Abstract
OBJECTIVE
The sodium-calcium exchanger isoform 1 (NCX1) regulates cytoplasmic calcium (Ca2+c) required for insulin secretion in β-cells. NCX1 is alternatively spliced, resulting in the expression of splice variants in different tissues such as NCX1.3 and -1.7 in β-cells. As pharmacological inhibitors of NCX1 splice variants are in development, the pharmacological profile of β-cell NCX1.3 and -1.7 and the cellular effects of NCX1 inhibition were investigated.
RESEARCH DESIGN AND METHODS
The patch-clamp technique was used to examine the pharmacological profile of the NCX1 inhibitor KB-R7943 on recombinant NCX1.3 and -1.7 activity. Ca2+ imaging and membrane capacitance were used to assess the effects of KB-R7943 on Ca2+c and insulin secretion in mouse and human β-cells and islets.
RESULTS
NCX1.3 and -1.7 calcium extrusion (forward-mode) activity was ∼16-fold more sensitive to KB-R7943 inhibition compared with cardiac NCX1.1 (IC50s = 2.9 and 2.4 vs. 43.0 μmol/l, respectively). In single mouse/human β-cells, 1 μmol/l KB-R7943 increased insulin granule exocytosis but was without effect on α-cell glucagon granule exocytosis. KB-R7943 also augmented sulfonylurea and glucose-stimulated Ca2+c levels and insulin secretion in mouse and human islets, although KB-R7943 was without effect under nonstimulated conditions.
CONCLUSIONS
Islet NCX1 splice variants display a markedly greater sensitivity to pharmacological inhibition than the cardiac NCX1.1 splice variant. NCX1 inhibition resulted in glucose-dependent increases in Ca2+c and insulin secretion in mouse and human islets. Thus, we identify β-cell NCX1 splice variants as targets for the development of novel glucose-sensitive insulinotropic drugs for type 2 diabetes.
It is now widely accepted that decreased β-cell function, resulting in inadequate insulin secretion, is a key component of type 2 diabetes pathophysiology (1,2). Indeed, pharmacological agents such as sulfonylureas are used clinically to stimulate insulin secretion in type 2 diabetes. However, there is no absolute requirement for elevated glucose in order for sulfonylureas to stimulate insulin secretion via inhibition of β-cell ATP-sensitive K+ channels (KATP channels). Consequently, hypoglycemia is a significant concern with sulfonylurea therapy (3,4), and there is much interest in the development of insulinotropic drugs with improved glucose sensitivity.
In the pancreatic β-cell, cytoplasmic calcium (Ca2+c) levels rise as a direct consequence of glucose metabolism, via closure of KATP channels, triggering Ca2+ entry and subsequent Ca2+-mediated exocytosis of insulin granules (5). Therefore, partial inhibition of any protein involved in the removal of Ca2+c during β-cell excitation should augment insulin secretion only when β-cells are stimulated. One potential candidate protein is the sodium-calcium exchanger isoform 1 (NCX1), which is a membrane protein involved in the extrusion of Ca2+c in many tissues, including the pancreatic β-cell (6–8). NCX1 is a bidirectional ion exchanger that predominately extrudes Ca2+c during forward-mode (FM) operation when Ca2+c is elevated. NCX1 may also operate in Ca2+ influx mode (reverse mode; RM) that contributes to the pathophysiological increases in Ca2+c and Ca2+c overload that occurs during cardiac ischemia/reperfusion injury (9–11). In this regard, pharmacological inhibitors have been developed as putative cardioprotective agents to reduce RM NCX1 activity and ameliorate the deleterious Ca2+c overload in cardiac tissue (12–14). While such NCX inhibitors favor pathophysiological cardiac RM NCX1 inhibition (13,15–18), their effects on the physiological Ca2+c extrusion via FM NCX1 activity in β-cells have not been determined. Theoretically, partial pharmacological inhibition of FM NCX1 activity in β-cells should delay Ca2+c clearance, leading to an increased Ca2+c exocytotic signal and enhanced insulin secretion that is sensitive to glucose.
NCX1 is encoded by the SLC8A1 gene (6,19), which is alternatively spliced, leading to the expression of different splice variants in various tissues (20) with pancreatic β-cells expressing the NCX1.3 and -1.7 splice variants (8,21) compared with NCX1.1 in the heart. Importantly, we have recently shown that β-cell NCX1 splice variants display markedly different biophysical properties and acyl CoA sensitivity compared with the cardiac NCX1.1 splice variant (21). However, the pharmacological profile of β-cell NCX1.3 and -1.7 splice variants and the cellular effects of their inhibition have not been elucidated.
Therefore, the aims of this study were to determine 1) the sensitivities of the β-cell NCX1.3 and -1.7 splice variants to the NCX inhibitor KB-R7943 and 2) the effects of partial NCX1 inhibition on β-cell exocytosis, mouse and human islet Ca2+c, and insulin secretion under basal or glucose-/KCl sulfonylurea-stimulated conditions.
RESEARCH DESIGN AND METHODS
Molecular biology.
The rat NCX1.4 cDNA (22) and the Ad-shNCX1/Ad-scramble adenoviruses (23) were generously provided by Dr. J. Lytton (University of Calgary) and Dr. G.N. Pierce (University of Manitoba), respectively. Cloning and generation of the human NCX1 splice variants were described previously (21).
Cell culture and transfection.
tsA201 cells were maintained in DMEM supplemented with 25 mmol/l glucose, 2 mmol/l l-glutamine, 10% FCS, and 0.1% penicillin and streptomycin in a humidified incubator at 37°C with 5% CO2. tsA201 cells were cotransfected with an NCX1 cDNA clone and a green fluorescent protein plasmid using the calcium phosphate technique (24). Experiments were performed 24–72 h after transfection.
Islet isolation and dissociation.
Islets from BALB/c mice were isolated by collagenase (Sigma-Aldrich, Oakville, ON, CA) digestion of the pancreas, purified by Ficoll density gradient and then handpicked. Human islets from three donors were provided by Dr. James Shapiro (University of Alberta) and the Administrative and Bioinformatics Coordinating Center Human Islet Distribution Program at the University of Alberta. Islets were dispersed into single cells 2 h postisolation by gentle agitation in a Ca2+-free buffer containing 0.0025% trypsin and plated on poly-d-lysine glass coverslips. Mouse and human islet cells were cultured in medium 199 supplemented with 5.6 mmol/l glucose, 0.2% NaHCO3, 10% FCS, and 0.1% penicillin/streptomycin and in DMEM supplemented with 5.6 mmol/l glucose, 10% FCS, and 0.1% penicillin/streptomycin, respectively.
Electrophysiology.
The excised inside-out patch-clamp technique was used to measure recombinant FM NCX1 activity (22 ± 1°C) from transfected tsA201 cells as previously described (25). Unidirectional FM currents were elicited by rapidly changing the Ca2+c from ∼1 μmol/l to 3.2 mmol/l. The membrane patch was held at 0 mV, and NCX1 currents were measured and analyzed using the Axopatch 200B amplifier and Clampex 9.2 software (Axon Instruments, Foster City, CA). Peak current was defined as the maximal current recorded postactivation, and late current was taken as the current amplitude measured 1 min postactivation. Inactive exchangers were pre-exposed to KB-R7943 for 1.5 min prior to a 1-min activation in its presence. Total NCX1 current was measured as the area under the curve (AUC) during the entire activation period. Concentration-response curves were fitted with the Hill equation.
Calcium imaging.
Islets from BALB/c mice were cultured in medium 199 for 24–48 h at 37°C, 5% CO2. Islets were loaded for 60 min at room temperature with the Ca2+-sensitive fluorescent probe Fura-2AM (16 μmol/l, in a 1:1 vol/vol DMSO: pluronic acid mixture; Invitrogen, Burlington, ON, CA) in bath solution containing (in mmol/l) 140 NaCl, 5 KCl, 2 CaCl2, 1.4 MgCl2, 10 HEPES, and 2.8 glucose, pH 7.4. The KCl concentration was changed to 15 mmol/l when increased K+o was used as a depolarizing stimulus. The concentration of NaCl was adjusted accordingly to maintain osmolarity. Islets were washed and placed in an open chamber on the microscope stage and superfused with various bath solutions at 1 ml/min at 35 ± 1°C. A photomultiplier detection system (PTI, London, ON, CA) with FeliX32 software was used for data acquisition and analysis. Ratio-metric analysis was used to estimate changes in Ca2+c. Islets were excited at 340/380 nm (1 Hz), and the emitted 510-nm light was quantified. The average change in Ca2+c for tolbutamide experiments was taken as the AUC for 12 min (6-min drug application and 6-min washout). For the glucose experiments, the average change in Ca2+c was taken as the AUC for 20 min following peak onset. Changes in basal Ca2+ c were measured as the average 340/380 nm value over the last minute. The rate at which the average Ca2+c decreased once the stimulus was removed was taken as the time required for the fluorescent signal to drop to 10% of the peak value.
In situ NCX1 knockdown and pharmacology.
For in situ NCX1 knockdown, BALB/c islet cells were infected with an adenovirus (100 multiplicity of infection [MOI]) to deliver NCX1 short-hairpin RNA (shRNA) (Ad-shNCX1) or an adenoviral vector expressing a scrambled shRNA sequence as the control (Ad-scramble) and cultured in medium 199 for 72 h postinfection. For the in situ pharmacology experiments, cells were exposed to 1 μmol/l KB-R7943 for a minimum of 5 min.
Membrane capacitance.
To determine depolarization-induced exocytosis from single α- and β-cells, membrane capacitance changes were measured as described previously (21). Briefly, exocytosis was elicited with a train of 10 500-ms depolarizations (at 1 Hz) from −70 to 0 mV. The total exocytosis was taken as the sum of the capacitance increases during the depolarizing pulses. Capacitance changes were normalized to the initial cell size (as fF/pF). For calcium infusion experiments, the pipette calcium concentration was buffered to 200 nmol/l with EGTA, and capacitance was measured as previously described (26). Primary β-/α-cells were positively identified post hoc by immunostaining for insulin or glucagon, respectively.
Immunoblotting.
MIN6 cells were infected with 100 MOI of either Ad-shNCX1 or Ad-scramble and cultured for 72 h. A total of 40 μg of protein lysate were subjected to SDS-PAGE, transferred to polyvinylidene diflouride membranes (Millipore, Etobicoke, ON, CA), probed with the NCX-1 R3F1 monoclonal antibody (Swant, Bellinzona, CH), detected with peroxidase-conjugated secondary antibody (GE Healthcare, Mississauga, ON, CA), visualized by chemiluminescence (ECL-Plus; GE Healthcare), and exposed to X-ray film (Kodak, Rochester, NY). Protein-loading controls were β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) and total acetyl-CoA carboxylase (ACC) (Cell Signaling, Technology, Boston, MA). Quantification of NCX-1 protein expression was performed using densitometry and ImageJ software.
Glucose-stimulated insulin secretion assays.
Islets were preincubated for 2 h (with a fresh solution change after 1 h) at 37°C, in low-glucose Krebs-Ringer bicarbonate solution ± 1 μmol/l KB-R7943 (in mmol/l: 140 NaCl, 5 KCl, 2 CaCl2, 1.4 MgCl2, 10 HEPES, 24 NaHCO3, 0.5% BSA, and 2.8 glucose, pH 7.4). Groups of 25 islets were perifused for 10 min at 37°C with low-glucose KRB solution followed by 40 min with high-glucose (11.1 mmol/l) KRB at a rate of 0.25 ml/min using a Suprafusion 1000 System (Brandel, Gaithersburg, MD). Fractions were collected every 2 min, and changes in insulin secretion are expressed either as ng/ml per 25 islets or as a fold change (11.1/2.8 mmol/l). Insulin was quantified via enzyme-linked immunosorbent assay according to the manufacturer's instructions (ALPCO Diagnostics, Salem, NH).
Experimental compounds.
KB-R7943 (Tocris Bioscience, Ellisville, MO) was dissolved in DMSO as a 100 mmol/l stock. Tolbutamide (Sigma-Aldrich, Oakville, ON, CA) was dissolved in DMSO as a 20 mmol/l stock. Stock solutions were serially diluted in DMSO prior to use such that the final concentration of DMSO was 0.1% in all solutions when using KB-R7943 or tolbutamide.
Statistical analysis.
Statistical significance was assessed using the paired or unpaired Student t test or a one-way ANOVA with a Bonnferoni post hoc test, where required. P < 0.05 was considered significantly different, and data are expressed as means ± SE.
RESULTS
FM activity of β-cell NCX1 splice variants can be inhibited by KB-R7943.
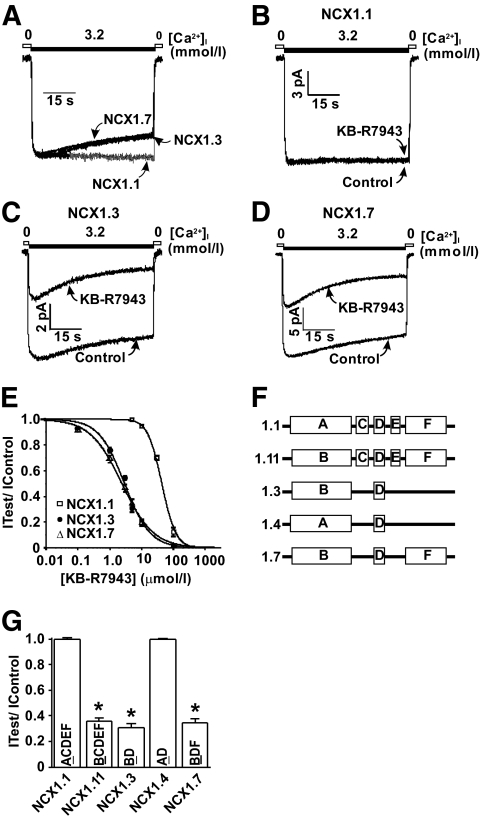
Past studies exploring the pharmacological inhibition of NCX1 have primarily focused on the cardiac splice variant (NCX1.1) and its RM of operation, as this mode contributes to calcium loading observed in ischemia/reperfusion injury. The general consensus is that NCX1 inhibitors preferentially inhibit the inactivating Ca2+ influx mode (RM) compared with the Ca2+ efflux mode (FM) (13,15–18) that displays no inactivation. We have recently reported that, in contrast to the cardiac NCX1.1 splice variant, β-cell NCX1 splice variants (NCX1.3 and -1.7) exhibit significant inactivation during FM operation (Fig. 1A) (21). Therefore, we tested the notion that NCX inhibitors, such as KB-R7943, may inhibit the FM activity of the β-cell NCX1.3 and -1.7 splice variants that display FM inactivation. As previously reported, 5 μmol/l KB-R7943 failed to inhibit total FM NCX1.1 activity (Fig. 1B and G) (27) but caused a significant reduction in total FM NCX1.3 (69.45 ± 3.12%, n = 10) (Fig. 1C and G) and NCX1.7 (65.57 ± 3.20%, n = 10) (Fig. 1D and G) activity. In addition, KB-R7943 displayed a concentration-dependent inhibition of FM activity (Fig. 1E), exhibiting a greater potency for inhibition of the β-cell splice variants, NCX1.3 (IC50 = 2.90 ± 0.27 μmol/l), and NCX1.7 (IC50 = 2.40 ± 0.15 μmol/l) compared with the cardiac NCX1.1 splice variant (IC50 = 42.97 ± 2.36 μmol/l).
FIG. 1.
KB-R7943 inhibits FM NCX1 activity in a splice variant–dependent manner. A: Representative current recordings of NCX1.1, -1.3, and -1.7 splice variants demonstrating that the β-cell NCX1 splice variants, NCX1.3 and -1.7, display FM inactivation, whereas the cardiac NCX1.1 splice variant does not. B–D: Representative current recordings illustrating that FM NCX1.1 activity is resistant to inhibition by 5 μmol/l KB-R7943, whereas NCX1.3 and -1.7 displayed marked KB-R7943 inhibition. E: KB-R7943 concentration-inhibition curves for NCX1.1, -1.3, and -1.7 FM currents. F: Diagrammatic representation of the alternative splicing region exon composition for various NCX1 splice variants. G: Grouped KB-R7943 (5 μmol/l) inhibition data for FM NCX1.1, -1.11, -1.3, -1.4, and -1.7 currents. *P < 0.05, n = 7–10 patches per group. 0 = <1 μmol/l Ca2+. NCX1 proteins were expressed in tsA201 cells.
Regions of the NCX1 protein that impart FM sensitivity to KB-R7943.
We have previously reported that the presence of exon B within the alternative splicing region of NCX1 proteins bestows FM inactivation (21). Since NCX1 splice variants differ only in the exon composition in the alternative splicing region, this region likely confers FM sensitivity to KB-R7943. Examination of the exon composition between NCX1.1 (ACDEF) and NCX1.3 (BD) and NCX1.7 (BDF) points to the role of exon B in bestowing the increased KB-R7943 sensitivity (Fig. 1F). To examine this directly, exon A in NCX1.1 was substituted for exon B, generating NCX1.11 (BCDEF) (Fig. 1F). NCX1.11 displays FM KB-R7943 sensitivity similar to that observed for NCX1.3 and NCX1.7 (64.3 ± 2.92% reduction in total current, n = 8) (Fig. 1G). Similarly, replacing exon B with A in rat NCX1.3 (NCX1.4) (Fig. 1F) dramatically decreased FM KB-R7943 sensitivity (Fig. 1G). Therefore, the presence of exon B in NCX1 splice variants bestows the increased KB-R7943 sensitivity to FM inhibition.
KB-R7943 is a more potent blocker of the late component of FM β-cell NCX1 splice variants.
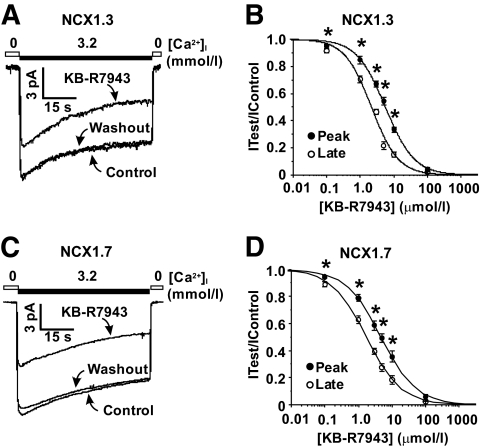
Previous reports show that KB-R7943 preferentially blocks the late (or steady-state) component of RM NCX1 current, presumably by either stabilizing the I1 inactivated state or by increasing the rate of inactivation (27,28). To examine whether KB-R7943 exhibits a similar effect on FM currents that inactivate, concentration-response curves for NCX1.3 and -1.7 were generated. KB-R7943 exhibits a significantly greater potency for FM late current (IC50 = 2.15 ± 0.26 μmol/l, NCX1.3; 1.72 ± 0.09 μmol/l, NCX1.7) compared with peak current (IC50 = 5.59 ± 0.28 μmol/l, NCX1.3; 4.71 ± 0.15 μmol/l, NCX1.7) for both NCX1.3 (Fig. 2A and B) and NCX1.7 (Fig. 2C and D), and these effects readily wash out (Fig. 2A and C).
FIG. 2.
The late FM NCX1.3 and -1.7 currents are more sensitive to KB-R7943 inhibition (3 μmol/l) compared with peak currents. Representative recordings of NCX1.3 (A) and -1.7 (C) FM currents before (control) and after the application of 3 μmol/l KB-R7943. Concentration-response curves for NCX1.3 (B) and -1.7 (D) late and peak KB-R7943 FM current inhibition demonstrating that KB-R7943 preferentially inhibits the late FM currents compared with peak FM currents. *P < 0.05, n = 4–10 patches per concentration. 0 = <1 μmol/l Ca2+. NCX1 proteins were expressed in tsA201 cells.
KB-R7943 inhibits β-cell l-type calcium channels.
KB-R7943 is not selective for NCX1 and is known to inhibit the cardiac l-type calcium channel at higher concentrations (IC50s 3–7 μmol/l) (29,30). Consequently, the effect of KB-R7943 was tested on endogenous l-type calcium channels in β-cells using the whole-cell patch-clamp technique. Application of 1 μmol/l KB-R7943 to mouse and human β-cells caused a 32.66 ± 3.74% (n = 7) and 29.54 ± 3.18% reduction in total Ba2+ current, respectively. Therefore, the possibility exists that this nonspecific effect of KB-R7943 on the β-cell l-type calcium channel may paradoxically reduce calcium entry and subsequent insulin secretion. Accordingly, the precise cellular consequences of NCX1 inhibition on calcium signaling and exocytosis were determined in the following experiments.
NCX1 inhibition increases stimulated, but not basal, islet cytoplasmic calcium levels.
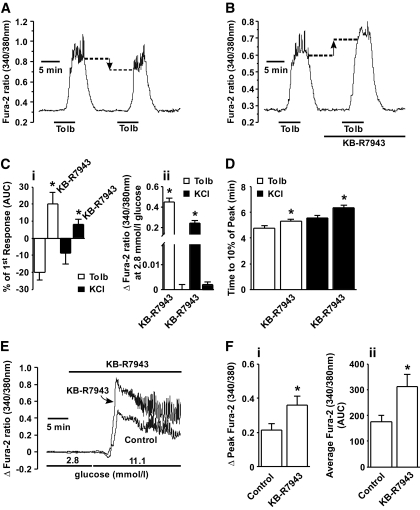
To determine whether partial inhibition of β-cell NCX1 is effective at increasing islet Ca2+c levels, changes in Ca2+c were monitored by Fura-2 ratiometric fluorometry. Upon the application of 20 μmol/l tolbutamide, mouse islets constantly superfused with 2.8 mmol/l glucose exhibited a significant increase in Ca2+c (a 0.45 ± 0.04 increase in the 340/380 nm ratio, n = 9) (Fig. 3A and B), confirming in this system that sulfonylurea drugs stimulate β-cells even at low glucose concentrations. Upon a second tolbutamide challenge, the elevation in Ca2+c decreased by 21.87 ± 4.58% (n = 11) (Fig. 3A). In contrast, application of 1 μmol/l KB-R7943 (a concentration that yields a partial ∼25% inhibition of recombinant β-cell NCX1.3 and 1.7 currents) (Fig. 1E) in the presence of 2.8 mmol/l glucose failed to significantly increase Ca2+c levels (Fig. 3B) but significantly augmented Ca2+c when added concurrently with tolbutamide (19.95 ± 6.92% increase in Ca2+c n = 9) (Fig. 3B and C). Similar results were obtained when the extracellular concentration of KCl was raised to 15 mmol/l (Fig. 3C). One μmol/l KB-R7943 also significantly slowed the rate of calcium clearance after removal of the tolbutamide/KCl challenge (Fig. 3D). Application of 1 μmol/l KB-R7943 alone at low (2.8 mmol/l) glucose did not alter Ca2+c (Fig. 3E) yet caused a significant 83.44 ± 18.27% increase to the peak Ca2+c response (Fig. 3E and F) as well as increased average Ca2+c by 79.98 ± 8.73% (n = 9) (Fig. 3F) during an 11.1 mmol/l glucose challenge.
FIG. 3.
Calcium imaging of mouse islets. A: Application of 20 μmol/l tolbutamide (Tolb) to islets superfused with 2.8 mmol/l glucose increases Ca2+c levels that are lower upon a second tolbutamide application. B: In contrast, the addition of 1 μmol/l KB-R7943 significantly increases the magnitude of Ca2+c elevation observed during the second application of tolbutamide. KB-R7943 does not increase Ca2+c levels when applied alone in the presence of 2.8 mmol/l glucose. Ci: Grouped data showing that KB-R7943 significantly increases Ca2+c levels (*P < 0.05, n = 6–11 islets) in the presence of tolbutamide or KCl. Cii: Grouped data showing that in the absence of the tolbutamide or KCl stimulatory signal, KB-R7943 does not increase Ca2+c (n = 6–9 islets). D: Grouped data indicating that the rate at which Ca2+c returned to basal levels is slower in the presence of KB-R7943 for both tolbutamide and KCl (*P < 0.05, n = 6–9 islets). E: Increasing glucose from 2.8 to 11.1 mmol/l elicited increases in Ca2+c, whereas 1 μmol/l KB-R7943 did not increase Ca2+c at 2.8 mmol/l glucose but significantly increased Ca2+c in 11.1 mmol/l glucose when compared with 11.1 mmol/l glucose alone. Grouped data showing that 1 μmol/l KB-R7943 significantly increased the peak (Δpeak) (Fi) and average Ca2+c (AUC) in response to 11.1 mmol/l glucose (Fii). *P < 0.05, n = 9 islets per group.
NCX1 inhibition increases calcium-dependent exocytosis in β-cells but not α-cells.
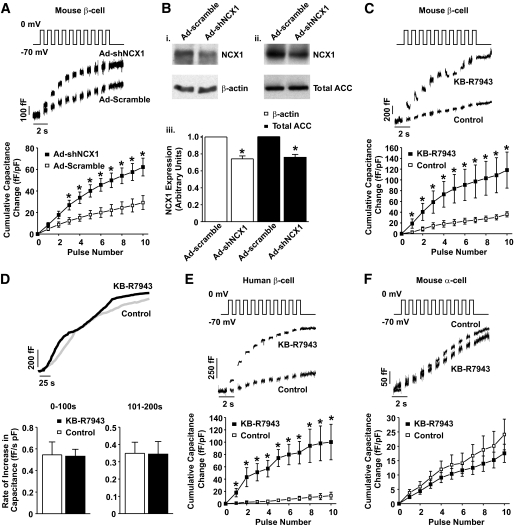
To examine the cellular consequences of decreased FM NCX1 activity, we measured whole-cell capacitance changes elicited by a train of depolarizing pulses in mouse and human β-cells with a reduction in NCX1 activity (Fig. 4). Adenoviral delivery of shRNA for NCX1 (Ad-shNCX1) increased the capacitance response of the mouse β-cells (Fig. 4A) when compared with adenoviral delivery of the scrambled shNCX1 sequence (Ad-scramble). This resulted in an overall increase in the total capacitance change from 29.22 ± 6.44 (n = 8) to 62.22 ± 8.25 (n = 12) fF/pF (Fig. 4A). Independent Western blot analysis on MIN6 cell lysates demonstrates that infection of MIN6 cells with Ad-shNCX1 results in a ∼25% reduction of NCX1 protein expression (25.76 ± 3.36% and 24.09 ± 3.46% [n = 3] vs. noninfected control with β-actin and total ACC used as loading controls, respectively) (Fig. 4B). Similarly, partial inhibition of FM NCX1 activity with 1 μmol/l KB-R7943 increased the capacitance response (Fig. 4C) and increased the change in total capacitance from 36.04 ± 4.87 (n = 10) to 118.22 ± 33.48 (n = 6) fF/pF (Fig. 4C).
FIG. 4.
NCX1 inhibition and Ca2+-dependent exocytosis in β- and α-cells. Representative capacitance measurements and grouped data of cumulative changes at individual pulse numbers from mouse and human β-cells with reduced NCX1 expression (Ad-shNCX1), scrambled control vector (Ad-scramble) (A), or in the presence of 1μmol/l KB-R7943 (C and E) in response to a depolarizing train of membrane potential pulses. B: Western blot analysis (i, ii) showing that NCX1 protein expression is only partially reduced as a result of Ad-shNCX1 infection in MIN6 cells (i–iii). β-actin (i) and total ACC (ii) were used as loading controls. D: Representative capacitance measurements and grouped data for calcium infusion experiments in the presence and absence of 1μmol/l KB-R7943. F: Representative capacitance measurements and grouped data from mouse α-cells in response to 1 μmol/l KB-R7943. *P < 0.05, n = 8–20 cells per group or three separate lysates (B).
To determine whether KB-R7943 elicits any indirect effects on the exocytotic process independently of NCX1 inhibition, exocytosis was stimulated by direct infusion of 200 nmol/l free Ca2+ in the presence or absence of KB-R7943, and the corresponding increase in capacitance was measured. In either condition, the rate at which the capacitance increased was not significantly different in the presence or absence of KB-R7943 (Fig. 4D). These results suggest that KB-R7943 does not enhance exocytosis when Ca2+c is infinitely buffered, thus minimizing the contribution of calcium extrusion via NCX1.
The effect observed with mouse β-cells was also seen with human β-cells, where 1 μmol/l KB-R7943 increased the capacitance response (Fig. 4E) and increased the total change in capacitance from 13.01 ± 5.64 (n = 6) to 100.20 ± 28.82 ((n = 4) fF/pF (Fig. 4E). In direct contrast, application of 1 μmol/l KB-R7943 did not significantly alter the depolarization-induced capacitance response observed in mouse α-cells (Fig. 4F).
NCX1 inhibition augments insulin secretion in a glucose-dependent manner.
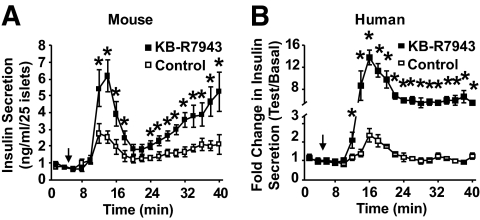
The calcium and exocytosis data indicate that partial NCX1 inhibition should stimulate islet insulin secretion in a glucose-sensitive manner. To test this notion directly, mouse and human islet perifusion experiments were performed in the presence or absence of 1 μmol/l KB-R7943 and insulin secretion measured in response to increasing glucose from 2.8 to 11.1 mmol/l. In both mouse and human islets, KB-R7943 significantly enhanced first- and second-phase insulin secretion stimulated by 11.1 mmol/l glucose (Fig. 5A and B) but was without effect at 2.8 mmol/l glucose.
FIG. 5.
Glucose-stimulated insulin secretion from mouse and human islets in response to elevations in glucose from 2.8 to 11.1 mmol/l (denoted by arrows). KB-R7943 (1 μmol/l) augments insulin secretion from mouse (A) and human (B) islets only in the presence of elevated glucose (11.1 mmol/l). *P < 0.05, n = 5–6 for mouse and 2–3 for humans.
DISCUSSION
Our data represents the first pharmacological characterization of β-cell/islet NCX1.3 and -1.7 FM currents. Interestingly, we observed that the FM activity of these β-cell/islet NCX1 splice variants can be potently inhibited by KB-R7943 and that this block is dependent upon the presence of FM inactivation (Fig 1C and D). To investigate this directly, we examined the KB-R7943 sensitivity of various NCX1 splice variants. NCX1 splice variants that contain exon A (NCX1.1 [ACDEF] and NCX1.4 [AD]) do not exhibit FM inactivation (21) and thus exhibit a resistance to block by KB-R7943 (Fig 1F and G). In contrast, NCX1 splice variants that contain exon B (NCX1.3 [BD], NCX1.7 [BDF], and NCX1.11 [BCDEF]) exhibit FM inactivation (21) and are significantly more sensitive to block by KB-R7943 (Fig. 1F and G). This observed exon A/B dependence of KB-R7943 inhibition suggests that tissue-specific pharmacological separation may be achieved as NCX1 splice variants are selectively expressed depending on tissue type. For example, the cardiac splice variant, NCX1.1, does not exhibit FM inactivation, and, thus, its physiologically relevant mode of activity is ∼16-fold less sensitive to KB-R7943 inhibition compared with the β-cell NCX1.3 and -1.7 splice variants. It should be noted that NCX1.3 and -1.7 are not exclusively expressed in the endocrine pancreas but are also expressed in vasculature and other tissues (20). Inhibition of FM NCX1 activity in vascular smooth muscle may cause Ca2+-dependent vasoconstriction and increased blood pressure, although KB-R7943 has been recently shown to reduce vasoconstriction via inhibition of reverse-mode NCX1 activity and reduced Ca2+ entry (31). Therefore, the extrapancreatic effects of selective NCX1.3 inhibition and -1.7 will require further examination.
It has previously been shown that KB-R7943 preferentially blocks the late (or steady-state) component of RM NCX1.1 current by either increasing the rate of inactivation or by stabilizing the I1 inactivated state (27,28). Our results demonstrate that the same may be true for the inactivating FM currents from NCX1.3 and -1.7 (Fig. 2). Although the exact mechanism of FM inactivation has not been determined, the two different types of inactivation may involve similar regions of the NCX1 protein that may explain these observed effects of KB-R7943.
The pancreatic β-cell expresses a number of intracellular and plasma membrane–bound proteins that regulate Ca2+c, including the plasma membrane calcium ATPase, the plasma membrane NCX, the endoplasmic reticulum calcium ATPase, and the mitochondrial uniporter. While the precise contributions of NCX1 have not been fully elucidated, it is clear that NCX1 plays an important role in maintaining Ca2+c homeostasis (32–35). Our results now confirm that the major role for NCX1 is the facilitation of Ca2+c efflux during periods of increased β-cell excitability, namely that partial inhibition of β-cell NCX1 with KB-R7943 further increases Ca2+c only when Ca2+c is already elevated by either tolbutamide, glucose, or KCl (Fig. 3), leading to increased exocytosis (Fig. 4) and enhanced insulin secretion only in the presence of such a stimulus (Fig. 5). While these results have been attributed to KB-R7943 inhibiting FM NCX1 activity and directly verified by shRNA, possible effects of KB-R7943 on other calcium-handling proteins cannot be ruled out.
One additional key finding from this study is the lack of effect of KB-R7943 on pancreatic α-cell exocytosis (Fig. 4). This suggests that glucagon secretion would be unaffected by pharmacological inhibition of NCX1 splice variants within the endocrine pancreas and indicate that either 1) NCX1 does not play a major role in regulating Ca2+c homeostasis and hence glucagon release from α-cells or 2) α-cells express an NCX1 splice variant insensitive to pharmacological inhibition. Thus, these findings identify a novel glucose-sensitive mechanism for insulin, but not glucagon, secretion and provide a rationale for the development of selective β-cell NCX1 splice variant inhibitors as a new class of glucose-sensitive insulinotropic drugs.
KB-R7943 is not exclusively selective for NCX1, and at higher concentrations it also affects other ion channels (36–38). For example, KB-R7943 has been shown to inhibit both the cardiac (IC50s 3–7 μmol/l [29,30]) and β-cell l-type calcium channels at higher concentrations (this study). Furthermore, complete NCX1 inhibition may lead to deleterious effects on β-cell function though excess Ca2+c accumulation. Therefore, we selected a concentration of KB-R7943 (1 μmol/l) that obtained the desired partial inhibition of β-cell NCX1 FM activity (Fig. 1E) with the least nonspecific inhibition of l-type calcium channel currents. It is likely that the insulinotropic efficacy of KB-R7943 would be even greater if this molecule lacked l-type calcium channel inhibition. It is important to note that the marked effects on insulin secretion were observed at 1 μmol/l KB-R7943, a concentration that only inhibits recombinant β-cell NCX1.3/1.7 FM currents by ∼25% (Fig. 1E), suggesting that NCX1 plays an important role in Ca2+c clearance and that partial inhibition is sufficient to enhance Ca2+c and elicit stimulatory effects on insulin secretion.
Currently available NCX1 inhibitors, such as KB-R7943, also affect a number of ion transport processes at higher concentrations and therefore should be considered as “pharmacological tools” rather than as viable therapeutic agents. Indeed, results from our preliminary in vivo intraperitoneal glucose tolerance tests in mice only showed a trend toward KB-R7943 lowering blood glucose (data not shown). Thus, the identification of drugs with a greater selectivity for NCX1 inhibition over other ion transport pathways in the β-cell is a necessary first step in the advancement of β-cell NCX1 splice variants as novel therapeutic targets. In this latter regard, newer NCX inhibitors with a reported greater selectivity for NCX1 over other ion transport processes are in development for cardiovascular disease, and these inhibitors may also warrant further investigation for their splice variant selectivity and insulinotropic efficacy (13,15–18).
Summary.
Findings from this study demonstrate for the first time that β-cell/islet NCX1 splice variants display a markedly increased sensitivity to pharmacological inhibitors, resulting in the glucose-sensitive augmentation of insulin secretion. These results identify a novel glucose-dependent pathway that possesses desirable pharmacological properties for future drug discovery.
ACKNOWLEDGMENTS
This work was supported by operating grants from the Canadian Diabetes Association (CDA) to P.E.L. and J.R.B.D. P.E.M. was supported by an operating grant from the Canadian Institutes of Health Research (CIHR). K.S.C.H. received support from the CIHR Strategic Training Initiative for the study of Membrane Proteins. N.J.W. and M.J.R. were supported by the Alberta Heritage Foundation for Medical Research (AHFMR) and the CDA Trainee Awards. T.P., D.S., G.J.S., and X.Q.D. were supported by AHFMR Trainee Awards. D.A.L. was supported by a Muttart-Collip Diabetes Research and Training Center studentship.
P.E.M. received salary support as a CDA Scholar, AHFMR Scholar, and the Canada Research Chair in Islet Biology. P.E.L. and J.R.B.D. received salary support as AHFMR Senior Scholars. J.R.B.D. is a Canada Research Chair in Heart Disease and Metabolism.
No potential conflicts of interest relevant to this article were reported.
K.S.C.H. performed experiments, contributed to the discussion, wrote/reviewed and edited the manuscript. D.S. performed experiments, analyzed data, and contributed to the interpretation of data and the discussion. N.J.W., G.J.S., L.C.M., D.A.L., X.Q.D., T.P., and M.J.R. performed experiments and analyzed data. J.R.B.D. contributed to the design of some experiments and the discussion. P.E.M. contributed to the design of some experiments, the interpretation of data, and the discussion. P.E.L. initiated the study, contributed to the design of experiments, the interpretation of data, and wrote/reviewed/edited the manuscript.
We thank D.E. Dixon and N. Smith for the isolation/dispersion of mouse islets, Dr. J. Manning Fox for assistance with islet perifusions, and S. Rahman for technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Festa A, Williams K, Hanley AJ, Haffner SM: β-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes 2008;57:1638–1644 [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group U.K. Prospective Diabetes Study 16: overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 3.Del Prato S, Aragona M, Coppelli A: Sulfonylureas and hypoglycaemia. Diabetes Nutr Metab 2002;15:444–450; discussion 450–441 [PubMed] [Google Scholar]
- 4.Amiel SA, Dixon T, Mann R, Jameson K: Hypoglycaemia in type 2 diabetes. Diabet Med 2008;25:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rorsman P, Renstrom E: Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029–1045 [DOI] [PubMed] [Google Scholar]
- 6.Lytton J: Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 2007;406:365–382 [DOI] [PubMed] [Google Scholar]
- 7.Herchuelz A, Diaz-Horta O, Van Eylen F: Na/Ca exchange in function, growth, and demise of beta-cells. Ann N Y Acad Sci 2002;976:315–324 [DOI] [PubMed] [Google Scholar]
- 8.Herchuelz A, Diaz-Horta O, van Eylen F: Na/Ca exchange and Ca2+ homeostasis in the pancreatic beta-cell. Diabetes Metab 2002;28:3S54–60; discussion 3S108–3S112 [PubMed] [Google Scholar]
- 9.Shigekawa M, Iwamoto T: Cardiac Na(+)-Ca(2+) exchange: molecular and pharmacological aspects. Circ Res 2001;88:864–876 [DOI] [PubMed] [Google Scholar]
- 10.Reuter H, Pott C, Goldhaber JI, Henderson SA, Philipson KD, Schwinger RH: Na(+)–Ca2+ exchange in the regulation of cardiac excitation-contraction coupling. Cardiovasc Res 2005;67:198–207 [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto T, Kita S: Topics on the Na+/Ca2+ exchanger: role of vascular NCX1 in salt-dependent hypertension. J Pharmacol Sci 2006;102:32–36 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura A, Harada K, Sugimoto H, Nakajima F, Nishimura N: [Effects of KB-R7943, a novel Na+/Ca2+ exchange inhibitor, on myocardial ischemia/reperfusion injury]. Nippon Yakurigaku Zasshi 1998;111:105–115 [article in Japanese] [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hamano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A: SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther 2001;298:249–256 [PubMed] [Google Scholar]
- 14.Lee C, Dhalla NS, Hryshko LV: Therapeutic potential of novel Na+-Ca2+ exchange inhibitors in attenuating ischemia-reperfusion injury. Can J Cardiol 2005;21:509–516 [PubMed] [Google Scholar]
- 15.Iwamoto T, Watano T, Shigekawa M: A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem 1996;271:22391–22397 [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto T, Inoue Y, Ito K, Sakaue T, Kita S, Katsuragi T: The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4-(4-nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol Pharmacol 2004;66:45–55 [DOI] [PubMed] [Google Scholar]
- 17.Kuramochi T, Kakefuda A, Yamada H, Ogiyama T, Taguchi T, Sakamoto S: Discovery of N-(3-{4-[(3-fluorobenzyl)oxy]phenoxy}propyl)-2-pyridin-4-ylacetamide as a potent and selective reverse NCX inhibitor. Chem Pharm Bull (Tokyo) 2005;53:1043–1047 [DOI] [PubMed] [Google Scholar]
- 18.Kuramochi T, Kakefuda A, Yamada H, Tsukamoto I, Taguchi T, Sakamoto S: Discovery of an N-(2-aminopyridin-4-ylmethyl)nicotinamide derivative: a potent and orally bioavailable NCX inhibitor. Bioorg Med Chem 2005;13:4022–4036 [DOI] [PubMed] [Google Scholar]
- 19.Philipson KD, Nicoll DA, Ottolia M, Quednau BD, Reuter H, John S, Qiu Z: The Na+/Ca2+ exchange molecule: an overview. Ann N Y Acad Sci 2002;976:1–10 [DOI] [PubMed] [Google Scholar]
- 20.Quednau BD, Nicoll DA, Philipson KD: Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol 1997;272:C1250–C1261 [DOI] [PubMed] [Google Scholar]
- 21.Hamming KS, Riedel MJ, Soliman D, Matemisz LC, Webster NJ, Searle GJ, MacDonald PE, Light PE: Splice variant-dependent regulation of beta-cell sodium-calcium exchange by acyl-coenzyme As. Mol Endocrinol 2008;22:2293–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn J, Elias CL, Le HD, Omelchenko A, Hryshko LV, Lytton J: The molecular determinants of ionic regulatory differences between brain and kidney Na+/Ca2+ exchanger (NCX1) isoforms. J Biol Chem 2002;277:33957–33962 [DOI] [PubMed] [Google Scholar]
- 23.Hurtado C, Ander BP, Maddaford TG, Lukas A, Hryshko LV, Pierce GN: Adenovirally delivered shRNA strongly inhibits Na+-Ca2+ exchanger expression but does not prevent contraction of neonatal cardiomyocytes. J Mol Cell Cardiol 2005;38:647–654 [DOI] [PubMed] [Google Scholar]
- 24.Jordan M, Wurm F: Transfection of adherent and suspended cells by calcium phosphate. Methods 2004;33:136–143 [DOI] [PubMed] [Google Scholar]
- 25.Riedel MJ, Baczko I, Searle GJ, Webster N, Fercho M, Jones L, Lang J, Lytton J, Dyck JR, Light PE: Metabolic regulation of sodium-calcium exchange by intracellular acyl CoAs. EMBO J 2006;25:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pigeau GM, Kolic J, Ball BJ, Hoppa MB, Wang YW, Ruckle T, Woo M, Manning Fox JE, MacDonald PE: Insulin granule recruitment and exocytosis is dependent on p110gamma in insulinoma and human β-cells. Diabetes 2009;58:2084–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias CL, Lukas A, Shurraw S, Scott J, Omelchenko A, Gross GJ, Hnatowich M, Hryshko LV: Inhibition of Na+/Ca2+ exchange by KB-R7943: transport mode selectivity and antiarrhythmic consequences. Am J Physiol Heart Circ Physiol 2001;281:H1334–H1345 [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto T: Na+/Ca2+ exchange as a drug target: insights from molecular pharmacology and genetic engineering. Ann N Y Acad Sci 2007;1099:516–528 [DOI] [PubMed] [Google Scholar]
- 29.Birinyi P, Acsai K, Banyasz T, Toth A, Horvath B, Virag L, Szentandrassy N, Magyar J, Varro A, Fulop F, Nanasi PP: Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 2005;372:63–70 [DOI] [PubMed] [Google Scholar]
- 30.Ouardouz M, Zamponi GW, Barr W, Kiedrowski L, Stys PK: Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacology 2005;48:566–575 [DOI] [PubMed] [Google Scholar]
- 31.Raina H, Ella SR, Hill MA: Decreased activity of the smooth muscle Na+/Ca2+ exchanger impairs arteriolar myogenic reactivity. J Physiol 2008;586:1669–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes E, Lee AK, Tse A: Dominant role of sarcoendoplasmic reticulum Ca2+-ATPase pump in Ca2+ homeostasis and exocytosis in rat pancreatic β-cells. Endocrinology 2006;147:1396–1407 [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Koh DS, Hille B: Dynamics of calcium clearance in mouse pancreatic β-cells. Diabetes 2003;52:1723–1731 [DOI] [PubMed] [Google Scholar]
- 34.Van Eylen F, Horta OD, Barez A, Kamagate A, Flatt PR, Macianskiene R, Mubagwa K, Herchuelz A: Overexpression of the Na/Ca exchanger shapes stimulus-induced cytosolic Ca(2+) oscillations in insulin-producing BRIN-BD11 cells. Diabetes 2002;51:366–375 [DOI] [PubMed] [Google Scholar]
- 35.Van Eylen F, Lebeau C, Albuquerque-Silva J, Herchuelz A: Contribution of Na/Ca exchange to Ca2+ outflow and entry in the rat pancreatic β-cell: studies with antisense oligonucleotides. Diabetes 1998;47:1873–1880 [DOI] [PubMed] [Google Scholar]
- 36.Pintado AJ, Herrero CJ, Garcia AG, Montiel C: The novel Na(+)/Ca(2+) exchange inhibitor KB-R7943 also blocks native and expressed neuronal nicotinic receptors. Br J Pharmacol 2000;130:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobolevsky AI, Khodorov BI: Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+ exchange inhibitor KB-R7943. Neuropharmacology 1999;38:1235–1242 [DOI] [PubMed] [Google Scholar]
- 38.Watano T, Kimura J, Morita T, Nakanishi H: A novel antagonist, No. 7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br J Pharmacol 1996;119:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]