hyperglycemia via VPAC2R.
Intracerebroventricular infusion of the VPAC1R agonist (1 nmol/h, n = 5) or the VPAC2R agonist (1 nmol/h, n = 6) resulted in quite different effects on plasma glucose concentration and EGP (Fig. 1D and E). Intracerebroventricular infusion of the VPAC2R agonist caused a significant increase in plasma glucose concentrations (∼40%, Pt < 0.001), which was higher than the effect of vehicle (Pg < 0.001) or the VPAC1R agonist (Pg = 0.001); it also significantly increased EGP (∼65%, Pt < 0.001), an effect that was again significantly stronger than that of vehicle (Pg < 0.001) and VPAC1R agonist (Pg = 0.02). Although the effects were shorter lasting and less pronounced than those of PACAP-38, no significant differences were found between the VPAC2R agonist and PACAP-38 with regard to their effects on plasma glucose concentration and EGP. Intracerebroventricular infusion of the VPAC1R agonist also increased plasma glucose concentrations, especially during later stages (Pt = 0.002) and differed significantly from vehicle (Pg = 0.02), but this increase was significantly lower than that of PACAP-38 (Pg = 0.01) and that of VPAC2R agonist (Pg = 0.001). On the other hand, the VPAC1R agonist did not significantly change EGP; its effect did not differ significantly from the vehicle group and was significantly lower than that of the VPAC2R agonist (Pg < 0.001) and PACAP-38 (Pg = 0.004) (Fig. 1C and D). MCR during VPAC1R agonist decreased (Pt = 0.001) significantly but did not differ from vehicle and PACAP-38. During VPAC2R agonist infusion, MCR increased (Pt < 0.001) significantly; it was significantly higher than vehicle (Pg = 0.03) and not different from PACAP-38. VPAC1R and VPAC2R are also expressed in peripheral tissues, such as pancreas and adipose tissue (30). To exclude the possibility that the glucoregulatory effects of PACAP/VIP resulted from leakage of brain infusates into the systemic circulation, we also infused the VPAC1R and VPAC2R agonists (with the same conditions as used for the intracerebroventricular administration) directly into the systemic circulation. No significant changes in either plasma glucose concentrations or EGP were detected (supplementary Fig. 1A and B, available in the online appendix).
Because no specific PAC1R agonist was available, we tested the possible involvement of the PAC1R in the glucoregulatory effects of PACAP-38 by intracerebroventricular preinfusion of the PAC1R-specific antagonist PACAP-6-38 (31) (5 nmol/h) together with PACAP-38 (1 nmol/h) (n = 4). The coinfusion of PACAP-6-38 with PACAP-38 still significantly increased plasma glucose concentrations (Pt < 0.001, Pg = 0.70 vs. PACAP-38), EGP (Pt < 0.001, Pg = 0.80 vs. PACAP-38), and MCR (Pt = 0.049, Pg = 0.80 vs. PACAP-38) (Fig. 1G–I).
To test further the role of the VPAC2R, the VPAC2R-specific antagonist myristoyl-K12-VIP(<1–26)-KKGGT (32) was preinfused (5 nmol/h) together with PACAP-38 (1 nmol/h) (n = 6). Plasma glucose levels did not rise significantly but were still significantly higher than the vehicle control (Pg = 0.003). EGP showed a significant increase over time (Pt = 0.003) but was not different from its own vehicle control. Compared with the single intracerebroventricular PACAP-38 infusion group, the coinfusion almost completely blocked the increases of plasma glucose (Pg = 0.03) and EGP (Pg < 0.001) evoked by PACAP-38. MCR increased as well (Pt < 0.001) but significantly less than single PACAP-38 (Pg = 0.02) and not different from its own vehicle control (Fig. 1G–I).
The inhibitory effect of intracerebroventricularly administered PACAP-38 on food intake involves proopiomelanocortin-containing neurons in the ARC and can be blocked by the coadministration of the MC3-R/MC4-R specific antagonist SHU9119 (18). Here, coinfusion of the MC3-R/MC4-R antagonist SHU9119 (5 nmol/h) with PACAP-38 (1 nmol/h) still increased plasma glucose concentrations (Pt < 0.001), EGP (Pt < 0.001), and MCR (Pt = 0.049) and did not significantly change the hyperglycemic, EGP, and MCR stimulatory effects of a single intracerebroventricular PACAP-38 infusion (Fig. 1G–I).
Intracerebroventricular administration of PAC1R (n = 4), VPAC2R (n = 5), or SHU9119 (n = 6) on its own did not significantly affect plasma glucose concentrations or EGP (supplementary Fig. 1C and D).
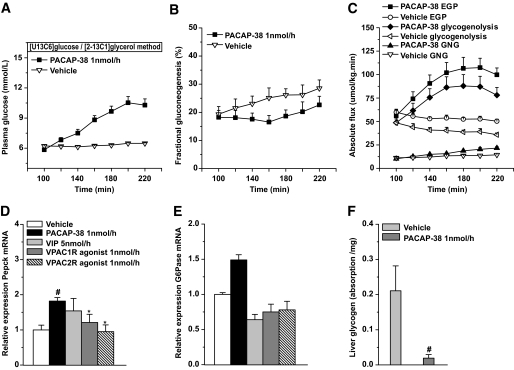
Contribution of glycogenolysis and GNG to the hyperglycemic action of PACAP-38.
In animals receiving dual-isotope tracer infusions for analyzing glycogenolysis and GNG, intracerebroventricular administration of PACAP-38 (n = 6) significantly increased plasma glucose concentrations and EGP (Pt < 0.001 and Pt < 0.002, respectively), that is, comparable to the previous results with the [6.6-2H2]glucose method (compare Fig. 2A and C and Fig. 1A and B). In the vehicle group (n = 5), EGP decreased slightly during the study period (Pt = 0.034). In both groups (i.e., vehicle and PACAP-38), the fractional contribution from GNG to EGP slowly increased along the 120-min intracerebroventricular infusion (from 19 to 28% in vehicle [Pt = 0.002] and from 18 to 23% in the PACAP group [Pt = 0.008]), without significant differences between the two groups (Fig. 2B). The absolute rate of GNG, however, increased significantly more in the PACAP-38 group than in the vehicle group (Pg = 0.027). Consistently, RT-PCR showed significantly increased mRNA expression of Pepck compared with vehicle and VPAC1R and VPAC2R agonists (Fig. 2D); mRNA expression of G6Pase in PACAP-38 showed a trend toward an increase in comparison with vehicle (Fig. 2E). In the PACAP-38 group, glycogenolysis significantly increased by 61% (Pt < 0.001), whereas in the vehicle group, glycogenolysis significantly decreased by 27% (Pt = 0.005) (Fig. 2C). In line with the increased glycogenolysis, liver glycogen stores were significantly lower in the PACAP-38 animals (Fig. 2F).
FIG. 2.
Fractional contributions of gluconeogenesis and glycogenolysis to the hyperglycemic action of intracerebroventricular PACAP-38. A: The changes in plasma glucose concentrations in animals with the dual-isotope tracer experiment are very comparable to those of the single-isotope tracer experiments. B: The fractional GNG of the PACAP-38 group increases slightly, but not significantly, slower than that in the vehicle group. C: The absolute flux of GNG and glycogenolysis contribute very differently to the PACAP-38-induced changes in total EGP. D and E: Relative expression of hepatic Pepck and G6Pase mRNA after drug infusions. PACAP-38 profoundly increased Pepck mRNA expressions, whereas vehicle, VPAC1R, and VPAC2R did not. F: Liver glycogen content at the end of the experiment is significantly lower in PACAP-38 compared with vehicle-treated animals, indicating more pronounced glycogen depletion in the PACAP-38-treated animals. Data are means ± SEM. #P < 0.05 vs. vehicle; *P < 0.05 vs. PACAP-38.
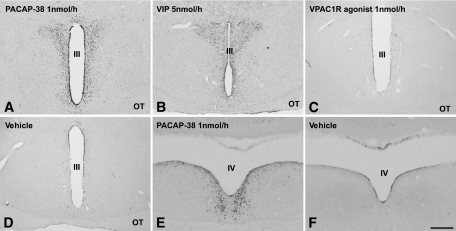
PACAP-38 induces Fos immunopositive nuclei in sympathetic preautonomic PVN neurons.
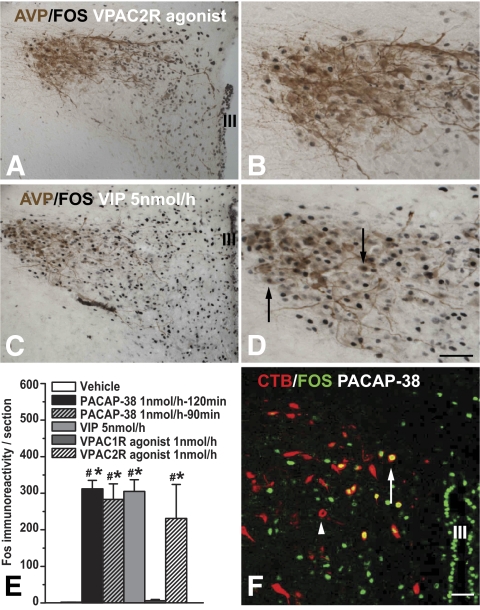
Intracerebroventricular administration of PACAP-38, VPAC2R agonist, or VIP (5 nmol/h), but not the VPAC1R agonist or vehicle, induced Fos-ir in the PVN, periventricular nucleus, and ARC in the hypothalamus, as well as the nucleus incertus in the pons (Figs. 3 and 4). In the PVN, the PACAP-38 and VPAC2R-agonist induced Fos-ir nuclei mainly located in its rostromedial part, with considerably fewer labeled nuclei extending into the lateral magnocellular compartment of the PVN. Fos/AVP double staining showed that few of the VPAC2R agonist-induced Fos-ir neurons contained AVP-ir (Fig. 4A and B). In contrast, VIP induced strong Fos-ir both in the rostromedial and lateral magnocellular compartment and colocalization with AVP (Fig. 4C and D). Counting Fos-ir showed that PACAP-38, VIP, and VPAC2R agonist similarly induced significant more Fos-ir in PVN neurons than in vehicle and VPAC1R agonist groups (Fig. 4E). This specific Fos-ir pattern was also observed after a 90 min intracerebroventricular infusion of PACAP-38, with similar numbers of Fos-ir nuclei in the PVN (Fig. 4E).
FIG. 3.
Intracerebroventricular infusion of PACAP-38, VIP, or their receptor agonists results in different Fos-ir patterns in different brain areas. A: PACAP-38 (1 nmol/h) induced Fos-ir in PVN and the periventricular area. A similar Fos-ir pattern can be seen after VIP (5 nmol/h) (B, and Fig. 4C and D) and VPAC2R (1 nmol/h) (Fig. 4A and B) but not VIP (1 nmol/h) (not shown), VPAC1R (1 nmol/h) (C), or vehicle (D). E and F: Intracerebroventricular administration of PACAP-38 (E), VIP (5 nmol/h), and VPAC2R agonist also induced Fos-ir in the nucleus incertus located in the pons. No such Fos-ir was visible in the nucleus incertus with vehicle (F) or VPAC1R agonist infusion (not shown). OT, optic tract; III, third cerebral ventricle; IV, fourth cerebral ventricle. Scale bar: A–D, 400 μm; E and F, 200 μm.
FIG. 4.
Fos-ir neurons induced by intracerebroventricular administration of PACAP-38 and the VPAC2R agonist mainly locate in the medial PVN. VIP-induced Fos-ir also extends to the lateral PVN. A: Few of the VPAC2R agonist-induced Fos-ir neurons contain AVP (high magnification in B). C: VIP induced strong Fos-ir both in the rostromedial and lateral magnocellular compartment of the PVN with considerable colocalization with AVP (arrow in D, with high magnification). E: The number of Fos-ir–positive nuclei in the PVN was very comparable between the PACAP-38, VIP (5 nmol/h), and VPAC2R agonist groups. Moreover, animals perfused 90 min instead of 120 min after the start of the intracerebroventricular PACAP-38 infusion had identical numbers of Fos-ir nuclei in the PVN. F: Retrogradely labeled preautonomic neurons in the PVN (red, arrowhead) after an injection of CTB into the thoracic IML. Some of the retrogradely labeled neurons are also activated by the intracerebroventricular administration of PACAP-38 as indicated by the colocalized Fos-ir (green, arrow). III, third cerebral ventricle. Scale bar: A and C, 100 μm; B, D, and F, 50 μm. (A high-quality color representation of this figure is available in the online issue.)
Among the animals with CTB-AF555 injections in the IML, three showed clear CTB labeling in the CNS, including the corticospinal projection neurons, lateral hypothalamus, PVN, locus coeruleus, parabrachial nucleus, and the nucleus ambiguous. In the PVN, CTB-labeled neurons were concentrated in the dorsomedial and less in the ventrolateral subdivision. Several CTB-labeled neurons colocalized Fos-ir induced by PACAP-38. Among the total CTB-labeled neurons, 33 ± 3% per section also contained Fos-ir nuclei. The percentage of CTB-labeled neurons among the total of Fos-ir nuclei in PVN per section is ∼3%. This colocalization was not observed in other brain areas (Fig. 4F).
Hepatic sympathetic denervation blocks the hyperglycemic action of PACAP-38.
Basal plasma glucose concentrations, EGP, and MCR were not influenced by hepatic sympathetic denervation (HSX), parasympathetic denervation (HPX), or sham denervation (shamX) (Figs. 5A–C). After HSX, the hyperglycemic and EGP, but not MCR, stimulatory effects of PACAP-38 were significantly reduced. Plasma glucose concentrations of HSX animals no longer showed a significant increase and significantly lower than HPX (Pg = 0.02) and shamX groups (Pg = 0.01). Although EGP still showed a significant increase (Pt < 0.001) in the HSX group and was higher than that of vehicle-treated nondenervated animals (Pg = 0.02), it was also significantly lower than that of PACAP-38–treated nondenervated (Pg = 0.005), shamX (Pg = 0.036), and HPX (Pg = 0.036) animals. No differences were found between HPX and shamX animals in plasma glucose concentration and EGP nor between shamX or HPX and the PACAP-38–treated nondenervated animals in experiment 1. The PACAP-38–induced increase in MCR was not affected in any of the denervated groups. The effectiveness of HSX was validated by a significantly reduced noradrenalin content in the liver of HSX (26.17 ± 1.32 ng/g) compared with HPX (57.93 ± 5.88 ng/g) (P < 0.001) and shamX (58.00 ± 4.30 ng/g) (P < 0.001) animals. No difference in hepatic noradrenalin content was found between HPX and shamX groups. In addition, noradrenalin in both HPX and shamX groups was not different from that in intact rats without abdominal surgery (58.96 ± 9.24 ng/g).
FIG. 5.
Selective denervation of the HSX, but not the HPX to the liver or shamX, largely blocks the hyperglycemic and EGP stimulatory effects of PACAP-38. MCR, however, was not influenced by any type of denervation. The starting and ending time points for the intracerebroventricular (i.c.v.) infusion of PACAP-38 or vehicle in all groups are illustrated in B.
Local infusion of PACAP-38 into the PVN increases plasma glucose concentration and EGP.
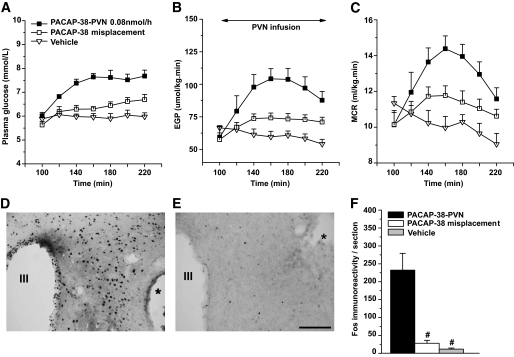
To verify that intracerebroventricular PACAP-38 induced changes in glucose metabolism largely depend on its direct effects on PVN neurons, we infused PACAP-38 locally into the PVN (n = 5). Plasma glucose concentration and EGP (Fig. 6A and B) increased significantly compared with vehicle control (n = 5) (P < 0.001 and P = 0.01, respectively) and compared with animals that had their cannulas placed outside the boundaries of the PVN area (n = 8) (P = 0.001 and P = 0.02, respectively). MCR increased significantly compared with vehicle control (P = 0.02) (Fig. 6C) but did not reach significance compared with the misplaced group (P = 0.07). The number of Fos-ir nuclei in PVN only increased when PACAP-38 was injected into the PVN (Fig. 6D–F).
FIG. 6.
Local infusion of PACAP-38 into the PVN increases plasma glucose concentration (A), EGP (B), and MCR (C). The starting and ending time points for the PVN infusion of PACAP or vehicle in all groups is illustrated in B. Misplacement of infusion cannula outside the boundaries of the PVN area produced a significantly smaller response. The effects of local administration of PACAP-38 or vehicle in the PVN on Fos-ir in the PVN are indicated in D and E, respectively. Local infusion of PACAP-38 produced a Fos-ir response in the PVN that was very similar to the one produced by the intracerebroventricular administration of PACAP-38 (compare Figs. 6D and 3A). Cannula positions are indicated by * in D and E. F: Counting of Fos-ir nuclei in PVN from the three injection groups. #P < 0.001 vs. PACAP-38–PVN group. Scale bar: 100 μm.
Plasma glucoregulatory hormones.
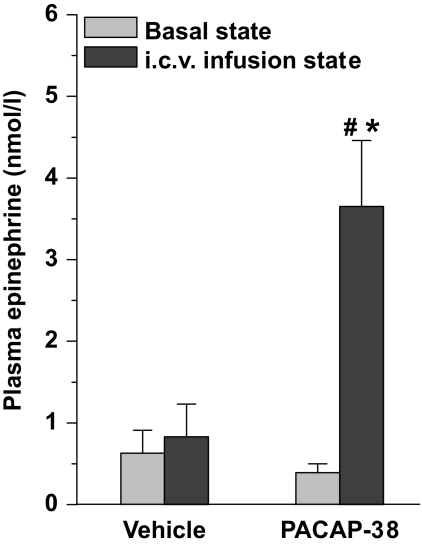
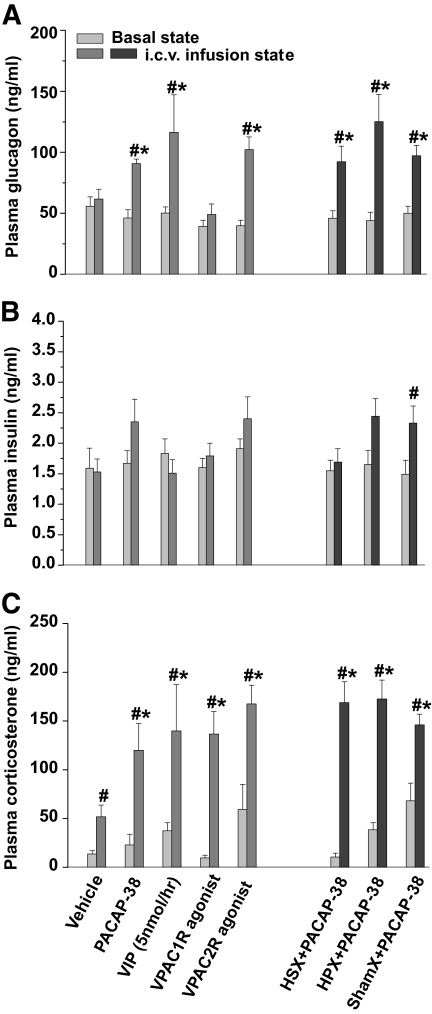
Plasma epinephrine concentrations increased significantly after intracerebroventricular infusion of PACAP-38 (Fig. 7). The average concentration of plasma glucagon increased significantly after PACAP-38, VIP (5 nmol/h), and VPAC2R but not after the lower concentration of VIP (1 nmol/h; not shown), VPAC1R agonist, or vehicle infusion (Fig. 8A). Furthermore, no significant difference was found between the glucagon responses of the VIP (5 nmol/h), PACAP-38, and VPAC2R-agonist treated groups. Mean plasma insulin concentrations were mildly elevated upon intracerebroventricular administration of PACAP-38 and the VPAC2R agonist, but neither of them reached statistical significance (Fig. 8B). In HSX, HPX, and shamX animals, PACAP-38 infusion increased plasma glucagon concentrations to a level similar to that for PACAP-38, VIP, or VPAC2R-agonist in nondenervated animals. After HSX, mean plasma insulin levels were not increased during the administration of PACAP-38. In HPX and shamX animals, only the increase in the shamX group reached significance. Plasma corticosterone concentrations showed a significant increase in all groups, with all drug-induced responses significantly higher than those of the vehicle controls. No significant differences were found between PACAP-38, VIP, and their receptor-specific agonists, or between HSX, HPX, and shamX groups (Fig. 8C).
FIG. 7.
Plasma epinephrine concentrations increased significantly after a 90-min intracerebroventricular (i.c.v.) infusion of PACAP-38. Data are means ± SEM. #P < 0.05 vs. basal state, *P < 0.05 vs. vehicle control.
FIG. 8.
Plasma insulin, glucagon, and corticosterone responses to the intracerebroventricular (i.c.v.)infusion of PACAP-38, VIP, and their receptor agonists in liver-intact and denervated animals. A: Intracerebroventricular infusion of PACAP-38, VIP, and VPAC2R agonist significantly increased plasma glucagon concentrations compared with the basal state and compared with the vehicle control. HSX, HPX, or shamX denervation has no influence on the stimulatory effect of PACAP-38 on plasma glucagon. B: In the same animals, only the plasma insulin increase in the sham-denervated animals reached statistical significance. C: All groups showed increased plasma corticosterone concentrations (compared with basal), but no difference was found among the different drug infusion groups, although all differed significantly from the vehicle infusion. Data are means ± SEM. #P < 0.05 vs. basal state, *P < 0.05 vs. vehicle control.
DISCUSSION
The present results clearly show that hypothalamic PACAP signaling is tightly involved in the control of glucose metabolism. Intracerebroventricular administration of PACAP-38 causes a pronounced increase in EGP and lasting hyperglycemia. Follow-up experiments using intranuclear infusions, coinfusions of specific VIP/PACAP receptor (ant)agonists, Fos immunohistochemistry, retrograde tracing, and specific denervations of the autonomic liver innervation suggest a role for the hypothalamic PACAP system in the control of glucose metabolism via a specific central pathway involving VPAC2R and preautonomic neurons in the PVN.
Although PACAP and VIP share several similar functions, including stimulating the release of prolactin and other pituitary hormones (4), they are basically distinct peptides, with different origins and distributions in both brain and periphery. Hypothalamic VIP-ir fibers, including those in the PVN, almost solely derive from the SCN (33). On the other hand, PACAP-ir fibers are widely distributed within the hypothalamus, especially in its medial part (34). Moreover, the hypothalamic PACAP innervation is derived from different origins, including intrahypothalamic sources such as the VMH and extrahypothalamic sources such as the bed nucleus of the stria terminalis (9,35), brainstem (9,36), and retina (10). Despite the equal affinity of PACAP and VIP for the VPAC2R as shown by in vitro receptor binding studies (13), we found a quite different central effect of PACAP-38 and VIP on glucose metabolism. Similar differences were observed in other studies. In the adrenal gland, PACAP is ∼100-fold more potent than VIP in evoking secretion of catecholamine (37), and in the SCN, PACAP is 1,000-fold more potent than VIP at altering the phasing of the circadian rhythm (10) despite both tissues expressing VPAC2R (38,39).
Specific denervation of the sympathetic input to the liver obliterated the EGP increase produced by intracerebroventricular administration of PACAP-38. This indicates that PACAP-38 stimulation of hepatic glucose production partly depends on an intact hepatic sympathetic autonomic innervation. On the other hand, the only partial suppression of EGP in HSX indicates that PACAP-38 also uses other pathways to stimulate liver glucose production, such as increased corticosterone, epinephrine, and glucagon release by adrenal and pancreas. PACAP activation of the thoracic IML projecting preautonomic neurons in the PVN strongly suggests that the EGP stimulating action involves activation of liver projecting preautonomic neurons by PACAP-38 (40). This idea is supported by the presence of VPAC2R immunoreactivity in the dorsal and ventral PVN (41), the stimulatory effects of focal PVN infusions of PACAP-38 on plasma glucose concentrations and EGP, and the effects of HSX.
PACAP-38 also has stimulatory effects on glucose uptake, as the MCR induced by PACAP-38 was higher than in vehicle, and this probably indicates that PVN preautonomic neurons projecting to glucose-using tissues such as muscle and adipose tissue also are stimulated. MCR was not influenced by any of the denervation procedures; thus, when EGP was suppressed by HSX, the plasma glucose concentration also was lowered in the PACAP-38+HSX group (Fig. 5A). The VPAC2R-agonist induced the highest MCR response, and blocking VPAC2R, but not PAC1R, suppressed the PACAP-38-induced increase of MCR, suggesting that, in the CNS, PACAP-38 stimulates glucose disposal mainly via VPAC2R. Systemically, however, PAC1R seems to play a major role by regulating glucose disposal via direct actions in the pancreas and by changing insulin activity as based on data from PAC1R knockout animals (42). Thus, different CNS and peripheral PACAP/VIP receptors may play roles in retaining glucose homeostasis.
Glucose is produced in the liver by glycogenolysis and GNG. It is largely unknown which specific signals, besides circulating glucoregulatory hormones, control the balance between glycogenolysis and GNG. The present study shows that PACAP-38 increased glycogenolysis in line with early studies on the stimulatory effects of electrically stimulated sympathetic nerves on liver glycogenolysis (43).
At present it is unclear which group(s) of PACAP-containing neurons is responsible for the brain control of glucose metabolism. PACAP knockout animals have been shown to have an impaired counterregulatory response to hypoglycemia (44). Recovery from hypoglycemia is believed to be mainly processed by the VMH and the sympathoadrenal pathway (45). Because PACAP mRNA is highly expressed in the VMH (34), it is possible that activation of PACAP neurons in the VMH, and subsequently the PACAP-containing projections to the PVN, are involved in the hypothalamic mechanism necessary to stimulate hepatic glucose production upon hypoglycemic challenges. PACAP is also involved in acute and chronic stress responses via the PVN–corticotropin-releasing hormone neurons, the hypothalamic-adrenal–pituitary axis, and the sympathetic nervous system (46–49). The PACAP system could thus be an important gateway to control hepatic glucose production during stress (46–49). Finally, as PACAP expression in the VMH is also under the influence of estrogen (50) and leptin (51), the currently revealed glucoregulatory effects of PACAP might also be part of a brain circuit that connects the reproductive and adiposity systems with energy metabolism.
In summary, we present a neuronal pathway by which PACAP-38 activates hypothalamic preautonomic neurons that control sympathetic nerves innervating the liver, resulting in a hyperglycemia almost entirely due to an increase in hepatic glycogenolysis. Future experiments should reveal the specific physiological stimuli and PACAP neurons responsible for activation of these glucoregulatory neurons.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Dutch Diabetes Fonds (2004.00.027) and a grant from the Royal Netherlands Academy of Arts and Sciences for a joint project between the Netherlands and China (07CDP034).
No potential conflicts of interest relevant to this article were reported.
We thank Wilma Verweij for her help with correcting the English, An Ruiter, Hannah M Eggink, and Cathy Cailotto for their technical assistance, and Jilles Timmer for the husbandry of the experimental animals.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Horvath TL, Andrews ZB, Diano S: Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab 2009;20:78–87 [DOI] [PubMed] [Google Scholar]
- 2.Levin BE: Glucosensing neurons do more than just sense glucose. Int J Obes Relat Metab Disord 2001;25Suppl. 5:S68–72 [DOI] [PubMed] [Google Scholar]
- 3.Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS: Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 2009;58:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH: Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 1989;164:567–574 [DOI] [PubMed] [Google Scholar]
- 5.Sherwood NM, Adams BA, Isaac ER, Wu S, Fradinger EA: Knocked down and out: PACAP in development, reproduction and feeding. Peptides 2007;28:1680–1687 [DOI] [PubMed] [Google Scholar]
- 6.Sherwood NM, Krueckl SL, McRory JE: The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 2000;21:619–670 [DOI] [PubMed] [Google Scholar]
- 7.Arimura A, Somogyvári-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C: Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 1991;129:2787–2789 [DOI] [PubMed] [Google Scholar]
- 8.Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M: Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res 1993;602:57–63 [DOI] [PubMed] [Google Scholar]
- 9.Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM: Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci 2005;25:4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD: Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci 1997;17:2637–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto H, Ishihara T, Shigemoto R, Mori K, Nagata S: Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron 1993;11:333–342 [DOI] [PubMed] [Google Scholar]
- 12.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S: Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron 1992;8:811–819 [DOI] [PubMed] [Google Scholar]
- 13.Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ: The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett 1993;334:3–8 [DOI] [PubMed] [Google Scholar]
- 14.Couvineau A, Maoret JJ, Rouyer-Fessard C, Carrero I, Laburthe M: Cloning and functional characterization of the human VIP1/PACAP receptor promoter. Ann N Y Acad Sci 1998;865:59–63 [DOI] [PubMed] [Google Scholar]
- 15.Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL: N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J Neuroendocrinol 1999;11:941–949 [DOI] [PubMed] [Google Scholar]
- 16.Usdin TB, Bonner TI, Mezey E: Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology 1994;135:2662–2680 [DOI] [PubMed] [Google Scholar]
- 17.Nomura M, Ueta Y, Serino R, Yamamoto Y, Shibuya I, Yamashita H: Effects of centrally administered pituitary adenylate cyclase-activating polypeptide on c-fos gene expression and heteronuclear RNA for vasopressin in rat paraventricular and supraoptic nuclei. Neuroendocrinology 1999;69:167–180 [DOI] [PubMed] [Google Scholar]
- 18.Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jégou S: Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 2009;34:424–435 [DOI] [PubMed] [Google Scholar]
- 19.Yi CX, Serlie MJ, Ackermans MT, Foppen E, Buijs RM, Sauerwein HP, Fliers E, Kalsbeek A: A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes 2009;58:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM: Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 2004;24:7604–7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, Spanswick D: Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol 2003;549:809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellerstein MK, Neese RA, Linfoot P, Christiansen M, Turner S, Letscher A: Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J Clin Invest 1997;100:1305–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermans MT, Pereira Arias AM, Bisschop PH, Endert E, Sauerwein HP, Romijn JA: The quantification of gluconeogenesis in healthy men by 2H2O and [2-13C]glycerol yields different results: rates of gluconeogenesis in healthy men measured with 2H2O are higher than those measured with [2-13C]glycerol. J Clin Endocrinol Metab 2001;86:2220–2226 [DOI] [PubMed] [Google Scholar]
- 24.Bandsma RH, Grefhorst A, van Dijk TH, van der Sluijs FH, Hammer A, Reijngoud DJ, Kuipers F: Enhanced glucose cycling and suppressed de novo synthesis of glucose-6-phosphate result in a net unchanged hepatic glucose output in ob/ob mice. Diabetologia 2004;47:2022–2031 [DOI] [PubMed] [Google Scholar]
- 25.Hellerstein MK, Neese RA: Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. Am J Physiol 1992;263:E988–1001 [DOI] [PubMed] [Google Scholar]
- 26.Yi CX, Challet E, Pévet P, Kalsbeek A, Escobar C, Buijs RM: A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats. Eur J Neurosci 2008;27:1965–1972 [DOI] [PubMed] [Google Scholar]
- 27.Steele R: Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959;82:420–430 [DOI] [PubMed] [Google Scholar]
- 28.Gourlet P, Vandermeers A, Vertongen P, Rathe J, De Neef P, Cnudde J, Waelbroeck M, Robberecht P: Development of high affinity selective VIP1 receptor agonists. Peptides 1997;18:1539–1545 [DOI] [PubMed] [Google Scholar]
- 29.Langer I, Gregoire F, Nachtergael I, De Neef P, Vertongen P, Robberecht P: Hexanoylation of a VPAC2 receptor-preferring ligand markedly increased its selectivity and potency. Peptides 2004;25:275–278 [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Mojsov S: Multiple human receptors for pituitary adenylyl cyclase-activating polypeptide and vasoactive intestinal peptide are expressed in a tissue-specific manner. Ann N Y Acad Sci 1996;805:624–627 [DOI] [PubMed] [Google Scholar]
- 31.Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J: Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem 1992;207:239–246 [DOI] [PubMed] [Google Scholar]
- 32.Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P: Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC(2) receptor. Peptides 2000;21:1543–1549 [DOI] [PubMed] [Google Scholar]
- 33.Card JP, Brecha N, Karten HJ, Moore RY: Immunocytochemical localization of vasoactive intestinal polypeptide-containing cells and processes in the suprachiasmatic nucleus of the rat: light and electron microscopic analysis. J Neurosci 1981;1:1289–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannibal J: Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 2002;453:389–417 [DOI] [PubMed] [Google Scholar]
- 35.Köves K, Arimura A, Görcs TG, Somogyvári-Vigh A: Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology 1991;54:159–169 [DOI] [PubMed] [Google Scholar]
- 36.Das M, Vihlen CS, Legradi G: Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol 2007;500:761–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo X, Wakade AR: Differential secretion of catecholamines in response to peptidergic and cholinergic transmitters in rat adrenals. J Physiol 1994;475:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC: Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology 2004;145:1203–1210 [DOI] [PubMed] [Google Scholar]
- 39.Ajpru S, McArthur AJ, Piggins HD, Sugden D: Identification of PAC1 receptor isoform mRNAs by real-time PCR in rat suprachiasmatic nucleus. Brain Res Mol Brain Res 2002;105:29–37 [DOI] [PubMed] [Google Scholar]
- 40.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM: Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res 2000;871:50–56 [DOI] [PubMed] [Google Scholar]
- 41.Kalló I, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW: Transgenic approach reveals expression of the VPAC2 receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur J Neurosci 2004;19:2201–2211 [DOI] [PubMed] [Google Scholar]
- 42.Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, Ahrén B, Brabet P: PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest 2000;105:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimazu T, Fukuda A: Increased activities of glycogenolytic enzymes in liver after splanchnic-nerve stimulation. Science 1965;150:1607–1608 [DOI] [PubMed] [Google Scholar]
- 44.Gray SL, Cummings KJ, Jirik FR, Sherwood NM: Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol 2001;15:1739–1747 [DOI] [PubMed] [Google Scholar]
- 45.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI: Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 1994;93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal A, Halvorson LM, Legradi G: Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 2005;138:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinevich V, Fournier A, Pelletier G: Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res 1997;773:190–196 [DOI] [PubMed] [Google Scholar]
- 48.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V: Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 2009;34:833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Légrádi G, Hannibal J, Lechan RM: Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett 1998;246:145–148 [DOI] [PubMed] [Google Scholar]
- 50.Apostolakis EM, Lanz R, O'Malley BW: Pituitary adenylate cyclase-activating peptide: a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol Endocrinol 2004;18:173–183 [DOI] [PubMed] [Google Scholar]
- 51.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM: PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 2009;29:14828–14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.