Abstract
OBJECTIVE
Peptide YY3–36 (PYY3–36), a Y2 receptor agonist, and oxyntomodulin, a glucagon-like peptide 1 (GLP-1) receptor agonist, are cosecreted by intestinal L-cells after each meal. Separately each hormone acts as an endogenous satiety signal and reduces appetite in humans when infused intravenously. The aim of the current study was to investigate whether the anorectic effects of PYY3–36 and oxyntomodulin can be additive.
RESEARCH DESIGN AND METHODS
Twelve overweight or obese human volunteers underwent a randomized, double-blinded, placebo-controlled study. An ad libitum test meal was used to measure energy intake during intravenous infusions of either PYY3–36 or oxyntomodulin or combined PYY3–36/oxyntomodulin.
RESULTS
Energy intake during coadministration of PYY3–36 and oxyntomodulin was reduced by 42.7% in comparison with the saline control and was significantly lower than that during infusions of either hormone alone.
CONCLUSIONS
The anorectic effects of PYY3–36 and oxyntomodulin can be additive in overweight and obese humans. Coadministration of Y2 receptor agonists and GLP-1 receptor agonists may be a useful treatment strategy for obesity.
Obesity is a major risk factor for the development of type 2 diabetes and its prevalence is increasing rapidly throughout the world (1,2). Weight loss reduces that risk substantially (3) but is difficult to achieve and sustain. Bariatric surgery is the only obesity treatment proven to reduce mortality (4,5). Furthermore, the rapid improvement in glucose homeostasis after Roux-en-Y gastric bypass surgery (6,7) has led some to question whether type 2 diabetes, as well as obesity, should be considered a surgically curable disease (8,9). However, operative mortality and delayed complications are not uncommon (10,11). There is therefore a pressing need to develop safe, effective, nonsurgical treatments for obesity.
The main proposed mechanism by which Roux-en-Y gastric bypass causes weight loss is through altering the secretion of gut hormones (12). Two important such hormones, peptide YY3–36 (PYY3–36) and oxyntomodulin, are physiologically cosecreted after meals (13,14). Postprandial concentrations of both PYY3–36 and oxyntomodulin are increased by Roux-en-Y gastric bypass (12,15–17). Intravenous infusion of each hormone individually has been shown to reduce appetite in humans (18–20). Furthermore, oxyntomodulin causes weight loss in obese human volunteers when administered by repeated subcutaneous injection (21). However, one proposed physiologic function of these hormones at higher concentrations is nausea (22). Thus, if significant appetite reduction is attempted by giving a large dose of a single hormone, nausea or even vomiting may result (20–23). We hypothesized that coadministered PYY3–36 and oxyntomodulin would mimic the natural postprandial situation and have additive effects on appetite, but that neither hormone would reach concentrations associated with nausea.
RESEARCH DESIGN AND METHODS
Healthy male and female volunteers aged ≥18 years with a stable BMI of 25–40 kg/m2 were recruited by advertisement. Potential participants were screened and determined to be healthy by medical history, physical examination, routine blood tests, and 12-lead electrocardiogram. The SCOFF questionnaire (24), the Dutch Eating Behavior Questionnaire (25), and a 3-day diet diary were used to exclude those with disordered eating or a high level of restrained eating. Palatability of the study meal was assessed using a 9-point hedonic scale. It was calculated that, for 90% power to detect a difference in energy intake of 10% between treatments, 12 participants would be required, assuming a within-subject SD of 6% and a significance level of 0.05. Thus, 12 volunteers were selected for the study (Table 1). Women of child-bearing age were advised to avoid pregnancy during the study and underwent urine tests to exclude pregnancy before each infusion.
TABLE 1.
Baseline characteristics of participants
| Age (years) | 33.74 ± 2.32 |
| Sex: female/male | 7/5 |
| Height (m) | 1.67 ± 0.03 |
| Weight (kg) | 86.13 ± 3.48 |
| BMI (kg/m2) | 30.94 ± 1.03 |
| Fasting insulin (pmol/l) | 57.4 ± 0.86 |
| Fasting plasma glucose (mmol/l) | 4.96 ± 0.05 |
| Fasting PYY3–36 (pmol/l) | 21.6 ± 0.98 |
| Fasting OLI (pmol/l) | 85.1 ± 5.94 |
Data are means ± SE or n.
The study was approved by the Hammersmith and Queen Charlotte's and Chelsea Research Ethics Committee (reference number 06/Q0406/50). All participants gave written informed consent, and the study was planned and performed in accordance with the Declaration of Helsinki.
The study followed a randomized, double-blind, placebo-controlled crossover protocol comparing the effect on energy intake of six different pairs of infusions, as shown in Table 2. Each subject received two 110-min intravenous infusions, A and B, simultaneously, at each visit. Infusion A consisted of either PYY3–36 or saline control. Infusion B consisted of either oxyntomodulin or saline control. Infusion doses were based on previously established doses, with a twofold difference between high and low doses for each peptide. The infusion rate for high-dose PYY3–36 was based on previous work by Batterham et al. (26). The infusion rate for high-dose oxyntomodulin was similar to that used by Cohen et al. (19). The duration of infusion was chosen to allow steady state to be reached and sustained during test meals. Peptides were synthesized by Bachem U.K. and were sterile on culture and negative for pyrogen, as previously described (26). The amino acid content of representative peptide vials was measured independently (Alta Bioscience, Edgbaston, Birmingham, U.K.). Control vials were prepared with sterile saline and were indistinguishable visually from those containing peptide. To reduce adsorption of peptide onto the walls of syringes and infusion lines, the contents of randomized vials were dissolved in Gelofusine (B. Braun Medical, Sheffield, U.K.).
TABLE 2.
Summary of infusions
| Infusion pair designation | Infusion A | Infusion B |
|---|---|---|
| Saline | Saline | Saline |
| Low-dose PYY3–36 | 0.25 pmol/kg/min PYY3–36 | Saline |
| Low-dose OXM | Saline | 1.5 pmol/kg/min OXM |
| High-dose PYY3–36 | 0.5 pmol/kg/min PYY3–36 | Saline |
| High-dose OXM | Saline | 3.0 pmol/kg/min OXM |
| PYY3–36 + OXM | 0.25 pmol/kg/min PYY3–36 | 1.5 pmol/kg/min OXM |
OXM, oxyntomodulin.
Study visits were scheduled a minimum of 3 days apart. Subjects were asked to standardize their diet, abstain from alcohol, and avoid strenuous exercise for 24 h before each visit. Food diaries were used to monitor dietary compliance. Subjects fasted and drank only water from 9:00 p.m. on the night before each visit. After arrival at 9:00 a.m., peripheral venous cannulae were inserted in both of the patient's forearms, one for infusions and one for blood sampling. A three-way tap with low internal volume (Becton Dickinson, Franklin Lakes, NJ) was attached to the infusion cannula to allow connection of two separate infusion lines. Subjects then relaxed for 30 min before the start of the infusions. All time cues were removed from the study room, and subjects were encouraged to relax by reading or watching films on DVD.
Blood samples were collected at −30, 0, 30, 60, 75, 90, 120, and 135 min into lithium-heparin–coated tubes containing 2,000 kallikrein inhibitor units (0.2 ml) aprotinin (Bayer Schering Pharma, Berlin, Germany). Samples were stored on ice until centrifugation at 4°C, after which plasma was separated immediately and stored at −20°C until analysis. Immediately before each blood sample was taken, subjects completed visual analog scales (VAS) rating hunger, satiety, prospective food consumption, and nausea (27). Pulse and blood pressure were measured every 30 min and at the end of each study visit.
Ninety minutes after the start of the infusions, subjects were offered a meal that was provided in excess and were asked to eat until they were comfortably full. Water was freely available. Both food and water were weighed pre- and postprandially, and energy intake was calculated. The test meal procedure was identical to that used in previous studies with PYY3–36 and oxyntomodulin (18,19,26). At the end of the meal, the infusions were discontinued, and the subjects were asked to rate the palatability of the food using VAS.
Hormone assays.
Plasma PYY3–36 and oxyntomodulin-like immunoreactivity (OLI) concentrations were measured using established in-house radioimmunoassays. The PYY assay (28,29) could detect changes of 4.4 pmol/l (95% confidence limit) with an intra-assay variation of 11.5%. The OLI assay (14) could detect changes of 10 pmol/l (95% confidence limit) with an intra-assay variation of 5.7%. Because the radioimmunoassay technique is comparative and not absolute, all samples were assayed in duplicate and within a single assay to eliminate interassay variation. Plasma insulin and glucose concentrations at 0, 60, and 90 min were measured on an Olympus analyzer in the Department of Clinical Biochemistry, Hammersmith Hospital.
Statistical analysis.
Combined data are represented as means ± SEM. Comparisons of energy intake were by repeated-measures ANOVA with a Tukey multiple comparison posttest. Effects on changes in energy intake of subjects' BMI and sex were analyzed by linear regression and repeated-measures two-way ANOVA, respectively. VAS scores were adjusted for baseline, and differences were compared by a repeated-measures nonparametric Friedman test with a Dunn multiple comparison posttest. Comparisons at each time point of plasma insulin and glucose levels and of cardiovascular parameters were made by one-way ANOVA with a Tukey multiple comparison posttest. Analyses were performed using Prism (version 4.03; GraphPad Software, San Diego, CA).
RESULTS
Adverse effects.
On one visit each, the first three participants experienced severe nausea and sweating. As a result, the randomization code on this single occasion was examined by an independent medical colleague appointed for the purpose before the study and not directly connected with the investigation. It was identified that each case of nausea had occurred during high-dose PYY3–36 infusion, with symptoms commencing ∼50 min after the start of the infusion. In each case, symptoms settled within 30 min of stopping the infusions, and the participants were able to leave the investigation ward as normal after the last blood sample. Mean peak plasma PYY3–36 concentration achieved during these infusions was 156.5 ± 56.9 pmol/l (n = 3). It was not felt possible thereafter to continue the high-dose PYY3–36 infusion arm, and the study then proceeded as a five-way crossover, maintaining the randomized, double-blind, placebo-controlled design. During the remainder of the study, four participants reported nausea, requiring early termination of their infusions, at one visit each. However, because the nausea was not associated with vomiting, the randomization code was not examined again until the end of the study.
Effect of PYY3–36 and oxyntomodulin infusions on energy intake and appetite.
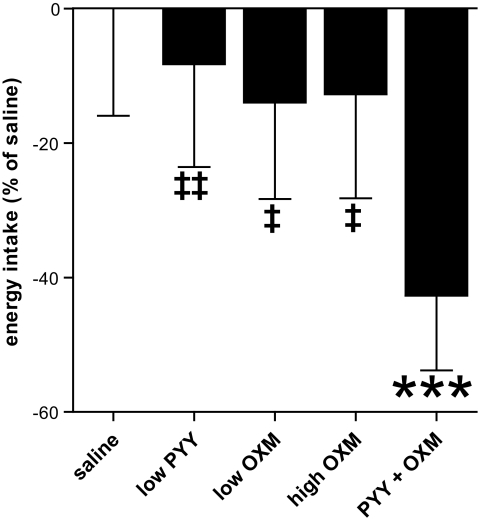
In comparison with saline control infusion, energy intake during combined PYY3–36 + oxyntomodulin infusion was reduced by 42.7% at the study meal (P < 0.001) and was also significantly lower than during infusions of either hormone alone (mean energy intake at buffet meal: 557 ± 88.9 kcal [saline], 511 ± 85.2 kcal [low-dose PYY3–36], 480 ± 80.0 kcal [low-dose oxyntomodulin], 486 ± 86.2 kcal [high-dose oxyntomodulin], and 319 ± 61.9 kcal [combined PYY3–36 + oxyntomodulin]; P < 0.001 vs. saline, P < 0.01 vs. low-dose PYY3–36, and P < 0.05 vs. low-dose oxyntomodulin and high-dose oxyntomodulin; n = 12) (Fig. 1). There was no evidence that change in energy intake varied with either BMI or sex of the subjects.
FIG. 1.
Percent reduction in energy intake at the buffet meal, with reference to the mean intake during saline infusion (all subjects included, n = 12). ***P < 0.001 vs. saline. ‡P < 0.05 vs. PYY3–36 + oxyntomodulin. ‡‡P < 0.01 vs. PYY3–36 + oxyntomodulin.
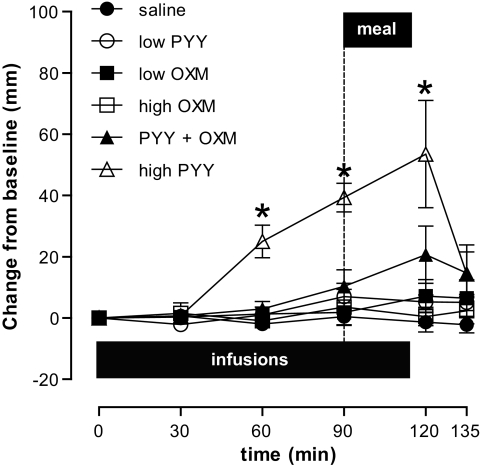
Neither the palatability of the buffet meal nor other satiety-related VAS responses were altered significantly by any infusion except high-dose PYY3–36. In particular, there were no significant differences in nausea scores between the five completed arms of the study at any time point (Fig. 2). However, four participants did report mild nausea, one during a high-dose oxyntomodulin infusion and the other three during combined PYY3–36 + oxyntomodulin infusion. Even though the nausea settled rapidly in each case after the infusion was (prematurely) stopped, it may have reduced energy intake at the subsequent buffet meal. The energy intake data were therefore analyzed further, excluding all data from the four affected participants. In this analysis (n = 8), the combined PYY3–36 + oxyntomodulin infusion significantly reduced energy intake by 33% in comparison with saline control (P < 0.05). However, the control low-dose PYY3–36, low-dose oxyntomodulin, and high-dose oxyntomodulin infusions for these subjects did not reduce food intake significantly (mean energy intake: 593 ± 132.7 kcal [saline], 524 ± 129.9 kcal [low-dose PYY3–36], 503 ± 121.3 kcal [low-dose oxyntomodulin], 532 ± 113.6 kcal [high-dose oxyntomodulin], and 398 ± 77.3 kcal [combined PYY3–36 + oxyntomodulin]; P < 0.05 vs. saline; n = 8).
FIG. 2.
Subjective rating of nausea, as measured by VAS response. Scores are depicted as change from baseline value (millimeters) (n = 12 for each treatment except high-dose PYY3–36 where n = 3). ●, saline; ○, low-dose PYY3–36; ■, low-dose oxyntomodulin (OXM); □, high-dose oxyntomodulin; ▲, PYY3–36 + oxyntomodulin; ▵, high-dose PYY3–36. *P < 0.05 versus saline. Horizontal black bar, infusion duration; dotted vertical line, meal time.
Plasma concentrations of PYY3–36, OLI, insulin, and glucose.
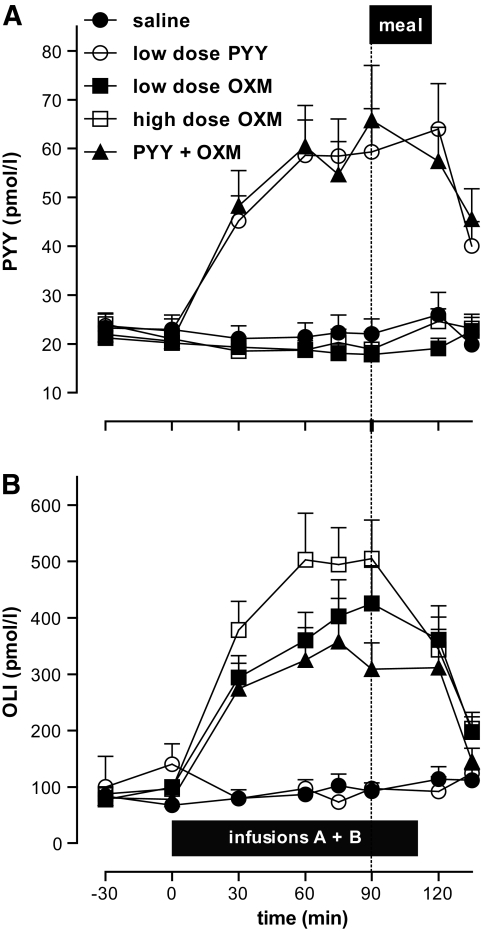
Basal plasma concentration of PYY3–36 was 22.2 ± 0.7 pmol/l. Infusion of low-dose PYY caused a threefold elevation in plasma PYY3–36 concentration to a peak of 62.9 ± 7.2 pmol/l and had no effect on plasma OLI concentration. Basal plasma concentration of OLI was 83.6 ± 4.1 pmol/l. Infusion of low-dose oxyntomodulin caused a fivefold elevation in plasma OLI concentration to a peak of 381.2 ± 49.0 pmol/l, whereas infusion of high-dose oxyntomodulin caused a sixfold elevation to a peak of 505.3 ± 68.3 pmol/l (Fig. 3). Plasma PYY3–36 concentration remained at basal levels during low- and high-dose oxyntomodulin infusions. There were no statistically significant differences between treatments in insulin or glucose concentrations before the meal. Plasma insulin and glucose were not measured postprandially because energy intake was not fixed.
FIG. 3.
Plasma concentrations of PYY3–36 (A) and OLI (B) during study infusions. ●, saline; ○, low-dose PYY3–36; ■, low-dose oxyntomodulin; □, high-dose oxyntomodulin; ▲, PYY3–36 + oxyntomodulin. Horizontal black bar, infusion duration; dotted vertical line, meal time.
Effect of PYY3–36 and oxyntomodulin infusions on cardiovascular parameters.
No statistically significant differences in pulse or blood pressure were detected between treatments at any time point.
DISCUSSION
Combined administration of PYY3–36 and oxyntomodulin at low dose resulted in a statistically significant reduction in energy intake of 42.7% in comparison with that on the saline control day. In contrast, mean energy intake during low-dose infusion of either PYY3–36 or oxyntomodulin was 8.3 and 14% lower, respectively, than during saline infusion, but in neither case was this difference statistically significant from the food intake during saline infusion. In a separate analysis that excluded all data from subjects who had experienced nausea at any point during the study, the reductions in energy intake achieved by low-dose infusion of either hormone alone (12 and 15% for PYY3–36 and oxyntomodulin, respectively) were again nonsignificant, but combined low-dose infusions of PYY3–36 and oxyntomodulin reduced mean energy intake significantly by 33% compared with saline. This indicates that the combination of PYY3–36 and oxyntomodulin reduces food intake to a greater extent than either hormone infused separately at this same dose.
Although not directly comparable, because the procedures were different, the anorectic effect of low-dose PYY3–36 infusion in the current study was less than that observed by Batterham et al. (26) using a somewhat higher dose. The anorectic effects of both low- and high-dose oxyntomodulin infusions were also less than those previously observed by Cohen et al. (19). Furthermore, there was no difference in effect between these low- and high-dose oxyntomodulin infusions, despite the twofold difference in dose. However, the peak plasma OLI concentrations in the current study were substantially lower than those achieved previously, which may reflect differences in infusion preparation (19).
During high-dose PYY3–36 infusion, the mean peak plasma PYY3–36 concentration was sufficient to cause sweating and severe nausea in all subjects, in keeping with previous reports (20,22,23). In contrast, nausea did not occur with low-dose PYY3–36 infusion, during which the peak plasma PYY3–36 concentration was similar to that achieved in obese subjects by Batterham et al. (26). High-dose oxyntomodulin infusion resulted in a mean plasma OLI concentration ∼60% of that achieved previously by intravenous infusion (19) and considerably lower than that previously reported to cause nausea (21). Nevertheless, 1 of the 12 subjects experienced nausea during high-dose oxyntomodulin infusion, suggesting that the threshold for oxyntomodulin-induced nausea may vary between individuals. There were no adverse effects with low-dose oxyntomodulin. However, combined low-dose infusions of PYY3–36 and oxyntomodulin caused nausea in 3 of 12 subjects. Thus, although coadministration of PYY3–36 and oxyntomodulin can produce a robust reduction in energy intake, this combination may increase the incidence of side effects.
When satiety-inducing hormones that act via different receptors are administered in combination, it can be hypothesized that the effects on appetite should be additive. However, studies performed on lean human volunteers do not always support this. Neary et al. (30) reported that pancreatic polypeptide, a Y4 receptor agonist, and PYY3–36, a selective Y2 receptor agonist, did not reduce food intake when infused together. Others have found that, although cholecystokinin and glucagon-like peptide 1 (GLP-1) synergistically reduced hunger sensations, the combination did not reduce energy intake to a greater extent than infusion of either hormone separately (31). It is possible that the absence of additive effects results either from duplication of the principal mode of action or from unsuspected, mutually antagonistic actions within each pair of hormones. In contrast, and in agreement with the current study, Neary et al. (32) demonstrated that intravenous infusion of PYY3–36 with GLP-1 reduced food intake to a greater extent than either hormone administered separately. Furthermore, exendin-4, which, like GLP-1 and oxyntomodulin, is a GLP-1 receptor agonist, acts synergistically with PYY3–36 to reduce food intake in mice (33). The current study thus supports the concept that Y2 receptor agonists and GLP-1 receptor agonists have distinct and additive effects on appetite.
The plasma concentrations of PYY3–36 and OLI during these infusions are within the range of those occurring after Roux-en-Y gastric bypass surgery. Measurement of oxyntomodulin concentration in plasma presents particular difficulties because of cross-reactivity of total glucagon (enteroglucagon) antibodies with several circulating products of preproglucagon cleavage (14). This may account for the 10-fold difference in postprandial levels reported after Roux-en-Y gastric bypass (16,17). Notwithstanding this discrepancy and differences in antibody specificity, the mean plasma OLI concentration achieved during the current study was similar to postprandial enteroglucagon levels reported after Roux-en-Y gastric bypass (15). With regard to plasma PYY3–36 concentration, the mean peak level achieved during low-dose infusion in the current study was ∼50% higher than the peak concentration reported after a 420-kcal mixed meal (34), but slightly lower than that measured after a 398-kcal liquid meal (35), both studies being performed on patients who had undergone Roux-en-Y gastric bypass. Thus, the results of the current study may throw light on the mechanism of food intake reduction after Roux-en-Y gastric bypass.
In summary, we have shown that combined infusion of PYY3–36 and oxyntomodulin appear to have an additive anorectic effect in overweight and obese humans. These results and data from other recent studies suggest that Y2 receptor agonists and GLP-1 receptor agonists may be particularly suited to coadministration for the treatment of obesity. However, further studies are required to establish whether chronic coadministration of gut hormones can increase the potential anorectic effect without inducing a parallel increase in nausea.
ACKNOWLEDGMENTS
B.C.T.F. is the recipient of an Medical Research Council (MRC) Clinical Research Training Fellowship, K.W. is the recipient of a Wellcome Trust Research Training Fellowship, and both B.C.T.F. and K.W. are recipients of National Institute for Health Research Clinical Lectureships. A.M.W. is the recipient of a Department of Health Clinician Scientist Award. V.P. is funded through a European Union Marie Curie Sixth Framework Fellowship (NuSISCO). N.M.M. is funded by a Higher Education Funding Council for England Clinical Senior Lecturer Award. This research is funded by program grants from the MRC (G7811974) and Wellcome Trust (072643/Z/03/Z) and by an European Union FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme and an Integrative Mammalian Biology (IMB) Capacity Building Award.
M.A.G. and S.R.B. are inventors for patents describing the use of gut hormones and their analogs and derivatives in the treatment of obesity. S.R.B. is a consultant for Thiakis, a subsidiary of Wyeth Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 102nd annual meeting of the Association of Physicians of U.K. and Ireland, Leicester, U.K., 10–11 April 2008.
We are indebted to the volunteers who participated in the study. We are also grateful to the staff of the Sir John McMichael Clinical Investigation Centre and the Department of Clinical Biochemistry at Hammersmith Hospital and to Professor Gordon Stamp.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.World Health Organization Global InfoBase Team The SuRF Report 2. Surveillance of Chronic Disease Risk Factors: Country-Level Data and Comparable Estimates Geneva, World Health Org, 2007 [Google Scholar]
- 2.Colditz GA, Willett WC, Rotnitzky A, Manson JE: Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–486 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC: Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 5.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM: Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 6.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS: Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–481 [DOI] [PubMed] [Google Scholar]
- 7.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E: The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004;240:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubino F: Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 2008;31:S290–S296 [DOI] [PubMed] [Google Scholar]
- 9.Pories WJ, MacDonald KG, Jr, Flickinger EG, Dohm GL, Sinha MK, Barakat HA, May HJ, Khazanie P, Swanson MS, Morgan E: Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992;215:633–642; discussion 643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L: Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005;294:1903–1908 [DOI] [PubMed] [Google Scholar]
- 11.Zingmond DS, McGory ML, Ko CY: Hospitalization before and after gastric bypass surgery. JAMA 2005;294:1918–1924 [DOI] [PubMed] [Google Scholar]
- 12.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T: Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007;246:780–785 [DOI] [PubMed] [Google Scholar]
- 13.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR: Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985;89:1070–1077 [DOI] [PubMed] [Google Scholar]
- 14.Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR: Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab 1983;57:488–495 [DOI] [PubMed] [Google Scholar]
- 15.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ: Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 2006;93:210–215 [DOI] [PubMed] [Google Scholar]
- 16.Meryn S, Stein D, Straus EW: Pancreatic polypeptide, pancreatic glucagon and enteroglucagon in morbid obesity and following gastric bypass operation. Int J Obes 1986;10:37–42 [PubMed] [Google Scholar]
- 17.Kellum JM, Kuemmerle JF, O'Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ: Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 1990;211:763–770; discussion 770–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR: Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 2002;418:650–654 [DOI] [PubMed] [Google Scholar]
- 19.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR: Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 2003;88:4696–4701 [DOI] [PubMed] [Google Scholar]
- 20.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C: Effect of peptide YY3–36 on food intake in humans. Gastroenterology 2005;129:1430–1436 [DOI] [PubMed] [Google Scholar]
- 21.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR: Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 2005;54:2390–2395 [DOI] [PubMed] [Google Scholar]
- 22.le Roux CW, Borg CM, Murphy KG, Vincent RP, Ghatei MA, Bloom SR: Supraphysiological doses of intravenous PYY3–36 cause nausea, but no additional reduction in food intake. Ann Clin Biochem 2008;45:93–95 [DOI] [PubMed] [Google Scholar]
- 23.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A: Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab 2007;292:E1062–E1068 [DOI] [PubMed] [Google Scholar]
- 24.Morgan JF, Reid F, Lacey JH: The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ 1999;319:1467–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Strien T, Rookus MA, Bergers GP, Frijters JE, Defares PB: Life events, emotional eating and change in body mass index. Int J Obes 1986;10:29–35 [PubMed] [Google Scholar]
- 26.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR: Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 2003;349:941–948 [DOI] [PubMed] [Google Scholar]
- 27.Flint A, Raben A, Blundell JE, Astrup A: Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48 [DOI] [PubMed] [Google Scholar]
- 28.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR: Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006;147:3–8 [DOI] [PubMed] [Google Scholar]
- 29.Adrian TE, Savage AP, Sagor GR, Allen JM, Bacarese-Hamilton AJ, Tatemoto K, Polak JM, Bloom SR: Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology 1985;89:494–499 [DOI] [PubMed] [Google Scholar]
- 30.Neary NM, McGowan BM, Monteiro MP, Jesudason DR, Ghatei MA, Bloom SR: No evidence of an additive inhibitory feeding effect following PP and PYY 3–36 administration. Int J Obes (Lond) 2008;32:1438–1440 [DOI] [PubMed] [Google Scholar]
- 31.Gutzwiller JP, Degen L, Matzinger D, Prestin S, Beglinger C: Interaction between GLP-1 and CCK-33 in inhibiting food intake and appetite in men. Am J Physiol Regul Integr Comp Physiol 2004;287:R562–R567 [DOI] [PubMed] [Google Scholar]
- 32.Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Ghatei MA, Bloom SR: Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 2005;146:5120–5127 [DOI] [PubMed] [Google Scholar]
- 33.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL: Peripheral exendin-4 and peptide YY3–36 synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005;146:3748–3756 [DOI] [PubMed] [Google Scholar]
- 34.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR: Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J: Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006;91:1735–1740 [DOI] [PubMed] [Google Scholar]