Abstract
OBJECTIVE
Depletion of body fat stores during uncontrolled, insulin-deficient diabetes (uDM) results in markedly reduced plasma leptin levels. This study investigated the role of leptin deficiency in the genesis of severe insulin resistance and related metabolic and neuroendocrine derangements induced by uDM.
RESEARCH DESIGN AND METHODS
Adult male Wistar rats remained nondiabetic or were injected with the β-cell toxin, streptozotocin (STZ) to induce uDM and subsequently underwent subcutaneous implantation of an osmotic minipump containing either vehicle or leptin at a dose (150 μg/kg/day) designed to replace leptin at nondiabetic plasma levels. To control for leptin effects on food intake, another group of STZ-injected animals were pair fed to the intake of those receiving leptin. Food intake, body weight, and blood glucose levels were measured daily, with body composition and indirect calorimetry performed on day 11, and an insulin tolerance test to measure insulin sensitivity performed on day 16. Plasma hormone and substrate levels, hepatic gluconeogenic gene expression, and measures of tissue insulin signal transduction were also measured.
RESULTS
Physiologic leptin replacement prevented insulin resistance in uDM via a mechanism unrelated to changes in food intake or body weight. This effect was associated with reduced total body fat and hepatic triglyceride content, preservation of lean mass, and improved insulin signal transduction via the insulin receptor substrate–phosphatidylinositol-3-hydroxy kinase pathway in the liver, but not in skeletal muscle or adipose tissue. Although physiologic leptin replacement lowered blood glucose levels only slightly, it fully normalized elevated plasma glucagon and corticosterone levels and reversed the increased hepatic expression of gluconeogenic enzymes characteristic of rats with uDM.
CONCLUSIONS
We conclude that leptin deficiency plays a key role in the pathogenesis of severe insulin resistance and related endocrine disorders in uDM. Treatment of diabetes in humans may benefit from correction of leptin deficiency as well as insulin deficiency.
Recent evidence implicates leptin not only in the regulation of energy balance but also in glucose homeostasis as well. In addition to hyperphagia and obesity, insulin resistance is a prominent feature of animal models characterized by reduced leptin signaling (1), and leptin administration improves insulin sensitivity and glucose metabolism in these models (2,3) independently of its effects on energy homeostasis (4). Investigation into the role of leptin in glucose metabolism has focused largely on genetic models of impaired leptin signaling (e.g., leptin-deficient ob/ob mice), whereas other studies have used pharmacologic doses of leptin (1–6). In this study we investigated the physiologic role of leptin in glucose metabolism by determining the contribution made by leptin deficiency to the severe insulin resistance and associated metabolic and endocrine dysfunction characteristic of uncontrolled, insulin-deficient diabetes (uDM).
Severe leptin deficiency is a well-documented consequence of uDM that occurs after destruction of insulin-secreting β-cells (7,8). Because insulin is required for the synthesis and storage of triglyceride in adipose tissue, weight gain cannot occur in uDM, and the associated loss of body fat is accompanied by markedly reduced plasma leptin levels. This effect, in turn, is implicated in the mechanism whereby uDM increases food intake (9), because exogenous leptin administration at doses that prevent a fall in plasma leptin levels also prevent hyperphagia in uDM (8). Another feature of uDM in humans is progressive, severe insulin resistance (10–12), an effect also observed in streptozotocin (STZ)-induced diabetes in rats (13). Although insulin deficiency clearly underlies hyperglycemia and weight loss in uDM, the contribution of markedly reduced plasma leptin levels to insulin resistance and related metabolic and endocrine derangements in this setting remains to be determined. Because plasma levels of leptin as well as of insulin are normalized by insulin treatment of STZ-induced diabetes, at least some of the beneficial effects that have been ascribed to insulin treatment could result from restoring leptin action to normal (7). Indeed, hyperleptinemia generated either by pharmacologic administration of leptin (5) or with adenoviral gene therapy (14) ameliorates hyperglycemia and associated increases of plasma glucagon levels in STZ-induced diabetes, despite persistently low insulin levels. These data raise the possibility that deficient endogenous leptin signaling may underlie at least some manifestations of uDM.
Based on these considerations, we sought to determine the extent to which deficiency of endogenous leptin contributes to insulin resistance and related endocrine dysfunction in STZ-induced diabetes. To accomplish this goal, we subcutaneously infused either vehicle or leptin at a dose that prevents leptin deficiency in rats with STZ-induced uDM. We found that physiologic leptin replacement prevented the development of insulin resistance in uDM via a mechanism independent of its effects on food intake and body weight. Moreover, this leptin effect was associated with normalization of elevated plasma levels of glucagon and corticosterone, with the reversal of increased hepatic expression of the gluconeogenic genes, glucose-6-phosphatase (G6Pase), and phosphoenolpyruvate kinase (Pepck), and with improved insulin signal transduction via the insulin receptor substrate–phosphatidylinositol-3-hydroxy kinase (IRS-PI3K) pathway in the liver, but not in skeletal muscle or adipose tissue. In comparison, physiologic leptin replacement only modestly reduced hyperglycemia in STZ-induced diabetic rats and did not alter the potent upregulation of hepatic Igfbp2 mRNA levels previously reported (15). Taken together, these data suggest that reduced leptin levels contribute to the progressive, severe insulin resistance characteristic of uDM via a mechanism that appears to predominantly involve the liver.
RESEARCH DESIGN AND METHODS
Adult male Wistar rats (Harlan, Indianapolis, IN) were housed in individual cages under specific pathogen–free conditions, maintained in a temperature-controlled room with a 12:12-h light/dark cycle and provided with ad libitum access to water and standard laboratory chow (PMI Nutrition International) unless otherwise stated. All procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Care Committee at the University of Washington.
Effect of physiologic leptin replacement during STZ-induced leptin deficiency.
To induce uDM, rats received two consecutive daily subcutaneous injections (40 mg/kg) of freshly prepared STZ (Sigma) dissolved in ice-cold sodium citrate (pH 4.5). Subsequently, under isoflurane anesthesia, two groups of diabetic animals (n = 7–9 per group) received a subcutaneously implanted osmotic minipump (Alzet model 2ML4; DURECT) containing either vehicle (STZ-veh) or leptin (STZ-lep) at a dosage (150 μg/kg/day) calculated to restore plasma leptin levels to nondiabetic control values. Leptin was obtained from Dr. A.F. Parlow (National Hormone and Peptide Program) and diluted in PBS (pH 7.9). A control nondiabetic group (veh-veh) received sodium citrate rather than STZ, followed by implantation of a subcutaneous osmotic minipump containing vehicle (PBS). Finally, to control for the effect of leptin to reduce food intake in rats with STZ-induced diabetes (8), an additional group of diabetic animals receiving vehicle were pair fed (STZ-veh-PF) to match the intake of STZ-lep–treated animals as previously described (6). Food intake, body weight, and blood glucose levels were measured daily during the mid-light cycle for 18 days.
The experimental paradigm described above was subsequently repeated in separate groups of animals, with the exception that an indwelling catheter was inserted into the left carotid artery as previously described (16), before STZ administration to permit blood sampling from conscious, unstressed rats for determination of plasma glucagon, catecholamine, and corticosterone levels.
Body composition and indirect calorimetry.
Determinations of body lean and fat mass were made on a separate group of animals using quantitative magnetic resonance 11 days after STZ administration (EchoMRI-700TM; Echo Medical Systems, Houston, TX). Locomotor activity was assessed by the infrared beam breaks using an Opto-Varimetrix-3 sensor system (Columbus Instruments, Columbus, OH). Indirect calorimetry was performed on this same group of animals using a computer-controlled calorimetry system (Oxymax; Columbus Instruments) in habituated animals as previously described (17).
Measurement of insulin sensitivity.
To determine the effect of leptin replacement on STZ-induced insulin resistance, animals from each group were subjected to an insulin tolerance test (ITT) on day 16 after STZ injection. Briefly, after a 3-h fast mid-light cycle, animals received an injection of insulin (2 units/kg i.p., Humulin R; Lilly), and glucose levels were determined on tail-vein blood samples using a hand-held glucometer (Accu-Chek; Roche) at time 0 and at 30-min intervals over 120 min.
Blood and urine collection and assay.
Urinary glucose was measured using a GM9D glucose direct analyzer (Analox Instruments, London, U.K.) on urine samples obtained from animals 14 days after STZ administration. Tail-vein blood samples were collected in chilled EDTA-treated tubes for measurement of plasma insulin and leptin on days 0, 1, 2, 3, 4, 7, and 16, and nonesterified free fatty acids (NEFAs) on day 16. On day 16, arterial blood for catecholamine, corticosterone, and glucagon assays was collected from conscious, freely moving animals and placed into tubes containing EGTA/glutathione, EDTA, and benzamidine (10 μl of 1 mol/l)/heparin (1 unit), respectively. Whole blood was centrifuged at 1,500 rpm for 20 min, plasma was removed, aliquoted, and stored at −20°C for subsequent analysis. Plasma immunoreactive insulin and leptin levels were determined by ELISA (Crystal Chem). Free fatty acids were measured using a colorimetric assay kit that relies on fatty acid as substrate for enzymatic acylation of CoA (Wako Chemicals). Catecholamine levels were measured in duplicate using a sensitive and specific radioenzymatic assay (18). Glucagon was assayed with a glucagon radioimmunoassay kit (Linco Research), and plasma corticosterone levels were measured using enzyme immunosorbent assay (Diagnostics Systems Laboratories).
Tissue processing and biochemical analysis.
To measure tissue insulin sensitivity, 18 days after STZ injection, animals were fasted for a 4-h mid-light cycle, injected with either vehicle or insulin (2 units/kg i.p.), and killed 15 min later. Liver, skeletal muscle (tibialis anterior), and white adipose tissue (WAT) (epididymal depot) were excised and snap frozen for subsequent analysis.
Tissues were homogenized in T-Per lysis buffer (10 μl/mg tissue) (Pierce) supplemented with protease and phosphatase inhibitor cocktails (Roche). Homogenates were centrifuged, pellets were discarded, supernatants were retained for determination of protein content using a Micro BCA protein assay kit (Pierce), and equal amounts of protein were used for each condition in each assay. Insulin-induced activation of PI3K was assessed in tissues by measuring serine phosphorylation of Akt (residue 473) using an ELISA assay (Invitrogen). Liver tissue triglyceride content was determined using quantitative magnetic resonance with the Echo 3-in-1 MRI machine (Echo Medical Systems).
RT-PCR.
Total RNA was extracted from liver and pancreas using TRIzol B (MRC), quantified by spectrophotometry (NanoDrop 1000; Thermo Scientific, IL), and reverse-transcribed (1 μg) with avian myeloblastosis virus reverse transcriptase (Promega). RT-PCR was then performed on an ABI Prism 7900 HT (Applied Biosystems) using SYBR Green Master Mix (Applied Biosystems). PCR data were analyzed using the Sequence Detection System software (SDS version 2.2; Applied Biosystems). Expression levels of each gene were normalized to a housekeeping gene (18S RNA) and expressed as a percentage of veh-veh controls. Nontemplate controls were incorporated into each PCR run.
Statistical analysis.
All results are expressed as means ± SEM. Statistical analyses were performed using Statistica (version 7.1; StatSoft). A one-way analysis of variance with a least significant difference post hoc test was used to compare mean values among multiple groups, and a two-sample unpaired Student t test was used for two-group comparisons. In all instances, P < 0.05 was considered significant.
RESULTS
Effect of physiologic leptin replacement on glucose and energy homeostasis during STZ-induced diabetes.
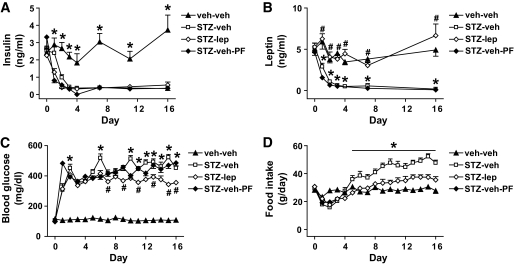
As expected (8), plasma insulin levels were dramatically reduced by day 2 in all animals that received STZ relative to nondiabetic controls, and they remained very low throughout the duration of the study (Fig. 1A). The effect of STZ on plasma leptin levels closely paralleled its effect on plasma insulin levels, with marked reductions observed by day 2 in diabetic animals receiving subcutaneous vehicle (Fig. 1B). By design, STZ-induced diabetic animals that received continuous subcutaneous leptin exhibited plasma leptin levels comparable with nondiabetic control values throughout the study (Fig. 1B).
FIG. 1.
Physiologic leptin replacement attenuates diabetic hyperglycemia and diabetic hyperphagia in STZ-treated rats. Plasma insulin (A), plasma leptin (B), blood glucose (C), and mean daily food intake (D) in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (□) or pair fed (♦), a physiologic replacement dose of leptin (♢) or nondiabetic controls (▲). *P < 0.05 vs. STZ-lep; #P < 0.05 vs. STZ-veh-PF.
As expected, the reduction of plasma insulin levels in animals made diabetic by STZ caused marked hyperglycemia (Fig. 1C). Although marked hyperglycemia was also observed in STZ-treated animals that received subcutaneous leptin, the magnitude of this effect was modestly reduced compared with that in diabetic animals receiving vehicle (P < 0.05), indicating that leptin deficiency exacerbates (but is not a major cause of) hyperglycemia in rats with uDM. This modest glucose-lowering effect of leptin replacement, however, cannot be explained by reduced food intake, because blood glucose was not reduced in STZ-induced diabetic animals that were pair fed to the intake of STZ-induced diabetic rats receiving subcutaneous leptin. By comparison, blood glucose levels remained within the normal range in nondiabetic controls.
In diabetic animals receiving subcutaneous vehicle, food intake was increased by day 6 after STZ administration and remained elevated compared with nondiabetic controls (Fig. 1D). In comparison, consistent with previous reports (8), diabetic hyperphagia was prevented by maintaining plasma leptin levels in the physiologic range, such that food intake was comparable with that of nondiabetic controls (8) (Fig. 1D).
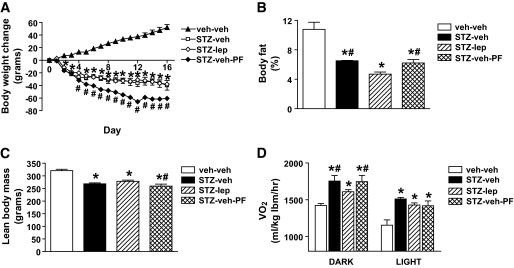
Although body weight gradually increased in nondiabetic rats during the study, it decreased immediately after diabetes onset in STZ-treated rats receiving vehicle and remained significantly below baseline values despite their pronounced hyperphagia (Fig. 2A). STZ-induced diabetic animals that were pair fed to the intake of leptin-treated diabetic rats exhibited even greater weight loss than diabetic rats fed ad libitum, owing to their reduced food intake. Interestingly, STZ-induced diabetic rats that received subcutaneous leptin lost significantly less weight than STZ-veh-PF animals, maintaining weight comparable with diabetic rats fed ad libitum, despite consuming less food.
FIG. 2.
Physiologic leptin replacement reduces fat mass and attenuates increased energy expenditure in STZ-treated rats. Body weight change (A), percent body fat mass (B), lean body mass (C), and Vo2 (D) measured using quantitative magnetic resonance and indirect calorimetry, respectively, in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (□) or pair fed (♦), a physiologic replacement dose of leptin (♢) or nondiabetic controls (▲). *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep. lbm, lean body mass.
To explore the basis for these body weight changes, we subjected a separate cohort of animals to the same protocol and measured body composition analysis and indirect calorimetry 11 days after STZ administration. We found that leptin treatment caused a greater loss of body fat mass in STZ-induced diabetic animals than was observed in those receiving vehicle (Fig. 2B), but also spared lean body mass relative to STZ-veh-PF animals (Fig. 2C). Moreover, using indirect calorimetry, we found that Vo2 was markedly increased in vehicle-treated STZ-induced diabetic animals relative to nondiabetic controls, regardless of whether they were fed ad libitum or pair fed and that this hypermetabolic effect was attenuated by physiologic leptin replacement (Fig. 2D). However, the increased metabolic rate in STZ-induced diabetic animals could not be attributed to changes in ambulatory activity (number of beam breaks: 2,953 ± 243 for veh-veh vs. 1,562 ± 87 for STZ-veh vs. 2,215 ± 163 for STZ-lep vs. 2,927 ± 207 for STZ-veh-PF). Respiratory quotient and metabolic heat production are not reported because the assumptions involved in using the respiratory quotient to estimate heat production are not met in abnormal metabolic states, including those characterized by a shift to ketone utilization as a metabolic substrate or during rapid depletion of protein and fat stores (19,20). Nonetheless, these data collectively suggest that increased energy expenditure in STZ-veh-PF relative to STZ-lep-treated animals probably contributes to their excessive weight loss and depletion of lean mass, despite consuming equal amounts of food.
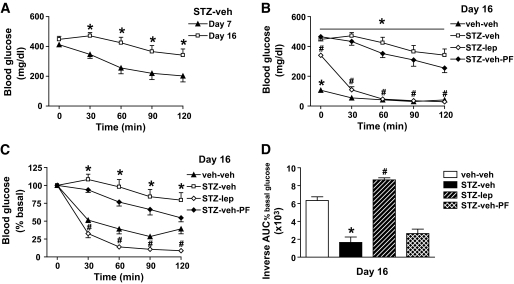
Effect of physiologic leptin replacement on insulin sensitivity in rats with STZ-induced diabetes.
Consistent with a previous report that insulin resistance is progressive over time in rats with STZ-induced diabetes (13), we confirmed that the glucose-lowering effect of insulin was markedly diminished on day 16 compared with day 7 after STZ administration (P < 0.05) (Fig. 3A). To determine whether reduced plasma leptin levels contribute to this progressive insulin resistance, animals that received either vehicle or a physiologic replacement dose of leptin were subjected to an ITT 16 days after induction of uDM with STZ. Relative to STZ-treated animals receiving vehicle, the ability of insulin to reduce blood glucose was dramatically enhanced in those that received leptin (P < 0.05) (Fig. 3B). To account for differences in the level of hyperglycemia at the onset of the ITT, insulin-induced changes of blood glucose were also analyzed as a percentage of basal values (Fig. 3C). Regardless of whether blood glucose levels were analyzed as absolute values or as percent basal, physiologic leptin replacement in STZ-induced diabetes markedly increased insulin sensitivity via a mechanism that could not be attributed to changes in food intake, since it was not observed in STZ-veh-PF animals (Fig. 3B–D).
FIG. 3.
Physiologic leptin replacement improves insulin sensitivity in STZ-treated rats. A: Blood glucose levels in STZ-induced diabetic animals on day 7 (▲) and 16 (□) after STZ-injection during an insulin tolerance test (2 units/kg). *P < 0.05 vs. day 7. Blood glucose levels (B), percent basal blood glucose levels (C), and the inverse integrated area under the percent basal glucose curve (D) in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (□) or pair fed (♦), a physiologic replacement dose of leptin (♢) or nondiabetic controls (▲) during an ITT (2 units/kg) *P < 0.05 vs. STZ-lep; #P < 0.05 vs. STZ-veh-PF.
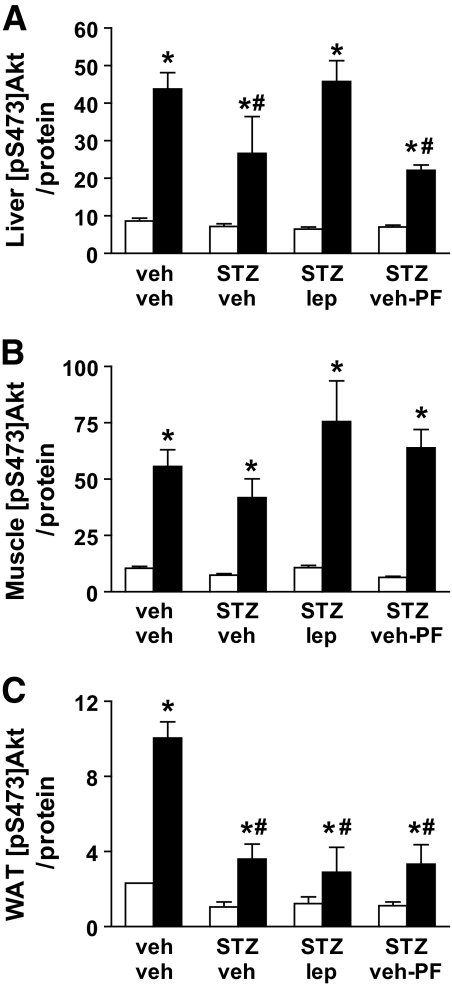
Effects of STZ-induced diabetes with or without physiologic leptin replacement on insulin signal transduction.
To further characterize the impact of physiologic leptin replacement on insulin action in uDM, we examined insulin-induced activation of the IRS-PI3K-Akt signal transduction pathway in liver, muscle, and WAT. After intraperitoneal saline, there were no differences in levels of pS473-Akt in any tissue among treatment groups, but, as expected, systemic insulin injection significantly increased levels of pS473-Akt in liver, muscle, and WAT in all groups compared with saline (P < 0.05) (Fig. 3). In liver tissue, the ability of insulin to increase levels of pS473-Akt was reduced in STZ-treated animals receiving vehicle compared with nondiabetic controls, and this effect was prevented by physiologic leptin replacement (Fig. 4A). Furthermore, because insulin-stimulated production of pS473-Akt was also reduced in STZ-veh-PF livers, the effect of leptin to normalize this response cannot be explained by reduced food intake. In contrast, insulin-induced activation of pS473-Akt in skeletal muscle was not affected by either STZ-induced diabetes or by leptin administration (Fig. 4B). Finally, although insulin significantly increased pS473-Akt in WAT of animals in each group relative to saline-injected controls, the response to insulin was significantly attenuated in all STZ-induced diabetic animals relative to that in nondiabetic controls (Fig. 4C).
FIG. 4.
Physiologic leptin replacement increases hepatic insulin signal transduction. Effect of intraperitoneal (2 units/kg) insulin (■)-induced activation of serine phosphorylation of Akt compared with vehicle (□) in liver (A), muscle (tibialis anterior) (B), and WAT (epididymal fat) (C) in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (STZ-veh) or pair fed (STZ-veh-PF) a physiologic replacement dose of leptin (STZ-lep) or nondiabetic controls (veh-veh). *P < 0.05 vs. veh-veh-veh; #P < 0.05 vs. veh-veh-ins.
Potential mechanisms whereby leptin deficiency causes insulin resistance in STZ-induced diabetes.
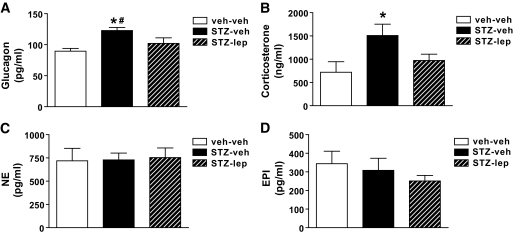
Uncontrolled diabetes is associated with increased circulating levels of both glucagon and corticosterone, and these responses are implicated in diabetes manifestations including insulin resistance, hyperglycemia, and hyperphagia (21–23). Based on recent evidence that adenovirally induced hyperleptinemia ameliorates hyperglycemia via normalization of elevated plasma glucagon levels (14), we determined whether maintaining physiologic leptin levels also is sufficient to prevent diabetes-induced increases of plasma glucagon and broadened the hypothesis to include other counterregulatory hormones. Consistent with the aforementioned findings (14), plasma glucagon levels were elevated in STZ-treated animals receiving vehicle relative to that in nondiabetic controls, and this elevation was prevented by physiologic leptin replacement (Fig. 5A). Thus, leptin deficiency appears to be required for the effect of uDM to raise glucagon levels. Similarly, plasma corticosterone levels were increased in STZ-treated rats, and this effect was also attenuated by leptin replacement (Fig. 5B). In contrast, plasma norepinephrine and epinephrine levels were similar across treatment groups (Fig. 5C and D). Normalization of both plasma glucagon and corticosterone levels may therefore contribute to improved hepatic insulin sensitivity and modest reduction of plasma glucose induced by leptin replacement, but catecholamines are unlikely to be involved.
FIG. 5.
Physiologic leptin replacement reduces hyperglucagonemia and hypercorticosteronemia. Arterial plasma glucagon (A), corticosterone (B), norepinephrine (NE) (C), and epinephrine (EPI) (D) levels in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving either vehicle (STZ-veh) or a physiologic replacement dose of leptin (STZ-lep). *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep.
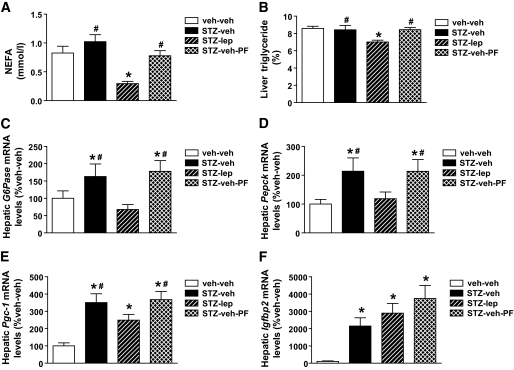
To investigate whether changes in plasma or tissue lipid accumulation might contribute to the effect of leptin on insulin sensitivity, we measured both plasma NEFA levels and triglyceride content in liver. We found no differences in either plasma NEFA levels (day 16) or liver triglyceride content in STZ-veh–treated animals compared with nondiabetic controls, regardless of whether they were fed ad libitum or pair fed, but these levels were both reduced in diabetic animals that received leptin (Fig. 6A and B).
FIG. 6.
Physiologic leptin replacement reduces plasma and hepatic lipid content and gluconeogenic gene expression. Plasma NEFAs obtained from tail-vein samples (A), hepatic triglyceride content (B) and expression of G6Pase (C), Pepck (D), Pgc-1α (E), and Igfbp2 (F) using real-time PCR in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (STZ-veh) or pair fed (STZ-veh-PF) or a physiologic replacement dose of leptin (STZ-lep). *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep.
To investigate the role of leptin deficiency in the effect of uDM to increase hepatic glucose production and hepatic insulin resistance (24), we used real-time PCR to measure hepatic expression of mRNA encoding G6Pase and Pepck in the absence of insulin injection. As expected, levels of both mRNA species were elevated in diabetic animals that received vehicle relative to nondiabetic controls (Fig. 6C and D). In contrast, hepatic expression of both G6Pase and Pepck genes was comparable between diabetic animals receiving leptin and nondiabetic controls and was significantly below vehicle-treated diabetic animals. This effect is not attributable to reduced food intake, since pair feeding was without effect (Fig. 6C and D). A similar pattern of hepatic expression was seen for mRNA encoding peroxisome proliferator–activated receptor-γ–coactivator-1α (PGC-1α), a transcriptional coregulator implicated in the activation of both G6Pase and Pepck. Thus, leptin deficiency is required for increased hepatic expression of gluconeogenic genes in uDM. By comparison, Igfbp2, a recently reported hepatic gene implicated in the action of leptin to suppress hepatic glucose production (15), was increased >20-fold in STZ-induced diabetic rats relative to nondiabetic controls, and this diabetes-induced upregulation was not affected by leptin treatment (Fig. 6F).
DISCUSSION
Growing evidence suggests that either impaired or deficient leptin signaling results in the development of insulin resistance and impaired glucose metabolism (1,4–6,25). Here, we report that leptin deficiency also contributes to the development of progressive insulin resistance and associated neuroendocrine derangements in uDM. We found that systemic administration of exogenous leptin at a dose that maintains normal physiologic plasma leptin levels prevented the development of severe, progressive insulin resistance in rats with uDM and that this effect could not be explained by leptin-induced changes in food intake or body weight. Moreover, the mechanism underlying this effect appears to preferentially involve the liver, as physiologic leptin replacement in uDM reduced hepatic triglyceride content and gluconeogenic gene expression and also restored insulin signal transduction to normal in liver, but not in skeletal muscle or WAT. In addition, physiologic leptin replacement in uDM reduced body fat while sparing lean mass, attenuated the increased energy expenditure that accompanies uDM (26), and normalized elevated levels of plasma glucagon and corticosterone. Taken together, these findings implicate leptin deficiency as a cause of wide-ranging metabolic and neuroendocrine derangements associated with uDM.
Although insulin resistance is a well-documented complication of uDM (10–12,27), few studies have sought to identify the underlying mechanism. Some investigators have postulated a role for hyperglycemia, based on a model in which “glucose toxicity” impairs insulin signal transduction in peripheral tissues (28,29). Consistent with this, insulin resistance in individuals with type 1 diabetes is attenuated when glycemic control is improved (30,31), whereas uncontrolled hyperglycemia induces insulin resistance in these individuals (32,33). Our finding that physiologic leptin replacement prevented uDM-induced insulin resistance with only very modest effects to reduce diabetic hyperglycemia, however, suggests that in this study, hyperglycemia per se is unlikely to play a major role. Although reduced food intake might also be expected to contribute to the insulin-sensitizing effects of leptin, this possibility is inconsistent with our finding that diabetic animals pair fed to the intake of those receiving leptin failed to exhibit an improvement of insulin sensitivity.
In light of the potent insulin-sensitizing effect of leptin replacement in rats with uDM, it is reasonable to ask why the effect on hyperglycemia was so modest. Presumably this reflects the severe insulin deficiency characteristic of this model, such that improved insulin sensitivity has little impact on ambient glucose levels in the absence of insulin therapy. This observation also highlights an important distinction between the effect of physiologic leptin replacement we observed in the current work, and recent work examining the effect of pharmacologic hyperleptinemia in uDM (5,14). In the latter, induction of very high circulating leptin levels in rodents with uDM resulted in full normalization of hyperglycemia, whereas this clearly was not the case in our study. Thus, normalization of hyperglycemia in uDM cannot be achieved simply by physiologic replacement of endogenous leptin, but apparently requires pharmacologic levels of leptin.
Complementing our finding that physiologic leptin replacement enhances insulin-mediated glucose lowering in STZ-induced diabetic animals, we found that this intervention also restored insulin signaling in liver, but not in skeletal muscle or adipose tissue, as measured by insulin-induction of pAkt. The mechanisms underlying this improvement in hepatic insulin signaling remain uncertain but could involve reductions of body fat mass and/or liver triglyceride content. Alternatively, we recently reported that leptin action in the hypothalamic arcuate nucleus improves hepatic insulin action via a mechanism involving the hepatic vagus nerve (6). Thus, additional studies are warranted to determine whether this autonomic mechanism might also contribute to the improved insulin sensitivity after physiologic leptin replacement in uDM.
Studies have shown that uDM is associated with increased plasma glucagon levels and elevated hepatic expression of the gluconeogenic genes G6Pase and Pepck, and these responses are implicated in the pathogenesis of insulin resistance (34,35) and diabetic hyperglycemia (21,22). Our finding that physiologic leptin replacement in uDM dramatically improved insulin sensitivity and normalized elevated levels of both plasma glucagon and hepatic expression of these gluconeogenic genes is consistent with this hypothesis. A recent study reported that hyperleptinemia induced by an adenoviral gene therapy approach ameliorated hyperglycemia in STZ-induced diabetes, and this was hypothesized to occur via a suppression of hyperglucagonemia and consequent reduction of hepatic gluconeogenic gene expression (14). Previous studies, however, have not established the extent to which hyperglycemia in uDM might be driven by increases of glucagon and associated changes of glucose production. In this context, we note that although plasma glucagon levels and hepatic expression of G6Pase and Pepck were normalized by physiologic leptin replacement in our study, hyperglycemia was only slightly improved compared with that in diabetic animals that received vehicle. These data suggest that normalization of plasma glucagon levels plays only a minor role in leptin's antidiabetic effects, although it remains possible that hyperglucagonemia contributes to progressive insulin resistance in uDM. Further, our findings identify for the first time a role for leptin deficiency in the effect of uDM to raise circulating glucagon levels.
Hyperglucagonemia and the energy cost associated with increased gluconeogenesis are implicated in the increased metabolic rate in patients with poorly controlled type 1 diabetes, an effect that is reversed by insulin treatment (26). Such a mechanism may also explain the marked increase in energy expenditure in STZ-induced diabetic rats relative to that of nondiabetic controls, as this cannot be explained by changes in activity. Changes in food intake are also unlikely to explain this metabolic response to uDM, because metabolic rate was increased similarly in STZ-veh and STZ-veh-PF groups. Although leptin has previously been demonstrated to increase energy expenditure, at least in part via sympathetic activation of brown adipose tissue (36), we found that the elevated metabolic rate induced by uDM was attenuated by leptin replacement. Although the mechanism underlying this leptin effect awaits further study, suppression of elevated rates of gluconeogenesis and perhaps other futile cycles seems likely. To our knowledge, this is the first demonstration of an effect of leptin to lower metabolic rate, and it reinforces the concept that the effect of leptin on metabolic regulation is highly context dependent. These data also help explain why leptin-treated diabetic animals lose weight in amounts similar to those for animals receiving vehicle, despite consuming much less food. Indeed, matching the intake of STZ-veh animals by pair feeding to that of leptin-replaced rats produced excessive weight loss. These findings collectively suggest that leptin deficiency is a major determinant of hypermetabolism induced by uDM.
It has been shown that uDM is also characterized by increased glucocorticoid secretion and activation of the hypothalamic-adrenal-pituitary (HPA) axis (37,38). Because glucocorticoids both inhibit peripheral glucose uptake in muscle and adipose tissue and induce hepatic expression of G6Pase and Pepck (39), HPA activation in uDM probably contributes to insulin resistance in this setting. Our finding that leptin replacement normalized elevated plasma corticosterone levels in STZ-induced diabetic rats provides a plausible mechanism to explain the observed improvement of insulin sensitivity but again suggests that increased circulating glucocorticoid levels are not a dominant cause of hyperglycemia in uDM. Also of interest here is the implication that leptin deficiency causes HPA activation in uDM, as has been suggested in other conditions (40). In contrast, we observed no effect of leptin replacement on plasma catecholamines.
Recently, it was reported that systemic administration of leptin upregulates hepatic expression of Igfbp2 and adenovirus-induced overexpression of IGFBP2 normalizes blood glucose levels in insulin-resistant and uDM mice (15). Our finding that hepatic expression of Igfpb2 was increased >20-fold in leptin-deficient STZ-induced diabetic animals relative to that in nondiabetic controls, however, suggests that increased hepatic expression of this gene is unlikely to exert salutary effects in the setting of uDM, an impression confirmed by the absence of any effect of leptin replacement on Igfpb2 mRNA levels. Thus, hepatic Igfbp2 is unlikely to explain the effect of physiologic leptin replacement to improve insulin sensitivity in uDM.
Several lines of evidence suggest that insulin resistance occurs in patients with type 1 diabetes (10–12,27) and is an independent risk factor for micro- and macrovascular complications in these patients (41,42). However, therapeutic approaches currently available for treating insulin resistance in type 1 diabetes are quite limited. Although intensive insulin therapy in individuals with type 1 diabetes improves insulin sensitivity and glycemic control, the Diabetes Control and Complication Trial (DCCT) reported that although intensive insulin therapy preserves pancreatic β-cell function and improves retinopathy and neuropathy, these benefits are offset by an increased frequency of severe hypoglycemia and weight gain (43–45). In contrast, treating type 1 diabetic subjects with lifestyle modifications, such as diet (46) and exercise (47), or pharmaceutical approaches using thiazolidinediones (48) or metformin (49), yield improvements in insulin sensitivity in type 1 diabetic subjects, but not glycemic control. Our current data raise the possibility that a combination therapy approach using both insulin and leptin treatment in type 1 diabetic patients may reduce the amount of insulin required to achieve good glycemic control and also limit weight gain (50).
In summary, we report that STZ-induced diabetes causes a rapid and severe reduction of plasma leptin levels that precedes the development of severe insulin resistance. The latter effect was prevented by restoration of plasma leptin to normal physiologic levels which also reduced hepatic triglyceride content and restored normal insulin signal transduction in the liver. Moreover, physiologic replacement of plasma leptin levels suppressed the characteristic increase of plasma glucagon and corticosterone levels and elevated hepatic expression of the gluconeogenic genes Pepck and G6Pase in uDM but only modestly reduced the extent of hyperglycemia. These findings also implicate leptin deficiency as a cause of hyperglucagonemia and suggest that although normalization of elevated plasma glucagon levels may contribute to the antidiabetic effects of pharmacologic leptin therapy, other factors must also be involved. We conclude that leptin deficiency plays a major role in the progressive, severe insulin resistance characteristic of uDM. This novel finding also raises the therapeutic possibility that supplementing insulin treatment with leptin may be a useful adjunct in the management of type 1 diabetes.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (grants DK-083042 and DK-052989 to M.W.S. and DK-050154-13 to G.J.T.), by the Clinical Nutrition Research Unit (grant DK-035816) at the University of Washington, by research funding from AstraZeneca, and by a Scientist Development Grant from the American Heart Association (to G.J.M.).
No potential conflicts of interest relevant to this article were reported.
Author contributions: J.P.G. and S.O.: research data, contributed to discussion, and reviewed/edited manuscript; B.E.W., J.P.T., D.A.S., G.J.T., and M.W.S.: contributed to discussion and reviewed/edited manuscript; K.O. and K.J.K.: research data and reviewed/edited manuscript; J.D.F. and M.E.M.: research data; G.J.M. research data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript.
Parts of this study were presented in poster form at the 70th annual meeting of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
We acknowledge the excellent technical assistance provided by Alex Cubelo, Charles Davis, and Iaela David at the University of Washington and Howard Chang from the VA Puget Sound Health Care System for performing the plasma glucagon and catecholamine measurements.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 2.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM: Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A 1997;94:8878–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P: Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546–549 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS: Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 1996;45:531–535 [DOI] [PubMed] [Google Scholar]
- 5.Chinookoswong N, Wang JL, Shi ZQ: Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes 1999;48:1487–1492 [DOI] [PubMed] [Google Scholar]
- 6.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW: Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2005;2:411–420 [DOI] [PubMed] [Google Scholar]
- 7.Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahrén B: Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol 1998;274:R1482–1491 [DOI] [PubMed] [Google Scholar]
- 8.Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW: Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes 1999;48:1275–1280 [DOI] [PubMed] [Google Scholar]
- 9.Booth DA: Some characteristics of feeding during streptoxotocin-induced diabetes in the rat. J Comp Physiol Psychol 1972;80:238–249 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Simonson D, Ferrannini E: Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1982;23:313–319 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Hendler R, Simonson D: Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 1982;31:795–801 [DOI] [PubMed] [Google Scholar]
- 12.Yki-Järvinen H, Koivisto VA: Natural course of insulin resistance in type I diabetes. N Engl J Med 1986;315:224–230 [DOI] [PubMed] [Google Scholar]
- 13.Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Jr, Rhodes CJ, Schwartz MW: Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 2006;3:67–73 [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Park BH, Wang MY, Wang ZV, Unger RH: Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A 2008;105:14070–14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM: Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 2010;11:11–22 [DOI] [PubMed] [Google Scholar]
- 16.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ: Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelling RW, Yan W, Al-Noori S, Pardini A, Morton GJ, Ogimoto K, Schwartz MW, Dempsey PJ: Deficiency of TNFα converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 2008;149:6053–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans MI, Halter JB, Porte D, Jr: Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem 1978;24:567–570 [PubMed] [Google Scholar]
- 19.Ferrannini E: The theoretical bases of indirect calorimetry: a review. Metabolism 1988;37:287–301 [DOI] [PubMed] [Google Scholar]
- 20.Schutz Y: On problems of calculating energy expenditure and substrate utilization from respiratory exchange data. Z Ernahrungswiss 1997;36:255–262 [DOI] [PubMed] [Google Scholar]
- 21.Müller WA, Faloona GR, Unger RH: The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest 1971;50:1992–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobbs R, Sakurai H, Sasaki H, Faloona G, Valverde I, Baetens D, Orci L, Unger R: Glucagon: role in the hyperglycemia of diabetes mellitus. Science 1975;187:544–547 [DOI] [PubMed] [Google Scholar]
- 23.Scribner KA, Walker CD, Cascio CS, Dallman MF: Chronic streptozotocin diabetes in rats facilitates the acute stress response without altering pituitary or adrenal responsiveness to secretagogues. Endocrinology 1991;129:99–108 [DOI] [PubMed] [Google Scholar]
- 24.Wahren J, Ekberg K: Splanchnic regulation of glucose production. Annu Rev Nutr 2007;27:329–345 [DOI] [PubMed] [Google Scholar]
- 25.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK: The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 2005;1:63–72 [DOI] [PubMed] [Google Scholar]
- 26.Nair KS, Halliday D, Garrow JS: Increased energy expenditure in poorly controlled type 1 (insulin-dependent) diabetic patients. Diabetologia 1984;27:13–16 [DOI] [PubMed] [Google Scholar]
- 27.Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De Cobelli F, Battezzati A, Secchi A, Del Maschio A, Luzi L: Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 2003;285:E1174–1181 [DOI] [PubMed] [Google Scholar]
- 28.Unger RH, Grundy S: Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 1985;28:119–121 [DOI] [PubMed] [Google Scholar]
- 29.Poitout V, Robertson RP: Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lager I, Lonnroth P, von Schenck H, Smith U: Reversal of insulin resistance in type I diabetes after treatment with continuous subcutaneous insulin infusion. Br Med J (Clin Res Ed) 1983;287:1661–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yki-Järvinen H, Koivisto VA: Continuous subcutaneous insulin infusion therapy decreases insulin resistance in type 1 diabetes. J Clin Endocrinol Metab 1984;58:659–666 [DOI] [PubMed] [Google Scholar]
- 32.Vuorinen-Markkola H, Koivisto VA, Yki-Jarvinen H: Mechanisms of hyperglycemia-induced insulin resistance in whole body and skeletal muscle of type I diabetic patients. Diabetes 1992;41:571–580 [DOI] [PubMed] [Google Scholar]
- 33.Yki-Järvinen H, Helve E, Koivisto VA: Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes 1987;36:892–896 [DOI] [PubMed] [Google Scholar]
- 34.Trinh KY, O'Doherty RM, Anderson P, Lange AJ, Newgard CB: Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J Biol Chem 1998;273:31615–31620 [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Liu S, Ferguson S, Wang L, Klepcyk P, Yun JS, Friedman JE: Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem 2002;277:23301–23307 [DOI] [PubMed] [Google Scholar]
- 36.Scarpace PJ, Matheny M, Pollock BH, Tümer N: Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol 1997;273:E226–230 [DOI] [PubMed] [Google Scholar]
- 37.Strack AM, Sebastian RJ, Schwartz MW, Dallman MF: Glucocorticoids and insulin: reciprocal signals for energy balance. Am J Physiol 1995;268:R142–R149 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MW, Strack AM, Dallman MF: Evidence that elevated plasma corticosterone levels are the cause of reduced hypothalamic corticotrophin-releasing hormone gene expression in diabetes. Regul Pept 1997;72:105–112 [DOI] [PubMed] [Google Scholar]
- 39.Exton JH: Regulation of gluconeogenesis by glucocorticoids. Monogr Endocrinol 1979;12:535–546 [DOI] [PubMed] [Google Scholar]
- 40.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS: Role of leptin in the neuroendocrince response to fasting. Nature 1996;382:250–252 [DOI] [PubMed] [Google Scholar]
- 41.Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ, Fuller JH, Songini M, Kohner EM, EURODIAB Prospective Complications Study Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 2001;24:284–289 [DOI] [PubMed] [Google Scholar]
- 42.Soedamah-Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, Manes C, Fuller JH: Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care 2004;27:530–537 [DOI] [PubMed] [Google Scholar]
- 43.The DCCT Research Group Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care 1988;11:567–573 [DOI] [PubMed] [Google Scholar]
- 44.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 45.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 46.Rosenfalck AM, Almdal T, Viggers L, Madsbad S, Hilsted J: A low-fat diet improves peripheral insulin sensitivity in patients with type 1 diabetes. Diabet Med 2006;23:384–392 [DOI] [PubMed] [Google Scholar]
- 47.Yki-Järvinen H, DeFronzo RA, Koivisto VA: Normalization of insulin sensitivity in type I diabetic subjects by physical training during insulin pump therapy. Diabetes Care 1984;7:520–527 [DOI] [PubMed] [Google Scholar]
- 48.Strowig SM, Raskin P: The effect of rosiglitazone on overweight subjects with type 1 diabetes. Diabetes Care 2005;28:1562–1567 [DOI] [PubMed] [Google Scholar]
- 49.Pang TT, Narendran P: Addressing insulin resistance in type 1 diabetes. Diabet Med 2008;25:1015–1024 [DOI] [PubMed] [Google Scholar]
- 50.Miyanaga F, Ogawa Y, Ebihara K, Hidaka S, Tanaka T, Hayashi S, Masuzaki H, Nakao K: Leptin as an adjunct of insulin therapy in insulin-deficient diabetes. Diabetologia 2003;46:1329–1337 [DOI] [PubMed] [Google Scholar]