Abstract
OBJECTIVE
Pancreatic islets of perinatal mice lacking the transcription factor Rfx3 exhibit a marked reduction in insulin-producing β-cells. The objective of this work was to unravel the cellular and molecular mechanisms underlying this deficiency.
RESEARCH DESIGN AND METHODS
Immunofluorescence studies and quantitative RT-PCR experiments were used to study the emergence of insulin-positive cells, the expression of transcription factors implicated in the differentiation of β-cells from endocrine progenitors, and the expression of mature β-cell markers during development in Rfx3−/− and pancreas-specific Rfx3-knockout mice. RNA interference experiments were performed to document the consequences of downregulating Rfx3 expression in Min6 β-cells. Quantitative chromatin immunoprecipitation (ChIP), ChIP sequencing, and bandshift experiments were used to identify Rfx3 target genes.
RESULTS
Reduced development of insulin-positive cells in Rfx3−/− mice was not due to deficiencies in endocrine progenitors or β-lineage specification, but reflected the accumulation of insulin-positive β-cell precursors and defective β-cells exhibiting reduced insulin, Glut-2, and Gck expression. Similar incompletely differentiated β-cells developed in pancreas-specific Rfx3-deficient embryos. Defective β-cells lacking Glut-2 and Gck expression dominate in Rfx3-deficent adults, leading to glucose intolerance. Attenuated Glut-2 and glucokinase expression, and impaired glucose-stimulated insulin secretion, were also induced by RNA interference–mediated inhibition of Rfx3 expression in Min6 cells. Finally, Rfx3 was found to bind in Min6 cells and human islets to two well-known regulatory sequences, Pal-1 and Pal-2, in the neuroendocrine promoter of the glucokinase gene.
CONCLUSIONS
Our results show that Rfx3 is required for the differentiation and function of mature β-cells and regulates the β-cell promoter of the glucokinase gene.
Pancreatic endocrine cells are organized into clusters called islets of Langerhans. Mature mouse islets contain a central core of insulin-producing β-cells surrounded by glucagon-producing α-cells, somatostatin-producing δ-cells, and pancreatic polypeptide (PP)-producing cells. During development, these endocrine cells arise in the primitive pancreatic epithelium from progenitor cells expressing the transcription factor Ngn3 (1). Ngn3 regulates specification of the four endocrine cell lineages as a function of specific developmental time windows (2). A complex network of transcription factors directs the differentiation of Ngn3+ progenitors into mature endocrine cells (3). Key factors implicated in β-cell development include NeuroD1, Nkx2.2, Pax4, Nkx6.1, MafA, and Pdx1 (3). NeuroD1, encoded by an Ngn3-regulated gene, is required for the formation of β-cells (4). Nkx2.2 functions downstream of NeuroD1 and promotes commitment of cells to the α, β, and PP lineages at the expense of the ε-cell lineage (5,6). A balance between Arx and Pax4 expression controls specification of α/ε versus β/δ precursors (7). Nkx6.1 is expressed in cells committed to the β-lineage and participates in the developmental program leading to the generation of mature β-cells (8).
Mature β-cells acquire the capacity to synthesize and secrete insulin in response to variations in blood glucose levels. Key components of the glucose-sensing and insulin secretion machinery include the Glut-2 glucose transporter and the glucose sensor glucokinase. Several transcription factors have been implicated in the acquisition of mature β-cell functions, including Pdx1, MafA, and NeuroD1 (4,9,10).
There is growing evidence that Rfx transcription factors are implicated in islet development. There are seven Rfx factors (Rfx1–Rfx7) in mammals (11–13). With the exception of Rfx5, which is a well-known regulator in the immune system (14), the functions of mammalian Rfx factors have only started to emerge recently (15–19). Rfx6 was recently demonstrated to be crucial for islet development in zebra fish, mice, and humans (18,19). We had reported earlier that pancreatic Rfx3 expression is restricted to islets and detected in Ngn3+ progenitors and α, β, δ, and PP cells (20). Islets of perinatal Rfx3−/− mice contain reduced numbers of cells expressing insulin, glucagon, and ghrelin, whereas PP-producing cells are increased (20). Adult Rfx3−/− mice exhibit impaired glucose tolerance (20). These findings suggested that Rfx3 is, like Rfx6, implicated in the development of pancreatic endocrine cells, including β-cells. However, the mechanisms involved remained unknown.
We have performed a detailed analysis of β-cell development in Rfx3−/− mice and conditional pancreas-specific Rfx3-deficient mice. β-Cell development was impaired from E15.5 onward and characterized by the accumulation of β-cell precursors and defective β-cells having reduced insulin, Glut-2, and glucokinase expression. Impaired glucose-stimulated insulin secretion and reductions in Glut-2 and glucokinase expression were induced in Min6 β-cells by RNA interference–mediated inhibition of Rfx3 expression. Finally, we identified the glucokinase gene as a direct target of Rfx3. These results show that Rfx3 is required for the differentiation and function of mature β-cells, and that it is a key regulator of glucokinase expression.
RESEARCH DESIGN AND METHODS
Mice.
Data for Rfx3−/− mice, and mice carrying an Rfx3 allele in which exon 3 is flanked by loxP sequences (Rfx3loxP), have been reported (16). Pancreas-specific Rfx3 deletion (Rfx3ΔP) was obtained by crossing mice carrying Rfx3loxP with Pdx1-Cre mice (21). Rfx3ΔP/− mice and Rfx3loxP/− littermates were obtained by mating Rfx3loxP/− and Rfx3+/− /Pdx1-Cre mice. E0.5 was defined as the morning when a vaginal plug was detected. Genotyping was done as described (16). Mice were on a C57BL/6 background. Experiments were approved by the Federal and Cantonal veterinary authorities.
Staining of sections and morphometry.
For E13.5 and E15.5, pancreases were cut, respectively, into three or five consecutive series of ∼10 sections. For E17.5 and E19.5, pancreases were cut into seven consecutive series of ∼10 sections. Measurements were performed using one section from each series. Immunostaining of frozen sections was performed by standard procedures. Antibodies and secondary reagents are indicated in supplementary Table 1, available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0986/DC1. Apoptotic cells were revealed by Tdt-mediated dUTP nick end labeling (TUNEL) staining (Roche). Stained sections were visualized by confocal microscopy. Cell counting and morphometry were performed using Mertamorph v6.2 (Universal Imaging Corporation). Labeled cells were quantified within Pdx1+ cells (E13.5 and E15.5) or 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI)-stained cells (E17.5 and E19.5).
Islet purification.
Mouse islets were isolated as described (22). Human islets (purity >90%, viability >95%) were provided by the Islet Cell Resource Center of Geneva (Juvenile Diabetes Research Foundation, European islet distribution program).
Quantitative RT-PCR.
Total RNA was extracted from pancreas with RNeasy kits (QIAGEN, Switzerland) and from Min6 cells and purified islets with TRIzol (Invitrogen). Quantitative RT-PCR (qRT-PCR) was performed as described (20). Results were normalized using TATA-binding protein (Tbp) mRNA. Primers are provided in supplementary Table 2.
RNA interference.
Double-stranded oligonucleotides encoding Rfx3-specific and control (23) small interfering RNAs (siRNAs) were cloned into the pLVTHM lentiviral vector (24). Five siRNA-encoding oligonucleotides were tested for Rfx3. The best results were obtained with the following: 5′-CGCGTCCCCAACACTGGAGGAAATTACTTTCAAGAGAAGTAATTTCCTCCAGTGTTTTTTTGGAAAT-3′ (sense) and 5′-CGATTTCCAAAAAAACACTGGAGGAAATTACTTCTCTTGAAAGTAATTTCCTCCAGTGTTGGGGA-3′ (antisense). Min6 cells were transduced as described (24). GFP expression was used for assessing transduction efficiencies and purifying transduced cells (FACS Vantage Sorter, Becton Dickinson).
Glucose-stimulated insulin secretion.
Glucose-stimulated insulin secretion assays with Min6 cells were performed as described (25).
Rfx3 binding studies.
ChIP was performed as described (26) using antibodies specific for RFX3 (13). Primer sequences are provided in supplementary Table 3. The ChIP-sequencing library was prepared from 10 ng of ChIP sample (quantified by a Qubit fluorometer; Invitrogen) using the ChIP-seq Sample Preparation Kit (Illumina). Sequencing was performed with the Genome Analyzer II (Illumina) using the 36 Cycle Sequencing Kit v2. Sequences were derived from a single channel of the flow cell. Data were processed using the Illumina Pipeline Software package v1.0. Bandshift experiments were performed as described (13) using in vitro translated Rfx3 and Min6 extracts. Oligonucleotides used as probes and competitors are provided in supplementary Table 4.
Statistical analysis.
Significance was evaluated using a t test for independent samples, one-way ANOVA followed by post-test comparisons, and Kolmogorov-Smirnov or median nonparametric tests.
RESULTS
Impaired pancreatic endocrine-cell development in Rfx3−/− embryos.
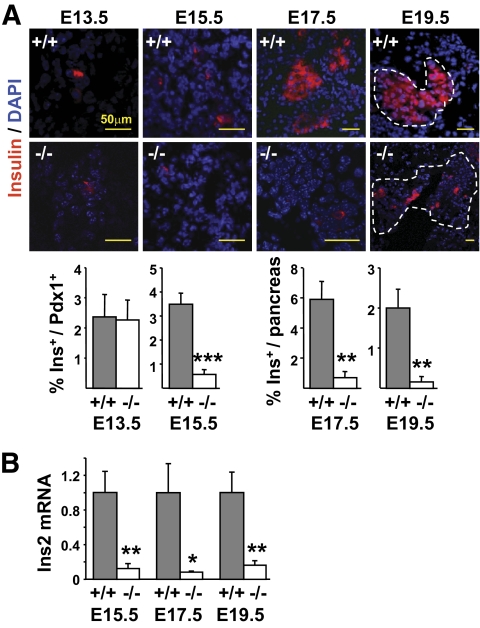
Pancreas sections from E13.5, E15.5, E17.5, and E19.5 embryos were stained with antibodies against insulin (Fig. 1A). No differences in insulin-positive cell numbers were observed between Rfx3−/− and wild-type (WT) embryos at E13.5. A fourfold reduction in insulin-positive cells was observed in Rfx3−/− embryos by E15.5, and this reduction became accentuated at E17.5 (6-fold) and E19.5 (10-fold). A 10-fold decrease in insulin (Ins) mRNA expression was revealed by qRT-PCR in the pancreas of E15.5, E17.5, and E19.5 embryos (Fig. 1B).
FIG. 1.
Development of insulin-positive cells is severely impaired in Rfx3−/− embryos. A: Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stages E13.5, E15.5, E17.5, and E19.5 were stained with antibodies against insulin. Nuclei were labeled with DAPI. Representative sections are shown (top). The percentages of insulin-positive cells were quantified in total Pdx1+ cells (E13.5 and E15.5) or in the entire pancreas (E17.5 and E19.5) of WT (+/+) and Rfx3−/− (−/−) embryos (bottom). B: Ins2 mRNA abundance was measured by qRT-PCR in total pancreas RNA from WT (+/+) and Rfx3−/− (−/−) embryos at stages E15.5, E17.5, and E19.5. The results were normalized using Tbp mRNA and are expressed relative to the levels found in WT embryos. The mean ± SEM derived from five embryos is shown for each stage and genotype; ***P < 0.0001; **P < 0.001; *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
Other islet defects described previously in perinatal Rfx3−/− mice (20) also appeared early during development (supplementary Figs. 1 and 2). Reductions in glucagon-positive cells, and glucagon and ghrelin mRNA expression, became evident by E15.5. Increases in PP+ cells and PP mRNA levels were observed at E13.5. Somatostatin expression remained unchanged throughout development. In agreement with the reduction in glucagon- and insulin-positive cells, the number of cells expressing MafB—a transcription factor implicated in α- and β-cell development—and MafB mRNA expression were decreased throughout development (supplementary Fig. 3).
Early pancreatic development is normal in Rfx3−/− embryos.
To determine whether the reduction in insulin-positive cells in Rfx3−/− embryos might be secondary to a global defect in pancreas formation, we examined the pancreas at critical developmental stages. Pancreatic tissue in Rfx3−/− embryos was normal in size and gross morphology at E12.5, E15.5, and E19.5 (supplementary Fig. 4).
We next quantified the expression of transcription factors playing pivotal roles in multipotent pancreatic-progenitor cells (Pdx1 and Ptf1a), developing exocrine cells (Ptf1a), and endocrine-progenitor cells (Ngn3) (supplementary Figs. 5 and 6) (3). At E13.5, the developing pancreas of Rfx3−/− embryos appeared well branched and contained WT numbers of Pdx1+ cells. The pancreas of E13.5 and E15.5 Rfx3−/− embryos also contained WT numbers of Ptf1a+ cells concentrated in growing acini. qRT-PCR experiments confirmed that Ptf1a mRNA levels were unaffected throughout development. Finally, the number of cells expressing Ngn3 was not altered in Rfx3−/− embryos at E13.5, E15.5, or E19.5. qRT-PCR experiments confirmed that Ngn3 mRNA was expressed at WT levels at all stages in Rfx3−/− embryos. The deficiency in Rfx3 does thus not affect early morphogenesis of the pancreatic epithelium, impair development of the exocrine pancreas, or lead to altered numbers of Ngn3+ progenitors.
Normal β-lineage specification in Rfx3−/− embryos.
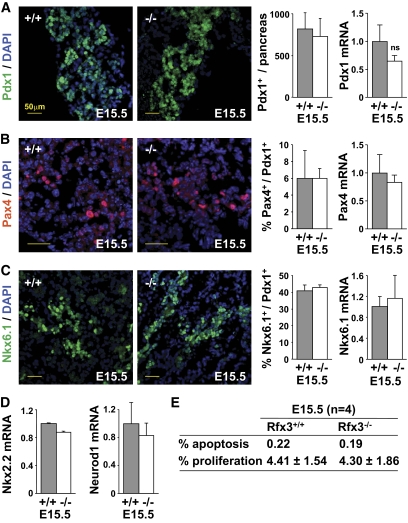
As the deficiency in insulin-positive cells and reduced Ins2 mRNA expression first became evident in Rfx3−/− embryos at E15.5, this stage was examined for the expression of transcription factors implicated in β-cell development (Fig. 2A–D). Cells expressing Pdx1, Pax4, and Nkx6.1 were not reduced in E15.5 Rfx3−/− embryos. No significant decreases in Pdx1, Pax4, Nkx6.1, Nkx2.2, or Neurod1 mRNAs were observed at E15.5. These findings suggest that the emergence of cells committed to the β-lineage is not affected in Rfx3−/− embryos.
FIG. 2.
The expression of key transcription factors implicated in β-cell development is normal in Rfx3−/− embryos. A: Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stage E15.5 were stained with DAPI and antibodies against Pdx1. Representative sections are shown (left). Pdx1+ cells and Pdx1 mRNA levels were quantified in the pancreases of WT (+/+) and Rfx3−/− (−/−) embryos (right). B: Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stage E15.5 were stained with DAPI and antibodies against Pax4. Representative sections are shown (left). Pax4+ cells and Pax4 mRNA levels were quantified in the pancreases of WT (+/+) and Rfx3−/− (−/−) embryos (right). Pax4+ cells were expressed as percentage of Pdx1+ cells (counted on serial sections). C: Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stage E15.5 were stained with DAPI and antibodies against Nkx6.1. Representative sections are shown (left). Nkx6.1+ cells and Nkx6.1 mRNA levels were quantified in the pancreases of WT (+/+) and Rfx3−/− (−/−) embryos (right). Nkx6.1+ cells were expressed as percentage of Pdx1+ cells (counted on serial sections). D: Nkx2.2 and Neurod1 mRNA levels were quantified in total pancreas RNA from WT (+/+) and Rfx3−/− (−/−) embryos at stage E15.5. The results were normalized using Tbp mRNA and are expressed relative to the levels found in WT embryos. The mean ± SEM derived from five embryos is shown for each stage and genotype. ns, not significant. E: TUNEL staining and labeling with anti–phospho-histone H3 antibodies were used to quantify the percentages of apoptotic and proliferating islet cells in pancreatic sections from E15.5 Rfx3+/+ and Rfx3−/− embryos. Means are shown for four embryos of each genotype. (A high-quality color representation of this figure is available in the online issue.)
The frequencies of apoptotic or proliferating cells were not affected in the islets of E15.5 Rfx3−/− embryos (Fig. 2E). Altered islet development is therefore not due to increased apoptosis or decreased proliferation.
Defective β-cell differentiation in Rfx3−/− mice.
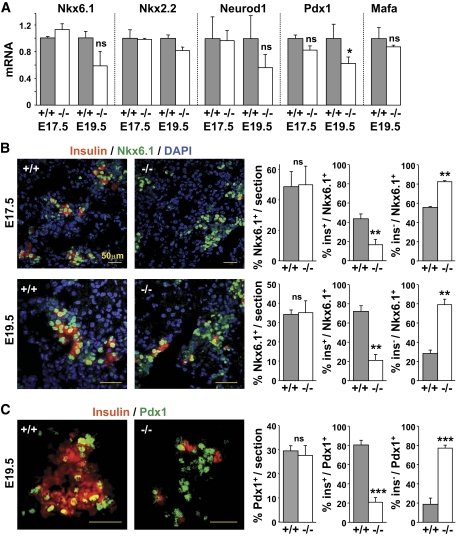
To clarify the phenotype of the β-lineage cells that develop in Rfx3−/− embryos, we examined later developmental stages for the expression of transcription factors required for β-cell differentiation and function. Nkx2.2, Nkx6.1, Neurod1, and Mafa mRNA levels were not reduced in the pancreas of Rfx3−/− embryos at E17.5 and E19.5 (Fig. 3A). Pdx1 mRNA expression was reduced modestly at E19.5 but not affected at E17.5 (Fig. 3A). Nkx6.1+ and Pdx1+ cell numbers were indistinguishable between WT and Rfx3−/− embryos (Fig. 3B–C).
FIG. 3.
Block in β-cell differentiation in Rfx3−/− embryos. A: Nkx6.1, Nkx2.2, Neurod1, Pdx1, and Mafa mRNA levels were quantified in the pancreases of Rfx3+/+ (+/+) and Rfx3−/− (−/−) embryos at stages E17.5 and E19.5. The results were normalized using Tbp mRNA and are expressed relative to the levels found in WT embryos. B: Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stages E17.5 and E19.5 were co-stained with antibodies against Nkx6.1 and insulin. Nuclei were labeled with DAPI. Representative sections are shown (left). The percentages of total Nkx6.1+ cells, Nkx6.1+insulin+ cells, and Nkx6.1+insulin− cells were quantified (right). C: Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stage E19.5 were co-stained with DAPI and antibodies against Pdx1 and insulin. Representative sections are shown (left). The percentages of total Pdx1+ cells, Pdx1+insulin+ cells, and Pdx1+insulin− cells were quantified (right). The mean ± SE derived from five embryos is shown for each stage and genotype; ***P < 0.0001; **P < 0.001; ns, not significant. (A high-quality color representation of this figure is available in the online issue.)
We next analyzed the differentiation status of Nkx6.1+ and Pdx1+ β-lineage cells in WT and Rfx3−/− embryos at E17.5 and E19.5 (Fig. 3B and C). These markers become restricted to β-cells as pancreas development progresses (8,27). The fractions of Nkx6.1+ cells that express or lack insulin were quantified independently at E17.5 and E19.5. In WT embryos, Nkx6.1+insulin+ cells increased from 44% at E17.5 to 68% at E19.5. Conversely, Nkx6.1+ insulin− cells decreased from 56% at E17.5 to 42% at E19.5. In Rfx3−/− embryos, Nkx6.1+insulin+ cells attained only 22% at E19.5, whereas Nkx6.1+insulin− cells constituted the major β-cell fraction (78–88%). Similar results were obtained by quantifying Pdx1+insulin+ and Pdx1+insulin− cells at E19.5. Whereas Pdx1+insulin+ cells constituted the major fraction (80%) in WT embryos, Pdx1+insulin− cells predominated (78%) in Rfx3−/− embryos.
A significant fraction of Nkx6.1+ cells in Rfx3−/− embryos exhibited ectopic PP expression (supplementary Fig. 7A). These Nkx6.1+PP+ cells do not co-express insulin (supplementary Fig. 7B). Lineage-tracing experiments have suggested that β-cells develop from precursors expressing PP (28). The increase in PP+ cells in Rfx3-deficient mice might thus reflect the persistence of PP-expressing β-cell precursors.
Defective β-cell differentiation in pancreas-specific Rfx3-knockout mice.
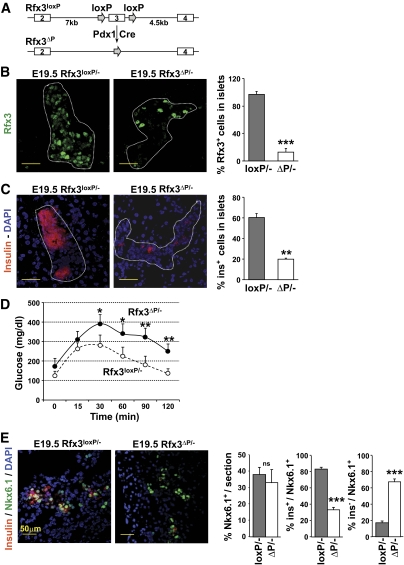
Rfx3 is required for the formation of cilia on the embryonic node, which direct the establishment of left-right asymmetry (29). Most Rfx3−/− mice therefore die from developmental defects involving abnormal specification of the left-right body axis (16). We could consequently not exclude that the pancreatic phenotype observed in Rfx3−/− embryos might be an indirect consequence of body-patterning abnormalities. We therefore generated pancreas-specific Rfx3 knockout mice. Mice carrying an Rfx3 allele containing an essential exon flanked by loxP sequences (Rfx3loxP) were crossed with transgenic mice expressing Cre recombinase under control of the Pdx1 promoter (Pdx1-Cre) (Fig. 4A). Rfx3loxP/−/Pdx1-Cre mice carrying a pancreas-specific Rfx3 deletion (Rfx3ΔP/− mice) were born according to expected Mendelian frequencies and did not exhibit any overt developmental anomalies. Immunofluorescence staining of pancreas sections from E19.5 embryos demonstrated that the number of Rfx3+ nuclei was reduced over fivefold in the islets of Rfx3ΔP/− mice (Fig. 4B).
FIG. 4.
β-Cell differentiation is impaired in conditional pancreas-specific Rfx3 knockout mice. A: Mice carrying a pancreas-specific deletion of exon 3 of the Rfx3 gene (Rfx3ΔP) were generated by crossing mice carrying an Rfx3 allele in which exon 3 is flanked by loxP sequences (Rfx3loxP) with transgenic mice expressing Cre recombinase under control of the Pdx1 promoter (Pdx1-cre). B: The efficiency of Rfx3 deletion was monitored by staining pancreas sections with antibodies specific for Rfx3. Representative islets (dashed contours) from Rfx3loxP/− and Rfx3ΔP/− E19.5 embryos are shown (left). The bar graph (right) shows quantifications of the percentages of Rfx3+ cells in the islets of Rfx3loxP/− and Rfx3ΔP/− E19.5 embryos. The mean ± SEM derived from five embryos is shown for each genotype; ***P < 0.0001. C: Pancreas sections from Rfx3loxP/− and Rfx3ΔP/− E19.5 embryos were stained with antibodies against insulin. Representative islets (dashed contours) are shown (left). The bar graph (right) shows quantifications of the percentages of insulin-positive cells in the islets of Rfx3loxP/− and Rfx3ΔP/− E19 embryos. The mean ± SEM derived from five embryos is shown for each genotype; **P < 0.001. D: Rfx3ΔP/− mice exhibit impaired glucose tolerance. Blood glucose concentrations were measured in Rfx3loxP/− and Rfx3ΔP/− mice at the indicated times after intraperitoneal injection of glucose. The mean ± SEM is derived from the analysis of five mice of each genotype; *P < 0.05; **P < 0.001. E: Pancreas sections from Rfx3loxP/− and Rfx3ΔP/− embryos at stage E19.5 were co-stained with DAPI and antibodies against Nkx6.1 and insulin. Representative sections are shown (left). The percentages of total Nkx6.1+ cells, Nkx6.1+insulin+ cells, and Nkx6.1+insulin− cells were quantified (right). The mean ± SEM derived from five embryos is shown for each stage and genotype; ***P < 0.0001; **P < 0.001; ns, not significant. (A high-quality color representation of this figure is available in the online issue.)
Insulin-positive islet cells were reduced threefold in E19.5 Rfx3ΔP/− embryos compared with Rfx3loxP/− controls (Fig. 4C). As in Rfx3−/− mice (24), the β-cell defect in Rfx3ΔP/− mice leads to impaired glucose tolerance in adults (Fig. 4D). Furthermore, the islets of E19.5 Rfx3ΔP/− embryos contained fewer glucagon-positive cells and increased numbers of PP+ cells (supplementary Fig. 8). Finally, cilia in the islets were shorter and reduced in number (supplementary Fig. 9). These results confirmed that all of the islet cell defects documented in Rfx3−/− mice result from a pancreas-intrinsic deficiency in Rfx3.
We next quantified Nkx6.1+, Nkx6.1+insulin+, and Nkx6.1+insulin− cells in pancreatic sections from Rfx3ΔP/− and Rfx3loxP/− embryos at E19.5 (Fig. 4E). As in Rfx3−/− mice, the pancreas of Rfx3ΔP/− embryos contained WT numbers of total Nkx6.1 cells, a marked reduction in Nkx6.1+insulin+ cells, and a corresponding increase in Nkx6.1+insulin− cells (Fig. 4E).
Rfx3−/− mice exhibit a block in β-cell maturation.
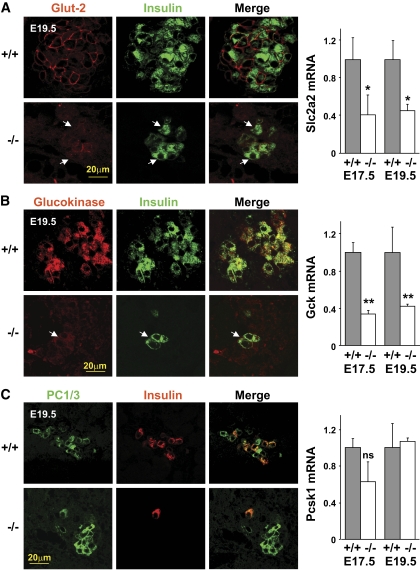
The accumulation of Nkx6.1+insulin− and Pdx1+insulin− cells in Rfx3-deficient islets suggested that there is a developmental block in the β-cell lineage. We therefore examined the expression of key mature β-cell markers in Rfx3−/− (Fig. 5) and Rfx3ΔP/− (supplementary Fig. 10A) embryos. Glut-2 (Slc2a2) and glucokinase (Gck) mRNA levels were significantly reduced in E17.5 and E19.5 Rfx3−/− embryos, whereas there was no reduction in PC1/3 (Pcsk1) mRNA. There was a marked reduction in cells exhibiting strong Glut-2 and glucokinase expression in the islets of E19.5 Rfx3−/− and Rfx3ΔP/− embryos. The residual insulin-positive cells exhibited weak or no Glut-2 and glucokinase staining. No change in the number of PC1/3+ cells was evident. These findings confirmed that the deficiency in Rfx3 leads to the accumulation of cells characterized by normal expression of certain β-cell markers (Nkx6.1, Pdx1, PC1/3) but impaired expression of key proteins required for β-cell function (Glut-2, glucokinase, insulin).
FIG. 5.
β-Cells in Rfx3−/− embryos exhibit strongly decreased Glut-2 and glucokinase expression. Pancreas sections from WT (+/+) and Rfx3−/− (−/−) embryos at stage E19.5 were stained with antibodies against insulin and Glut-2 (A), glucokinase (B), or PC1/3 (C). Representative sections are shown (left). Residual insulin-positive cells in Rfx3−/− embryos show only weak Glut-2 and glucokinase expression (arrows). Slc2a2 (A), Gck (B), and Pcsk1 (C) mRNA levels were quantified in the pancreases of WT (+/+) and Rfx3−/− (−/−) embryos at stages E17.5 and E19.5 (right). The results were normalized using Tbp mRNA and are expressed relative to the levels found in WT embryos. The mean ± SEM derived from five embryos is shown for each stage and genotype; **P < 0.001; *P < 0.05; ns, not significant. (A high-quality digital representation of this figure is available in the online issue.)
Insulin-positive cell numbers were not reduced markedly in Rfx3ΔP/− or Rfx3−/− adults, indicating that the β-cell mass is restored in postnatal mice (supplementary Fig. 10B). These adult β-cells remain defective with respect to Glut-2 and glucokinase expression (supplementary Fig. 10B). This defect is associated with impaired glucose tolerance in Rfx3ΔP/− (Fig. 4D) and Rfx3−/− (20) mice.
Rfx3 is required for mature β-cell function.
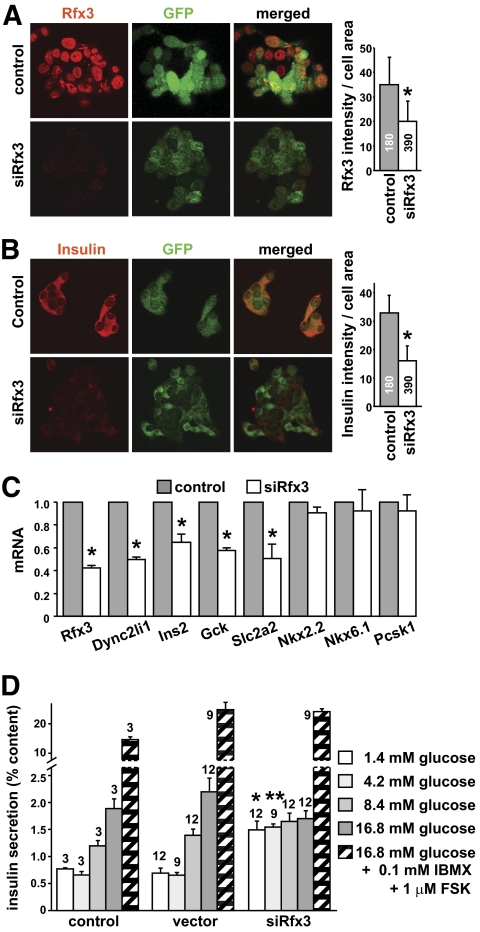
To document a potential role of Rfx3 in mature β-cells, we inhibited Rfx3 expression by RNA interference in the Min6 cell line. Min6 cells express Rfx3 mRNA at levels similar to those observed in mouse islets (supplementary Fig. 11A). Min6 cells were transduced with lentiviral vectors encoding Rfx3-specific or control siRNAs. Significant (albeit partial) reductions in Rfx3 protein and mRNA were induced in cells expressing the Rfx3 siRNA (Fig. 6A, supplementary Fig. 11B).
FIG. 6.
Rfx3 is required for maintaining β-cell maturity. Min6 cells were transduced with lentiviral vectors expressing control or Rfx3-specific siRNAs (siRfx3). The vectors also express GFP. A: Transduced cells were stained with antibodies against Rfx3 and examined by fluorescence microscopy to visualize Rfx3 (red) and GFP (green) expression. Representative fields are shown (left). The bar graph (right) shows the quantification of Rfx3 expression (fluorescence intensity). The mean ± SEM was derived from analysis of the indicated numbers of cells; *P < 0.05. B: Transduced cells were stained with antibodies against insulin and examined by fluorescence microscopy to visualize insulin (red) and GFP (green) expression. Representative fields are shown (left). The bar graph (right) shows the quantification of insulin expression (fluorescence intensity). The mean ± SEM was derived from analysis of the indicated numbers of cells; *P < 0.05. C: Rfx3, Dync2li1, Ins2, Gck, Slc2a2, Nkx2.2, Nkx6.1, and Pcsk1 mRNA levels were quantified in cells transduced with the control and Rfx3-specific siRNA vectors. The results were normalized using Tbp mRNA and are expressed relative to cells transduced with the control vector. The mean ± SEM derived from three independent experiments is shown; *P < 0.05. D: Insulin secretion in response to the indicated conditions was measured for Min6 cells transduced with the empty vector (vector), the control siRNA vector (control), or the Rfx3-specific siRNA vector (siRfx3). Results are expressed as percent of total insulin content and show the mean ± SEM derived from the number of measurements indicated at the top of the bars: *P < 0.03, **P < 0.001 vs. corresponding control value. FSK, forskolin; IBMX, isomethyl butyl xanthine. (A high-quality digital representation of this figure is available in the online issue.)
The impact of inhibiting Rfx3 expression on insulin production was assessed by three complementary approaches: quantifying insulin-staining intensity of individual cells by confocal microscopy, measurement of total insulin content by acid-ethanol extraction, and quantification of Ins2 mRNA. Although there were modest reductions in insulin staining and Ins2 mRNA in cells expressing the Rfx3 siRNA (Fig. 6B and C, supplementary Fig. 11B), this was not paralleled by a significant decrease in total insulin content (data not shown). Slc2a2 and Gck mRNAs were significantly decreased in cells expressing the Rfx3 siRNA (Fig. 6C). The reductions in Slc2a2 and Gck mRNAs were similar to that observed for a gene (Dync2li1) known to be regulated by Rfx3 (Fig. 6C) (16,20). In contrast, as observed in Rfx3−/− embryos, there was no decrease in Nkx6.1, Nkx2.2, and Pcsk1 mRNAs (Fig. 6C).
To test the effect of inhibiting Rfx3 expression on the secretory function of the transduced Min6 cells, we examined their insulin secretion in response to glucose or glucose plus agents raising cAMP (IBMX and forskolin) (Fig. 6D). The inhibition of Rfx3 expression did not affect insulin content (data not shown), but led to significantly elevated basal insulin release. As a result, stimulatory levels of glucose were unable to increase insulin output, as was observed in cells transduced with an empty vector or a vector encoding an irrelevant siRNA. This change was not due to the transduction protocol, since normal basal and glucose-stimulated insulin secretion was observed with the two control cell types. It could also not be ascribed to nonspecific inhibition of the secretory machinery, since stimulation with glucose in the presence of isomethyl butyl xanthine (IBMX) plus forskolin increased insulin release similarly in the three types of Min6 cells (Fig. 6D).
The β-cell promoter of the Gck gene is regulated by Rfx3.
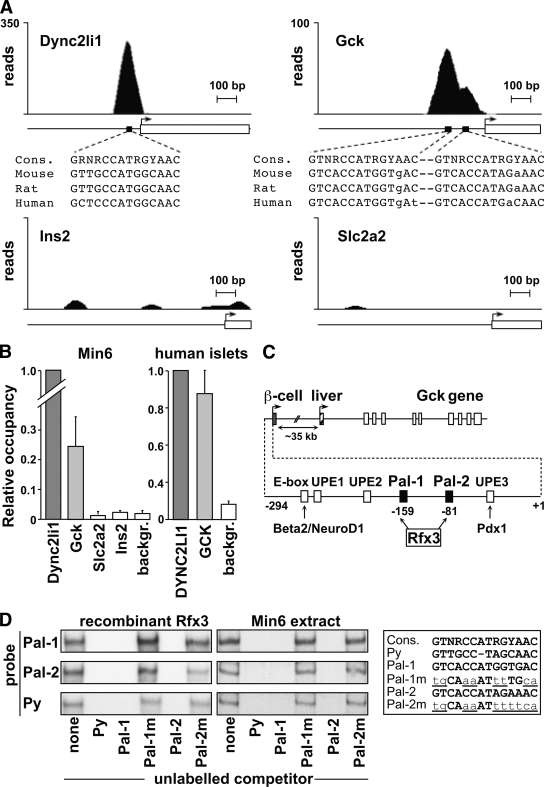
To determine if the Ins2, Gck, or Slc2a2 genes might be direct targets of Rfx3, we analyzed the results of a chromatin immunoprecipitation sequencing (ChIP-seq) experiment performed with Rfx3-specific antibodies and chromatin from Min6 cells. Two peaks were observed in the promoter that drives Gck expression in β-cells (Fig. 7A). This double peak coincided with two highly conserved sequences showing homology to the consensus Rfx binding site (30). Binding of Rfx3 to the Gck promoter was as evident as at the control Dync2li1 promoter. No signals were observed at the liver-specific Gck promoter, the Ins2 and Slc2a2 genes, or genes that are not expected to be targets of Rfx3 on the basis of our siRNA experiments and the analysis of Rfx3−/− mice (Fig. 7A, supplementary Fig. 12, data not shown).
FIG. 7.
Rfx3 binds to two key regulatory elements in the β-cell–specific promoter of the Gck gene. A: The profiles show the number of ChIP-seq reads mapping to the upstream regions of the Dync2li1, Gck, Slc2a2, and Ins2 genes. Transcription start sites (arrows), transcribed regions (open boxes), and scales in base pairs (bp) are indicated. Peaks corresponding to binding of Rfx3 were observed in the promoters of the Dync2li1 and Gck genes. The positions of these peaks coincide with conserved Rfx binding motifs (black boxes). The motifs found in the promoters of the mouse, rat, and human Dync2li1 and Gck genes are shown aligned with the consensus Rfx-binding site. No binding of Rfx3 was observed at the promoters of the Ins2 or Slc2a2 genes. B: Binding of Rfx3 to the promoters of the Dync2li1, Gck, Slc2a2, and Ins2 genes was assessed by quantitative ChIP in Min6 cells (left) or purified human islets (right). Control regions were included as background (backgr.) references. Results are expressed relative to binding of Rfx3 at the Dync2li1 promoter and show the mean ± SEM derived from three independent experiments. C: Schematic map of the Gck gene. Transcription of the Gck gene is regulated by two alternative cell type–specific promoters. The first promoter drives Gck expression in β-cells. The second promoter, situated ∼35 kb further downstream, is used in the liver. The two Rfx3 binding sites correspond to the previously identified Pal-1 and Pal-2 sequences centered on nucleotides −159 and −81 of the β-cell promoter. The approximate positions of other known regulatory elements (E-box, UPE-1, UPE-2, UPE-3) and binding sites for transcription factors (Beta2/NeuroD1, Pdx1) implicated in Gck expression in β-cells are indicated. The map of the Gck promoter is based on the study by Im et al. (36). D: Bandshift experiments were performed with recombinant Rfx3 (left gels) and Min6 extracts (right gels) using double-stranded oligonucleotide probes containing Pal-1, Pal-2, and a known Rfx binding site (Py) from the polyoma-virus enhancer. The regions of the gels containing the major band corresponding to binding of Rfx3 are shown (see supplementary Fig. 11). Binding reactions were supplemented as indicated below with unlabeled competitor oligonucleotides containing the Py, Pal-1, mutated Pal-1 (Pal-1m), Pal-2, or mutated Pal-2 (Pal-2m) sites. The corresponding sequences are shown aligned with the consensus Rfx binding site. Mutated nucleotides are underlined and in lower case.
Quantitative ChIP (qChIP) experiments confirmed that Rfx3 binds to the Gck promoter in Min6 cells, albeit not as strongly as at the Dync2li1 promoter (Fig. 7B). Binding of Rfx3 was not observed at the Slc2a2 or Ins2 genes. qChIP experiments performed with purified human islets demonstrated that RFX3 also binds to the human GCK promoter (Fig. 7B).
The Rfx3 binding motifs in the Gck promoter lie within a region that is essential and sufficient for driving β-cell expression in vitro and in vivo (Fig. 7C) (31–34). They correspond to two palindromic sequences (Pal-1 and Pal-2) known to be critical for activation of the Gck promoter in β-cells (34,35). That Rfx3 can bind specifically to the Pal-1 and Pal-2 motifs was confirmed by bandshift experiments performed with recombinant Rfx3 and Min6 extracts (Fig. 7D, supplementary Fig. 13). These findings indicate that Rfx3 is a bona fide transcriptional regulator of the human and mouse glucokinase gene.
DISCUSSION
Strongly decreased numbers of insulin-positive cells became evident in Rfx3−/− embryos by E15.5 and persisted until birth. This could not be attributed to a deficiency in Ngn3+ progenitors, altered islet cell apoptosis or proliferation, or defective β-lineage specification. Instead, β-lineage cells develop in Rfx3−/− embryos, but remain incompletely differentiated. Few, if any, of these cells have a mature β-cell phenotype. A minority (∼20%) correspond to insulin-positive β-cells exhibiting defective glucokinase and Glut-2 expression. The majority (∼80%) have a phenotype that could correspond to late β-cell precursors (Ngn3−, NKx6.1+, Pdx1+, insulin−, glucokinase−, Glut-2−). The latter are not simply endocrine progenitors since they lack Ngn3 expression (3,21). A similar skewed distribution of β-lineage cells is observed in pancreas-specific Rfx3-knockout embryos, indicating that it results from an endocrine cell–intrinsic deficiency in Rfx3. Defective β-cells lacking glucokinase and Glut-2 expression persist in adult Rfx3−/− and conditional Rfx3-deficient mice, leading to intolerance to glucose. Downregulating Rfx3 expression in Min6 cells provoked reduced Glut-2 and glucokinase expression and an elevation of basal insulin release, which prevented the detection of a glucose-stimulated increase in insulin output. Collectively, these findings indicate that Rfx3 is critical for both β-cell differentiation and the function of mature β-cells.
The Gck gene is regulated by two cell type–specific promoters (36). The most upstream promoter drives expression in β-cells and various other cell types, including glucose-sensitive neurons in the central nervous system and endocrine cells in the gut and pituitary gland (31,37,38). This neuroendocrine promoter is essential and sufficient for driving glucokinase expression in β-cells in vitro and in vivo (31–34). Rfx3 binds to two sequences in the neuroendocrine promoter in Min6 cells. RFX3 also binds to this promoter in human islets. The Rfx3 binding sites correspond to two palindromic sequences (Pal-1 and Pal-2) that are essential for regulating β-cell activity of the neuroendocrine promoter in rodents and humans (34,35,39). Gel-retardation, footprint, methylation-interference, and cross-linking experiments have demonstrated that Pal-1 and Pal-2 are recognized by nuclear factors in β-cells (33–35,39). Until now, the identity of these factors remained unknown. Our results show that the function of Pal-1 and Pal-2 is mediated by binding of Rfx3. Rfx3 thus collaborates with other β-cell–specific transcription factors, such as Pdx1 and NeuroD1 (36,40,41), in regulating Gck expression in β-cells.
Mice heterozygous for a deletion of the neuroendocrine promoter (which present only a 50% reduction in glucokinase expression in islets) exhibit hyperglycemia, intolerance to glucose, reduced glucose-stimulated hyperinsulinemia, and impaired glucose-stimulated insulin secretion (42). Similarly, mice heterozygous for a pancreas-specific Gck deletion exhibit an impaired ability to raise plasma insulin concentrations in response to glucose (43). A modest decrease in Gck expression is thus sufficient for perturbing glucose-stimulated insulin secretion and the maintenance of glucose homeostasis. Attenuated glucokinase expression in β-cells can thus explain, at least in part, why Rfx3−/− and Rfx3ΔP/− mice are intolerant to glucose.
Loss-of-function mutations in the human GCK gene cause one form of maturity-onset diabetes of the young (MODY-2) and permanent neonatal diabetes (44). A mutation in the neuroendocrine GCK promoter was recently found to be responsible for reduced glucokinase expression in certain MODY patients (45). Interestingly, this mutation lies immediately adjacent to the Pal-1 motif. Other forms of MODY result from mutations in genes encoding transcription factors that regulate the expression of GCK and/or play other key roles in β-cell function (45). Because we have identified Rfx3 as a critical regulator of Gck expression and mature β-cell function, it is tempting to speculate that mutations within the RFX3 gene, or in its regulatory regions, could give rise to MODY or other diseases characterized by pancreas dysfunction, defective insulin secretion, and/or perturbed glucose homeostasis.
We have as yet not elucidated the mechanisms responsible for reduced insulin and Glut-2 expression in β-lineage cells of Rfx3−/− mice. Neither gene is a direct target of Rfx3. Binding of Rfx3 was also not observed at the genes encoding NeuroD1, Pdx1, or MafA, which are known to activate insulin gene transcription (46), and the expression of these transcription factors was not affected in Rfx3−/− mice. Furthermore, analysis of our ChIP-seq data has not revealed other target genes known to be implicated in insulin or Glut-2 expression. Finally, the deficiencies in insulin-positive cells and insulin expression in Rfx3−/− mice are unlikely to be indirect consequences of reduced glucokinase expression, because no reductions in β-cell mass or pancreatic insulin content were documented in Gck-deficient mice (42).
Our ChIP-seq data have indicated that Rfx3 binds to the promoters of hundreds of genes implicated in the formation and function of cilia (data not shown). This is consistent with the fact that Rfx3 is required for the biogenesis of cilia on various cell types, including pancreatic endocrine cells (20,47). However, the functions of cilia in islets are unknown. No defects were observed in mice in which cilia were ablated in the pancreas by deletion of the Kif3a gene (47). It therefore remains to be determined whether the ciliary defects in Rfx3−/− mice contribute directly or indirectly to perturbed islet cell development and/or function.
The islet defects in Rfx3-deficient mice are strikingly similar, albeit not identical, to those observed in Rfx6-deficient mice (18). Shared features include impaired development of mature endocrine cells expressing insulin, glucagon, and ghrelin; increased numbers of cells expressing PP; normal expression of transcription factors implicated in β-cell specification (Nkx6-1, Nkx2-2); the accumulation of Nkx6.1+ cells lacking insulin; the appearance of Nkx6.1+ cells expressing PP; and reduced Glut-2 and Gck expression. Notable differences include the development of cells expressing somatostatin, which is defective in Rfx6−/− mice but normal in Rfx3-deficient mice, and the development of cilia on islet cells, which is impaired in Rfx3-deficent mice but unaffected in Rfx6−/− mice. A likely explanation for the partially overlapping phenotype is that Rfx3 and Rfx6 collaborate to regulate a common set of genes by binding as Rfx3-Rfx6 heterodimers and distinct sets of genes by binding as either Rfx3 and Rfx6 homodimers, or heterodimers with other Rfx factors. One common target gene is likely to be Gck, since preliminary ChIP experiments have suggested that its β-cell promoter is bound by both Rfx3 and Rfx6.
Detailed understanding of the programs governing β-cell differentiation is a prerequisite for the development of β-cell replacement therapies for diabetes relying on β-cells generated by guided differentiation of stem or progenitor cells, or trans-differentiation of other mature cells (3,48,49). Although major progress has been made, successful generation of mature β-cells has so far been mitigated because the program controlling β-cell differentiation is only partially understood. Our results contribute significantly to elucidation of this program and may therefore be valuable for the generation of functional β-cells in vitro.
Supplementary Material
ACKNOWLEDGMENTS
Work in the laboratory of W.R. was supported by the Swiss National Science Foundation, the Geneva Cancer League, the Swiss Multiple Sclerosis Society, the National Center of Competence in Research on Neural Plasticity and Repair (NCCR-NEURO), the Boninchi Foundation, and the Fondation Romande pour la Recherche sur le Diabète. A.A.-L. was supported by fellowships from the Roche Research Foundation, the Jules Thorne Foundation, the Association de Langue Francaise pour l'Etude du Diabète et des Maladies Métaboliques (ALFEDIAM), and the European Foundation for the Study of Diabetes (EFSD). Work in the laboratory of P.M. was supported by the Swiss National Science Foundation (310000-109402; CR32I3-129987), the Juvenile Diabetes Research Foundation (1-2007-158), and the EU (FP7 BETAIMAGE 222980; IMI IMIDIA C2008-T7). Work in the laboratory of P.L.H. was supported by the Juvenile Diabetes Research Foundation (5-2005-12; 1-2005-31), the NIH/NIDDK (DK072522-01, Beta Cell Biology Consortium), the Swiss National Science Foundation (3100A0-103867/1), the NCCR Frontiers in Genetics, and the EU (FP6 Integrated Project Beta Cell Therapy for Diabetes).
No potential conflicts of interest relevant to this article were reported.
A.A.-L. generated the bulk of the results. Q.S.-E., C.D.S., and P.B. performed ChIP-seq data analysis. C.B., Q.S.-E., and B.D. provided advice, expertise, mice, and reagents. A.A.-L. and W.R. wrote the manuscript. C.B., P.L.H., and P.M. reviewed and edited the manuscript. A.A.-L., P.M., and W.R. conceived and designed the experiments. P.L.H., P.M., and W.R. provided financial support.
We are grateful to E. Barras, A. Charollais, D. Rigo, and A. Gjinobci for expert technical assistance and I. Dunand-Sauthier for help with lentiviral vectors. We thank P. Halban for providing Min6 cells, B. Sosa-Pineda for providing the Pax4 antibody and B. Polat for help with islet purification.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gradwohl G, Dierich A, LeMeur M, Guillemot F: Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 2000;97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A: Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12:457–465 [DOI] [PubMed] [Google Scholar]
- 3.Oliver-Krasinski JM, Stoffers DA: On the origin of the beta cell. Genes Dev 2008;22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ: Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 1997;11:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L: Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A 2004;101:2924–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS: Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 1998;125:2213–2221 [DOI] [PubMed] [Google Scholar]
- 7.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P: Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003;17:2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M: Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 2000;127:5533–5540 [DOI] [PubMed] [Google Scholar]
- 9.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC: Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 2002;277:11225–11232 [DOI] [PubMed] [Google Scholar]
- 10.Holland AM, Gonez LJ, Naselli G, Macdonald RJ, Harrison LC: Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes 2005;54:2586–2595 [DOI] [PubMed] [Google Scholar]
- 11.Aftab S, Semenec L, Chu JS, Chen N: Identification and characterization of novel human tissue-specific RFX transcription factors. BMC Evol Biol 2008;8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery P, Durand B, Mach B, Reith W: RFX proteins, a novel family of DNA binding proteins conserved in the eukaryotic kingdom. Nucleic Acid Res 1996;24:803–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reith W, Ucla C, Barras E, Gaud A, Durand B, Herrero-Sanchez C, Kobr M, Mach B: RFX1, a transactivator of hepatitis B virus enhancer I, belongs to a novel family of homodimeric and heterodimeric DNA-binding proteins. Mol Cell Biol 1994;14:1230–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reith W, Mach B: The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 2001;19:331–373 [DOI] [PubMed] [Google Scholar]
- 15.Blackshear PJ, Graves JP, Stumpo DJ, Cobos I, Rubenstein JL, Zeldin DC: Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development 2003;130:4539–4552 [DOI] [PubMed] [Google Scholar]
- 16.Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W: The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol 2004;24:4417–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Stumpo DJ, Graves JP, DeGraff LM, Grissom SF, Collins JB, Li L, Zeldin DC, Blackshear PJ: Identification of potential target genes for RFX4_v3, a transcription factor critical for brain development. J Neurochem 2006;98:860–875 [DOI] [PubMed] [Google Scholar]
- 18.Smith SB, Qu HQ, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch AM, Grabs R, Wang J, Lynn FC, Miyatsuka T, Mitchell J, Seerke R, Desir J, Eijnden SV, Abramowicz M, Kacet N, Weill J, Renard ME, Gentile M, Hansen I, Dewar K, Hattersley AT, Wang R, Wilson ME, Johnson JD, Polychronakos C, German MS: Rfx6 directs islet formation and insulin production in mice and humans. Nature 463:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soyer J, Flasse L, Raffelsberger W, Beucher A, Orvain C, Peers B, Ravassard P, Vermot J, Voz ML, Mellitzer G, Gradwohl G: Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development 137:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ait-Lounis A, Baas D, Barras E, Benadiba C, Charollais A, Nlend Nlend R, Liegeois D, Meda P, Durand B, Reith W: Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes 2007;56:950–959 [DOI] [PubMed] [Google Scholar]
- 21.Gu G, Dubauskaite J, Melton DA: Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002;129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 22.Giordano E, Cirulli V, Bosco D, Rouiller D, Halban P, Meda P: B-cell size influences glucose-stimulated insulin secretion. Am J Physiol 1993;265:C358–C364 [DOI] [PubMed] [Google Scholar]
- 23.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR: Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell 2007;13:391–404 [DOI] [PubMed] [Google Scholar]
- 24.Wiznerowicz M, Trono D: Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol 2003;77:8957–8961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese A, Zhang M, Serre-Beinier V, Caton D, Mas C, Satin LS, Meda P: Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells. Diabetes 2003;52:417–424 [DOI] [PubMed] [Google Scholar]
- 26.Masternak K, Peyraud N, Krawczyk M, Barras E, Reith W: Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat Immunol 2003;4:132–137 [DOI] [PubMed] [Google Scholar]
- 27.Rudnick A, Ling TY, Odagiri H, Rutter WJ, German MS: Pancreatic beta cells express a diverse set of homeobox genes. Proc Natl Acad Sci U S A 1994;91:12203–12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrera PL: Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000;127:2317–2322 [DOI] [PubMed] [Google Scholar]
- 29.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N: Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998;95:829–837 [DOI] [PubMed] [Google Scholar]
- 30.Emery P, Strubin M, Hofmann K, Bucher P, Mach B, Reith W: A consensus motif in the RFX DNA binding domain and binding domain mutants with altered specificity. Mol Cell Biol 1996;16:4486–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jetton TL, Liang Y, Pettepher CC, Zimmerman EC, Cox FG, Horvath K, Matschinsky FM, Magnuson MA: Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem 1994;269:3641–3654 [PubMed] [Google Scholar]
- 32.Jetton TL, Moates JM, Lindner J, Wright CV, Magnuson MA: Targeted oncogenesis of hormone-negative pancreatic islet progenitor cells. Proc Natl Acad Sci U S A 1998;95:8654–8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibiger IB, Walther R, Pett U, Leibiger B: Positive and negative regulatory elements are involved in transcriptional control of the rat glucokinase gene in the insulin producing cell line HIT M2.2.2. FEBS Lett 1994;337:161–166 [DOI] [PubMed] [Google Scholar]
- 34.Shelton KD, Franklin AJ, Khoor A, Beechem J, Magnuson MA: Multiple elements in the upstream glucokinase promoter contribute to transcription in insulinoma cells. Mol Cell Biol 1992;12:4578–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moates JM, Shelton KD, Magnuson MA: Characterization of the Pal motifs in the upstream glucokinase promoter: binding of a cell type-specific protein complex correlates with transcriptional activation. Mol Endocrinol 1996;10:723–731 [DOI] [PubMed] [Google Scholar]
- 36.Im SS, Kim SY, Kim HI, Ahn YH: Transcriptional regulation of glucose sensors in pancreatic beta cells and liver. Curr Diabetes Rev 2006;2:11–18 [DOI] [PubMed] [Google Scholar]
- 37.Sorenson RL, Stout LE, Brelje TC, Jetton TL, Matschinsky FM: Immunohistochemical evidence for the presence of glucokinase in the gonadotropes and thyrotropes of the anterior pituitary gland of rat and monkey. J Histochem Cytochem 2007;55:555–566 [DOI] [PubMed] [Google Scholar]
- 38.Zelent D, Golson ML, Koeberlein B, Quintens R, van Lommel L, Buettger C, Weik-Collins H, Taub R, Grimsby J, Schuit F, Kaestner KH, Matschinsky FM: A glucose sensor role for glucokinase in anterior pituitary cells. Diabetes 2006;55:1923–1929 [DOI] [PubMed] [Google Scholar]
- 39.Moates JM, Magnuson MA: The Pal elements in the upstream glucokinase promoter exhibit dyad symmetry and display cell-specific enhancer activity when multimerised. Diabetologia 2004;47:1632–1640 [DOI] [PubMed] [Google Scholar]
- 40.Moates JM, Nanda S, Cissell MA, Tsai MJ, Stein R: BETA2 activates transcription from the upstream glucokinase gene promoter in islet beta-cells and gut endocrine cells. Diabetes 2003;52:403–408 [DOI] [PubMed] [Google Scholar]
- 41.Watada H, Kajimoto Y, Umayahara Y, Matsuoka T, Kaneto H, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y: The human glucokinase gene beta-cell-type promoter: an essential role of insulin promoter factor 1/PDX-1 in its activation in HIT-T15 cells. Diabetes 1996;45:1478–1488 [DOI] [PubMed] [Google Scholar]
- 42.Terauchi Y, Sakura H, Yasuda K, Iwamoto K, Takahashi N, Ito K, Kasai H, Suzuki H, Ueda O, Kamada N, et al. : Pancreatic beta-cell-specific targeted disruption of glucokinase gene: diabetes mellitus due to defective insulin secretion to glucose. J Biol Chem 1995;270:30253–30256 [DOI] [PubMed] [Google Scholar]
- 43.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA: Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999;274:305–315 [DOI] [PubMed] [Google Scholar]
- 44.Gloyn AL: Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat 2003;22:353–362 [DOI] [PubMed] [Google Scholar]
- 45.Gat-Yablonski G, Shalitin S, Phillip M: Maturity onset diabetes of the young: review. Pediatr Endocrinol Rev 2006;3(Suppl. 3):514–520 [PubMed] [Google Scholar]
- 46.Andrali SS, Sampley ML, Vanderford NL, Ozcan S: Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J 2008;415:1–10 [DOI] [PubMed] [Google Scholar]
- 47.Cano DA, Sekine S, Hebrok M: Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 2006;131:1856–1869 [DOI] [PubMed] [Google Scholar]
- 48.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE: Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 2005;23:1534–1541 [DOI] [PubMed] [Google Scholar]
- 49.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE: Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.